Abstract
Charcot–Marie–Tooth disease (CMT) is a genetically heterogeneous group of peripheral neuropathies most of which are associated with mutations in four genes including peripheral myelin protein-22 (PMP22), myelin protein zero (MPZ), gap junction protein beta1 (GJB1) and mitofusin2 (MFN2). This current case report describes the clinical and genetic characteristics of a 6-year-old male proband. A physical examination revealed muscular hypotonia. He started walking on his own at 18 months. A nerve conduction study with needle electromyography revealed conduction block. A novel MPZ mutation (c.398C > T, p.Pro133Leu) was revealed in the proband. This mutation was also found in the 32-year-old father of the proband. The father had had deformity of the feet and distal muscle weakness since childhood. The novel p.Pro133Leu pathogenic mutation was responsible for early onset but slowly progressive CMT1B. We assume that this site is an intolerant to change region in the MPZ gene. This variant in the MPZ gene is an important contributor to hereditary neuropathy with reduced nerve conduction velocity in the Russian population. This case highlights the importance of whole exome sequencing for a proper clinical diagnosis of CMT associated with a mutation in the MPZ gene.
Keywords: Charcot-Marie-Tooth disease, MPZ gene, neuropathy
Introduction
Charcot–Marie–Tooth (CMT) disease is an inherited peripheral neuropathy that has an estimated prevalence in the population of 1 in 1214.1 It is a clinically and genetically heterogeneous group of disorders characterized by distal muscle weakness and atrophy leading to motor handicap. Sensory loss is another symptom of CMT.2 The disease is characterized by orthopaedic problems (e.g. pes cavus, scoliosis, hip dysplasia), pain, sleep disturbances and muscle cramps.2–7
Charcot–Marie–Tooth disease can be divided into several phenotypes as follows: (i) CMT1B with early/late onset and reduced nerve conduction velocity (NCV) (demyelinating type); (ii) CMT2I with a manifestation in adulthood and normal NCV (axonal type); (iii) Dejerine–Sottas syndrome (DSS) with infant-onset and reduced NCV.8 CMT1, CMT2 and autosomal dominant intermediate CMT are typically inherited in an autosomal dominant manner.9 CMT4 refers to autosomal recessive types and CMTX refers to X-linked types of CMT.10
The myelin protein zero (MPZ) gene is the major structural protein in peripheral nerve myelin and it is involved in myelin formation and the maintenance of myelin integrity.11,12 MPZ mutations have been associated with several hereditary neuropathies.13 Due to the high level of heterogeneity of the phenotypic manifestations of MPZ mutations, it is difficult to identify any regularities for this gene.
This current case report describes a Russian family in which both the father and son have muscle weakness, foot deformities and gait abnormalities. Molecular analysis in the father and son identified a novel heterozygous variant c.398C > T (p.Pro133Leu) in exon 3 of the MPZ gene that segregated with the phenotype. This part of the gene is highly conserved between species and a new pathogenic variant in it can be the cause of the development of neuropathy. This family case series exemplifies the phenotypic variants that are based on mutations in the MPZ gene.
Case report
In July 2017, the proband, a 6-year-old boy from the Komi Republic, Russia, presented to the Department of Psychoneurology, State Institution Republican Children's Clinical Hospital, Syktyvkar, Komi Republic, Russia with gait abnormality and coordination impairment. His medical history showed that he was born from the first pregnancy as a full-term baby with a birth weight of 3640 g. Apgar scores were 8 and 9 at the 1st and 5th min, respectively. A physical examination at birth revealed muscular hypotonia. He started walking on his own at the age of 18 months.
When the proband was 3 years old, a nerve conduction study with needle electromyography (EMG) of the distal parts of the medial and tibial nerves revealed conduction block. The EMG from the hand muscles revealed spinal and neural types of muscle electrogenesis damage. An orthopaedic examination revealed congenital flat valgus feet. Blood (including creatine phosphokinase) and urine tests were normal. Electroencephalography (EEG) data showed diffuse dysrhythmia but without foci of epileptic activity. Electrocardiography (ECG) revealed tachycardia and demonstrated no signs of cardiomyopathy. The presence of neuropathy was suggested, but investigations did not identify duplication of the peripheral myelin protein 22 (PMP22) gene.
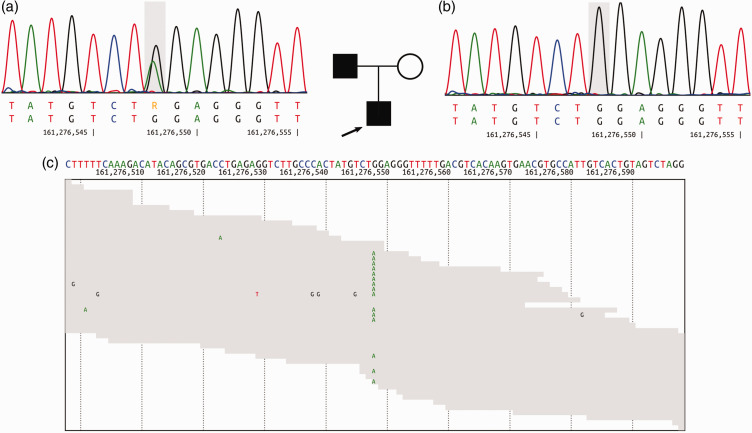
After admission to hospital when he was 6 years old, the patient responded appropriately to the examination and did not have any cerebral symptoms. An orthopaedic examination identified poor posture. The neurological examination revealed muscle hypotonia, symmetrical reduction of knee and Achilles tendon reflexes, moderate reflexes from the hands and severe ataxia. There was instability in the Romberg’s position. Magnetic resonance imaging of the brain and spinal cord could not be performed due to the increased motor activity of the child. During electromyoneurography (EMNG) of the motor fibres of the medial, fibular and tibial nerves, low amplitude M-waves were obtained from the tibialis anterior muscle on the right side with a conduction velocity 12 m/s (normal range 58–60 m/s). Conduction block along the examined nerves was detected. Developmental delay was not documented. A speech therapist detected dyslalia that did not include speech impairment due to neurological factors. When the proband was 6 years old, MPZ gene sequencing revealed a novel heterozygous mutation c.398C > T (p.Pro133Leu) in the MPZ gene (NM_000530.6) (Figure 1).
Figure 1.
Pedigree of the 6-year-old male proband’s family: (a) sequencing chromatogram of the proband’s father showing heterozygous missense mutation c.398C>T in the myelin protein zero (MPZ) gene; (b) sequencing chromatograms of the proband’s mother showed no mutation and (c) next-generation sequencing reads aligned against a reference genome hg19 (GRCh37.p13). The proband and his father were both heterozygous for the c.398C>T pathogenic variant. Circle – female, square – male, open symbols – unaffected, filled symbols – affected, arrow – proband. The bases in the grey frame are mutational sites. The colour version of this figure is available at: http://imr.sagepub.com.
The 32-year-old father of the proband had had deformity of the feet since childhood and did not start walking until the age of 4. By the age of 20, he had weakness in his legs and was diagnosed with neuralgic amyotrophy. A neurological examination performed at the age of 22 years revealed conduction block along nerves of the lower limbs (NCV 0 m/s in the lower limbs and 8–19 m/s in the upper limbs). EEG revealed insufficient organization of alpha activity in the form of a rhythm with amplitude up to 114 μV, index up to 77% and frequency spread of 9.7–11.8 Hz. At the age of 31 years, he had pes cavus, weakness and numbness of his arms and legs, hypotrophy of the interosseous muscles of the hands and scoliosis of the thoracolumbar spine. ECG showed an incomplete right bundle branch block. Tendon reflexes were absent in his arms and legs, hypoaesthesia was observed on his arms from the elbows, and on his legs from the knees. During EMNG of the distal parts of the medial, fibular and tibial nerves, the M-waves were not obtained at maximum stimulation. The father was able to walk on his own. He has a healthy 9-year-old son from his first marriage.
As CMT is an autosomal dominant disease, to identify the genetic cause of CMT in this family whole exome sequencing (WES) was undertaken for the proband and Sanger sequencing for his parents. The WES (Genotek Limited, Moscow, Russia) was performed after informed consent from the patient’s parents. Genomic DNA from peripheral blood samples was isolated using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). DNA libraries were prepared using a QIAseq FX DNA Library Combinatorial Dual-Index Kit (Qiagen). The samples were enriched with SureSelect XT2 kit (Agilent Technologies, Santa Clara, CA, USA) and sequenced using a HiSeq 2500 sequencer (Illumina, San Diego, CA, USA), generating 2 × 100 base pair reads (see supplementary materials, Supplementary methods). After sequencing, 3′-nucleotides with read quality below 10 were trimmed using Cutadapt.14 Illumina raw reads were aligned to a reference genome hg19 (GRCh37.p13) using the Burrows–Wheeler Aligner Maximum Entropy Method. The aligned file was used for variant calling using a Genome Analysis Toolkit (GATK) according to GATK best practices.15 FastQС was used for data quality control. The proband and his father had a novel p.Pro133Leu substitution within the MPZ protein, which is a mutation (NM_000530.6:c.398C > T) (Figure 1) that has not been identified previously in ExAC, 1000 Genomes Browser and in our 2000 in-house exomes. This variant was classified as ‘likely pathogenic’ according to the variant interpretation guidelines of The American College of Medical Genetics and Genomics as follows: three pathogenic moderate (PM) criteria (PM1, PM2, PM5); and three pathogenic supporting (PP) criteria (PP2, PP3, PP5).16 The variant was classified as a ‘pathogenic’ according to the predictive programs SIFT, PolyPhen2 and Mutation Taster (PolyPhen2 HumDiv score 1.00; PolyPhen2 Hum Var score 0.96; SIFT score 0.00; Mutation Taster score 1.0) (see supplementary materials, Supplementary methods). The mother of the proband is healthy and does not carry the mutation in the MPZ gene (Figure 1).
This case report conforms to CARE guidelines.17 The Ethics Committee of Genotek Limited approved this research (no. 01/2019). The patient's parents provided written informed consent for the research and for publication of the clinical information and sequencing data.
Discussion
The diagnosis of CMT1B in the proband was confirmed by the age of manifestation, clinical examination and the presence of the MPZ gene mutation. Previously, the father of the proband had not been diagnosed, however a detailed medical history of the father revealed signs of neuropathy at the age of 20, which was later than in his son. He had similar symptoms to his son, presenting with muscle weakness and EMNG revealed conduction block. In the proband, the same new mutation resulted in manifestation at an early age. According to previous data, CMT1B is the most common type in the Russian population and there is a greater contribution of the MPZ mutations in reducing NCV.18 If CMT1B manifests at an early age, then there is a significant decrease in the NCV.19 If the disease manifests later, then there is a slight decrease in the NCV.19 A strategy of focused genetic testing for the CMT has been proposed.20 This diagnostic algorithm is based on conduction velocity and severity of manifestation.20 At a conduction velocity <15 m/s, the algorithm suggests initial PMP22 duplication testing.20 Mutations in the MPZ gene are assumed to occur in a smaller percentage of cases (e.g. 3.3% among 427 Taiwanese CMT patients).21 However, data suggests a greater contribution from MPZ mutations in reducing conduction velocity in the Russian population.18 This conclusion was supported by three heterozygous mutations in the MPZ gene that were previously described in ClinVar and were found in four Genotek Limited patients that belong to the Russian population: c.186C > G (p.Ile62Met), c.270C > A (p.Asp90Glu), and c.235-1_235delGA (p.Ile79fs).
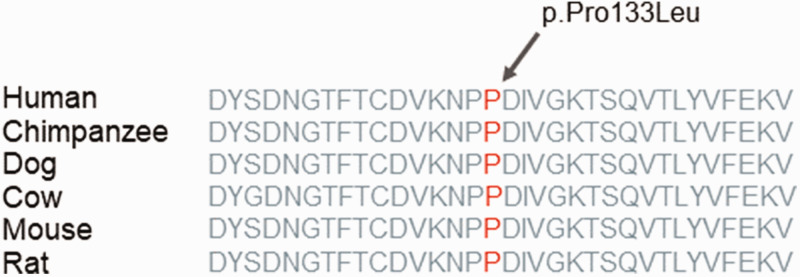
Myelin protein zero is a myelin sheath protein that is found in peripheral nerves, which consists of three domains: extracellular, intracellular and transmembrane.22 The intracellular domain ensures adhesion of the myelin sheath with the nerve axial cylinder and individual myelin fibres to each other.23 The extracellular domain forms the structure of the myelin sheath and its compaction.24 Numerous mutations leading to a change in the extracellular MPZ domain have been described previously that can give rise to highly variable clinical phenotypes.13 Therefore, a review of the literature was undertaken to investigate the effect of the MPZ mutations in this region on the clinical characteristics of CMT patients. According to the literature, mutations that lead to substitution on the extracellular domain of the MPZ protein had a significant effect on the CMT disease severity.25 Despite this, no dependence of the NCV on the localization of the mutation within the extracellular domain of MPZ protein was found. Several patients were identified with a mutation in the MPZ gene that leads to an amino acid substitution in the protein structure,26,27 similar to that observed in the current case. Therefore, an additional comparison of this parameter was undertaken among the patients presented in this case report, which identified a mutation leading to a substitution at positions 123–143 of the MPZ protein. Unfortunately, for some patients, it was not possible to find any description of their symptoms of the disease in the published articles, which makes it impossible to make a detailed comparison.28–30 The phenotype of patients with CMT and DSS that have missense mutations in exon 3 of the MPZ gene varies from early to late onset of the disease with a different score of conduction velocity.28 Exon 3 is highly conserved between species and mutation in this segment results in a severe form of CMT with a severe slowdown in the NCV (Figure 2). It was found that a similar amino acid substitution at codon 132 (p.Pro132Leu) resulted in the focally folded myelin with degenerative changes.31
Figure 2.
Analysis of the mutation and protein domains of myelin protein zero gene. The affected amino acid locates in the highly conserved amino acid region in different species of mammals. The arrow and red letters show the affected site. The colour version of this figure is available at: http://imr.sagepub.com.
Mutations in positions close to those described in this current case report were found in ClinVar and patients carrying these pathogenic variants had similar symptoms to those identified in the proband with the novel p.Pro133Leu substitution. A previous study described the p.Asn131Lys mutation in exon 3 of the MPZ gene, which is responsible for its adhesive properties.26 The case of a 5-year-old affected boy was similar to that of the current proband.26 He demonstrated delayed motor milestones, tremor of the fingers, weakness of the distal muscles, pes cavus in the lower limbs, bilateral moderate distal muscle wasting and weakness, his tendon reflexes were depressed and the NCV was 3.8 m/s.26 The proband’s father had the same symptoms but it was diagnosed at the age of 34 years.26 The phenotypic expression in patients with CMT may depend on genetic, environmental factors or the number of normal genes in an affected patient.27 This was confirmed with the phenotypic variability observed in a pair of genetically identical twins with CMT (Aspl28Asn).27 The proband had an earlier and more severe disease onset than his brother.27
In conclusion, this current case report describes two cases of CMT in a Russian family. These two cases, a father and his son, demonstrate that the same mutation leading to the development of CMT1B can have different phenotypic manifestations even within the same family. This report highlights the importance of WES for a proper clinical diagnosis of the CMT associated with a mutation in the MPZ gene. Molecular genetic testing of the MPZ gene should be considered to confirm the diagnosis of CMT in patients with neuropathy. This case report also emphasizes the significance of WES in the molecular diagnosis of inherited Mendelian disorders with phenotypic heterogeneity.
Research Data
Research Data for Novel mutation in the MPZ gene causes early-onset but slow-progressive Charcot–Marie–Tooth disease in a Russian family: a case report by Anastasiya Aleksandrovna Kozina, Natalia Vladimirovna Baryshnikova, Anna Yurievna Ilinskaya, Anna Alexandrovna Kim, Nikolay Alekseevich Plotnikov, Nadezhda Andreevna Pogodina, Ekaterina Ivanovna Surkova, Peter Alekseevich Shatalov and Valery Vladimirovich Ilinsky in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605221139718 for Novel mutation in the MPZ gene causes early-onset but slow-progressive Charcot–Marie–Tooth disease in a Russian family: a case report by Anastasiya Aleksandrovna Kozina, Natalia Vladimirovna Baryshnikova, Anna Yurievna Ilinskaya, Anna Alexandrovna Kim, Nikolay Alekseevich Plotnikov, Nadezhda Andreevna Pogodina, Ekaterina Ivanovna Surkova, Peter Alekseevich Shatalov and Valery Vladimirovich Ilinsky in Journal of International Medical Research
Acknowledgements
Anastasiya Alexandrovna Kozina and Valery Vladimirovich Ilinsky acknowledge the Leading Scientific School of Professor Andrey Lisitsa (grant of the President the Russian Federation Nsh-6313.2018.4). The authors gratefully acknowledge and thank the proband and his family for their participation in the study.
Author contributions: Kozina A.A., Baryshnikova N.V., Ilinskaya A.Y., Kim A.A., Plotnikov N.A., Pogodina N.A., Surkova E.I., Shatalov P.A. and Ilinsky V.V. met the International Committee of Medical Journal Editors criteria for authorship. Kozina A.A., Baryshnikova N.V. and Shatalov P.A. collected, analysed and interpreted the clinical data. Ilinskaya A.Y., Kim A.A., Plotnikov N.A. and Pogodina N.A. contributed to sequencing data collection and carried out the mutation analysis. Kozina A.A., Baryshnikova N.V., Ilinskaya A.Y., Pogodina N.A., Surkova E.I. and Shatalov P.A. drafted the manuscript. Ilinsky V.V. guided the completion of this article, supervised and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Programme for Basic Research in the Russian Federation for a long-term period (2021–2030) (grant no. 122030100168-2).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request. The novel mutation was approved by ClinVar (VCV000636232.1).
ORCID iDs
Nikolay Alekseevich Plotnikov https://orcid.org/0000-0001-5585-0035
Ekaterina Ivanovna Surkova https://orcid.org/0000-0002-9414-4202
Supplemental material
Supplemental material for this article is available online.
References
- 1.Braathen GJ. Genetic epidemiology of Charcot-Marie-Tooth disease. Acta Neurol Scand Supp 2012; 126: iv–22. [DOI] [PubMed] [Google Scholar]
- 2.Pisciotta C, Shy ME. Neuropathy. In: Geschwind DH, Paulson HL and Klein C (eds) Handbook of Clinical Neurology – Neurogenetics, Part II – Volume 148. Elsevier, 2018, pp.653–665. [DOI] [PubMed]
- 3.Karol LA, Elerson E. Scoliosis in patients with Charcot-Marie-tooth disease. J Bone Joint Surg Am 2007; 89: 1504–1510. [DOI] [PubMed] [Google Scholar]
- 4.Novais EN, Bixby SD, Rennick J, et al. Hip dysplasia is more severe in Charcot-Marie-Tooth disease than in developmental dysplasia of the hip. Clin Orthop Relat Res 2014; 472: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazzaglia C, Vollono C, Ferraro D, et al. Mechanisms of neuropathic pain in patients with Charcot-Marie-Tooth 1 A: A laser-evoked potential study. Pain 2010; 149: 379–385. [DOI] [PubMed] [Google Scholar]
- 6.Boentert M, Knop K, Schuhmacher C, et al. Sleep disorders in Charcot-Marie-Tooth disease type 1. J Neurol Neurosurg Psychiatry 2014; 85: 319–325. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NE, Sowden J, Dilek N, et al. Prospective study of muscle cramps in Charcot-Marie-Tooth disease. Muscle Nerve 2015; 51: 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner LE, Hilz MJ, Appel SH, et al. Clinical phenotypes of different MPZ (P0) mutations may include Charcot-Marie-Tooth type 1B, Dejerine-Sottas, and congenital hypomyelination. Neuron 1996; 17: 451–460. [DOI] [PubMed] [Google Scholar]
- 9.Saporta MA, Shy ME. Inherited peripheral neuropathies. Neurol Clin 2013; 31: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delague V, Bareil C, Tuffery S, Bouvagnet P, et al. Mapping of a new locus for autosomal recessive demyelinating Charcot-Marie-Tooth disease to 19q13.1-13.3 in a large consanguineous Lebanese family: exclusion of MAG as a candidate gene. Am J Hum Genet 2000; 67: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martini R, Mohajeri MH, Kasper S, et al. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J Neurosci 1995; 15: 4488–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martini R, Schachner M. Molecular bases of myelin formation as revealed by investigations on mice deficient in glial cell surface molecules. Glia 1997; 19: 298–310. [PubMed] [Google Scholar]
- 13.Timmerman V, Strickland AV, Züchner S. Genetics of Charcot-Marie-Tooth (CMT) Disease within the Frame of the Human Genome Project Success. Genes (Basel) 2014; 5: 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011; 17: 10–12. [Google Scholar]
- 15.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: Consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 18.Milovidova TB, Dadali EL, Fedotov VP, et al. Clinical-genetic correlations in the hereditary motor-sensor neuropathy caused by mutations in the MPZ (P0) gene. Zh Nevrol Psikhiatr Im S S Korsakova 2011; 111: 48–55 [Article in Russian, English abstract]. [PubMed] [Google Scholar]
- 19.Siskind CE, Panchal S, Smith CO, et al. A review of genetic counseling for Charcot Marie Tooth disease (CMT). J Genet Couns 2013; 22: 422–436. [DOI] [PubMed] [Google Scholar]
- 20.Saporta AS, Sottile SL, Miller LJ, et al. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol 2011; 69: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu YH, Lin KP, Guo YC, et al. Mutation spectrum of Charcot-Marie-Tooth disease among the Han Chinese in Taiwan. Ann Clin Transl Neurol 2019; 6: 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filbin MT, Walsh FS, Trapp BD, et al. Role of myelin P0 protein as a homophilic adhesion molecule. Nature 1990; 344: 871–872. [DOI] [PubMed] [Google Scholar]
- 23.Raasakka A, Ruskamo S, Kowal J, et al. Molecular structure and function of myelin protein P0 in membrane stacking. Sci Rep 2019; 9: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro L, Doyle JP, Hensley P, et al. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 1996; 17: 435–449. [DOI] [PubMed] [Google Scholar]
- 25.Hayasaka K, Himoro M, Sato W, et al. Charcot-Marie-Tooth neuropathy type 1B is associated with mutations of the myelin P0 gene. Nat Genet 1993; 5: 31–34. [DOI] [PubMed] [Google Scholar]
- 26.Kochański A, Drac H, Jȩdrzejowska H, et al. Focally folded myelin in Charcot-Marie-Tooth type 1B disease is associated with Asn131Lys mutation in myelin protein zero gene: Short report. Eur J Neurol 2003; 10: 547–549. [DOI] [PubMed] [Google Scholar]
- 27.Marques W, Hanna MG, Marques SR, et al. Phenotypic variation of a new PO mutation in genetically identical twins. J Neurol 1999; 246: 596–599. [DOI] [PubMed] [Google Scholar]
- 28.Sanmaneechai O, Feely S, Scherer SS, et al. Genotype-phenotype characteristics and baseline natural history of heritable neuropathies caused by mutations in the MPZ gene. Brain 2015; 138: 3180–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabreëls-Festen A. Dejerine-Sottas syndrome grown to maturity: overview of genetic and morphological heterogeneity and follow-up of 25 patients. J Anat 2002; 200: 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelis E, Timmerman V, De Jonghe P, et al. Rapid screening of myelin genes in CMT1 patients by SSCP analysis: identification of new mutations and polymorphisms in the P0 gene. Hum Genet 1994; 94: 653–657. [DOI] [PubMed] [Google Scholar]
- 31.Drac H, Kabzińska D, Moszyńska I, et al. Dysmyelinating and demyelinating Charcot-Marie-Tooth disease associated with two myelin protein zero gene mutations. J Appl Genet 2011; 52: 177–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Research Data for Novel mutation in the MPZ gene causes early-onset but slow-progressive Charcot–Marie–Tooth disease in a Russian family: a case report by Anastasiya Aleksandrovna Kozina, Natalia Vladimirovna Baryshnikova, Anna Yurievna Ilinskaya, Anna Alexandrovna Kim, Nikolay Alekseevich Plotnikov, Nadezhda Andreevna Pogodina, Ekaterina Ivanovna Surkova, Peter Alekseevich Shatalov and Valery Vladimirovich Ilinsky in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605221139718 for Novel mutation in the MPZ gene causes early-onset but slow-progressive Charcot–Marie–Tooth disease in a Russian family: a case report by Anastasiya Aleksandrovna Kozina, Natalia Vladimirovna Baryshnikova, Anna Yurievna Ilinskaya, Anna Alexandrovna Kim, Nikolay Alekseevich Plotnikov, Nadezhda Andreevna Pogodina, Ekaterina Ivanovna Surkova, Peter Alekseevich Shatalov and Valery Vladimirovich Ilinsky in Journal of International Medical Research
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request. The novel mutation was approved by ClinVar (VCV000636232.1).