Abstract
The aggregate index of systemic inflammation (AISI), systemic inflammation response index (SIRI), and neutrophil-to-lymphocyte*platelet ratio (NLRP) are novel indices that simultaneously reflect the inflammatory and immune status. However, the role of these indices in acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI) remains unclear. We aimed to elucidate the predictive value of AISI, SIRI, and NLRP in patients with ACS undergoing PCI. A total of 1558 patients with ACS undergoing PCI were consecutively enrolled from January 2016 to December 2018. The AISI, SIRI, NLRP, systemic immune-inflammatory index, derived neutrophil-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio cutoff values for predicting major adverse cardiovascular events (MACE) were calculated using receiver-operating characteristic curves, and Spearman's test was used to analyze correlations between these indices. Kaplan–Meier curves and Cox regression models were used for survival analyses, and the endpoint was a MACE, which included all-cause mortality and rehospitalization for severe heart failure during the follow-up period. The Kaplan–Meier curves showed that higher AISI, SIRI, and NLRP values were associated with a higher risk of MACE (all P < .001). The association between AISI, SIRI, and NLRP and ACS prognosis was stable in various subgroups according to sex, age, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, and heart failure (P for interaction > .05). Increasing tertiles of AISI, SIRI, and NLRP significantly increased the MACE risk (P for trend < .05). AISI, SIRI, and NLRP may be suitable laboratory markers for identifying high-risk patients with ACS after PCI.
Keywords: hematologic inflammatory markers, aggregate index of systemic inflammation, systemic inflammatory response index, neutrophil-to-lymphocyte*platelet ratio, acute coronary syndrome, percutaneous coronary intervention
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide.1 Acute coronary syndrome (ACS) is a severe CAD subtype characterized by a sudden reduction in the blood supply to the heart.2 Percutaneous coronary intervention (PCI) has become a universal ACS treatment strategy and has significantly reduced the incidence of major adverse cardiovascular events (MACE).3 However, patients with ACS undergoing PCI remain at high risk, and accurate and comprehensive risk assessment is particularly important in improving their prognosis.4
ACS mostly arises from the rupture or erosion of vulnerable atherosclerotic plaque.5 Inflammation plays an important role in the formation and development of atherosclerosis.6,7 Inflammatory responses are mainly reflected in changes in inflammatory cells and peripheral blood factors. As a reflection of inflammation level, the peripheral blood inflammatory cell count and its derived indicators have been widely used in clinical practice.8 A complete blood cell (CBC) count provides information on the number and morphology of various cells (ie, neutrophil, monocyte, platelet, and lymphocyte counts) and is inexpensive and readily available.9 Recently published studies have demonstrated that many combined ratios of CBC parameters, such as systemic immune-inflammatory index (SII), derived neutrophil-to-lymphocyte ratio (dNLR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), could be useful prognostic markers in ACS patients undergoing PCI.10,11 Furthermore, as more complicated and newly mentioned inflammatory markers, the aggregate index of systemic inflammation (AISI), systemic inflammation response index (SIRI), and neutrophil-to-lymphocyte*platelet ratio (NLRP) have only been proposed simultaneously as biomarkers for predicting mortality in COVID-19 patients.9,12,13 However, no studies have investigated the prognostic capacity of these 3 novel indices for ACS prognosis. We hypothesized that AISI, SIRI, and NLPR could also reflect the prognosis of patients with ACS as novel indicators of blood inflammatory cell counts. Therefore, the objective of this study was to analyze the association of AISI, SIRI, and NLPR with prognosis in ACS patients undergoing PCI and to compare the potential predictive value of AISI, SIRI, and NLPR indices with that of SII, dNLR, NLR, PLR, and MLR for prognosis of ACS patients. In addition, we performed a subgroup analysis (sex, age, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, and heart failure [HF]) to enhance the reliability of our conclusions.
Materials and Methods
Study Population
In total, 1773 patients with ACS who underwent PCI were consecutively enrolled in this prospective cohort study from January 2016 to December 2018 at the Affiliated Hospital of Chengde Medical University. The inclusion criteria were: patients aged ≥40 years; those with ACS (clinical types: unstable angina, non-ST elevation myocardial infarction [NSTEMI], and ST-elevation myocardial infarction [STEMI]); those with coronary arteriography showing ≥50% stenosis in one or more of the left main, left anterior descending, left circumflex, right coronary, or main branches; and those who underwent PCI (complete revascularization) for the first time. The exclusion criteria were as follows: coronary artery spasm or other secondary causes of angina or myocardial infarctions, infectious diseases, malignant tumors, blood system diseases (eg, anemia and leukopenia), severe heart diseases (eg, aortic dissection and hypertrophic cardiomyopathy), severe systemic disease, systemic inflammatory disorders, glucocorticoid therapies within 2 months, and chronic kidney disease (stage ≥3). This study was approved by the Institutional Review Board of the Affiliated Hospital of Chengde Medical University (number: LL2021036), and written informed consent was obtained from all participants.
Clinical Data Collection
Demographic and clinical characteristics, and information regarding typical ACS clinical risk factors, such as diabetes, hypertension, and dyslipidemia, were collected during hospitalization by postgraduate students. Hypertension was defined as a systolic blood pressure ≥140 mm Hg (1 mm Hg = 0.133 kPa) and diastolic blood pressure ≥90 mm Hg at rest or a previous hypertension diagnosis with antihypertensive therapy.14 Diabetes mellitus was defined according to the following American Diabetes Association guidelines: glycated hemoglobin ≥6.5%, fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), 2 h plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test using 75 g of glucose, classic hyperglycemia symptoms (eg, polyuria, polydipsia, and weight loss), or hyperglycemic crisis with random plasma glucose ≥200 mg/dL (11.1 mmol/L). In the absence of unequivocal hyperglycemia, the first 3 criteria were confirmed by repeat testing.15 Dyslipidemia was defined as a serum total cholesterol ≥5.18 mmol/L (200 mg/dL), high-density lipoprotein cholesterol ≤1.04 mmol/L, low-density lipoprotein cholesterol ≥3.37 mmol/L, triglycerides ≥1.7 mmol/L, or a previous dyslipidemia diagnosis with a prescribed medication.16 Experienced cardiologists performed PCI using the Judkins technique with 6F right and left heart catheters. Successful PCI was defined as residual stenosis less than 30% and final thrombolysis in myocardial infarction II or III flow in the treated artery. The angiographic characteristics of all the patients were determined and reported by the PCI team.
Laboratory Data
Fasting blood samples were collected within the first 24 h of admission before PCI. White blood cell (WBC), platelet, neutrophil, and lymphocyte counts were assessed using an automatic hematology analyzer (Sysmex XE-2100; Sysmex, Kobe, Japan). The hematologic inflammatory markers were calculated as follows: AISI = (neutrophil*platelet*monocyte)/lymphocyte, SIRI = (neutrophils*monocytes)/lymphocytes, NLPR = neutrophil/(lymphocyte*platelet), SII = (neutrophil*platelet)/lymphocyte, dNLR = neutrophil/(WBC–neutrophil), NLR = neutrophil/ lymphocyte, PLR = platelet/lymphocyte, MLR = monocyte/lymphocyte.12,17,18
Follow-up and Study Endpoints
The study endpoint was a MACE, which included all-cause mortality and re-hospitalization for severe heart failure during the follow-up period. All-cause mortality was defined as death from any cause. Severe heart failure was defined as New York Heart Association (NYHA) Classification class IV. Follow-up data were collected via a review of electronic medical records and/or clinic visits at 1, 3, 6, and 12 months and once a year thereafter. The reasons for loss to follow-up included missing or changes in patient contact information, population mobility, and withdrawal from the study. The research team was uniformly trained using the same inclusion criteria, measurement criteria, and follow-up protocols.
Statistical Analyses
Statistical analyses were performed using SPSS (version 26.0; SPSS Inc., Chicago, IL, USA), R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria), and GraphPad Prism (version 8.0; GraphPad Software Inc., La Jolla, CA, USA). The normality of continuous variables was determined using the Kolmogorov–Smirnov test. Continuous variables without a normal distribution were presented as medians (interquartile range) and were compared using the Mann–Whitney U-test. Categorical variables were presented as numbers (%) and compared using the chi-square or Fisher's exact test. Receiver-operating characteristic (ROC) curves were used to calculate the cutoff values for blood cell count-derived inflammation indices to predict MACE, and the area under the curve (AUC) with 95% confidence interval (95%CI), specificity, and sensitivity were also calculated to assess the differentiating capability of AISI, SIRI, NLRP, SII, dNLR, PLR, and MLR. The optimal cutoff value was determined as the value corresponding to the maximum Youden index (sensitivity + specificity − 1). Correlations between these inflammation indices were analyzed using the Spearman's rank correlation coefficient.
AISI, SIRI, and NLRP were comprehensively analyzed as nominal and continuous variables, respectively. As continuous variables, AISI, SIRI, and NLRP were standardized using Z-scores (individual value-mean population)/(standard deviation population). They were transformed into categorical variables according to tertiles (T1-T3) for survival analysis. Differences in survival outcomes were investigated using the log-rank test. The “Kaplan–Meier survival” and “survminer” packages of R software were used to estimate survival analysis according to the Kaplan–Meier curve. Cox proportional hazards models were used to identify the independent prognostic factors for MACE using the enter method. Model 1 was a crude model, and adjustments were made first for demographic factors, such as sex, age≥65 years, and BMI (model 2). Additional adjustments were made for clinical factors (ie, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, family history of CAD, cardiogenic shock [CGS], HF, creatinine [Cr], left ventricular ejection fraction [LVEF] <40%, and albumin [ALB] <40.72) (model 3). Interaction terms were used to investigate whether the association between these 3 novel indices and MACE differed, and subgroups (sex, age, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, and HF) were generated among patients. The hazard ratio (HR) was determined based on one standard deviation (1-SD) increases in AISI, SIRI, and NLRP levels and different tertiles (T2 and T3, T1 as reference), respectively. The “visreg” package of R software was used to visualize the possible interactions. Statistical significance was set at P < .05.
Results
Baseline Characteristics
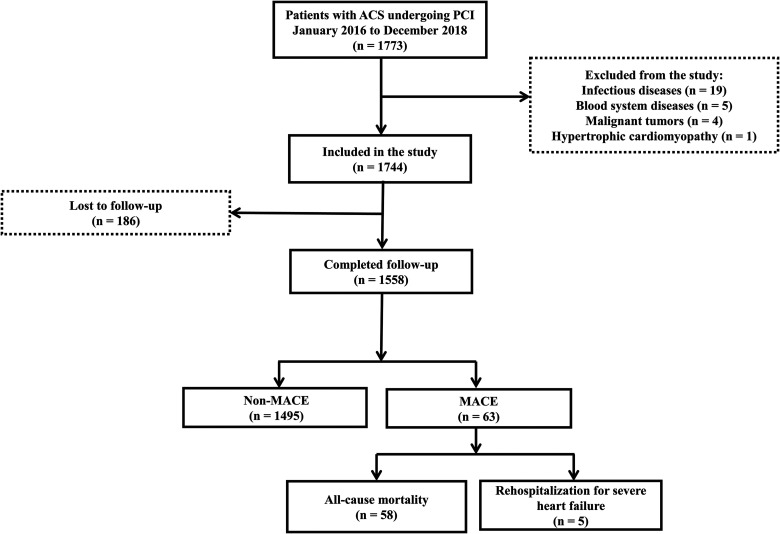
The median follow-up duration was 1142 days. A total of 1558 patients were assigned to the following groups: MACE group (n = 63; all-cause mortality [n = 58; death from any cause] and rehospitalization for severe heart failure [n = 5; heart function level IV based on the NYHA Classification]) and non-MACE group (n = 1495; no MACE occurred) (Figure 1). Patients were grouped by MACE for analysis. Patients with a MACE were more likely to be older than 65 years; have a history of stroke, HF, or CGS; have an enlarged left atrium, LVEF of <40%, AISI ≥293.46, SIRI ≥2.45, NLRP ≥0.018, SII ≥628.60, dNLR ≥2.30, NLR ≥2.67, PLR ≥225.49, and MLR ≥0.33 (all P < .05). The prevalence of STEMI and NSTEMI was also higher in the MACE group than in the non-MACE group, although the difference was not statistically significant. The neutrophil count and creatine kinase-MB and Cr levels (all P < .05) were higher, and the lymphocyte count and ALB levels were lower in the MACE group (all P < .05; Table 1).
Figure 1.
Patient enrollment and screening flowchart.
Table 1.
Baseline Clinical Characteristics Between the MACE and Non-MACE Groups.
| Variable | MACE (n = 63) |
Non-MACE (n = 1495) |
χ2/Z | P-value |
|---|---|---|---|---|
| Demographic and clinical data, n (%) | ||||
| Male | 46 (73.0) | 1120 (74.9) | 0.116 | .733 |
| Age ≥65 years | 32 (50.8) | 340 (22.7) | 26.171 | <.001 |
| BMI | 24.22 (22.67, 27.34) | 25.16 (23.05, 27.55) | −1.119 | .263 |
| Smoking | 29 (46.0) | 780 (52.2) | 0.914 | .339 |
| Dyslipidemia | 36 (57.1) | 846 (56.6) | 0.008 | .931 |
| Hypertension | 35 (55.6) | 880 (58.9) | 0.273 | .601 |
| Diabetes mellitus | 14 (22.2) | 380 (25.4) | 0.327 | .568 |
| History of stroke | 16 (25.4) | 206 (13.8) | 6.678 | .010 |
| History of TIA | 0 (0.0) | 5 (0.3) | 0.211 | .646 |
| HF | 20 (31.7) | 138 (9.2) | 33.629 | <.001 |
| CGS | 8 (12.7) | 17 (1.1) | 51.178 | <.001 |
| Family history of CAD | 5 (7.9) | 214 (14.3) | 2.036 | .154 |
| UA | 15 (23.8) | 601 (40.2) | 6.794 | .009 |
| STEMI | 34 (54.0) | 660 (44.1) | 2.360 | .124 |
| NSTEMI | 14 (22.2) | 234 (15.7) | 1.950 | .163 |
| Laboratory data, M(Q1, Q3) | ||||
| WBC count (109/L) | 8.95 (7.39, 11.35) | 8.00 (6.41, 10.49) | −1.707 | .088 |
| Platelet count (109/L) | 213.00 (176.00, 245.00) | 215.00 (179.50, 252.00) | −0.744 | .457 |
| Neutrophil count (109/L) | 6.82 (4.86, 9.22) | 5.47 (3.97, 8.29) | −2.522 | .012 |
| Lymphocyte count (109/L) | 1.29 (0.87, 1.72) | 1.67 (1.22, 2.29) | −3.464 | <.001 |
| Monocyte count (109/L) | 0.47 (0.34, 0.57) | 0.43 (0.32, 0.58) | −0.796 | .426 |
| AISI≥293.46 | 43 (70.5) | 711 (47.6) | 12.306 | <.001 |
| SIRI≥2.45 | 30 (49.2) | 393 (26.3) | 15.486 | <.001 |
| NLRP≥0.018 | 38 (62.3) | 619 (41.5) | 10.365 | <.001 |
| SII ≥628.60 | 46 (75.4) | 758 (50.7) | 14.288 | <.001 |
| dNLR ≥2.30 | 46 (75.4) | 735 (49.2) | 16.108 | <.001 |
| NLR ≥2.67 | 50 (82.0) | 860 (57.6) | 14.379 | <.001 |
| PLR ≥225.49 | 18 (29.5) | 214 (14.3) | 10.645 | <.001 |
| MLR ≥0.33 | 30 (50.0) | 429 (29.3) | 11.703 | <.001 |
| MPV (fL) | 10.40 (9.80, 10.90) | 10.40 (9.80, 11.00) | −0.547 | .585 |
| PDW (%) | 12.20 (11.00, 13.40) | 12.00 (10.90, 13.40) | −0.403 | .687 |
| ALB (g/L) | 39.60 (37.00, 41.40) | 41.39 (38.80, 43.70) | −3.305 | <.001 |
| CK-MB (U/L) | 36.00 (11.00, 123.49) | 16.37 (10.00, 48.00) | −2.702 | .007 |
| Cr (μmol/L) | 76.12 (63.20, 88.70) | 67.00 (58.50, 78.00) | −3.180 | .001 |
| Serum uric acid (μmol/L) | 333.10 (272.80, 391.10) | 327.00 (264.70, 384.45) | −0.669 | .504 |
| Echocardiography, n (%) | ||||
| LA↑ | 26 (45.6) | 431 (32.0) | 4.619 | .032 |
| LVEDD↑ | 18 (29.5) | 337 (22.6) | 1.600 | .206 |
| LVEF <40% | 8 (14.0) | 33 (2.4) | 25.971 | <.001 |
| Coronary angiography, n (%) | ||||
| 1 vessel | 16 (26.23) | 471 (31.57) | 0.776 | .378 |
| 2 vessels | 21 (34.43) | 473 (31.70) | 0.200 | .654 |
| 3 vessels | 24 (39.34) | 548 (36.73) | 0.172 | .678 |
Abbreviations: MACE, major adverse cardiovascular events; BMI, body mass index; TIA, transient ischemic attack; HF, heart failure; CGS, cardiogenic shock; CAD, coronary artery disease; UA, unstable angina; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; WBC, white blood cell; AISI, aggregate index of systemic inflammation (neutrophil*platelet*monocyte-to-lymphocyte ratio); SIRI, systemic inflammation response index (neutrophil*monocyte-to-lymphocyte ratio); NLRP, neutrophil-to-(lymphocyte*platelet) ratio; SII, systemic immune-inflammation index (neutrophil*platelet-to-lymphocyte ratio); dNLR, derived neutrophil-to-lymphocyte ratio (neutrophil/[white blood cell–neutrophil]); NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; MPV, mean platelet volume; PDW, platelet distribution width; ALB, albumin; CK-MB, creatine kinase-MB; Cr, creatinine; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction. ↑, above the upper limit of normal values.
ROC Curve Analyses
Table 2 presents the ROC curve analyses to determine the optimal cutoff values for blood cell count-derived inflammation indices for MACE evaluation in patients with ACS undergoing PCI. The AUC for AISI was 0.624 (P = .001, 95%CI: 0.533-0.694). The optimal diagnostic cut-off point was 293.46, with a sensitivity of 70.5% and a specificity of 52.4%. The AUC for SIRI was 0.625 (P < .001, 95%CI: 0.552-0.698), and the optimal diagnostic cutoff point was 2.45, with a sensitivity of 54.1% and a specificity of 71.8%. The AUC for NLRP was 0.625 (P < .001, 95%CI: 0.554-0.697), and the optimal diagnostic cut-off point was 0.018, with a sensitivity of 62.3% and a specificity of 59.4%. The AUC for SII was 0.626 (P < .001, 95%CI: 0.561-0.692), and the optimal diagnostic cutoff point was 628.60, with a sensitivity of 75.4% and a specificity of 49.3%. The AUC for dNLR was 0.638 (P < .001, 95%CI: 0.571-0.705), and the optimal diagnostic cutoff point was 2.30, with a sensitivity of 77.1% and a specificity of 50.6%. The AUC for NLR was 0.640 (P < .001, 95%CI: 0.570-0.709), and the optimal diagnostic cutoff point was 2.60, with a sensitivity of 83.6% and a specificity of 42.3%. The AUC for PLR was 0.593 (P = .014, 95%CI: 0.518-0.668), and the optimal diagnostic cutoff point was 225.49, with a sensitivity of 29.5% and a specificity of 85.7%. The AUC for MLR was 0.598 (P = .010, 95%CI: 0.516-0.680), and the optimal diagnostic cut-off point was 0.33 with a sensitivity of 50.8% and a specificity of 71.0% (Table 2).
Table 2.
ROC Curve Analyses of the Blood Cell Count-Derived Inflammation Indices Between MACE and non-MACE Groups.
| Variable | AUC | SE | P-value | 95%CI | Se (%) | Sp (%) | Cut off |
|---|---|---|---|---|---|---|---|
| AISI | 0.624 | 0.036 | .001 | 0.553-0.694 | 70.5 | 52.4 | 293.46 |
| SIRI | 0.625 | 0.037 | <.001 | 0.552-0.698 | 54.1 | 71.8 | 2.45 |
| NLRP | 0.625 | 0.037 | <.001 | 0.554-0.697 | 62.3 | 59.4 | 0.018 |
| SII | 0.626 | 0.034 | <.001 | 0.561-0.692 | 75.4 | 49.3 | 628.60 |
| dNLR | 0.638 | 0.034 | <.001 | 0.571-0.705 | 77.1 | 50.6 | 2.30 |
| NLR | 0.640 | 0.036 | <.001 | 0.570-0.709 | 83.6 | 42.3 | 2.60 |
| PLR | 0.593 | 0.038 | .014 | 0.518-0.668 | 29.5 | 85.7 | 225.49 |
| MLR | 0.598 | 0.042 | .010 | 0.516-0.680 | 50.8 | 71.0 | 0.33 |
Abbreviations: ROC, receiver-operating characteristic; MACE, major adverse cardiovascular events; AUC, area under the curve; SE, standard error; CI, confidence interval; Se, sensitivity; Sp, specificity; AISI, aggregate index of systemic inflammation (neutrophil*platelet*monocyte-to-lymphocyte ratio); SIRI, systemic inflammation response index (neutrophil*monocyte-to-lymphocyte ratio); NLRP, neutrophil-to-(lymphocyte*platelet) ratio; SII, systemic immune-inflammation index (neutrophil*platelet-to-lymphocyte ratio); dNLR, derived neutrophil-to-lymphocyte ratio (neutrophil/(white blood cell–neutrophil)); NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio.
Correlation Analysis
Spearman correlation analysis showed that AISI was significantly positively correlated with SIRI, NLRP, SII, dNLR, NLR, PLR, and MLR (all P < .001). SIRI was positively correlated with NLRP, SII, dNLR, NLR, PLR, and MLR (all P < .001). NLRP was positively correlated with the SII, dNLR, NLR, PLR, and MLR (all P < .001; Table 3).
Table 3.
Correlation Between Blood Cell Count-Derived Inflammation Indices.
| AISI | SIRI | NLRP | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| AISI | — | — | — | — | — | — |
| SIRI | 0.914 | <.001 | — | — | — | — |
| NLRP | 0.555 | <.001 | 0.760 | <.001 | — | — |
| SII | 0.860 | <.001 | 0.772 | <.001 | 0.686 | <.001 |
| dNLR | 0.696 | <.001 | 0.776 | <.001 | 0.882 | <.001 |
| NLR | 0.757 | <.001 | 0.843 | <.001 | 0.914 | <.001 |
| PLR | 0.582 | <.001 | 0.462 | <.001 | 0.498 | <.001 |
| MLR | 0.736 | <.001 | 0.840 | <.001 | 0.600 | <.001 |
Abbreviations: AISI, aggregate index of systemic inflammation (neutrophil*platelet*monocyte-to-lymphocyte ratio); SIRI, systemic inflammation response index (neutrophil*monocyte-to-lymphocyte ratio); NLRP, neutrophil-to-(lymphocyte*platelet) ratio; SII, systemic immune-inflammation index (neutrophil*platelet-to-lymphocyte ratio); dNLR, derived neutrophil-to-lymphocyte ratio (neutrophil/[white blood cell–neutrophil]); NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio.
Kaplan–Meier Analyses
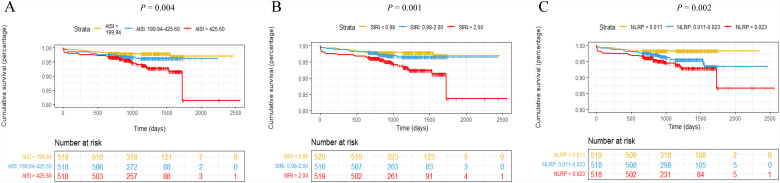
The Kaplan–Meier curve was plotted with the follow-up survival data. Patients in the AISI >425.60, SIRI >2.00, and NLRP >0.023 tertiles had significantly higher all-cause mortality and rehospitalization for severe heart failure than those in the other 2 tertiles (log-rank test, all P < .05; Figure 2).
Figure 2.
AISI, SIRI, and NLRP Kaplan–Meier survival curves for ACS patients undergoing PCI for MACE. (A) AISI curves for T1(<199.94), T2(199.94-425.60), and T3(>425.60) groups (log-rank test: P = .004). (B) SIRI curves for T1(<0.98), T2(0.98-2.00), and T3(>2.00) groups (log-rank test: P = .001). (C) NLRP curves for T1(<0.011), T2(0.011-0.023), and T3(>0.023) groups (log-rank test: P = .002).
Cox Regression Analyses
Univariate and multivariate Cox regression analyses were used to determine the independent predictors of MACE. As shown in Table 4, after adjusting for confounding factors in Model 2 (demographic factors), we found that a per 1-SD increase in AISI was independently associated with higher risk of MACE (HR [95%CI]: 1.271 [1.098, 1.471], P = .001), but the association became insignificant after adjusting for terms in Model 2 and clinical factors. After adjusting for confounding factors in Model 3 (demographic and clinical factors), we found that a per 1-SD increase in SIRI was independently associated with higher risk of MACE (HR [95%CI]: 1.208 [1.045-1.397], P = .011). However, a per 1-SD increase in NLRP failed to be a predictor of MACE (P >.05 in models 1-3). Table 5 shows the association between tertiles of these indices and the occurrence of MACE. After adjusting for confounding factors in Model 3, the risk of MACE increased gradually with increasing AISI (HR [95%CI]: T1: reference, T2: 1.587 [0.728, 3.457], T3: 2.345 [1.141, 4.819]; trend P < .05), SIRI (HR [95%CI]: T1: reference, T2: 1.189 [0.535, 2.644], T3: 2.135 [1.023, 4.455]; trend P < .05), and NLRP tertiles (HR [95%CI]: T1: reference, T2: 2.202 [0.955,5.074], T3: 2.599 [1.116,6.048]; trend P < .05).
Table 4.
Cox Regression Model for Predicting MACE in ACS Patients Undergoing PCI According to One Standard Deviation (SD) Increases in Blood Cell Count-Derived Inflammation Indices.
| Inflammation indices as a continuous variable (per 1-SD increase) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| AISI | 1.277 (1.101-1.481) | .001 | 1.271 (1.098-1.471) | .001 | 1.140 (0.955-1.360) | .146 |
| SIRI | 1.282 (1.153-1.426) | <.001 | 1.312 (1.172-1.470) | <.001 | 1.208 (1.045-1.397) | .011 |
| NLRP | 1.038 (0.898-1.201) | .611 | 1.014 (0.877-1.174) | .847 | 1.006 (0.854-1.185) | .941 |
Abbreviations: MACE, major adverse cardiovascular events; ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; HR, hazard ratio; CI, confidence interval; AISI, aggregate index of systemic inflammation (neutrophil*platelet* monocyte-to-lymphocyte ratio); SIRI, systemic inflammation response index (neutrophil*monocyte-to-lymphocyte ratio); NLRP, neutrophil-to-(lymphocyte*platelet) ratio; BMI, body mass index; CAD, coronary artery disease; CGS, cardiogenic shock; HF, heart failure; Cr, creatinine; LA, left atrium; LVEF, left ventricular ejection fraction; ALB, albumin.
Model 1: Unadjusted.
Model 2: Adjusted for sex, age>65 years, BMI.
Model 3: Adjusted for terms in Model 2 and smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, family history of CAD, CGS, HF, Cr, LVEF <40%, and ALB<40.72.
Table 5.
Cox Regression Model for Predicting MACE in ACS Patients Undergoing PCI According to Tertiles of Blood Cell Count-Derived Inflammation Indices.
| Inflammation indices as a nominal variable (tertiles 1-3) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| AISI | T1 | 1 (Reference) | — | 1 (Reference) | — | 1 (Reference) | — |
| T2 | 1.596 (0.768-3.317) | .210 | 1.570 (0.755-3.264) | .227 | 1.587(0.728-3.457) | .245 | |
| T3 | 2.808 (1.440-5.476) | .002 | 3.004 (1.535-5.880) | .001 | 2.345 (1.141-4.819) | .020 | |
| P for trend | .002 | <.001 | .017 | ||||
| SIRI | T1 | 1 (Reference) | — | 1 (Reference) | — | 1 (Reference) | — |
| T2 | 1.427 (0.675-3.017) | .352 | 1.436 (0.675-3.053) | .348 | 1.189 (0.535-2.644) | .671 | |
| T3 | 2.966 (1.531-5.746) | <.001 | 3.118 (1.586-6.131) | <.001 | 2.135 (1.023-4.455) | .043 | |
| P for trend | <.001 | <.001 | .025 | ||||
| NLRP | T1 | 1 (Reference) | — | 1 (Reference) | — | 1 (Reference) | — |
| T2 | 2.497(1.150-5.424) | .021 | 2.471(1.131-5.399) | .023 | 2.202(0.955-5.074) | .064 | |
| T3 | 3.606(1.711-7.600) | <.001 | 3.424(1.601-7.322) | .002 | 2.599(1.116-6.048) | .027 | |
| P for trend | <.001 | .001 | .033 | ||||
Abbreviations: MACE, major adverse cardiovascular events; ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; HR, hazard ratio; CI, confidence interval; AISI, aggregate index of systemic inflammation (neutrophil*platelet*monocyte-to-lymphocyte ratio); SIRI, systemic inflammation response index (neutrophil*monocyte-to-lymphocyte ratio); NLRP, neutrophil-to-(lymphocyte*platelet) ratio; BMI, body mass index; CAD, coronary artery disease; CGS, cardiogenic shock; HF, heart failure; Cr, creatinine; LA, left atrium; LVEF, left ventricular ejection fraction; ALB, albumin.
Model 1: Unadjusted.
Model 2: Adjusted for sex, age>65 years, BMI.
Model 3: Adjusted for terms in Model 2 and smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, family history of CAD, CGS, HF, Cr, LVEF <40%, and ALB<40.
AISI: T1 (<199.94), T2 (199.94-425.60), T3 (>425.60).
SIRI: T1 (<0.98), T2 (0.98-2.00), T3 (>2.00).
NLRP: T1 (<0.011), T2 (0.011-0.023), T3 (>0.023).
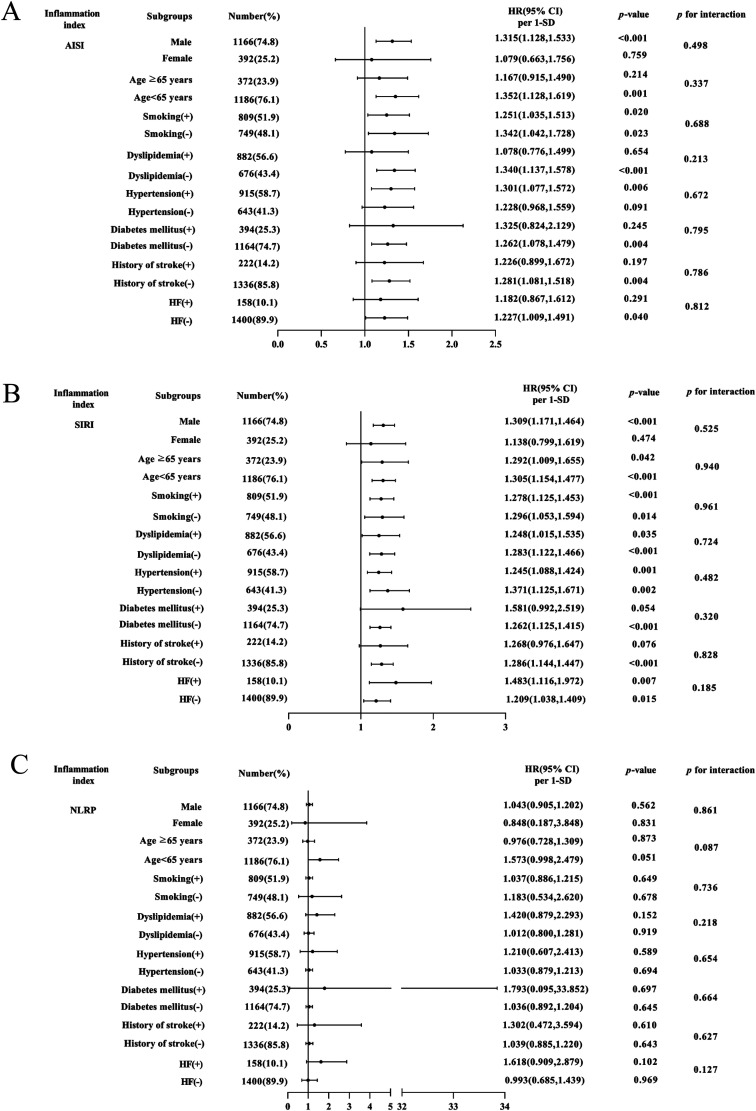
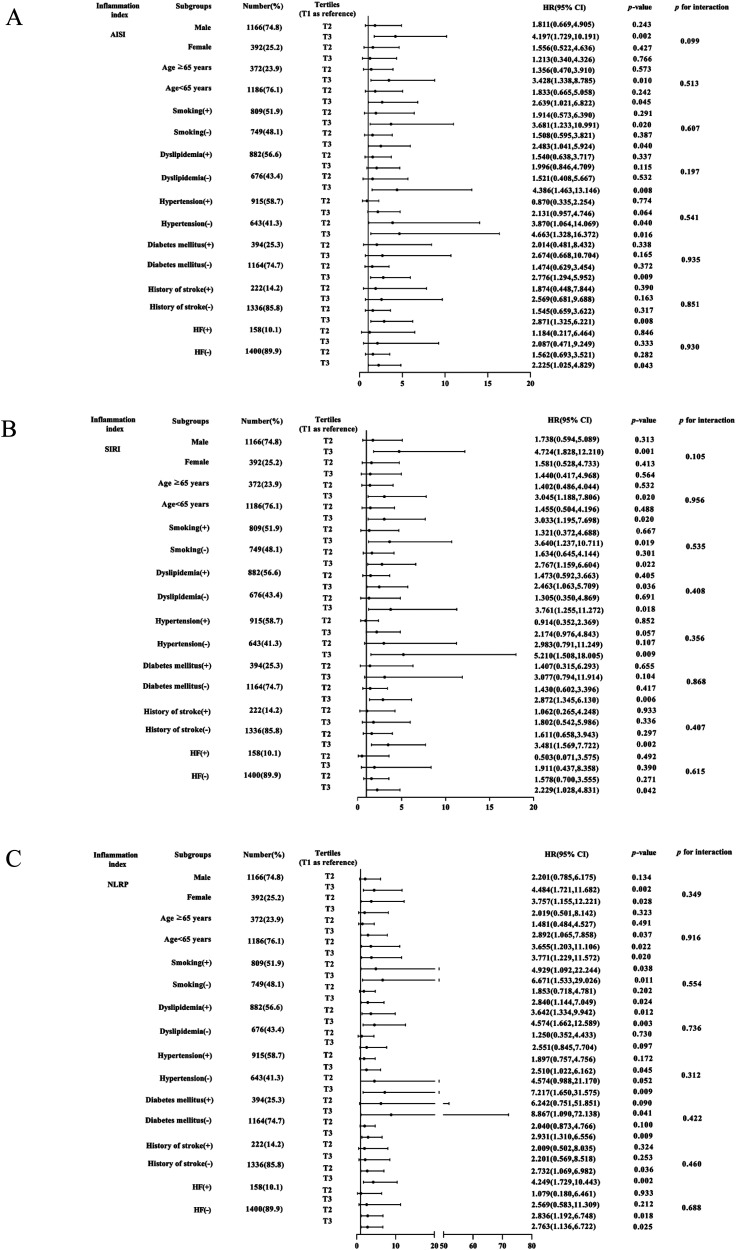
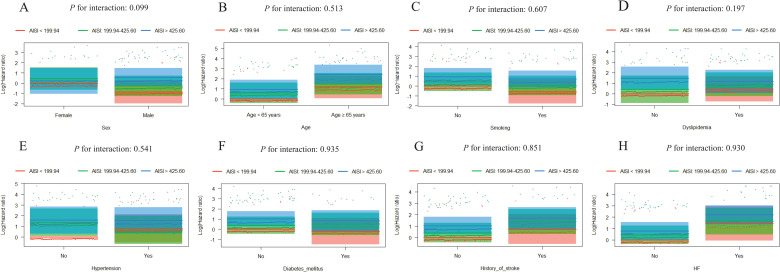
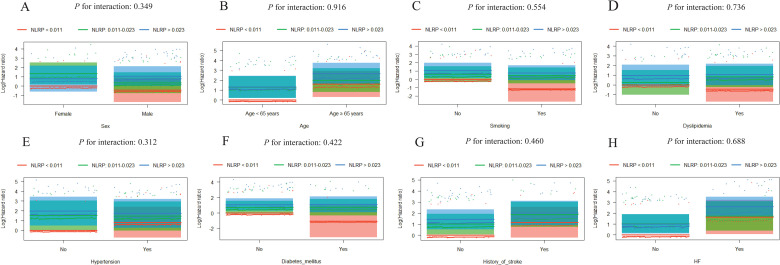
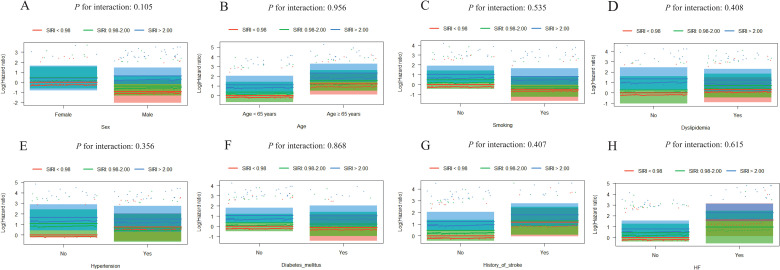
Further evaluation of the risk stratification value of these 3 indices for MACE was performed in various subgroups of the study population according to sex (male or female), age (≥ 65 or < 65 years), smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, and HF (yes or no). Increased AISI, SIRI, and NLRP were consistently related to MACE in various subgroups, despite these indices as nominal or continuous variables (all P for interaction > .05). The specific subgroup analysis results are shown in Figures 3 and 4. The independent association in different subgroups between AISI, SIRI, and NLRP (as a nominal variable) and MACE is shown in Figures 5 to 7.
Figure 3.
Forest graphs based on Cox proportional hazards analysis evaluating prognostic implication of AISI, SIRI, and NLRP in various stratifications, including sex, age, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, and HF. HR was evaluated based on 1-SD increase in these indexes. (A) Subgroup analysis of AISI, (B) SIRI, (C) and NLRP.
Figure 4.
Forest graphs based on Cox proportional hazards analysis evaluating prognostic implication of AISI, SIRI, and NLRP in various stratifications, including sex, age, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, and HF. HR was evaluated by different tertiles of these indexes. (A) Subgroup analysis of AISI, (B) SIRI, (C) and NLRP.
Figure 5.
Visualization of the possible interactions between AISI and different subgroups. Subgroup analysis according to (A) sex, (B) age, (C) smoking, (D) dyslipidemia, (E) hypertension, (F) diabetes mellitus, (G) history of stroke, and (H) HF. Column charts in each subgroup do not cross, which demonstrates the independent association between AISI and the prognosis of ACS patients undergoing PCI.
Figure 7.
Visualization of the possible interactions between NLRP and different subgroups. Subgroup analysis according to (A) sex, (B) age, (C) smoking, (D) dyslipidemia, (E) hypertension, (F) diabetes mellitus, (G) history of stroke, and (H) HF. Column charts in each subgroup do not cross, which demonstrates the independent association between NLRP and the prognosis of ACS patients undergoing PCI.
Figure 6.
Visualization of the possible interactions between SIRI and different subgroups. Subgroup analysis according to (A) sex, (B) age, (C) smoking, (D) dyslipidemia, (E) hypertension, (F) diabetes mellitus, (G) history of stroke, and (H) HF. Column charts in each subgroup do not cross, which demonstrates the independent association between SIRI and the prognosis of ACS patients undergoing PCI.
Discussion
In this study, we performed a comprehensive performance evaluation of different blood cell-derived indices in the prognosis of patients with ACS undergoing PCI. The major findings of our study were as follows: (1) AISI, SIRI, and NLPR were associated with the prognosis of ACS patients undergoing PCI, and patients with higher AISI, SIRI, and NLPR levels had a greater risk of long-term clinical cardiovascular outcomes; (2) increasing levels of AISI, SIRI, and NLPR showed a linear trend with the risk of MACE; (3) the association between AISI, SIRI, and NLPR and MACE was not significantly affected by sex, age, smoking, dyslipidemia, hypertension, diabetes mellitus, history of stroke, or HF; (4) AISI, SIRI, and NLPR had similar diagnostic values for MACE in patients with ACS after PCI compared with SII, dNLR, NLR, PLR, and MLR; (5) AISI, SIRI, and NLPR were all significantly positively correlated with SII, dNLR, NLR, PLR, and MLR, and the correlations between these indices were very strong. To the best of our knowledge, this is the first study to assess the value of the novel AISI, SIRI, and NLPR indices in the prognosis of ACS patients undergoing PCI. Moreover, there are no reports regarding the discriminatory power of these novel indices and the SII, dNLR, NLR, PLR, and MLR when distinguishing MACE from non-MACEs in patients with ACS after PCI.
Atherosclerosis is a lipoprotein-driven disease that leads to plaque formation at specific sites of the arterial tree through intimal inflammation, necrosis, fibrosis, and calcification.19 Overwhelming evidence shows that ACS is an inflammatory disease caused by the rupture or erosion of plaque with complex interactions of innate and adaptive immune systems.2,5,7 Atherosclerotic plaque destabilization, related to activation of neutrophils, monocytes, platelets, and lymphocytes, has also been postulated.20,21 WBC and their subsets are widely used in clinical practice. Previously, we described the SII, dNLR, NLR, PLR, and MLR as suitable laboratory markers for identifying high-risk ACS patients after PCI.10,11 In recent years, new systemic inflammation markers, such as AISI, SIRI, and NLPR, have attracted the attention of many researchers because they include multiple components of CBC and are also easy to obtain. Monocytes and neutrophils are part of the innate immune system and produce pro-inflammatory mediators. After activation, monocytes may transform into foam cells, destabilizing the atherosclerotic plaque and promoting dysfunctional and atherogenic lipoproteins.20,22 Monocytes can promote inflammatory and pro-thrombotic pathways by interacting with platelets.23,24 Platelets are considered to play a crucial role in vascular inflammation and can be rapidly depleted when vessels are in the acute inflammation stage.25 Moreover, platelets can capture and interact with neutrophils, which exacerbates the systemic inflammatory response.26 In contrast, lymphocytes play an important role in anti-inflammatory response.27,28 Our current study showed that patients with a MACE were more likely to have an AISI ≥293.46, SIRI ≥2.45, and NLRP ≥0.018. This phenomenon can be explained by the role of each blood cell type.
The AISI ([neutrophil*platelet*monocyte]/lymphocyte) is an inflammatory index that integrates the 4 types of cells involved in the immune response, including neutrophils, platelets, monocytes, and lymphocytes. Although AISI represents several inflammatory cells as ingredients and should provide a more precise prognostic value, no study has evaluated its association with ACS patients after PCI. Most recently, Urbanowicz et al (2022) found lower AISI levels in survivors who underwent off-pump coronary artery bypass surgery than in non-survivors.20 Zinellu et al (2021) underlined its prognostic significance in predicting mortality in patients with idiopathic pulmonary fibrosis.17 Hamad et al (2021) also studied its importance in patients with COVID-19.13 Furthermore, Erre et al (2020) evaluated the performance of the AISI in the diagnosis of rheumatoid arthritis and found that AISI was significantly associated with C-reactive protein (a classic marker of systemic inflammation).29 We found that the AISI was an independent predictor of poor outcomes in patients with ACS after PCI.
In 2016, Qi et al showed that the SIRI (neutrophil* monocyte/lymphocyte ratio) could be employed as an independent factor influencing the prognosis of patients with advanced pancreatic cancer.18 SIRI is based on the ratio of neutrophil, monocyte, and lymphocyte counts. SIRI combines all 3 to represent the balance between inflammatory activators and regulators in the host.30 These composite indicators are more stable and less susceptible than a single inflammatory indicator.31 Previous studies have shown that SIRI is a predictor of prognosis in various types of cancer.32 Very few studies have evaluated its association with patients with ACS after PCI. Cai et al (2022) conducted a retrospective study of 147 patients with infective endocarditis in the First Affiliated Hospital of Nanjing Medical University. They found that in infective endocarditis patients, SIRI was an independent predictor of poor outcome and a higher SIRI level was independently associated with the risk of in-hospital death.31 Jin et al (2021) found that a high risk of myocardial infarction was independently associated with high SIRI.33 Recently, Li et al (2022) conducted a prospective observational study in the cardiovascular center of Beijing Anzhen Hospital and found that SIRI ≥1.13 was significantly and independently associated with MACE in ACS patients who underwent PCI (HR: 2.561; 95%CI: 1.681-3.902; P < .001).4 In addition, SIRI was shown to be associated with C-reactive protein (CRP) levels, suggesting that SIRI is an indicator of inflammation levels in the body.31,34 It has also been verified that SIRI was relatively more stable than CRP, as the latter parameter had a greater risk of being impacted by blood specimen handling techniques and other infectious diseases.35 Our study also found that patients with higher SIRI levels had a greater risk of long-term clinical cardiovascular outcomes. A high SIRI status reflects a strong pro-inflammatory response mediated by monocytes and neutrophils and weak or inhibited lymphocyte-mediated anti-inflammatory response.8,31 Consequently, an elevated SIRI is linked to aggravated inflammation and poor ACS outcomes.
Previous studies have identified that NLPR is associated with abdominal surgery and cardiac surgery-associated acute kidney injury.36,37 However, no studies have assessed the prognostic ability of simple and novel NLRP for ACS patients. Recently, Nooh et al showed that an increased NLPR was significantly associated with poor survival in adult COVID-19 cancer patients. The optimal cutoff value for NLRP was 0.028 (AUC = 0.604).12 Similarly, Fois et al (2020) performed an analysis for inflammatory markers including NLRP in patients with COVID-19. The optimal cut-off value of NLRP was 0.019 (AUC = 0.706). A higher NLPR was associated with lower survival in COVID-19 survivors (HR = 4.21; 95%CI 1.94-9.13, P < .001).9 In our study, NLRP was an independent predictor of poor outcome in patients with ACS. In the ROC analysis, the optimal cut-off value of NLRP was 0.018 (AUC = 0.625). Patients with a higher NLRP showed more occurrences of MACE. Our NLRP values were similar to those of COVID-19 patients.
Limitations
Our study had several limitations. First, our data were obtained from a single center, and selection bias may have occurred. Second, the long duration of patient recruitment might lead to possible seasonal fluctuations in the blood cell ratio. Third, CRP was not evaluated due to the lack of some data. However, every effort was made to adjust for the possibility of spurious results. Finally, a single ACS subtype may have good specificity and homogeneity for study designs.
Conclusion
We found that higher AISI, SIRI, and NLRP were independently associated with a higher risk of all-cause mortality and rehospitalization for severe heart failure in patients with ACS undergoing PCI. AISI, SIRI, and NLRP had similar diagnostic values for MACE when compared with SII, dNLR, NLR, PLR, and MLR in patients with ACS after PCI. Therefore, we recommend AISI, SIRI, and NLRP as novel inflammatory biomarkers to demonstrate the prognosis of patients with ACS undergoing PCI, which could improve the prognosis of these patients.
Acknowledgments
The authors are grateful for the assistance of cardiologists, laboratory staff, and nurses at Chengde Medical University Affiliated Hospital. The authors thank Dr. Shuang Zhao for her assistance with interpreting the interaction results. They also would like to thank Editage (www.editage.cn) for the English language editing.
Footnotes
Authors’ Note: Ethical approval was obtained from the Institutional Review Board of The Affiliated Hospital of Chengde Medical University (Number: LL2021036). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Author Contributions: WF and LS designed the study. CW and YL designed the methodology. QS, YT, XW, JL, and YZ collected the clinical data. WF and LS wrote, reviewed, and edited the manuscript. All the authors have read and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Natural Science Foundation of Hebei Province (Grant no. H2021406071 to Lixian Sun), and Department of Education Graduate Innovation Funding of Hebei Province (Grant no. CXZZSS2021138 to Wenjun Fan).
ORCID iDs: Wenjun Fan https://orcid.org/0000-0002-9760-4331
Chen Wei https://orcid.org/0000-0001-7615-4215
Ying Zhang https://orcid.org/0000-0002-4822-4295
Yixiang Liu https://orcid.org/0000-0001-7185-0369
Jingyi Liu https://orcid.org/0000-0001-7123-0119
Lixian Sun https://orcid.org/0000-0001-9814-0965
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: A report from the American heart association. Circulation. 2022;145(8):e153-e639. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: A review. JAMA. 2022;327(7):662-675. [DOI] [PubMed] [Google Scholar]
- 3.Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020;106(18):1380-1386. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Ma X, Shao Q, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. 2022;9:811790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahed AC, Jang IK. Plaque erosion and acute coronary syndromes: Phenotype, molecular characteristics and future directions. Nat Rev Cardiol. 2021;18(10):724-734. [DOI] [PubMed] [Google Scholar]
- 6.Muhammad K, Ayoub MA, Iratni R. Vascular inflammation in cardiovascular disease: Is immune system protective or bystander? Curr Pharm Des. 2021;27(18):2141-2150. [DOI] [PubMed] [Google Scholar]
- 7.Engelen SE, Robinson AJB, Zurke YX, et al. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat Rev Cardiol. 2022;19(8):522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Z, Hao D, Song Y, et al. Systemic inflammatory response index as an independent risk factor for ischemic stroke in patients with rheumatoid arthritis: A retrospective study based on propensity score matching. Clin Rheumatol. 2021;40(10):3919-3927. [DOI] [PubMed] [Google Scholar]
- 9.Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23):5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X, Liu Y, Tian Y, et al. Prognostic value of peripheral blood inflammatory cell subsets in patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Int Med Res. 2021;49(4):3000605211010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan W, Zhang Y, Gao X, et al. The prognostic value of a derived neutrophil-lymphocyte ratio in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. 2021;27:10760296211034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nooh HA, Abdellateif MS, Refaat L, et al. The role of inflammatory indices in the outcome of COVID-19 cancer patients. Med Oncol. 2021;39(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamad DA, Aly MM, Abdelhameid MA, et al. Combined blood indexes of systemic inflammation as a mirror to admission to intensive care unit in COVID-19 patients: A multicentric study. J Epidemiol Glob Health. 2022;12(1):64-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334-1357. [DOI] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67-S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva PM, Duarte JS, von Hafe P, et al. Standardization of laboratory and lipid profile evaluation: A call for action with a special focus in 2016 ESC/EAS dyslipidaemia guidelines – full report. Atheroscler Suppl. 2018;31:e1-e12. [DOI] [PubMed] [Google Scholar]
- 17.Zinellu A, Collu C, Nasser M, et al. The aggregate index of systemic inflammation (AISI): A novel prognostic biomarker in idiopathic pulmonary fibrosis. J Clin Med. 2021;10(18):4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158-2167. [DOI] [PubMed] [Google Scholar]
- 19.Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852-1866. [DOI] [PubMed] [Google Scholar]
- 20.Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, et al. Neutrophil counts, neutrophil-to-lymphocyte ratio, and systemic inflammatory response index (SIRI) predict mortality after off-pump coronary artery bypass surgery. Cells. 2022;11(7):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinellu A, Paliogiannis P, Sotgiu E, et al. Blood cell count derived inflammation indexes in patients with idiopathic pulmonary fibrosis. Lung. 2020;198(5):821-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perros AJ, Esguerra-Lallen A, Rooks K, et al. Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. J Cell Mol Med. 2020;24(8):4791-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huilcaman R, Venturini W, Fuenzalida L, et al. Platelets, a key cell in inflammation and atherosclerosis progression. Cells. 2022;11(6):1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandel J, Casari M, Stepanyan M, et al. Beyond hemostasis: Platelet innate immune interactions and thromboinflammation. Int J Mol Sci. 2022;23(7):3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardon-Bounes F, Garcia C, Piton A, et al. Evolution of platelet activation parameters during septic shock in intensive care unit. Platelets. 2022;33(6):918-925. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Yu S, Qin J, et al. Prognostic value of platelet count-related ratios on admission in patients with pyogenic liver abscess. BMC Infect Dis. 2022;22(1):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Z, Cai G, Zhang P, et al. The value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as complementary diagnostic tools in the diagnosis of rheumatoid arthritis: A multicenter retrospective study. J Clin Lab Anal. 2021;35(1):e23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Si Y, Liu J, Shan W, et al. Association of lymphocyte-to-monocyte ratio with total coronary plaque burden in patients with coronary artery disease. Coron Artery Dis. 2020;31(7):650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erre GL, Buscetta G, Mangoni AA, et al. Diagnostic accuracy of different blood cells-derived indexes in rheumatoid arthritis: A cross-sectional study. Medicine (Baltimore). 2020;99(44):e22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao M, Duan X, Mi L, et al. Prognosis of hepatocellular carcinoma and its association with immune cells using systemic inflammatory response index. Future Oncol. 2022;18(18):2269-2288. [DOI] [PubMed] [Google Scholar]
- 31.Cai Z, Qiao T, Chen Y, et al. The association between systemic inflammatory response index and in-hospital mortality in patients with infective endocarditis. Clin Cardiol. 2022;45(6):664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Q, Su S, You W, et al. Systemic inflammation response index as a prognostic marker in cancer patients: A systematic review and meta-analysis of 38 cohorts. Dose Response. 2021;19(4):15593258211064744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: A ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva-Vaz P, Abrantes AM, Morgado-Nunes S, et al. Evaluation of prognostic factors of severity in acute biliary pancreatitis. Int J Mol Sci. 2020;21(12):4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, He H, Zang Y, et al. Systemic inflammation response index (SIRI) as a novel biomarker in patients with rheumatoid arthritis: A multi-center retrospective study. Clin Rheumatol. 2022;41(7):1989-2000. [DOI] [PubMed] [Google Scholar]
- 36.Gameiro J, Fonseca JA, Dias JM, et al. Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery. BMC Nephrol. 2018;19(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zou Z, Zhang Y, et al. Dynamics in perioperative neutrophil-to-lymphocyte*platelet ratio as a predictor of early acute kidney injury following cardiovascular surgery. Ren Fail. 2021;43(1):1012-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]