Abstract
Background:
Adipose-derived mesenchymal stem cells (ADMSCs) have recently been studied for the treatment of knee osteoarthritis. The goal is pain reduction and improvement of joint function leading to superior health-related quality of life.
Objectives:
The aim of this study was to provide a comprehensive meta-analysis assessing the evidence on the use of ADMSCs in knee osteoarthritis.
Design:
This is a Meta-analysis of randomised controlled trials.
Data Sources and Methods:
PubMed/MEDLINE, Embase, and Cochrane Databases were searched for randomized controlled trials using ADMSCs to treat patients with knee osteoarthritis. Only trials comparing ADMSCs to placebo or conservative treatment were included. The outcomes studied were improvement in functional, pain, and quality of life scores along with radiographic findings.
Results:
A total of four trials were included, representing 138 patients with knee osteoarthritis. WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) scores favored ADMSCs with a statistically and clinically significant difference over controls at 6- and 12-month follow-ups (p value < 0.0001). Pain, functional, and quality of life scores also favored ADMSCs at 12-month follow-up (p value < 0.0001).
Conclusion:
ADMSCs are effective in treating knee osteoarthritis symptoms as observed by functional and pain improvements. Furthermore, ADMSCs injection showed improvement of cartilage integrity, which indicates the potential for regenerating the knee cartilage. Future trials with larger number of patients and longer follow-up periods would help to elaborate further the therapeutic potential of ADMSCs.
Plain Language Summary
Adipose-derived mesenchymal stem cells use in knee osteoarthritis
Knee osteoarthritis is an extremely common disease that causes damage of the lining of the knee joint.
This will lead to pain and limited range of motion of the knee hence limited functionality.
Multiple treatments are used currently for knee osteoarthritis which all aim at slowing down the progression and limiting the need for knee replacement surgery.
Adipose-derived mesenchymal stem cells (ADMSCs) are stem cells harvested from the fat around the belly. These stem cells have the potential to be converted into cells of a certain origin (cartilage, muscle, fat).
Many studies are being performed to see whether these cells can transform to cartilage and repair the damaged knee joint.
In this study, we tried to find how the results of different studies comparing the usual treatments for knee osteoarthritis with that of ADMSCs compared.
We were mostly interested in the pain, functional, stiffness, and quality of life scores.
We also reviewed the MRI findings to find out whether the lining of the knee joint improved.
Four studies were included with 138 patients having knee osteoarthritis.
WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) score which is a self-administered questionnaire evaluating hip and knee osteoarthritis, showed better results in patients receiving ADMSC injections compared with other usual treatments at 12-month follow-up.
Pain, functional, stiffness, and quality of life scores also showed better results in ADMSCs at 12-month follow-up.
MRI images also showed better cartilage lining in the patients treated with ADMSCs.
We concluded that ADMSCs are both effective and safe to be used in treating knee osteoarthritis symptoms. However, studies with longer follow-up periods are needed to better assess the regenerative potential of ADMSCs.
Keywords: adipose-derived mesenchymal stem cells, cartilage, knee osteoarthritis, WOMAC score
Introduction
Osteoarthritis (OA) is characterized by chronic low-grade inflammation, involving the innate immune system.1 It affects around 250 million people worldwide with greater than 27 million in the United States alone.2,3 It involves the activation of multiple inflammatory markers within the synovium and the surrounding tissues. This induces matrix metalloproteinases, which cause cartilage breakdown secondary to proteoglycan and collagen destruction.4 The pain and stiffness associated with OA adversely impact patients’ quality of life (QoL) and activities of daily living.5 The knee is one of the most common joints affected by OA. The treatment goal in knee OA is pain reduction, improvement of joint function, and optimization of knee range of motion, thus leading to superior health-related QoL. The ultimate treatment of knee OA would be regeneration of the degraded cartilage.
Management of knee OA symptoms starts with patient education and structured exercises programs.6 This is followed by the use of topical non-steroidal anti-inflammatory (NSAID) drugs. A recent Meta-analysis of randomized controlled trials (RCTs) has shown the effectiveness of topical NSAIDs in relieving pain due to OA.7 For cases that do not improve on these modalities, other non-surgical options include intermittent oral NSAIDs or intra-articular steroid or hyaluronic acid injections.6
In the last three decades, mesenchymal stem cells (MSC) have emerged as a potential regenerative treatment for multiple diseases including OA.8 MSCs can be derived from multiple sites like bone marrow, umbilical cord, placenta, and many others. Nevertheless, the aforementioned locations are either not abundantly available or are associated with donor site complications and pain.9 To overcome these hurdles, adipose tissue transpires as a prospective source of MSCs because of its safety, availability, and accessibility.10
Several RCTs have investigated the role of ADMSCs in knee OA. To the best of our knowledge, no meta-analysis of RCT has yet been published. This meta-analysis aims at analyzing the RCTs available in the literature to draw a conclusion on the role of ADMSCs in knee OA, focusing on pain and radiological outcomes.
Methods
This meta-analysis was performed following the relevant requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.11 The following review was not registered on prospectively registered systematic reviews (PROSPERO). No protocol was prepared for study identification or data extraction.
A total of 1083 articles related to MSC were identified. After applying all exclusion criteria, only four RCTs were identified (Freitag et al., Lee et al., Lianging et al., and Hong et al.) which evaluated the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, WOMAC pain score, WOMAC stiffness score, Visual Analogue Scale (VAS), Numeric Pain Rating Scale (NPRS), cartilage integrity, Knee Injury and Osteoarthritis Outcome Score (KOOS) pain, symptoms, activities of daily living (ADL), sports, and QoL. Cartilage integrity and volume were assessed too. Each score was evaluated at 6-month and 12-month follow-up.
The results demonstrate the mean differences between pre-intervention and post-intervention scores for both the ADMSC injection and the control groups.
All the results were tabulated on an excel sheet that can be accessed on request.
Search strategy
A literature search was done using Cochrane Library, PubMed/MEDLINE, and Embase databases from inception till the second week of November 2021. The study design was limited to RCTs on all search engines with no restrictions on language or publication status. The search strategy used the following medical subject headings (MeSH) terms: [(‘Adipose-derived stem cell’ OR ‘Adipose-derived mesenchymal stem cell’ OR Lipoaspirate) AND (osteoarthritis OR ‘degenerative joint disease’ OR ‘degenerative arthritis’)].
Supplemental data were identified through a random search on Google and Google scholar. For further information about ongoing / current trials, the ClinicalTrials.gov Registry Platform was searched using the following three MeSH terms: ‘Adipose-derived stem cell’, ‘Adipose-derived mesenchymal stem cell’, and ‘osteoarthritis’.
Inclusion and exclusion criteria
Inclusion criteria included:
Studies done on human subjects.
Randomized controlled trials.
Studies on ADMSCs as treatment for knee OA with or without any surgical intervention, like arthroscopic debridement etc.
Studies reporting data of efficacy outcomes (radiological, clinical, and others), postoperative complications, and safety.
Exclusion criteria included:
Reviews or isolated case reports.
Studies of cartilage injury, lesions, and fractures.
Studies not including ADMSCs.
Study selection and data extraction
Two investigators (MI & AN) independently screened the retrieved database starting with the title and later the abstract. Full-text articles were reviewed according to the inclusion and exclusion criteria. Any disagreements were solved via discussion with the senior author (BS). A single author (NB) extracted and summarized data in a uniform excel sheet format (Table 1). To allow for comparability, several adjustments across all studies were taken. WOMAC total was multiplied by 1.042 in the studies by Lee et al. and Lu et al. to allow for comparison with WOMAC total in study by Freitag et al. VAS and NPRS scores were multiplied by 10 across all studies. WOMAC functional was multiplied by 1.47, WOMAC pain by 5, and WOMAC stiffness by 12.5. KOOS scores in the study by Freitag et al. were all reported as an inverse percentage to be more easily compared with the WOMAC and SF-36 scores where 100 indicates worst score.
Table 1.
Basic information of the studies included in the meta-analysis.
| Study | Country | Design | Sample Size | OA Grade using KL grade | Age (years) | Control group | Source (donor) | Intervention group | Follow-up | Results | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Arms | ||||||||||
| Freitag et al.12 | Australia | RCT | 30 | Knee OA Grade 2–3 | 53.6 (7.5) | Conventional Conservative treatment (simple analgesia, weight management and exercise) (n = 10) | Abdominal wall | 20 | Arm 1: single injection (100 × 106 ADMSCs) (n = 10) Arm 2: two injections (100 × 106 ADMSCs (n = 10) |
12 months | Both treatment groups had limited progression of the disease with no associated adverse events |
| Hong et al.13 | China | RCT (self-controlled) | 32 | Knee OA grade 2–3 | 52 (8.5) | HA (n = 16) | Abdominal wall | 16 | AD-MSCs | 12 months | ADMSCs is both safe and effective. pain relief, improved function, and repair of cartilage defects in patients with knee osteoarthritis |
| Lu et al.14 | China | RCT | 52 | Knee OA grade 1–3 | 57.33 (2.3) | 25 mg/2.5 ml with 1 % HA (n = 26: 13 left knees and 13 right knees) | Abdominal wall | 26 | 5 × 107 AD-MSCs | 12 months | Both the left and right knees articular cartilage volume increased significantly, along with pain alleviation and knee functioning |
| Lee et al.15 | South Korea | RCT | 24 | Knee OA grade 2–4 | 62.7 (00.5) | Normal saline 3 ml (NaCl 9 mg/ml) (n = 12) | Abdominal wall | 12 | 1 × 106 ADMSCs in 3 ml | 6 months | After 6 months, knee OA patients had pain reduction and improved function without any associated adverse events. |
ADMSC, adipose-derived mesenchymal stem cell; AD-MSCs, autologous adipose-derived mesenchymal stem cells; HA, hyaluronic acid; KL grade: Kellgren and Lawrence grading scale of severity of knee OA; MSC, mesenchymal stem cells; OA, osteoarthritis; RCT, randomized controlled trials.
All studies have follow-up time of 6 and 12 months except Lee et al.15 which has only 6-month follow-up.
Quality assessment
Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) was used independently by two of the authors to assess the risk of bias of the included studies.16 Rob 2 is structured into six domains of bias, focusing on different aspects of trial design, conduct, and reporting which are accompanied by signaling questions. The six domains are bias arising from the randomization process, bias due to deviations from intended interventions (effect of intervention assignment), bias due to deviations from intended interventions (effect of adhering to intervention), bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Each item was considered as ‘high risk’, ‘low risk’, or ‘some concern’.
Statistical analysis
Review Manager (RevMan 5.3) was used for statistical analysis. Two models of heterogeneity testing for meta-analyses were applied using the I2 and chi-square tests. The random-effect model was applied when the heterogeneity test was found to be significant with I2 greater than 50% and p value < 0.1, while the fixed-effect model was applied in the remaining instances.
Excluding assessment of heterogeneity, p values < 0.05 were considered statistically significant. Each variable included in the study was presented as an odds ratio (OR) with their 95% confidence interval, then plotted on one graph in addition to the summarized finding.
Results
Literature review results
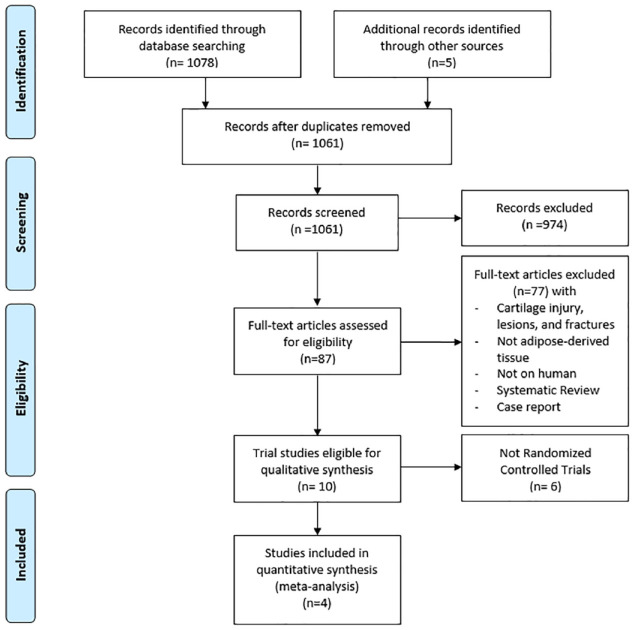
The initial search identified 1083 articles, 22 were excluded as duplicates. After screening the remaining 1061 articles for title and abstract, 974 were excluded. Of the 87 full-text articles assessed for eligibility, 77 were excluded. Of the 10 articles identified, 6 were excluded as they were not RCTs. The remaining 4 were included in the quantitative synthesis as only randomized clinical trials were to be included.12–15 The details of each article are displayed in Table 1. The search process followed the PRISMA flow chart (Figure 1).
Figure 1.
The PRISMA flow diagram showing the study selection process.
Quality of the studies
The risk of bias for the four included studies was evaluated using the ROB 2 tool (Figure 2). All 4 included articles were RCTs. Hong et al., Lu et al., and Lee et al. demonstrated a low risk of bias in all elements. However, Freitag et al. showed a low risk of bias in almost all elements except for bias arising from the randomization process and in the measurement of the outcome, since participants were not blinded to their treatment allocation. Therefore, the randomization process was not optimal.
Figure 2.

The risk of bias 2 (ROB2) assessments for randomized controlled trials of the included studies.
Characteristics of the studies
The characteristics of the included studies are presented in Table 1. All studies included in the meta-analysis were RCTs with only one study (Freitag et al.) lacking blinding. The meta-analysis included 138 knees across the studies, divided into two groups, ADMSCs 74 (53.6%) and control 64 (45.4%). The study by Freitag et al. included two arms in the ADMSCs group, receiving either one injection at baseline or two injections (one at baseline and another injection at 6 months). The degree of OA was classified according to Kellgren Lawrence criteria.17 The pain scores used in this meta-analysis were the WOMAC pain score, The VAS, and the NPRS. Patients were also assessed using MRI to confirm cartilage regeneration. A common approach of harvesting was performed using manual liposuction of abdominal subcutaneous tissue. In the studies included in this meta-analysis, MRI cartilage integrity was analyzed by calculating the difference in the size of the cartilage defect before and after ADMSC injection or the volume of cartilage thickness pre and post injection. Both methods of cartilage integrity evaluation were considered comparable and were regrouped under one forest plot: MRI cartilage integrity.
Outcomes of the studies
Western Ontario and McMaster Universities Osteoarthritis Index
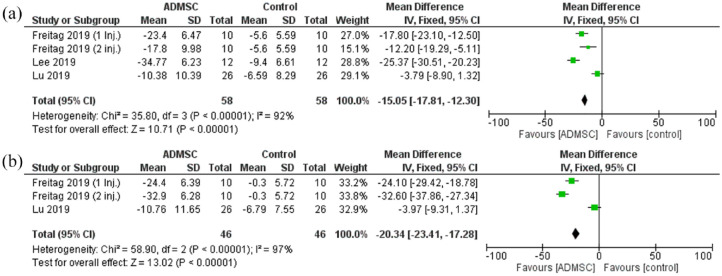
The WOMAC total score showed a statistically significant reduction in the ADMSC group as compared with the control after 6 months [Mean difference = −15.05, 95% CI (−17.81 to −12.30); p < 0.00001; Figure 3]. Pooled studies were heterogeneous (< 0.00001; I2 = 92%). Same statistically significant reduction was seen after 12 months as well [Mean difference = −20.34, 95% CI (−23.41 to −17.28); p < 0.00001; Figure 3]. Pooled studies were heterogeneous (p < 0.00001; I2 = 97%).
Figure 3.
The forest plot for the total WOMAC score at (a) 6- and (b) 12-month follow-up post-treatment.
The pain scores
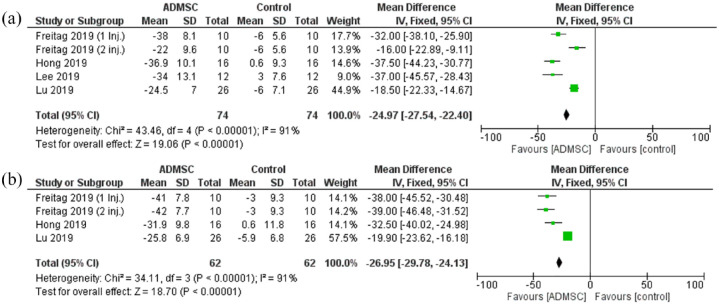
The pain scores assessed were the NPRS and the VAS score. The pain scores showed a statistically significant reduction in the ADMSC group as compared with the control after 6 months [Mean difference = −24.97, 95% CI (−27.54 to −22.40); p < 0.00001; Figure 4]. The analyzed studies were heterogeneous (p < 0.00001; I2 = 91%). A similar statistically significant reduction was seen after 12 months as well [Mean difference = −26.95, 95% CI (−29.78 to −24.13); P < 0.00001; Figure 4]. Again, the pooled studies were heterogeneous (p < 0.00001; I2 = 91%).
Figure 4.
The forest plot for the Pain scores at (a) 6- and (b) 12-month follow-up post-treatment.
The stiffness scores
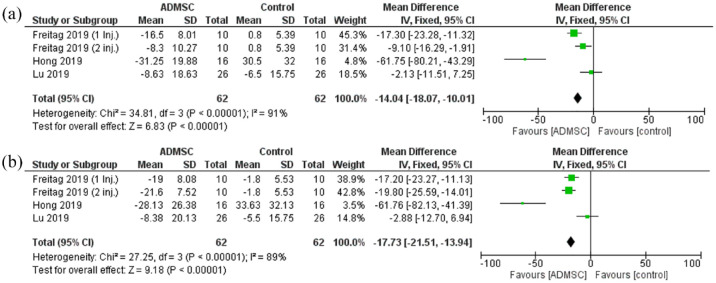
The Stiffness scores analyzed were the WOMAC stiffness scale and the KOOS symptoms score. The stiffness scores showed a statistically significant reduction in the ADMSC group as compared with the control after 6 months (Mean difference = −14.04, 95% CI [−18.07 to −10.01]; p < 0.00001; Figure 5). The pooled studies were heterogeneous (p < 0.00001; I2 = 91%). At 12 months, the stiffness scores showed a similar reduction favoring ADMSC group (Mean difference = −17.73, 95% CI [−21.51 to −13.94]; p < 0.00001; Figure 5). The pooled studies were heterogeneous as well (p < 0.00001; I2 = 89%).
Figure 5.
The forest plot for the Stiffness scores at (a) 6- and (b) 12-month follow-up post-treatment.
The functional scores
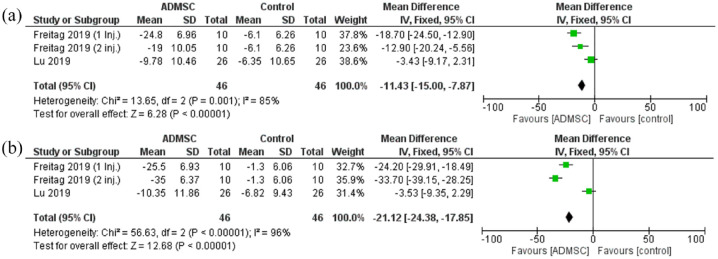
The functional scores considered were the WOMAC functional scale and the KOOS ADL scores. The functional scores showed a statistically significant reduction in the ADMSC group as compared with the control after 6 months [Mean difference = −11.43, 95% CI (−15.00 to −7.87); p < 0.00001; Figure 6]. The pooled studies were heterogeneous (p = 0.001; I2 = 85%). A similar statistically significant reduction was seen after 12 months as well [Mean difference = −21.12, 95% CI (−24.38 to −17.85); P < 0.00001; Figure 6]. The pooled studies were heterogeneous (p < 0.00001; I2 = 96%).
Figure 6.
The forest plot for the Functional scores at (a) 6- and (b) 12-month follow-up post-treatment.
The quality-of-life scores
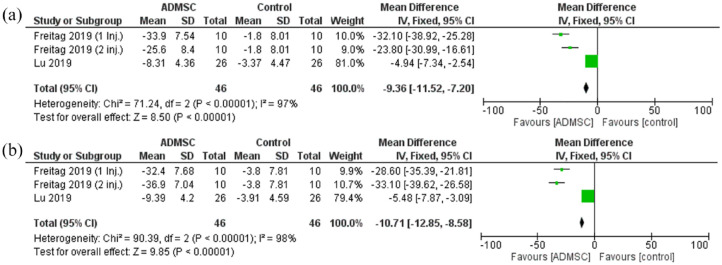
The quality-of-life scores used were the KOOS QoL score and the SF-36 scale. The quality-of-life scores exhibited a statistically significant reduction in the ADMSC group as compared with the control after 6 months [Mean difference = −9.36, 95% CI (−11.52 to −7.20); p < 0.00001; Figure 7]. The pooled studies were heterogeneous (p < 0.00001; I2 = 97%). A similar statistically significant reduction was seen after 12 months as well [Mean difference = −10.71, 95% CI (−12.85 to −8.58); p < 0.00001; Figure 7]. The pooled studies were heterogeneous as well (p < 0.00001; I2 = 98%).
Figure 7.
The forest plot for the quality of life scores at (a) 6- and (b) 12-month follow-up post-treatment.
MRI findings
The MRI cartilage integrity was reported as either a change in the cartilage defect or a change in cartilage volume showing a clinically significant improvement in the ADMSC group as compared with the control after 6 and 12 months. In the study by Lee et al.,15 the change in cartilage defect was significantly less in the ADMSC group after 6 months (2.39 ±14.54 mm2 versus 35.61 ± 58.80 mm2, p = 0.0051). After 12 months, Lu et al.14 showed a significant increase in cartilage volume in both right and left knees compared with control (193.36 ± 282.80 mm3, p = 0.0042 versus −101.88 ± 224.30 mm3, p = 0.0362 for the left knee; 108.70 ± 220.13 mm3, p = 0.0307 versus −23.47 ± 291.37 mm3, p = 0.6967 for right knee).
Discussion
Mesenchymal stem cells (MSCs) can differentiate both in vivo and in vitro into a variety of cell types. As multipotent stromal cells, MSCs depend on the milieu and the vascular niche to metamorphose into osteoblasts, chondrocytes, myocytes, or adipocytes.18–20 Among sources for MSCs, the adipose tissue in the human body has several advantages due to its abundance and accessibility using minimally invasive technique.21 It provides multipotent MSCs characterized by their viability, proliferative properties, and high potency for multi-lineage cell differentiation depending on the surrounding milieu.9,22 ADMSCs tackle the main components of OA by creating an anti-inflammatory and immunomodulatory response through the release of cytokines and growth factors, acting in a paracrine fashion in the damaged joint.23–26 In addition, ADMSCs stimulate local tissue repair through cytokines, intact vascular niche, and the differentiation of MSCs leading to pain relief, functional improvement, and cartilage regeneration.27–29 In our study, we analyzed 4 RCTs attempting to extrapolate the functional and radiological outcomes. Our aim was to assess whether the hypothesized regenerative potential of ADMSCs translates into better functional scores and improved QoL.
Knee OA is a disabling disease characterized by an inflammatory process in the knee due to constant wear and tear.30 This inflammatory process leads to cartilaginous degeneration affecting the biomechanics of the knee.31 Currently, no definite treatment for OA exists only modalities to help slow down the progression of the disease.30 These include weight loss, quadriceps & hamstring strengthening exercises, and analgesia. Once the disease has reached advanced stages, these modalities have proven to have minimal effect, and the only treatment available is a joint replacement surgery.32 Therefore, ADMSC has emerged as a viable alternative treatment. Our analysis found a statistically significant improvement in all the analyzed pain scores (WOMAC, VAS, KOOS, NPRS). The improvement in the WOMAC score at 6 and 12 months in patients receiving ADMSC injection compared with the control group can be explained by the attenuation of the chronic slow inflammation in OA. In the RCT by Freitag et al., the pain improvement was mainly attributed to the stabilization of OA progression by paracrine effects and secretion of growth factors by the vascular niche, rather than cartilage regeneration by mesenchymal stem cell differentiation into chondrocyte.8,12 On the other hand, other studies attribute this pain relief to the regeneration potential of the mesenchymal stem cells.27,33,34 What advocates for that are the chondral changes seen after intra-articular injection of MSC at 6-month follow-up as shown by Orozco et al.34 The improvement in functional scores in this study is also in line with previously described literature.35 These findings are encouraging enough to promote for the use of ADMSCs in the clinical setting. The non-invasive procedure and the ability to harvest the material in an outpatient clinical setting will prove to be vital in increasing its use and by that validate its efficacy and safety further.36 Thus, the focus for now should be the efficacy of ADMSCs in validated scoring system that emphasizes the symptomatic relief and the functional improvement. As for cartilage regeneration, more studies are needed to establish a clear consensus on the exact mechanism of this improvement.
This study assessed the MRI findings in patients receiving ADMSCs as well. The MRI results were reported as either changes in cartilage thickness or changes in cartilage defect. Lee et al. did not find any significant change in cartilage defect by MRI at 6 months post-ADMSC injection; however, they found a significant increase in the cartilage defect in the control group. The study by Hong et al. as well, noticed a decrease in the cartilage defect in the ADMSCs group compared with the control group. On the other hand, Lu et al. reported a clinically significant increase in the volume of cartilage post ADMSC injection compared with the control group. Therefore, cartilage regeneration is a conclusion that is difficult to be established from the above radiological findings and thus, more basic research investigating the molecular aspect of the cartilage post-ADMSCs is needed to analyze the integrity of the cartilage and if there are signs of ‘real’ regeneration. Jo et al.28 in his study used arthroscopy to show a decrease in cartilage defect of the medial femoral and tibial condyles at 6 months post-ADMSC injection for knee OA. This could prove to be a more reliable method to assess true regeneration as compared with MRI findings
ADMSCs’ potential for healing and differentiation into different cell lines has paved the way for its larger use with wide indications in several fields, including orthopedics.37 Concerns regarding the safety of ADMSCs remain despite their increased usage.38 The main concern regarding the use of stem cells has been their potential to differentiate into fast-growing cancerous cell lines. Multiple studies in the literature have addressed the tumorigenic potential of ADMSCs.39,40 In a study by Ra et al.,40 immunocompromised mice were injected with human ADMSCs showing no increased risk of transformation at doses as high as 2 × 108 cells/kg body weight. A meta-analysis by Lalu et al.41 revealed no increased risk of tumor formation attributed to mesenchymal cell infusion in adult or pediatric subjects. These studies were mostly concerned with the tumorigenicity of mesenchymal stem cells in vivo, when administered systematically. The risk of tumorigenicity following intra-articular injections particularly has been infrequently described in the literature with only one study by Peeters et al.42 reporting two occurrences of tumor (benign schwannoma and prostate carcinoma) following hip and knee injections with MSC, both unrelated to the harvesting or the injection sites. In our study, no surveillance for tumorigenicity was performed as previous studies already established the safety of intra-articular injection of ADMSCs.28,43,44 As for the adverse events related to the procedure, few patients described discomfort on activity and bruising at the abdominal harvesting site that resolved on its own after 1 week.12,13 Arthralgia and swelling at the injection site joint were the most common adverse events reported with all controlled analgesia and rest.28 Severe adverse events interfering with daily activity was reported in only four patients across the studies, three related to continuous knee pain beyond 2 weeks that resolved spontaneously after 1 month, and one patient with urinary stone related to the patient’s known history. Other side effects included nasopharyngitis, headache, and back pain all resolving on acetaminophen analgesia.28
This article has many strengths. First, the stage of the OA among the groups was similar with Kellgren–Lawrence between grade 2 and 3 knee OA with only one patient with grade 4 in the control group study by Lee et al. The absence of grade 4 was vital given the very advanced stage of OA that would make it difficult to improve or halt the progression at this point and thus reflect it on the functional aspect.12 Second, the harvesting procedure was done from the abdominal subcutaneous tissue by manual liposuction in all the studies removing the difference in stem cell yield between the various harvesting locations possible. Third, to the best of our knowledge, this study is the first meta-analysis to date of RCTs comparing ADMSCs with other injections in knee OA.
This meta-analysis has some limitations. First, there was no consensus on the control group in each study where it ranged from conservative treatment, saline injection to hyaluronic acid. Hyaluronic acid is a known treatment modality for knee OA and could improve the pain and functional scores.45 Therefore, the actual impact of ADMSCs would have on the native knee might differ based on the control groups used. Second, there were no common standardized scores for evaluating the change in pain and function. Functional and QoL scores were only reported in two studies (Lu et al. and Freitag et al.) making a conclusion on this topic difficult. The pain scores were adjusted to a score of 100 to allow better comparability. In addition, the change in KOOS score was reported in negative numbers to allow comparability as well. Third, the radiographic parameters were performed via MRI in all the studies; however, not all were assessed through a quantitative scoring system. Using validated and standardized imaging scores will prove to be essential to systematically assess the legitimacy of stem cell-based therapy and its repercussions.46 Fourth, the study by Freitag which lacked blinding was utilizing two study groups with one receiving two injections 6 months apart. We analyzed the two arms separately to allow differentiation of the outcomes based on the number of injections. Moreover, the article of Lee et al. included both patients with OA and local cartilage defects. Finally, only few forest plots were generated using two of the included studies, thus not affecting much the validity of our outcomes.
Conclusion
This meta-analysis showed that administration of ADMSCs is a safe procedure and a potentially effective therapy for the treatment of knee OA. ADSMCS has a short-term and possible long-term effect on pain relief and improvement of functional outcomes of patients with OA. Ongoing robust clinical trials assessing functional outcomes are needed to investigate the effectiveness of ADMSCs. Current RCTs cannot conclude a regenerative potential of ADMSCs. In addition, translational medicine integrating basic and clinical research would be of beneficial value for investigating the exact mechanistic pathophysiology of ADMSCs and if it has any potential for cartilage regeneration.
Acknowledgments
None.
Footnotes
ORCID iDs: Mohamad R. Issa  https://orcid.org/0000-0001-6223-7802
https://orcid.org/0000-0001-6223-7802
Bernard H. Sagherian  https://orcid.org/0000-0001-5359-9027
https://orcid.org/0000-0001-5359-9027
Contributor Information
Mohamad R. Issa, Division of Orthopaedic Surgery, Department of Surgery, Faculty of Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Ahmad S. Naja, Division of Orthopaedic Surgery, Department of Surgery, Faculty of Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Nour Z. Bouji, Clinical and Translational Science, School of Medicine, West Virginia University, Morgantown, WV, USA
Bernard H. Sagherian, Division of Orthopaedic Surgery, Department of Surgery, Faculty of Medicine, American University of Beirut Medical Center, P.O. Box 11-0236, Riad El-Solh, 1107 2020 Beirut, Lebanon.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Mohamad R. Issa: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Validation; Visualization; Writing – original draft; Writing – review & editing.
Ahmad S. Naja: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Visualization; Writing – original draft; Writing – review & editing.
Nour Z. Bouji: Conceptualization; Formal analysis; Methodology; Project administration; Resources; Software; Supervision; Writing – original draft.
Bernard H. Sagherian: Conceptualization; Formal analysis; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and material: Not applicable.
References
- 1. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res 2018; 11: 2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162: 46–54. [DOI] [PubMed] [Google Scholar]
- 3. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010; 6: 625–635. [DOI] [PubMed] [Google Scholar]
- 5. Newberry SJ, FitzGerald J, SooHoo NF, et al. Treatment of osteoarthritis of the knee: an update review. Rockville, MD: Agency for Healthcare Research and Quality, 2017. [PubMed] [Google Scholar]
- 6. Arden NK, Perry TA, Bannuru RR, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol 2021; 17: 59–66. [DOI] [PubMed] [Google Scholar]
- 7. Persson MSM, Stocks J, Varadi G, et al. Predicting response to topical non-steroidal anti-inflammatory drugs in osteoarthritis: an individual patient data meta-analysis of randomized controlled trials. Rheumatology 2020; 59: 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 2003; 48: 3464–3474. [DOI] [PubMed] [Google Scholar]
- 9. Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013; 22: 2063–2077. [DOI] [PubMed] [Google Scholar]
- 10. Alexander R. Overview of cellular stromal vascular fraction (cSVF) & biocellular uses of stem/stromal cells & matrix (tSVF+ HD-PRP) in regenerative medicine, aesthetic medicine and plastic surgery. J Stem Cell Res Dev Ther 2019; 2: 304–312. [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med 2019; 14: 213–230. [DOI] [PubMed] [Google Scholar]
- 13. Hong Z, Chen J, Zhang S, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 2019; 43: 1123–1134. [DOI] [PubMed] [Google Scholar]
- 14. Lu L, Dai C, Zhang Z, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther 2019; 10: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee WS, Kim HJ, Kim KI, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med 2019; 8: 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne J, Savović J, Page M, et al. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. BMJ 2019; 366: 1–24. [DOI] [PubMed] [Google Scholar]
- 17. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957; 16: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maioli M, Rinaldi S, Santaniello S, et al. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant 2014; 23: 1489–1500. [DOI] [PubMed] [Google Scholar]
- 19. Baglioni S, Francalanci M, Squecco R, et al. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J 2009; 23: 3494–3505. [DOI] [PubMed] [Google Scholar]
- 20. Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell 2008; 132: 544–548. [DOI] [PubMed] [Google Scholar]
- 21. Naja A, Mohammed H, Naja N, et al. Adipose tissue-derived mesenchymal stem cells: a novel therapy in orthopedics. J Orthopedics Rheumatol 2018; 5: 5. [Google Scholar]
- 22. Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep 2016; 2: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acharya C, Adesida A, Zajac P, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol 2012; 227: 88–97. [DOI] [PubMed] [Google Scholar]
- 24. Wu L, Prins HJ, Helder MN, et al. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A 2012; 18: 1542–1551. [DOI] [PubMed] [Google Scholar]
- 25. Wei C-C, Lin AB, Hung S-C. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant 2014; 23: 505–512. [DOI] [PubMed] [Google Scholar]
- 26. Pers Y-M, Ruiz M, Noël D, et al. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage 2015; 23: 2027–2035. [DOI] [PubMed] [Google Scholar]
- 27. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation 2013; 95: 1535–1541. [DOI] [PubMed] [Google Scholar]
- 28. Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 2014; 32: 1254–1266. [DOI] [PubMed] [Google Scholar]
- 29. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 2015; 99: 1681–1690. [DOI] [PubMed] [Google Scholar]
- 30. Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 2021; 325: 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 2017; 5: 16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruyère O, Cooper C, Pelletier J-P, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014; 44: 253–263. [DOI] [PubMed] [Google Scholar]
- 33. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 2013; 9: 584–594. [DOI] [PubMed] [Google Scholar]
- 34. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation 2014; 97: e66–e68. [DOI] [PubMed] [Google Scholar]
- 35. Maheshwer B, Polce EM, Paul K, et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy 2021; 37: 362–378. [DOI] [PubMed] [Google Scholar]
- 36. Kunze KN, Burnett RA, Wright-Chisem J, et al. Adipose-derived mesenchymal stem cell treatments and available formulations. Curr Rev Musculoskelet Med 2020; 13: 264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minteer D, Marra KG, Rubin JP. Adipose-derived mesenchymal stem cells: biology and potential applications. In: Weyand B, Dominici M, Hass R, et al. (eds) Mesenchymal stem cells-basics and clinical application I. Cham: Springer, 2012, pp. 59–71. [Google Scholar]
- 38. Toyserkani NM, Jørgensen MG, Tabatabaeifar S, et al. Concise review: a safety assessment of adipose-derived cell therapy in clinical trials: a systematic review of reported adverse events. Stem Cells Transl Med 2017; 6: 1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song Y, Du H, Dai C, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med 2018; 13: 295–307. [DOI] [PubMed] [Google Scholar]
- 40. Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011; 20: 1297–1308. [DOI] [PubMed] [Google Scholar]
- 41. Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (MSCs): a systematic review. Am J Respir Crit Care Med 2010; 181: A6043. [Google Scholar]
- 42. Peeters CM, Leijs MJ, Reijman M, et al. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Osteoarthritis Cartilage 2013; 21: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 43. Soler R, Orozco L, Munar A, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016; 23: 647–654. [DOI] [PubMed] [Google Scholar]
- 44. Spasovski D, Spasovski V, Bašcˇarević Z, et al. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med 2018; 20: e3002. [DOI] [PubMed] [Google Scholar]
- 45. Maheu E, Rannou F, Reginster J-Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016; 45: S28–S33. [DOI] [PubMed] [Google Scholar]
- 46. Tucker JD, Ericksen JJ, Goetz LL, et al. Should clinical studies involving ‘regenerative injection therapy’, strive to incorporate a triad of outcome measures instead of only including clinical outcome measures? Osteoarthritis Cartilage 2014; 22: 715–717. [DOI] [PubMed] [Google Scholar]