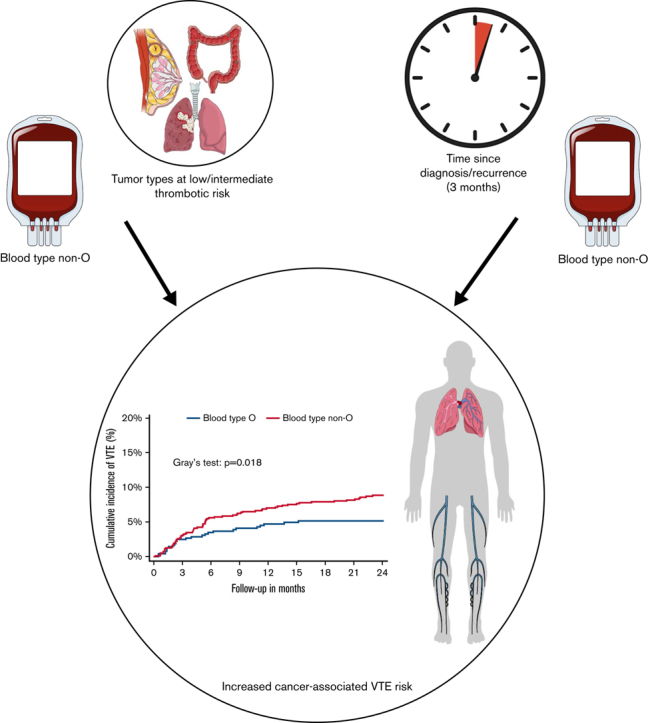
Key Points
-
•
Non-O blood type is a time-dependent predictor of cancer-associated VTE.
-
•
Non-O blood type is associated with an increased VTE risk in patients with intermediate- and low-risk tumor types.
Visual Abstract
Abstract
Venous thromboembolism (VTE) is common in patients with cancer. Although in the general population blood type non-O is associated with increased VTE risk, the impact of ABO blood type on risk of cancer-associated VTE has not been clarified. To determine the influence of ABO blood type on cancer-associated VTE risk, we conducted an analysis within the Vienna Cancer and Thrombosis Study, a prospective cohort study including patients with newly diagnosed or recurrent cancer observed for the primary outcome VTE. Restricted cubic spline analysis was performed and specific time-restricted subdistribution hazard ratios (SHR) were calculated to investigate the association between non-O blood type and VTE over time. One thousand, seven hundred and eight patients were included in the analysis (median follow-up time: 24 months; interquartile range: 10-24), and 151 patients developed VTE (8.8%). During the first 3 months of follow-up, there was no association between non-O blood type and VTE risk (SHR: 1.00; 95% confidence interval [CI]: 0.60-1.67). Thereafter, non-O blood type was associated with a higher VTE risk (SHR: 1.79; 95% CI: 1.12-2.85). Furthermore, non-O blood type was associated with increased VTE risk in patients with intermediate and low thrombotic risk tumor types (SHR: 1.73; 95% CI: 1.09-2.73) but not in very high-risk types (pancreatic, gastroesophageal, and brain cancer; SHR: 0.94; 95% CI: 0.55-1.61). This association was weakened after adjustment for factor VIII. Non-O blood type is a time-dependent predictor of VTE in patients with cancer. It is associated with increased VTE risk beyond 3 months of follow-up and in patients with intermediate- and low-risk tumor types.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer, and its occurrence is associated with poor prognosis.1 According to the latest evidence, patients with a cancer diagnosis are 9 -to 15-times more likely to develop VTE.2,3 However, as patients with cancer concomitantly have an increased risk of bleeding, routine thromboprophylaxis is not recommended in all patients. Current guidelines recommend pharmacological thromboprophylaxis in patients with high VTE risk that are most likely to benefit.4, 5, 6, 7 Therefore, a better understanding of risk profiles and identification of simple risk factors contributing to elevated risks of VTE is desired to aid in clinical decision making.
The ABO blood group type is a well-known and independent risk factor for VTE in the general population. ABO blood group represents one of the most common genetic risk factors, attributing to ∼30% of VTE events.8,9 In the noncancer population, individuals with non-O blood group (A, B, and AB) have an increased risk of thrombosis compared with those with blood group O.8, 9, 10 One proposed mechanism for the enhanced VTE risk is that individuals with blood groups A, B, and AB have higher levels of von Willebrand factor (vWF) and factor VIII (FVIII) than those with blood group O.11, 12, 13, 14 Elevated levels of FVIII again are associated with risk of VTE in patients without cancer and have also been reported to increase risk of VTE in patients with cancer.15,16
Interestingly, previous studies indicate that ABO blood type has an influence on platelet receptor glycosylation leading to an increased platelet adhesion to vWF in individuals with non-O blood group and increased risk of thrombosis development.17,18
The role of ABO blood groups for developing VTE in patients with cancer remains to be elucidated, and the studies available presently are conflicting and limited by their retrospective study design and small sample size. In a cohort of patients with cancer with mixed solid tumor types, increased risk of VTE was found in those with non-O blood group, whereas blood group B was associated with VTE in patients with glioblastoma and blood group A or AB in patients with pancreatic cancer.19, 20, 21, 22 Whether a potentially increased risk of VTE associated with the ABO blood groups is mediated by elevated vWF and FVIII levels is unclear because the presence of cancer itself might trigger elevation of both vWF and FVIII levels.23, 24, 25
Therefore, we aimed at investigating the association between ABO blood group types and risk of VTE occurrence in a prospective cohort study of patients with cancer, which has been designed to identify risk factors for cancer-associated VTE. We hypothesized that blood group non-O is also associated with increased risk of VTE in patients with cancer. Furthermore, we evaluated whether elevated levels of FVIII mediate the risk of VTE associated with ABO blood groups.
Methods
Study cohort
For this analysis, we used the dataset of the Vienna Cancer and Thrombosis study, a single-center prospective observational study that was initiated in 2003 at the Medical University of Vienna. The study has been approved by the University of Vienna’s ethics committee (EC number 126/2003; ethik-kom@meduniwien.ac.at) and was performed according to the Declaration of Helsinki. Detailed information about the study design had been published previously.26,27 In brief, the Vienna Cancer and Thrombosis study included patients >18 years old with histologically confirmed cancer who provided their written informed consent for study participation. Patients were either newly diagnosed with cancer or had disease progression after partial or complete remission. Exclusion criteria included chemotherapy or VTE in the last 3 months, indication for long-term anticoagulation, and overt viral or bacterial infection, radiotherapy, or surgery 2 weeks prior to study inclusion. Patients were followed prospectively for 2 years for the primary outcome of VTE and were contacted via postal mail or phone to gather information about their clinical course, especially regarding occurrence of VTE and anticancer treatment every ∼3 months. If the patient did not respond, her/his family doctor or relatives were contacted.
VTE was confirmed with objective imaging methods such as duplex/compression sonography or venography for deep vein thrombosis and computed tomography or ventilation/perfusion lung scan for pulmonary embolism. An adjudication committee, comprising independent specialists in vascular medicine (angiology), radiology, or nuclear medicine, verified all thrombotic events.
For the current analysis, ABO blood group was available in 1708 patients. Blood groups were evaluated at the General Hospital Vienna of the Medical University of Vienna, Vienna, Austria, in the routine clinical workup of patients. Patients were grouped into the blood types A, B, AB, and O. Patients with blood type A, B, and AB were combined into 1 group and referred to as non-O blood type for outcome analyses. Patients were further divided into risk groups depending on the thrombosis risk profile of their respective tumor type. Pancreatic, gastroesophageal, and brain cancer were considered very high VTE risk tumor types, whereas the others were considered to be intermediate or low risk.28
Laboratory analysis
Blood was drawn at study inclusion and collected in Vacutainer citrate tubes (Vacuette; Greiner-Bio One) by sterile, atraumatic venipuncture.
As previously described, vWF-Antigen (vWF-Ag) in patients’ plasma was measured with a commercially available enzyme-linked immunosorbent assay kit (Technozym).29 A Sysmex CA 7000 analyzer using FVIII-deficient plasma (Technoclone) and activated partial thromboplastin time Actin-FS (Dade Behring) was used to measure FVIII activity in plasma samples.16
Statistical analysis
All statistical analyses were performed with STATA 15.0 (Stata Corp, Houston, TX).
Standard summary statistics were used to report patient baseline characteristics (absolute frequencies, percentages, median, interquartile range [IQR]). All-cause mortality in the follow-up period was treated as a competing event, and therefore, VTE endpoints were studied in a competing risk framework. To compare the cumulative VTE incidence between groups, a proportional subhazard regression model according to Fine and Gray was conducted.
After visual inspection of data, a violation of the proportional subhazards assumption was suspected. Therefore, an exploratory analysis with restricted cubic spline analysis was performed to model the differences in risk estimates over follow-up time. Afterward, time-restricted subdistribution hazard ratios (SHR) specifically for the <3 months and ≥3 months follow-up time periods were calculated. A multivariable analysis was used to adjust for the covariates sex and age, disease stage (stage 4 vs others), and body mass index (BMI) at study inclusion. A second multivariable analysis adjusting for platelet count, soluble P-selectin (sP-selectin), and d-dimer levels was performed.
In a subgroup analysis, the impact of non-O blood type compared with O blood type on VTE risk in patients with very high and intermediate/low prothrombotic risk tumor types was analyzed. This analysis was adjusted for age, disease stage (stage 4 vs others), sex and BMI at study inclusion; and for platelet count, sP-selectin, and d-dimer levels in a second analysis as well.
A Mann-Whitney U test was used to compare vWF-Ag and FVIII activity levels between non-O blood type and O blood type patient groups. To assess if non-O blood type increases VTE risk independently or due to concomitantly elevated levels of FVIII, a multivariable model adjusted for continuous levels of FVIII was conducted.
A P value ≤.05 was considered to denote statistical significance.
Results
Patient characteristics
In total, 1708 patients were included in this analysis. Of those, 794 were female (46%), and the median age was 61 (IQR: 52-68) years. The most common tumor types were lung (19%), breast (16%), and brain (14%) cancer, and 32% of solid tumor patients presented with metastatic disease at study inclusion (Table 1).
Table 1.
Patient characteristics at study inclusion
| Patient characteristics | ||
|---|---|---|
| n (% missing value) | Median (IQR) / n (%) | |
| Age | 1708 (0%) | 61 (52-68) |
| Female | 1708 (0%) | 794 (46%) |
| Tumor type | 1708 (0%) | |
| Brain | — | 234 (14%) |
| Lymphoma | — | 221 (13%) |
| Others | — | 132 (8%) |
| Breast | — | 267 (16%) |
| Lung | — | 321 (19%) |
| Gastroesophageal | — | 53 (3%) |
| Colorectal | — | 159 (9%) |
| Pancreas | — | 117 (7%) |
| Renal | — | 37 (2%) |
| Prostate | — | 125 (7%) |
| Myeloma | — | 42 (2%) |
| Risk groups | 1708 (0%) | |
| Very high risk | — | 327 (19%) |
| Intermediate risk | — | 986 (58%) |
| Low risk | — | 395 (23%) |
| Metastatic disease (solid tumor patients) | 1445 (15%) | 548 (32%) |
| Median follow-up period (month) | 1708 (0%) | 24 (10-24) |
| Blood type (ABO) | 1708 (0%) | |
| O | — | 653 (38%) |
| A | — | 682 (40%) |
| B | — | 262 (15%) |
| AB | — | 111 (7%) |
| Biomarker levels at study inclusion | — | |
| FVIII (% activity) | 1618 (5%) | 186 (143-239) |
| vWF-Ag levels (IU/dL) | 626 (63%) | 177 (119-269) |
| Outcomes within 2-y follow-up | 1708 (0%) | |
| VTE | — | 151 (8.8%) |
| Death | — | 649 (38%) |
Blood type was available in 1708, FVIII activity in 1618, and vWF antigen in 626 patients.
The primary outcome VTE occurred in 151 patients during a median follow-up of 24 months (IQR: 10-24 months). The 3-, 6-, 12-, and 24-month cumulative VTE incidence was 3.6% (95% confidence interval [CI]: 2.7-4.5), 6.2% (95% CI: 5.1-7.5), 7.8% (95% CI: 6.6-9.2), and 9.2% (95% CI: 7.9-10.7), respectively (Table 2). Six hundred and forty-nine (2-year mortality: 38%) patients died during the 2-year observation period.
Table 2.
Cumulative incidence of VTE in patients with blood type O (n = 653) and non-O (n = 1055) divided in intermediate/low-risk and high-risk tumor types at 3-, 6-, 12-, and 24-month follow-up
| Cumulative VTE-incidence (%) [95% confidence interval] |
||||
|---|---|---|---|---|
| 3 mo | 6 mo | 12 mo | 24 mo | |
| Overall | ||||
| Blood type O | 3.8 [2.5-5.5] | 5.7 [4.1-7.7] | 7.0 [5.1-9.1] | 7.6 [5.7-9.9] |
| Blood type non-O | 3.4 [2.4-4.7] | 6.5 [5.1-8.1] | 8.4 [6.8-10.2] | 10.2 [8.4-12-2] |
| Intermediate/ low-risk cancer types | ||||
| Blood type O | 2.4 [1.3-4.0] | 3.4 [2.0-5.2] | 4.6 [3.0-6.7] | 5.0 [3.3-7.1] |
| Blood type non-O | 3.0 [2.0-4.3] | 5.4 [4.0-7.1] | 6.8 [5.2-8.6] | 8.6 [6.8-10.6] |
| High-risk cancer types | ||||
| Blood type O | 9.2 [5.0-14.9] | 14.7 [9.2-21.3] | 16.2 [10.5-23.1] | 17.9 [11.8-24.9] |
| Blood type non-O | 5.2 [2.7-9.0] | 11.1 [7.1-16.0] | 15.5 [10.7-21.1] | 17.3 [12.3-23.1] |
ABO blood types were distributed as follows in our cohort: 653 (38%) were blood type O, 682 (40%) A, 262 (15%) B, and 111 (7%) AB (Table 1), which is as expected in a European population. Biomarker levels of FVIII activity were available in 1618 and of vWF-Ag in 626 patients.
ABO blood group distribution did not vary in patients with different tumor types and tumor risk categories (supplemental Table 1). Further, blood group non-O vs O was not associated with an increased likelihood of having stage 4 disease at study inclusion (supplemental Table 2), and similarly, no association between ABO blood group type and survival was found (supplemental Figure 1).
Non-O vs O blood type and risk of VTE
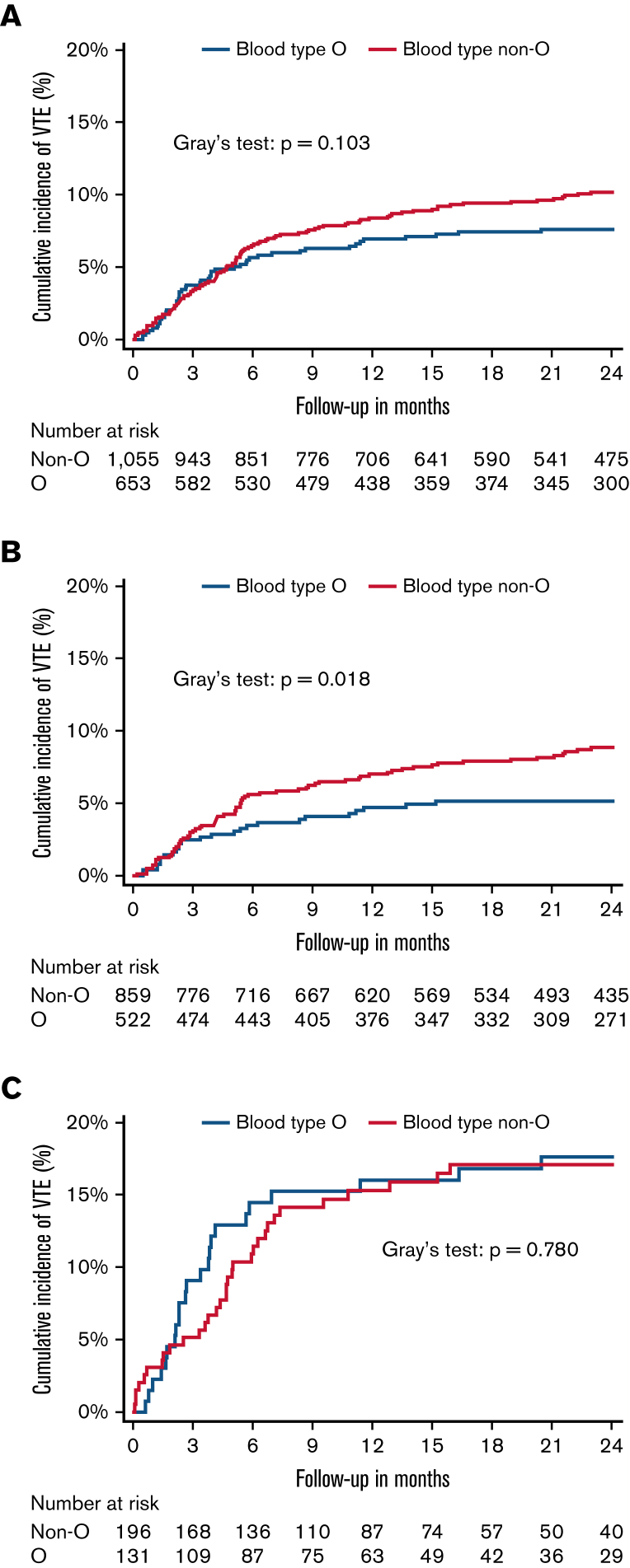
VTE risk was higher in patients with non-O blood type in competing risk regression analysis compared with patients with blood type O; however, this association was not statistically significant (SHR: 1.33; 95% CI: 0.95-1.88, P = .100) (Table 2). This remained nonsignificant after adjustment for age, sex, tumor type risk category, and disease stage (adjusted SHR: 1.36; 95% CI: 0.96-1.91, P = .082) (Figure 1).
Figure 1.
Cumulative incidence of VTE. (A) Cumulative VTE incidence in patients with blood type O (n = 653) compared with patients with blood type non-O (n = 1055). (B) Cumulative VTE incidence in patients with intermediate/low-risk tumor types with blood type O (n = 522) compared with patients with blood type non-O (n = 856). (C) Cumulative VTE incidence in patients with very high-risk tumor types with blood type O (n = 131) compared with patients with blood type non-O (n = 196). Cumulative incidence functions were obtained within a competing risk framework considering all-cause mortality as a competing event.
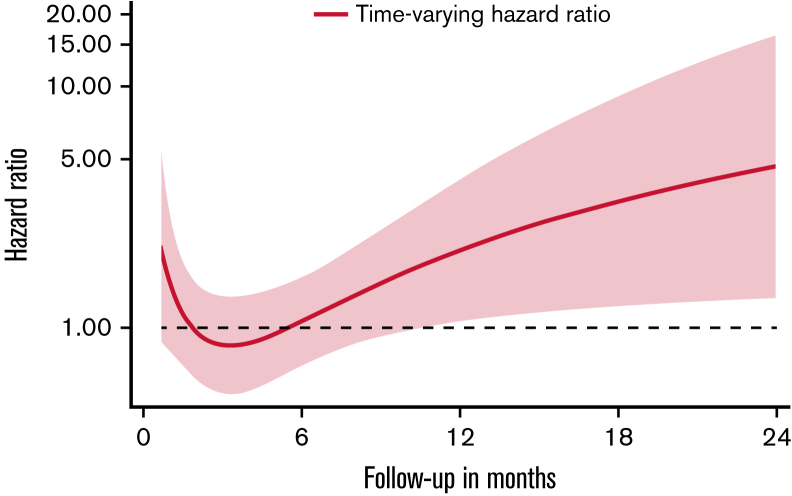
However, a violation of the proportional subhazard assumption was noted and assessed using a restricted cubic spline analysis to observe the change in hazard ratio for VTE of patients with blood type non-O compared with O over time. In this model, a time-varying effect of non-O blood type toward an increased VTE risk was observed (Figure 2). Therefore, time-restricted SHRs were obtained for <3 months (first 3 months) and ≥3 months (3-24 months) follow-up period. Patients with blood type non-O and O had a similar risk of VTE during the first 3 months of follow-up (SHR: 1.00; 95% CI: 0.60-1.67, P = .992). Beyond the initial 3 months (ie, during follow-up between month 3 and 24 after study inclusion), blood type non-O was significantly associated with increased VTE risk in patients with cancer (SHR: 1.79; 95% CI: 1.12-2.85, P = .015). This association prevailed upon multivariable adjustment for age, sex, disease stage (stage 4 vs others), and BMI (adjusted SHR: 1.77; 95% CI: 1.11-2.82, P = .017). In a second multivariable analysis, the association of non-O blood type and increased VTE risk beyond 3 months of follow-up was independent after adjustment for platelet count, sP-selectin levels, and D-dimer (adjusted SHR: 1.75; 95% CI: 1.07-2.87, P = .026; supplemental Table 4). In a subgroup analysis, in patients with very high VTE risk tumor types (pancreatic, gastroesophageal, glioblastoma), there was no difference in VTE risk between blood type non-O and O groups (SHR: 0.94; 95% CI: 0.55-1.61, P = .824). In contrast, in patients with tumors, considered to be at intermediate or low VTE risk, non-O blood type was associated with an increased risk for VTE compared with O blood type patients (SHR: 1.73; 95% CI: 1.09-2.73, P = .019). Again, this association remained significant upon multivariable adjustment for age, sex, stage, and BMI (adjusted SHR: 1.74; 95% CI: 1.09-2.74, P = .020) and upon adjustment for platelet counts, sP-selectin, and D-dimer (adjusted SHR: 1.68; 95% CI: 1.04-2.71, P = .034; supplemental Table 5). Furthermore, cumulative VTE incidence varied in patients with intermediate- and low-risk tumor types in patients with blood group O vs non-O (Figure 1B; Table 2) but not in patients with very high-risk tumor types (Figure 1C; Table 2).
Figure 2.
Time-varying hazard ratio for VTE in patients with blood type non-O compared with O over the 24-month follow-up period. Hazard ratios were obtained in a restricted cubic spline analysis. Dotted lines indicate 95% confidence interval.
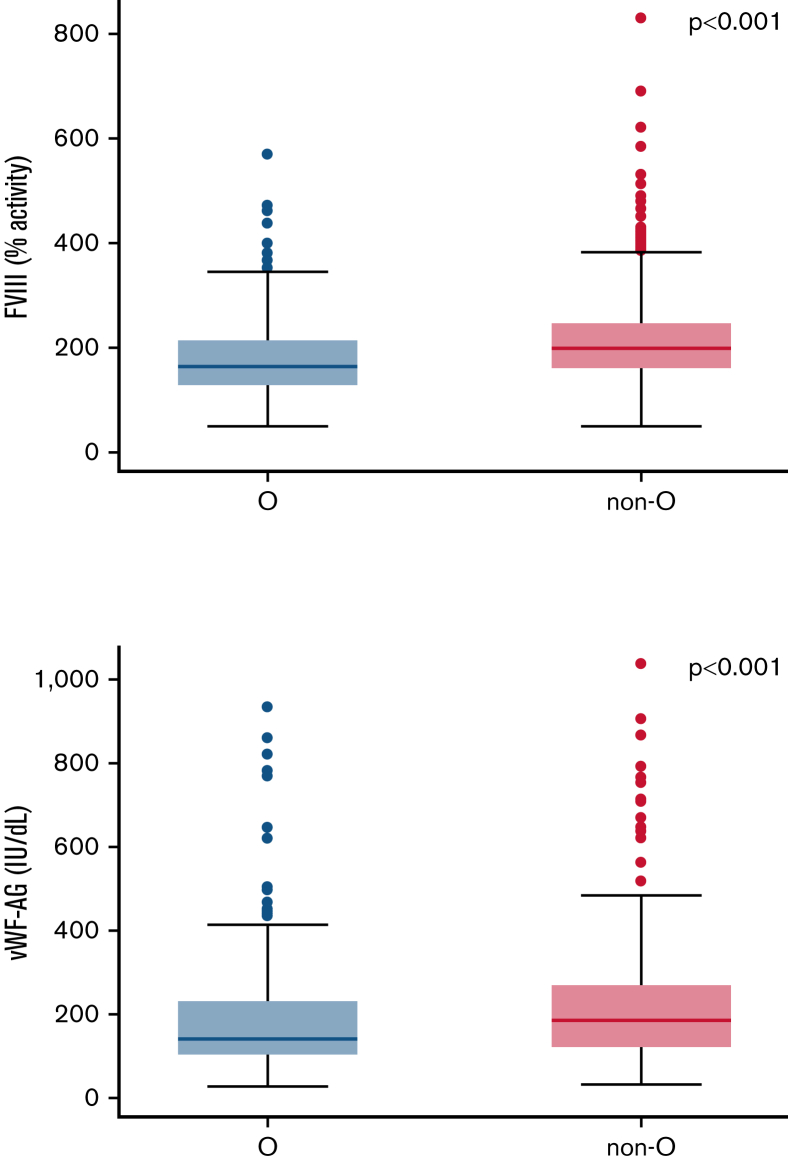
ABO blood types, vWF, and FVIII levels
Levels of vWF-Ag as well as FVIII activity in patients with cancer with blood type non-O were compared with blood type O patients. Patients with blood type non-O had higher levels of vWF-Ag (median: 198 [IQR: 158-249] vs 165 [IQR: 128-216], P < .001) and FVIII activity (median: 194 [IQR: 127-282] vs 148 [IQR: 106-244], P < .001) (Figure 3). After adjustment for continuous levels of FVIII in a multivariable model, the association of non-O blood type with VTE risk was weakened in strength and magnitude for the ≥3-month follow-up period (adjusted SHR: 1.47; 95% CI: 0.92-2.36, P = .109) and in the subgroup of patients with low- to intermediate-risk tumor types (adjusted SHR: 1.59; 95% CI: 0.99-2.55, P = .057).
Figure 3.
Differences in levels of FVII activity and vWF-Ag. (A) Levels of FVII activity (n = 1618) and (B) vWF-Ag (n = 626) in patients with blood type non-O compared with blood type O. Mann-Whitney U test was used to compare means.
Discussion
In this prospective observational cohort study, we have shown that ABO blood type is associated with risk of VTE in patients with cancer in a time-dependent manner. Non-O blood type was associated with an increased risk of VTE beyond 3 months of observation but not during the first 3 months. Further, non-O blood type was a predictor of occurrence of VTE in patients with intermediate and low thrombotic risk tumor types and not in tumor types at very high risk of VTE, such as pancreatic, gastroesophageal, and brain tumors.
These results indicate that cancer may be the main driver of the increased risk of VTE shortly after diagnosis or progression, depicting a time when the cancer is very active and patients are receiving anticancer therapies, putting them at high risk for VTE. This time period is also the one with the highest incidence of VTE in patients with cancer.30,31 Therefore, it seems logical that after this tumor- and treatment-driven risk period, patients return to a lower risk level. Thus, at this time point, known risk factors for VTE in the general (non-cancer) population also begin to have an influence on VTE risk in patients with cancer. Supporting this speculation is the fact that this association was present in patients with tumor types at intermediate and low VTE risk but not in tumor types at very high VTE risk (ie, pancreatic, gastroesophageal, and brain cancer). Interestingly, beyond 3 months of follow-up, the magnitude of VTE risk associated with non-O blood type in our cohort of patients with cancer was comparable to that previously observed in the general (non-cancer) population.8,10
Considering ABO blood group type for VTE risk assessment in patients with cancer seems to be promising for the following reasons: First of all, ABO blood group is often assessed in oncologic patients due to their high risk of developing anemia during their disease and potential need of transfusions. Otherwise, ABO typing is easily accessible worldwide. Further, ABO blood type is a constant factor, in contrast to other laboratory markers such as blood count values or d-dimer that only reflect a specific time point and are susceptible to external influences.32 Similar to ABO blood group type, tumor type is also a very quickly and easily assessable risk factor and therefore used in the Khorana score to promptly detect patients at high risk for VTE.33,34 Our findings suggest that considering ABO blood group might be especially promising when evaluating VTE risk in patients with intermediate- to low-risk tumor types and when assessing VTE risk after the initial high-risk period (first 3 months after diagnosis or progression of cancer).
In our present study, we could confirm also in patients with cancer that non-O blood type is associated with higher levels of FVIII activity and vWF-Ag. It is known that vWF and FVIII levels are significantly influenced by ABO blood type group (ie, vWF and FVIII levels are higher in patients with non-O blood type), and ∼30% of the variation in FVIII levels might be explained by ABO blood group type.9,11,13 It is thought that FVIII levels are higher in people with non-O blood type, mainly through increased vWF levels. This is most likely due to decreased clearance of vWF, and also because non-O blood group vWF is less rapidly cleaved by ADAMTS13.11 FVIII levels were also found to be greater in patients with cancer in general and with regard to non-O blood type.14,16,23, 24, 25,29,35 Importantly, elevated levels of FVIII and vWF are associated with an increased risk of VTE in patients with cancer.16,29 Thus, this might be the mechanism responsible for the observed increased risk in patients with non-O blood type, and we therefore investigated the association between blood type and VTE risk in a multivariable model, adjusted for continuous levels of FVIII. We found that the association of non-O blood type with VTE risk beyond 3 months follow-up and in cancer types at intermediate or low VTE risk was weakened and borderline statistically significant after adjustment of FVIII levels at study baseline. Thus, our findings support the speculation that non-O blood type might primarily increase VTE risk through elevated levels of FVIII. However, we had only a single point measurement of FVIII levels at study inclusion, and levels may change during the cancer journey.36 We also cannot exclude an increase of FVIII levels mediated by various other factors, such as chemotherapy or infections.
In addition, based on previous data on potential differences in platelet function according to ABO blood types, we evaluated the observed association between ABO and VTE risk beyond underlying levels of platelet counts and sP-selectin. The observed effect of non-O blood types and increased VTE risk in low/intermediate-risk cancer types and beyond 3 months of follow-up in the full study cohort was independent of platelet counts and levels of sP-selectin.
There are some limitations to our analysis that we would like to address. First of all, due to the design of our cohort study with no systematic screening for VTE, we cannot fully exclude that asymptomatic VTE may have been present in some patients. However, we believe that our systemic evaluation of patient records was complete, and thus, the likelihood of having missed such events is low. We believe that our cohort is representative of the Austrian population; however, we did not assess ethnicity, which could be of interest as ABO blood group distributions and frequencies of some cancers vary between patients with different genetic ethnical backgrounds. In addition, we did not have data regarding VTE risk factors such as chemotherapy, infections, or surgery in the follow-up period. Moreover, vWF-Ag and FVIII levels were not available in all patients. We could not implement vWF-Ag levels in the multivariable model to analyze whether the association of non-O blood types with VTE risk beyond 3 months and in cancer types at intermediate or low VTE risk was influenced by vWF. Unfortunately, we had vWF levels available in only a few patients in our cohort, and no samples were left to measure vWF levels. Therefore, the question of whether vWF mediates the risk of VTE associated with ABO blood groups in patients with cancer needs to be clarified in further studies. Furthermore, not all patients were therapy naïve at study inclusion, and interventions such as surgery and chemo- and radiotherapy are known to act as triggers for VTE as well.37, 38, 39 However, we excluded patients that underwent such interventions shortly before study inclusion to limit their influence. Lastly, as our analysis was exploratory in nature and evolved during the data analysis process, our findings have to be confirmed and validated in independent studies.
To conclude, beyond the first 3 months of follow-up, an increased risk of cancer-associated VTE in patients with non-O blood type was observed, comparably in magnitude of risk for VTE in the general non-cancer population. This association was also noted in patients with intermediate- and low-risk tumor types, suggesting that non-O blood type acts as a putative predictor of VTE in patients with cancer at comparatively low risk of thrombosis. ABO blood type group is an easily accessible VTE predictor that can help in the clinical practice during risk assessment in patients with cancer.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
Authorship
Contribution: C.E. and F.M. contributed to data acquisition, data analysis, data interpretation, and manuscript writing; S.N. contributed to data analysis and manuscript writing; M.R. contributed to data acquisition, data interpretation, and manuscript writing; I.P. contributed to study design, data acquisition, data interpretation, and manuscript writing; and C.A. contributed to study design, data acquisition, data interpretation, data analysis, and manuscript writing.
Footnotes
For original data, please contact the corresponding author, Cihan Ay (cihan.ay@meduniwien.ac.at).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 2.Grilz E, Posch F, Nopp S, et al. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer-a nationwide analysis. Eur Heart J. 2021;42(23):2299–2307. doi: 10.1093/eurheartj/ehab171. [DOI] [PubMed] [Google Scholar]
- 3.Mulder FI, Horváth-Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137(14):1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927–974. doi: 10.1182/bloodadvances.2020003442. [published correction appears in Blood Adv. 2021;5(7):1953] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 6.Farge D, Frere C, Connors JM, et al. International Initiative on Thrombosis and Cancer (ITAC) advisory panel 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566–e581. doi: 10.1016/S1470-2045(19)30336-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17(10):1772–1778. doi: 10.1111/jth.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasan SK, Rostgaard K, Majeed A, et al. ABO Blood Group and risk of thromboembolic and arterial disease: a study of 1.5 million blood donors. Circulation. 2016;133(15):1449–1457. doi: 10.1161/CIRCULATIONAHA.115.017563. [DOI] [PubMed] [Google Scholar]
- 9.Ohira T, Cushman M, Tsai MY, et al. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) J Thromb Haemost. 2007;5(7):1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 10.Dentali F, Sironi AP, Ageno W, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38(5):535–548. doi: 10.1055/s-0032-1315758. [DOI] [PubMed] [Google Scholar]
- 11.Ward SE, O’Sullivan JM, O’Donnell JS. The relationship between ABO blood group, von Willebrand factor, and primary hemostasis. Blood. 2020;136(25):2864–2874. doi: 10.1182/blood.2020005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schleef M, Strobel E, Dick A, Frank J, Schramm W, Spannagl M. Relationship between ABO and Secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br J Haematol. 2005;128(1):100–107. doi: 10.1111/j.1365-2141.2004.05249.x. [DOI] [PubMed] [Google Scholar]
- 13.Orstavik KH, Magnus P, Reisner H, Berg K, Graham JB, Nance W. Factor VIII and factor IX in a twin population. Evidence for a major effect of ABO locus on factor VIII level. Am J Hum Genet. 1985;37(1):89–101. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Chen X, Yang J, Guo R. Association of ABO blood groups with von Willebrand factor, factor VIII and ADAMTS-13 in patients with lung cancer. Oncol Lett. 2017;14(3):3787–3794. doi: 10.3892/ol.2017.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koster T, Rosendaal FR, Reitsma PH, van der Velden PA, Briët E, Vandenbroucke JP. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA polymorphisms-the Leiden Thrombophilia Study (LETS) Thromb Haemost. 1994;71(6):719–722. [PubMed] [Google Scholar]
- 16.Vormittag R, Simanek R, Ay C, et al. High factor VIII levels independently predict venous thromboembolism in cancer patients: the cancer and thrombosis study. Arterioscler Thromb Vasc Biol. 2009;29(12):2176–2181. doi: 10.1161/ATVBAHA.109.190827. [DOI] [PubMed] [Google Scholar]
- 17.Dunne E, Qi QM, Shaqfeh ES, et al. Blood group alters platelet binding kinetics to von Willebrand factor and consequently platelet function. Blood. 2019;133(12):1371–1377. doi: 10.1182/blood-2018-06-855528. [DOI] [PubMed] [Google Scholar]
- 18.Zhong M, Zhang H, Reilly JP, et al. ABO Blood Group as a model for platelet glycan modification in arterial thrombosis. Arterioscler Thromb Vasc Biol. 2015;35(7):1570–1578. doi: 10.1161/ATVBAHA.115.305337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100(8):1717–1723. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 20.Turan D, Yasar HA, Aksu OB, et al. Risk factors for thrombosis risk in patients with cancer. Journal of Oncological Sciences. 2018;4(3):130–133. [Google Scholar]
- 21.Heenkenda MK, Malmström A, Lysiak M, et al. Assessment of genetic and non-genetic risk factors for venous thromboembolism in glioblastoma - the predictive significance of B blood group. Thromb Res. 2019;183:136–142. doi: 10.1016/j.thromres.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Pise MN, Overman MJ, et al. ABO non-O type as a risk factor for thrombosis in patients with pancreatic cancer. Cancer Med. 2015;4(11):1651–1658. doi: 10.1002/cam4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yigit E, Gönüllü G, Yücel I, Turgut M, Erdem D, Cakar B. Relation between hemostatic parameters and prognostic/predictive factors in breast cancer. Eur J Intern Med. 2008;19(8):602–607. doi: 10.1016/j.ejim.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Battistelli S, Stefanoni M, Lorenzi B, et al. Coagulation factor levels in non-metastatic colorectal cancer patients. Int J Biol Markers. 2008;23(1):36–41. [PubMed] [Google Scholar]
- 25.Mannucci PM, Karimi M, Mosalaei A, Canciani MT, Peyvandi F. Patients with localized and disseminated tumors have reduced but measurable levels of ADAMTS-13 (von Willebrand factor cleaving protease) Haematologica. 2003;88(4):454–458. [PubMed] [Google Scholar]
- 26.Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Blood. 2008;112(7):2703–2708. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 27.Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27(25):4124–4129. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 28.Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5(7):e289–e298. doi: 10.1016/S2352-3026(18)30063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermeier HL, Riedl J, Ay C, et al. The role of ADAMTS-13 and von Willebrand factor in cancer patients: results from the Vienna Cancer and Thrombosis Study. Res Pract Thromb Haemost. 2019;3(3):503–514. doi: 10.1002/rth2.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blix K, Gran OV, Severinsen MT, et al. Impact of time since diagnosis and mortality rate on cancer-associated venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) cohort. J Thromb Haemost. 2018;16(7):1327–1335. doi: 10.1111/jth.14130. [DOI] [PubMed] [Google Scholar]
- 31.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Posch F, Riedl J, Reitter EM, et al. Dynamic assessment of venous thromboembolism risk in patients with cancer by longitudinal D-dimer analysis: A prospective study. J Thromb Haemost. 2020;18(6):1348–1356. doi: 10.1111/jth.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 35.Setiawan B, Permatadewi CO, de Samakto B, et al. Von Willebrand factor:antigen and ADAMTS-13 level, but not soluble P-selectin, are risk factors for the first asymptomatic deep vein thrombosis in cancer patients undergoing chemotherapy. Thromb J. 2020;18(1):33. doi: 10.1186/s12959-020-00247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reitter EM, Kaider A, Ay C, et al. Longitudinal analysis of hemostasis biomarkers in cancer patients during antitumor treatment. J Thromb Haemost. 2016;14(2):294–305. doi: 10.1111/jth.13218. [DOI] [PubMed] [Google Scholar]
- 37.Agnelli G, Becattini C, Meyer G, et al. Caravaggio Investigators Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 38.Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947–953. doi: 10.1038/sj.bjc.6605883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219–230. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.