Abstract
The major predisposing factors of developing oral cancer include smoking, alcohol drinking, and betel quid chewing. Betel quid chewing could cause the abrasion and damage of oral mucosa by crude fibers, chemical insults by additive slaked lime, and arecoline from areca nut. These would lead to the local consequence of oral submucosal fibrosis, which is regarded clinically as a precancer lesion and a major cause of trismus. In addition, the components and additives in betel quid contain chemical toxins and carcinogens, which would further affect the oral mucosa and gradually develop a malignancy. Following literature review, aside from having a greater total tumor burden and more local diseases in the oral cavity and digestive tract, patients with betel quid-related oral cancer also have more systemic diseases from metabolic syndrome, hypertension, cardiovascular disease, type II diabetes mellitus, and obesity than those without this habit. In conclusion, those patients who have the history of smoking, alcohol drinking, and betel quid chewing would present much more unique clinical characteristics than those who only have a history of smoking and alcohol drinking. More attention should therefore be paid to pretreatment evaluation, treatment strategy, and posttreatment follow-up among betel quid chewers.
Keywords: head and neck squamous cell carcinoma, oral cancer, submucosal fibrosis, betel nut, trismus, arecoline, areca nut, oral submucosal fibrosis, metabolic syndrome, cardiovascular disease
Introduction
Betel quid chewing plays a major role in the development of head and neck squamous cell carcinoma (HNSCC) in South Asia, Southeast Asia, Taiwan, and Pacific islands. The estimated highest prevalence rate of oral cancer in males is in Taiwan, followed by Papua New Guinea, Pakistan, India, Sri Lanka, and Bangladesh (Table 1), according to the available websites containing “2019 Taiwan Cancer Registry Annual Report”1 and 2020 GLOBOCAN (Global Cancer Observatory)2 from International Association of Cancer Registries (IACR). The clinical characteristics show some differences between the regions with betel quid chewing and those without. The components of betel quid usually consist of areca nut, betel leaf, slaked lime, and/or tobacco. Betel quid chewing is a traditional behavior, which has been prevalent in these regions for hundreds of years. The scientific name of areca palm is Areca catechu. Areca nut is the seed of Areca palm. Ancient findings of A. catechu in botanical remains were obtained from archaeological work from around 10 000 B.C. in Spirit Cave in Northwestern Thailand.3 In fact, the areca palms could be found extensively in these warm areas.
Table 1.
The Estimated Prevalence of Oral Cancer in Male in 2020.
| Region | Case Number | ASR/100 000 |
|---|---|---|
| Taiwan | 4776* | 27.01a |
| Papua New Guinea | 758 | 21.18 |
| Pakistan | 11 395 | 10.14 |
| India | 104 661 | 9.76 |
| Sri Lanka | 2202 | 9.67 |
| Bangladesh | 9356 | 9.5 |
| Namibia | 64 | 6.58 |
| Australia | 1862 | 6.46 |
| Hungary | 792 | 6.32 |
| Slovakia | 466 | 6.13 |
| Latvia | 183 | 6.01 |
| Poland | 2985 | 5.99 |
| Cabo Verde | 20 | 5.98 |
| Romania | 1607 | 5.37 |
| France | 4244 | 5.35 |
| Portugal | 804 | 5.26 |
| Cuba | 782 | 5.21 |
| United Kingdom | 3931 | 5.1 |
| Russian Federation | 9582 | 5.06 |
Abbreviation: ASR, age-standardized rate.
The report in Taiwan is the updated information in 2019.
Data source:
1. 2019 Taiwan Cancer Registry Annual Report. Website Available from: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=14913
2. 2020 GLOBOCAN (Global Cancer Observatory) from International Association of Cancer Registries (IACR). Website Available from: https://gco.iarc.fr/
There are several chemical components noted in areca nut. The major component, arecoline—which is a kind of alkaloid—is found to have significant effects on human organs. Arecoline could be classified as a psychoactive substance, which could readily cross the blood–brain barrier to stimulate the central nervous system. Aside from the partial muscarine effect, arecoline also shows part of nicotinic activity, which may play a role in the habitual use and additive character from this substance in humans.4 The main pharmacologic effect of arecoline could raise body excitability, decrease sleep time, and improve the abilities of learning and memory physiologically.5 Interestingly, workers or drivers in betel quid chewing regions show greater preference in betel quid chewing than taking coffee for the purposes of continuing to work more persistently and energetically. Arecoline is an alkaloid compound that can be absorbed into the body and could be detected in the saliva, blood, urine, hair, and breast milk in persons who chew betel nut.6 Generally, the systemic absorption of arecoline may cause systemic effects over human body physiologically. The gavage studies revealed that arecoline could increase the incidence of total tumors in mice.7 In addition, the reaction of arecoline with sodium nitrite could lead to the formation of N-nitrosamines. These N-nitrosamines, which are carcinogens to humans, could be identified in the saliva if people were to chew betel quid.6 Regarding the tumor-promoting effect of arecoline, it induces epithelial–mesenchymal transformation and promotes the metastasis of oral cancer by SAA1 expression.8 Therefore, chewing betel quid, or in combination with tobacco, will cause the carcinogenesis over the mucosa of the oral cavity, pharynx, and esophagus, which was well illustrated by “International Agency for Research on Cancer” (IARC) in 1978. The working group from IARC in 2003 made the conclusion that the ingredients from areca nut are carcinogenic to humans. There also exists sufficient evidence in humans for the carcinogenicity of betel quid without tobacco. Betel quid chewing alone, and without the habit of smoking, could cause oral cancer.9 Arecoline is currently classified as a “Group 1” carcinogen to humans, according to the report from IARC in 2012.7
Generally speaking, multiple habits with smoking, betel quid chewing, and alcohol drinking will lead to a more complex and severe consequence in cancer formation to these patients. It is estimated that those patients who have the habits of betel quid chewing, smoking, and alcohol drinking will have 123 folds to develop the oral cancer than those without, according to a hospital-based case–control study in Taiwan.10 The purpose of this review is to show the local and systemic conditions among patients with betel quid-related oral cancer.
Unique Molecular Characteristics of Betel Quid-Related Oral Cancer
Are the changes of genetic landscape in oral cancer cells different between those in betel quid chewing areas and those in nonbetel quid chewing areas? This is an interesting issue, because the carcinogens from areca nut may cause a different genetic landscape in HNSCC. Chen et al reported that the most frequently mutated genes were TP53 (65%), followed by PIK3CA (16.8%), CDKN2A (12.8%), HRAS (9.3%), BRAF (9.0%), EGFR (6.7%), and FGFR3 (5.8%) in a series of 345 cases of advanced oral cancer from Taiwan by next-generation sequencing (NGS) for whole-exome sequencing in 2015.11 These authors also compared these results to a dataset from The Cancer Genome Atlas (TCGA) HNSCC, which originated from the USA with a series of 279 cases.12 They found that the frequency of genetic variations in tumor suppressor genes was similar in both studies. However, CDKN2A (12.8% vs 22.6% in the TCGA dataset) and NOTCH1 (3.2% vs 18.6% in the TCGA dataset) are a much lower degree in sequence variation than those of the TCGA dataset.11 Regarding the mutation in oncogenes, it is much more in the Taiwanese dataset than those in the TCGA dataset. These oncogenes have a more than three-fold mutation rates in the Taiwanese dataset than those in the TCGA dataset, such as AKT1 (3.2% vs 0.7%), BRAF (9% vs 1.4%), CTNNB1 (2.3% vs 0.7%), FGFR1 (1.4% vs 0.4%), FGFR2 (4.3% vs 0.7%), KIT (4.1% vs 1.1%), KRAS (2.3% vs 0.4%), and MET (4.3% vs 1.1%).11
Betel quid chewing is traditional and popular in India. This habit leads to a serious problem in causing the oral cancer. Therefore, the “India Project Team of the International Cancer Genome Consortium” was proposed13 and funded by India government. In this project, 50 cases of gingivo-buccal cancer were enrolled to investigate the mutational landscape by direct exome sequencing and 60 cases were used as a validated set.13 All these patients were classified as stage III/IV tumor and 88% patients were male. Clinically, 96% patients had the exposure of tobacco (chewing ± smoking) in this study. Accordingly, the results showed the typical signature of tobacco in a preponderance of C:G > A:T transversion. The mutation spectrum in this cohort showed 54.3% patients with CASP8 mutations. FAT1 (60%) and/or NOTCH1 showed truncating mutation predominantly. Generally, TP53 was mutated in all patients. In addition, 55% patients showed mutation over MLL4 and USP9X.13 Interestingly, the authors divided the mutation genes into several subgroups to evaluate the survival impacts. They found patients in the subclusters C1.2 (with mutations in CASP8, NOTCH1, and FAT1), C1.4 (with mutations in CASP8, NOTCH1, and ARID2), and C3.2 (with mutations in MLL4 and other genes) had significantly better disease-free survival than other patients.13 In another report, Liao et al conducted a study in an area of betel quid chewing and proposed another different nine-gene signature (RYR1, HLA-B, TSHZ2, PCDH17, DNAH17, GRID1, SBNO2, KSR2, and GCN1L1) to predict the survival by whole-exome sequencing among 168 patients with oral cancer in 2021. This nine-gene signature is also validated by TCGA database in the prognostic value independently.2 These authors found that tumor cells harboring one or more mutated genes in this nine-gene panel would lead to a worse loco-regional control, a higher incidence of distant metastasis, a worse distant free survival, a worse disease-specific survival, and a worse overall survival in these patients than those without.14
Another research group in Taiwan enrolled 120 cases of oral cancer from Taiwanese patients to study the mutation spectrum by whole-exomes sequencing in 2017.15 They found well-known mutation spectrum over TP53 (43%; that is, 43% of tumors with TP53 mutations), FAT1 (35%), EPHA2 (15%), CDKN2A (17.5%), NOTCH1 (29.2%), CASP8 (23.3%), HRAS (11.7%), RASA1 (5%), and PIK3CA (17.5%). This mutational spectrum is similar to the Taiwanese study published in 2015.11 They also uncovered the novel mutation genes, CHUK (5%) and ELAVL1 (6.7%), which are not reported before. Additionally, the copy-number alterations in several gene clusters containing CCND1 and MAP4K2 are enriched in tongue cancer.15 These authors proposed the biological significance of these two genes, CHUK and ENAVL1, in the carcinogenesis of these Taiwanese patients. ELAVL1 is encoded an RNA-binding protein, which could stabilize many cancer-related genes.16 CHUK acts as the tumor suppressor and is involved in the activation of nuclear factor κB (NF-κB) by controlling the turnover of cyclin D1.17,18
Chai et al conducted the genome-wide Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) screens among 21 oral cancer cell lines in 2020.19 They used this gene editing technology to find the genes, which were essential for these oral cancer cells to survive. The authors identified 918 genes, which were essential in survival and spread of oral cancer cells in this study. Interestingly, 110 fitness genes were found to be unique in betel-quid-associated oral cancer cell lines and some fitness genes were significantly related to NF-kB signaling pathway, including NFKB2, TNFAIP3, CSNK2A1, and TRIM25.19 These results from oral cancer cell lines are compatible to the studies of tumor tissues from Taiwan and India where the betel quid-related oral cancer is common. Particularly, the extracted components from areca nut of betel quid could directly activate the NF-kB signaling pathway to promote the tumor survival according to the past studies.20,21 In another report from Malaysia, the authors investigated the genomic alterations among oral cancer cell lines.22 Aside from well-known mutated genes, TP53, CDKN2A, EPHA2, FAT1, NOTCH1, CASP8, and PIK3CA, the authors also identified mutations over MLL4, USP9 × , and ARID2 in oral cancer cell lines, which were derived from betel quid users. These results are similar to those found in “India Project.”13
Zhang et al conducted a study about the mutational signatures and the genomic landscape of betel quid-related tongue cancer (BQ-TC) by whole–exome sequencing technique in China. They also compared with the differences between the results of this study and those of TCGA. They found the mutation rate of TP53 in BQ-TC (47%) is much lower than that in TCGA-TC (77%). The RASA1 is a kind of tumor suppressor gene which mutation rate in this Chinese cohort (20%) is much more than that in TCGA dataset (4.48%). The genetic feature of BQ-TC showed the unique one in tongue cancer. Namely, the GCG (to GTG) pattern of the C > T mutations is the major mutation pattern in BQ–TC, but not in TCGA–TC. Furthermore, this pattern of C > T mutations contributes to more mutation rate over CpG islands in BQ-TC.23
Su et al also showed the similar results. The elevated BQ-related mutation rate was noted in tongue cancer, but not in overall oral cancer in a Taiwanese study, which is a quantitative survey of mutational signatures and genomic aberrations in BQ-related oral cancer by whole–exome sequencing technique. In addition, the numbers of small insertions and deletions and breakpoints derived from structural variations were increased but the extent of loss of heterozygosity was decreased in the genomes of BQ-related oral cancer.24 Additionally, Adhikari et al conducted a study about genome-wide analyses and quantitative methylation-specific PCR in oral cancer and they showed the methylation levels of DUSP4 were significantly higher in the betel quid-related oral cancer than those in the nonrelated one and controls. The authors concluded that hypermethylation of DUSP4 could be regarded as a specific marker in betel quid-related oral cancer.25
Clinical Characteristics
Age and Gender
There are 5340 cases of first diagnosis as oral cancer in Taiwan. The median age is 57 years in males and 65 years in females in 2019. The age-standardized rate is extremely high (Table 1) and is up to 27.01 per 100 000 persons in males, but only 2.79 per 100 000 persons in females. The male-to-female ratio is up to 9.68 to 1, according to “2019 Taiwan Cancer Registry Annual Report from the website of Health Promotion Administration, Ministry of Health and Welfare.”1 The mean age of oral cancer in most Asian countries between 2000 and 2012 is 51-55 years, according to a review from Rao et al.26 However, Ariyoshi et al27 reported that the mean age of oral cancer in Japan is 65.2 years. On the other hand, the mean age of diagnosis of oral cancer in USA is 64.2 years, and the younger patients (0-39 years) with oral cancer showed an increased trend, according to the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (USA) database between 1975 and 2016.28 Interestingly, the mean age of oral cancer would be 7 to 10 years younger among those patients who have the habit of betel quid chewing. The male-to-female ratio in USA is around 2.23 to 1, according to the SEER data between 2000 and 2010.29 Regarding the gender ratio in India, the male-to-female ratio is 135 989 to 48 849, which is around 2.78 to 1, according to the 2020 India National Cancer Registry Programme.30 In addition, the male-to-female ratio is only 1.45 to 1 in Japan, according to an epidemiologic report.27 However, the male-to-female ratio is extremely larger, at 9.68 to 1, in Taiwan.1 This accounts for the male patients who have very high prevalence rate in betel quid chewing than female patients. Indeed, most female patients do not have the habit of betel quid chewing in Taiwan. This reflects the fact that the female mean age of oral cancer in Taiwan is remarkably similar to that of countries of nonbetel quid chewing. Nonetheless, the male mean age of oral cancer in Taiwan is similar to that of countries of betel quid chewing in South Asia.
Local Conditions from Betel Quid Chewing
Oral Submucosal Fibrosis with Trismus
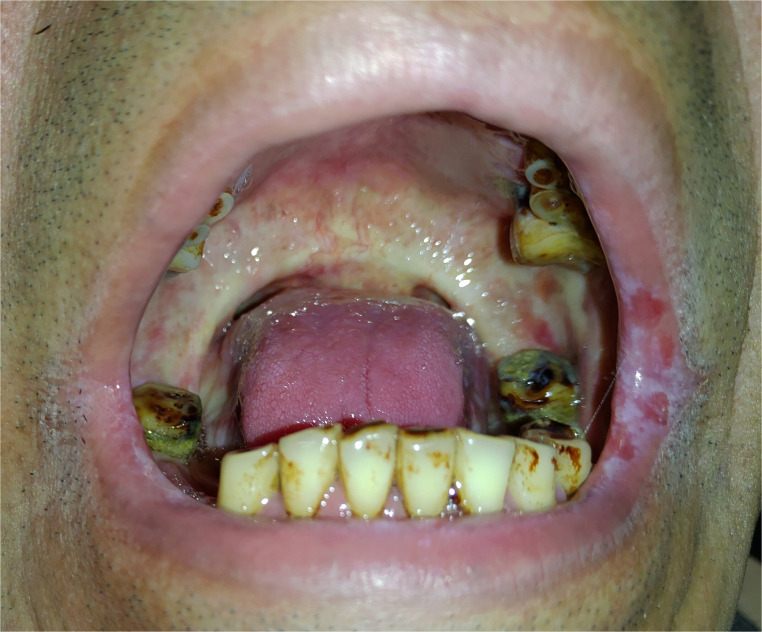
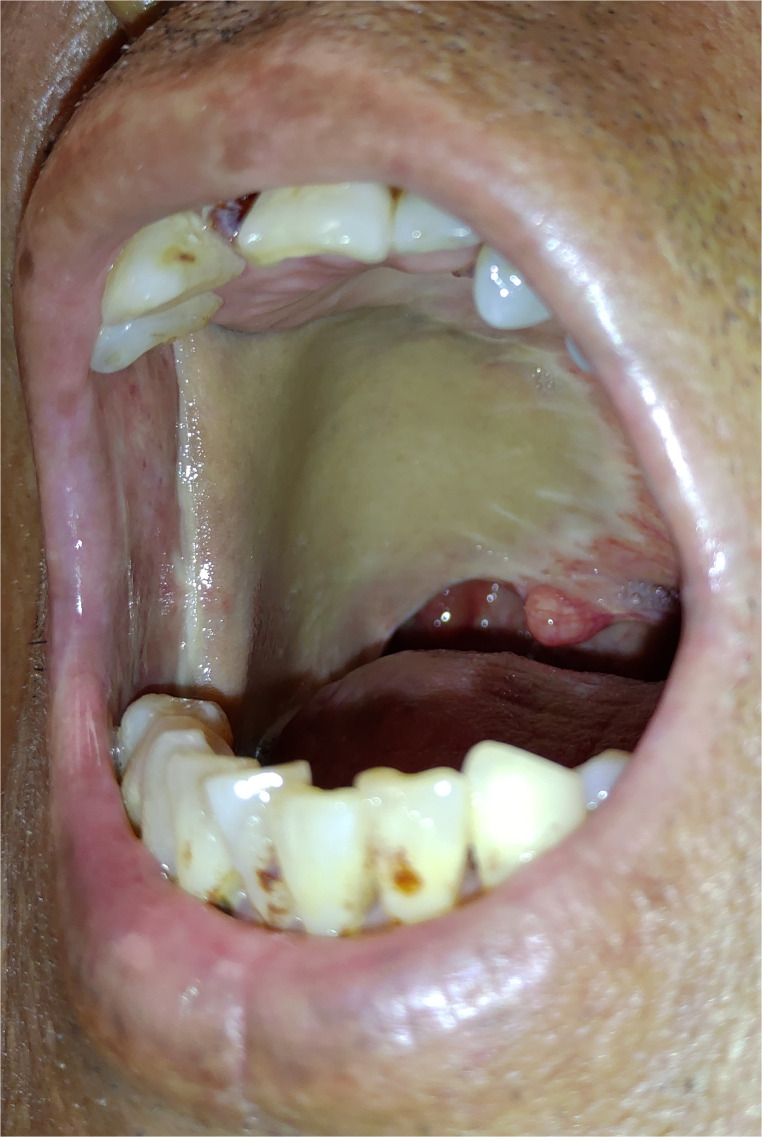
Oral submucosal fibrosis (OSMF; Figure 1) is common among patients who have the habit of betel quid chewing. Chewing betel quid for a long period of time would cause damage of oral mucosa significantly by the crude fibers in areca nut, the toxic effect from arecoline, and the corrosive character of slaked lime. Gradually, good compliance of oral mucosa will be replaced by stiff fibroblastic tissue, and this consequence may develop OSMF, which even leads to severe trismus in some patients (Figure 2). OSMF is a kind of oral potentially malignant disorder, and the pathological finding commonly reveals squamous cell hyperplasia with/without dysplasia. Patients with OSMF may develop a malignancy with an incidence of 4.5% after the initial diagnosis, in a report from India patients with a period of 8-year follow-up.31 The malignant transformation rate of OSMF in Taiwanese patients is 1.99% at a period of 10-year follow-up.32
Figure 1.
This patient shows submucosal fibrosis over the oral cavity and soft palate. Erythro-leukoplakia over mucosa of the left upper and lower lips is also noted. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
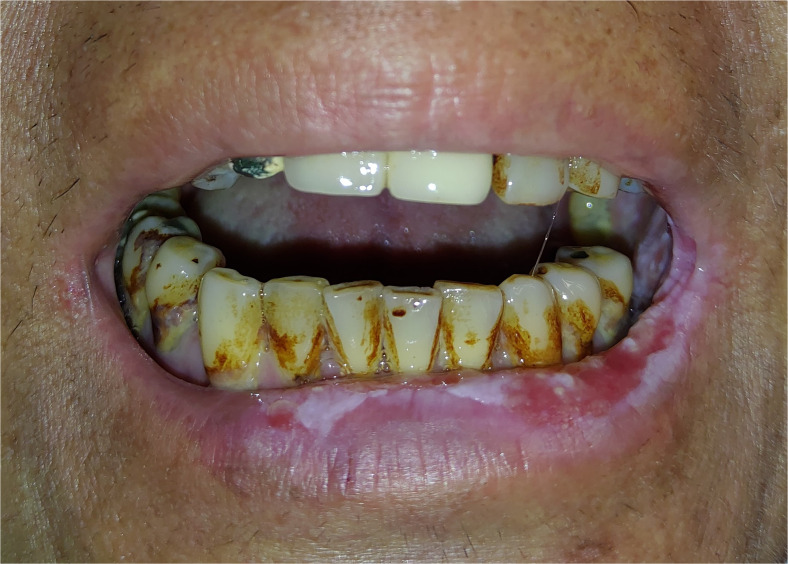
Figure 2.
This patient shows submucosal fibrosis with trismus over the oral cavity with limited maximal interincisal opening. Erythro-leukoplakia over mucosa of the lower lip is also noted. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
It is necessary to bring about long-term awareness in the mucosal change of the oral cavity among patients with OSMF, although the malignant transformation rate is less than 5% in a long period of follow-up.31,32 However, the persistent smoking, betel quid chewing, and liquor drinking will further increase the malignant transformation rate of OSMF. Clinically, the definition of trismus in patients with head and neck cancer is the maximal distance between upper and lower incisors, with a maximal interincisal opening (MIO) of less than 3.5 cm.33,34 Transoral excision of oral cancer or full inspection of oral lesion will be hampered if the distance of MIO is too short (Figure 2). The approach to oral cancer in this scenario is advised to shift to an external approach. It includes the lip splitting to remove buccal/buccogingival sulcus cancer and mandibulotomy or pull-through approach to remove tongue cancer. The oral rehabilitation in the dental field after treatment of oral cancer is a challenge in some patients because of the limitation in MIO due to OSMF and the consequences of treatment in oral cancer. Proper reconstruction for oral defect after ablation of oral cancer is essential in preventing further trismus after surgery. Local tongue flap35 (Figure 3), submental island flap,36 and radial forearm free flap (RFFF) (Figure 4)37 or trimmed anterior lateral thigh free flap38 (Figure 5) could be used for the defect of oral inner lining. Nasolabial myocutaneous flap39,40 is another reconstructive option for a buccal mucosal defect over anterior to middle part, although the facial scar is often a concern from patients. Wei et al reported that bilateral coronoidectomy, together with the oral reconstruction by RFFFs could, add more distance in the MIO after surgery in his series.37 However, the donor site morbidity is more by using RFFF for reconstruction of oral cavity defect.
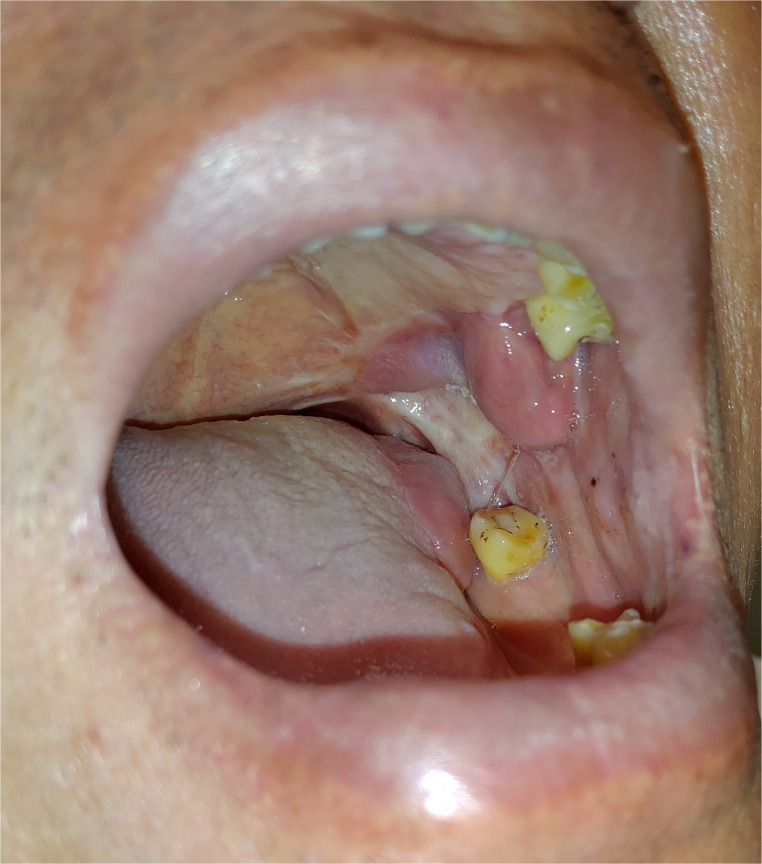
Figure 3.
This patient underwent excision of the left retromolar trigone cancer and reconstruction of oral soft tissue defect by tongue flap, which was divided about 6 weeks later. Transferred tongue tissue could be found over the left retromolar trigone. Original maximal interincisal opening is well preserved. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
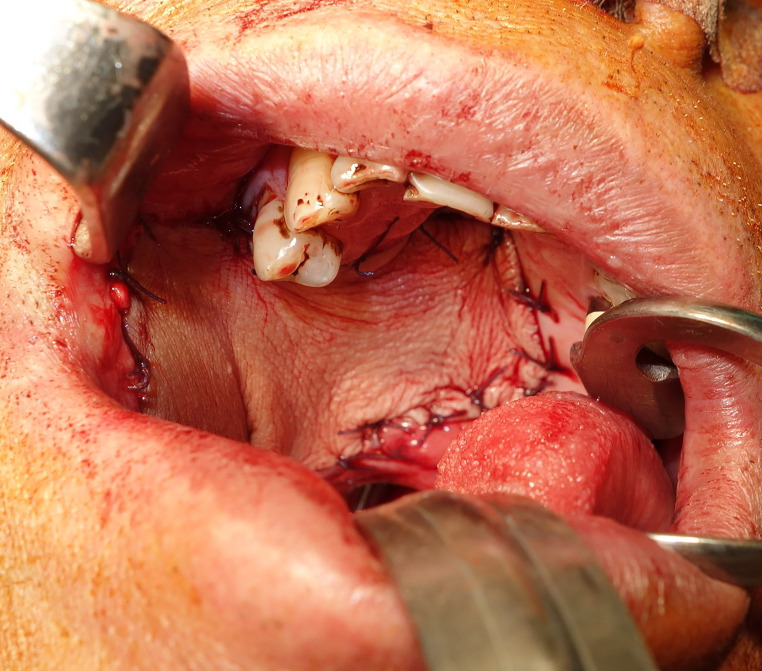
Figure 4.
This patient underwent excision of right buccal cancer, which extended to mucosa of the soft palate and hard palate. Radial forearm free flap was applied to reconstruct this defect. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
Figure 5.
This patient underwent excision of right buccal cancer, which extended to mucosa of the soft palate and hard palate. Trimmed anterior lateral thigh free flap was applied to reconstruct this defect. Original maximal interincisal opening is well preserved after surgery. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
There are clinical reports to evaluate the prognosis of oral cancer between those patients with OSMF and without. Chaturvedi et al suggested that oral cancer originating in background of OSMF reveals a distinct disease clinicopathologically. They found most patients of oral cancer arising from OSMF were young males. Their tumors showed thinner lesions, earlier tumor stage (pT), lesser incidence of neck metastases, better differentiated tumors, and lesser incidence of extracapsular spread (ECS) of lymph node than those patients without the OSMF. In addition, patients of oral cancer with OSMF even in advanced tumor stage (pT) would show lesser incidence of neck metastasis and ECS of lymph node than those without OSMF. All these distinct characters would contribute the better survival chance among patients with OSMF than those without.41,42 Another report from India confirmed the similar results that patients with oral cancer coming from OSMF have better survival chances than those without.43 Zhou et al also reported the similar findings. There is no lymph node metastasis in oral cancer among Chinese patients with OSMF. Additionally, the histologic type was also the well differentiated one.44 The lesser incidence in neck lymph node metastasis among patients with OSMF may come from the blockage of submucosal lymphatic channels and vascularity in fibrotic tissue.41 In fact, survival studies performed in different regions produced inconsistent results. Regarding the comparison of prognosis of oral cancer between betel quid chewers and nonbetel quid chewers, there is an interesting study of systemic review and meta-analysis focusing in this issue. Compared with nonchewers, the pooled hazard ratio (HR) among betel quid chewers was 1.26 for 5-year overall survival and 1.40 for 5-year disease-specific survival. Yang et al concluded that betel quid chewing is significantly associated with poor prognosis among patient with oral cancer. Of course, regarding the role of betel quid chewing on the prognosis of oral cancer, the current evidence is not adequate and controversial. Further studies are warranted.45
The treatment strategy among patients with OSMF is recommended to have a proper reconstruction as mentioned above after the removal of oral cancer to prevent further trismus, which would cause a worse condition in the quality of life and hamper a detailed inspection over oral mucosa after surgery clinically. Furthermore, the long-term follow-up of oral mucosa is necessary to detect the progressive change of abnormal mucosa in oral cavity or upper aerodigestive tract. Regarding the issue of surgical margin of oral cancer in patients with OSMF, the surgical margin is recommended to have 1-2 cm and the goal of pathological margin over 5 mm is also mandatory to achieve a better clinical outcome. Clinically, the application of intra-operative chemodetection for mucosal margin control by Lugol's staining is feasible and convenient for surgeon to detect abnormal mucosal margin with moderate dysplasia, carcinoma in situ or even early squamous cell carcinoma in Logol-voiding area.46
Chronic Periodontitis
Long-term betel quid chewing, smoking, and poor oral hygiene would lead to chronic periodontitis (Figure 6). Loosening of teeth due to periodontitis and gradual loss of teeth—or even becoming edentulous—is not rare, if patients do not keep oral hygiene and undergo adequate treatment of this disease. Currently, the association between betel quid chewing and periodontitis is well reported in the literature.47–49 Clinically, periodontitis is demonstrated to have a positive association with coronary artery disease50 and cerebrovascular disease51 in the epidemiological reports. These two medical conditions are advised to be thoroughly evaluated prior to treatment of oral cancer among these chewers. Furthermore, a recent report showed that chronic inflammatory status in chronic periodontal disease might have an association with oral cancer.52 Treatment of this chronic periodontal disease by a periodontist or an oral and maxillofacial surgeon following the complete treatment of oral cancer is essential among these patients.
Figure 6.
This betel quid chewer shows chronic periodontitis. Notably, submucosal fibrosis of oral mucosa with trismus is recorded. Right lower lip thick leukoplakia, central lower lip thin leukoplakia, and left anterior buccal small cancer are also noted simultaneously. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
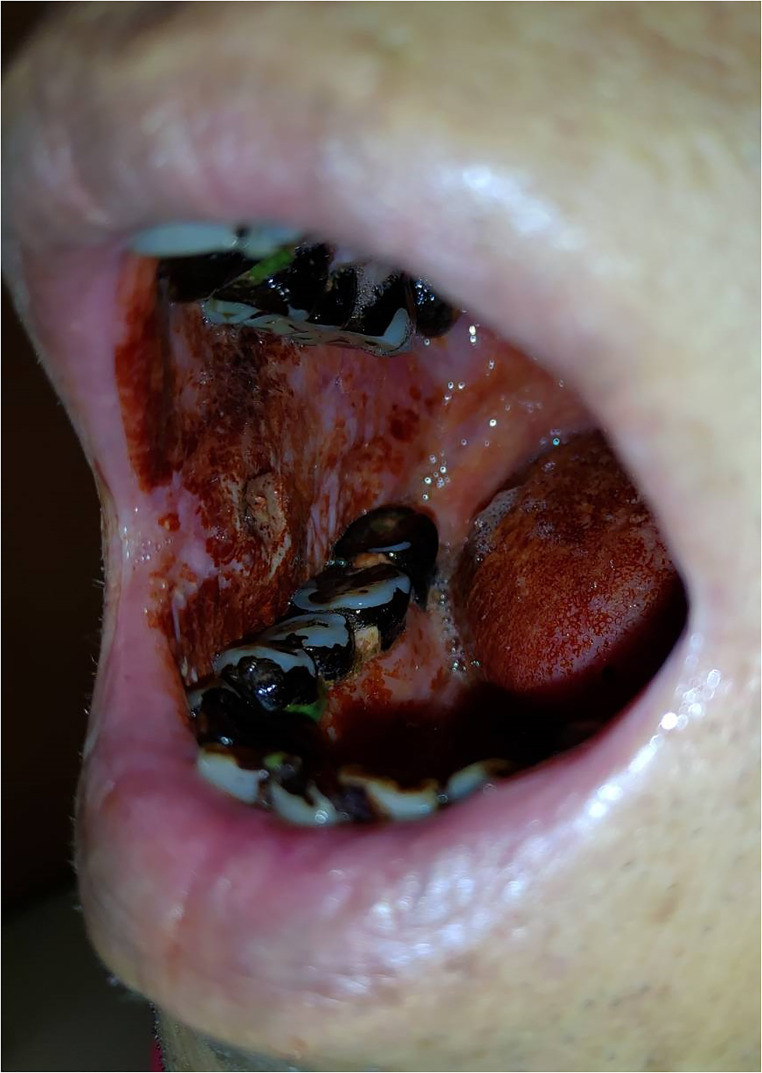
Extensive Tumor Burden Over Digestive Tract
Oral cancer patients with a combination of habits including betel quid chewing, smoking, and alcohol drinking would develop more extensive damage to the mucosa of aerodigestive tract than those with only the habits of smoking and alcohol drinking. Impressively, betel juice staining would soon coat over the mouth after betel quid chewing (Figure 7). In addition, the betel juice staining and residues of betel quid are swallowed all along the food pipe and the coatings are found all over the mucosa of base of tongue, epiglottis, pyriform sinus, whole esophagus, and stomach (Figure 8). Among patients with oral cancer, second primary site over esophageal cancer is poor prognosis in the late stage. In fact, the key component of areca nut—arecoline—could cause carcinogenesis over esophagus in mice.6 One population-based study in Taiwan shows that those patients who have the esophageal cancer diagnosed as the second primary cancer after the initial diagnosis of oral cancer in Taiwan are not rare. Compared with the general population, the standardized incidence ratio is up to 5.15 folds after a period of 5–10 years of follow-up.53 The other reports also revealed that the incidence of synchronous esophageal cancer would be 0.79%,54 and metachronous one would be 1.6%55 among patients with oral cancer in Taiwan. Betel quid chewing is also a risk factor for developing esophageal cancer, according to the reports from India.56,57
Figure 7.
This betel quid chewer had right side buccal cancer. He still chewed betel quid before his visit at our outpatient office for his malignancy. The betel juice could readily coat over the oral mucosa and this ulcerative tumor. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.).
Figure 8.
This betel quid chewer had left tonsil cancer with p16 negative tumor. He still chewed betel quid before his visit at our outpatient office for his malignancy. The betel juice and residues of areca nut could readily coat over the mucosa of base of tongue, epiglottis, hypopharynx, whole esophagus, and stomach. C.E., cervical esophagus; GE J, gastroesophageal junction; M/3, middle third esophagus. (This is an original figure and unpublished data. Informed consent was obtained for image use, although no personal identifying information is visible in the image.)
The most common second primary site from oral cancer is the oral cavity itself, or the mucosa of pharynx. Compared with the general population, the standardized incidence ratio is up to 14.67 folds to develop second primary site over the oral cavity/pharynx after a period of 5–10 years follow-up.53 Another report, which is a population-based study with one-million randomized sample size from Taiwan's National Health Insurance Research Database, showed that those patients with HNSCC in Taiwan would have a significantly higher incidence to develop esophageal cancer (HR: 12.34), gastric cancer (HR: 3.54), and colorectal cancer (HR: 1.51) compared to those who are not HNSCC.58 Generally, more than 75% patients who develop HNSCC in Taiwan would have the habit of betel quid chewing.59 The number of oral cancer accounts for nearly 60% of HNSCC in Taiwan annually, according to 2019 Taiwan Cancer Registry Annual Report. This large sample size with one-million population-based study could roughly reflect the higher incidence rate of second primary site development among patients with oral cancer in Taiwan. Regarding the carcinogenetic effect over the liver, the report from Taiwan showed that betel quid chewing would increase the risk of hepatocellular carcinoma (HCC) in a case–control study. Those persons who chewed betel quid would have a higher incidence (OR: 3.49) to develop HCC compared to those without betel quid chewing. Furthermore, there was a synergetic effect between betel quid chewing and the infection of chronic hepatitis B virus or hepatitis C virus.60 Basically, most studies illustrated in this manuscript are case–control studies from Taiwan. The data is produced from the oral cancer patients compared with the general population, but not from the comparison between the betel quid-related oral cancers and betel quid nonrelated oral cancers. The evidence was not enough to convince the readers. Indeed, it would be worthwhile to conduct the international multi-institute study in comparison of the conditions of digestive tract between patients with betel quid-related oral cancer and those with betel quid nonrelated oral cancer.
Medical Conditions from Betel Quid Chewers
Increased Incidence of Gastroesophageal Diseases
Betel quid chewers have a higher chance of causing irritation over the gastroesophageal mucosa directly. This could be observed in Figure 7 in a patient who swallowed the betel juice and residues into a food pipe. Hung et al reported in 1994 about the phenomenon of gastric mucosa damage by betel quid chewing.61 Additionally, the behavior of betel quid chewing would have a chance to bring these nondisinfected and raw substances with the notorious bacteria, Helicobacter pylori into gastrointestinal tract.62 Such kinds of bacteria may further aggravate the condition of gastric mucosa. A gastric ulcer—or even a gastric cancer—could happen several years later if patients do not undergo treatment. However, there is no exact data reported till now about the prevalence rate of H. pylori infection in gastric mucosa or peptic ulcer among persons who are chewers. Notably, most patients who have the habit of betel quid chewing also have the habit of smoking, and even alcohol drinking, in Taiwan. Clinically, patients who have concomitant these three habits will have 2.99-fold risk of Barrett's esophagus, 1.60-fold risk of grade A-B erosive esophagitis, 2.00-fold risk of gastric ulcer, 2.12-fold risk of duodenitis, and 1.29-fold risk of duodenal ulcer than those without, according to a large series of cross-sectional study.63
Increased Incidence of Hypertension and Cardiovascular Disease
Arecoline is a kind of partial agonist of muscarinic acetylcholine receptors and nicotinic receptor.4 Physiologically, the binding of arecoline to muscarine receptors would lead to parasympathomimetic effects. The behavior of betel quid chewing would increase the heart rate and systolic pressure in chewers due to central sympathetic response. However, decreased diastolic blood pressure could be noted among chewers due to peripheral cholinergic effects.64 The study from Bangladesh showed that chewing betel quid without tobacco was associated with general hypertension and systolic hypertension after adjusting other explanatory variables.65 Another epidemiologic report from Taiwan showed that betel quid chewing was significantly associated with hypertension in Taiwanese patients with type II diabetic mellitus and that the association was stronger in women.66 The chewers having a higher systolic blood pressure was based on a cross-sectional study in Taiwan.67 In addition, most betel chewers have the habit of smoking in Taiwan, and this is also a synergetic factor in leading to a higher incidence of hypertension in this cohort.
Betel chewers showed a higher incidence of cardiovascular diseases, which are shown in some epidemiologic reports. One epidemiologic study, which enrolled 56 116 participants with an 8-year follow-up, showed that relative risk of cardiovascular disease among the former betel quid chewers and current chewers was 1.56 and 2.02, respectively, compared to those without this habit after the adjustment of many variables. These authors concluded that betel nut chewing was independently associated with a greater risk of cardiovascular disease and all-cause mortality in Taiwanese men.68 Other reports also showed the similar result that betel chewers are independently associated with heart disease among women69 and men70 in Taiwan. Furthermore, chewers showed a significantly higher risk of having cardiovascular disease up to 24% after further adjustment for age, education, and other significant confounders in a community-population-registry-based integrated screening program in Taiwan.71 In the evidence from an animal model, the in vivo studies showed that arecoline from areca nut could lead to significant cardiotoxicity, heart damage, and cardiac fibrosis by inducing several hypertrophy-related signaling pathways in rats.72,73
Northeast India is one of the most prevalent in betel quid chewing region. Choudhury et al found that there may be a link between betel quid chewing and cerebral vascular disease such as stroke in Northeast India based on clinical observation. They conducted a computational study about arecoline and showed that arecoline has the potential to block the high-density lipoprotein (HDL) receptor. These authors suggested that arecoline may have a role in the contribution of atherosclerosis.74 This computational study for development of atherosclerosis model by arecoline should be further validated by in vivo and clinical studies.
Increased Incidence of Obesity, Metabolic Syndrome, and Type II Diabetes Mellitus
Patients with metabolic syndrome would have the concomitant conditions of higher levels of blood sugar, blood pressure, cholesterol, and triglyceride. The body shape with excess adipose tissue over the waist is also noted. In essence, those patients who have metabolic syndrome would have a greater chance of developing diabetes mellitus, stroke, and coronary artery disease. Regarding the obesity issue in betel chewers, this habit would increase the risk of obesity noted by a “2001 National Health Interview Survey” in Taiwan with an enrollment of 6126 males. In this cohort, 16.2% cases showed that the cause of obesity was suspected to be from the increased appetite after betel quid chewing.75 Another report from Taiwan also confirmed the relation between central obesity and betel chewers.76
Generally, betel chewers have a higher chance of developing metabolic syndrome. There are some case–control and epidemiologic studies aiming at this issue so far. A report of a case–control study showed that betel quid chewing was an independent risk factor for having the metabolic syndrome, with the odd ratio of 1.92 in males, and 1.60 in females.77 The case–control study from “National Cholesterol Education Program Adult Treatment Panel III” showed similar results in Taiwanese adults. The authors concluded that betel quid chewing could lead to a metabolic syndrome.78 Furthermore, the large series of population-based study with an enrollment of 19 839 Taiwanese participants also showed that there were independent predictive dose-response effects in betel-quid chewing to develop metabolic syndrome after adjustment of well-known risk factors.79 Hsu et al conducted an in vitro study, and they showed that arecoline could inhibit adipogenic differentiation and interfere with insulin-induced glucose uptake, which would further lead to hyperlipidemia and hyperglycemia/insulin-resistance due to fat cells dysfunction. These findings could provide more evidence about the development of metabolic syndrome from the habit of betel quid chewing.80
Essentially, those patients with metabolic syndrome would have a higher chance of developing type II diabetes mellitus. Tung et al conducted a population-based cross-sectional survey in Taiwan and showed that betel chewers had higher prevalence rates to have type II diabetes (10.3% vs 7.8%) than those without. Furthermore, this habit was an independent factor in developing type II diabetes with dose dependence and duration of chewing in Taiwanese males.81 Another population-based study in Taiwan revealed that betel chewers among patients with diabetes mellitus were younger and more obese than never-chewers. The author concluded that this betel chewing habit is diabetogenic.82
Increased Incidence of Liver Cirrhosis and HCC
Aside from hepatitis B virus, hepatitis C virus, and other chemical toxins, areca nut and betel quid would also cause a significant burden of liver diseases, because the main ingredient of areca nut—arecoline—is not only a carcinogen to the oral cavity mucosa, but also may have a role in the association with a higher risk in liver diseases such as liver cirrhosis83–86 and HCC.60, 86–88 This risk could be synergistically additive to those of hepatitis B or C virus infections.86 In fact, all of these studies mentioned above are community-based studies to support the relationship between liver diseases and betel quid chewing. In vitro and in vivo studies to further confirm the hepatocytes damage and carcinogenesis directly by this toxin from areca nut are few in the literature. In 1992, India researchers reported on the liver toxicity by pan masala (betel quid without betel leaf) from gavage studies in rats.89 They found that chronic feeding of pan masala could lead to the impairment of liver function. The mechanisms of liver toxicity by arecoline are not well known. In an in vivo study, arecoline induces hepatoxicity through the induction on rat hepatic CYP2E1 and 2B.90 Dasgupta at al. conducted an in vivo study about the arecoline toxicity in mice and found that arecoline could cause hepatotoxicity by disrupting the hepatocyte ultrastructure in mice and increase the hepatotoxic marker enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), in serum.91 Furthermore, Wang et al reported an in vitro study about the toxicity of arecoline and its oxidative metabolite, arecoline N-oxide, which is ingested by chewers and is a kind of primarily oxidized substance, in the normal liver cell lines. They found that arecoline N-oxide can produce a higher incidence of liver cells cytotoxicity, DNA damage, and mutation than its parent compound, arecoline, in liver cells.92
Clinical Implications
Patients with betel quid-related oral cancer suffer from the local and systemic conditions from betel quid chewing including the increased risk in developing metabolic syndrome, coronary artery disease, type II diabetes mellitus, and cerebral artery disease. An increased cancer burden is also noted clinically. Basically, these comorbidities are related to chewing dose and duration but the exact dose and duration in developing these comorbidities is not fully investigated. Pretreatment evaluation is recommended to have a more detail in systemic review for these medical conditions. Regarding the surveillance over digestive tract, gastro-esophagoscopy with narrow band image (NBI), and positron emission tomography (PET) are the recommended measurements for the detection of synchronous primary sites. Abdomen sonography is helpful to survey the liver or biliary tract diseases. Swallowing and speech problems are also common among these patients after oral cancer treatment. Earlier intervention through the assistance of speech pathologist is advocative. Additionally, synchronous cancer over aerodigestive tract and undetected coronary artery disease are not rare in these patients. Multidisciplinary consultation with other cancer team members, cardiologist, neurologist, or even endocrinologist is necessary to seek for the optimal treatment strategy.
Worse survival could be observed among patients with betel nut-related oral cancer if comorbidities and synchronous cancer93 happen in these patients. The metachronous cancer would show clinical impacts in the overall survival rate. Patients with metachronous cancer over oral cavity would be observed to have a decreased 10-year overall survival rate from 61% to 30% (calculated from the diagnosis of each primary site) if the fourth primary tumor is noted.94 Long-term follow-up are necessary among these patients to actively survey the possible recurrent tumor or metachronous tumor to increase the long-term overall survival. The follow-up period is recommended once per year among patients who complete the treatment of oral cancer for 5 years according to the American National Comprehensive Cancer Network (NCCN) guideline. However, the field of cancerization over oral mucosa is so extensive in these patients with betel quid-related oral cancer. The return visit is suggestive to be 6 months or sooner even after the 5-year treatment in these patients. In addition, the integration of cardiologist, endocrinologist, gastroenterologist, and neurologist to form a medical team for these patients after treatment is recommended for the survivorship care if the related comorbidities develop.
The target measurements in the return visit are suggestive to follow the NCCN guideline in Head and Neck Cancers (Version 2. 2022) among patients with betel quid-related oral cancer although there are unique clinical presentations among these patients. Besides the cessation program of smoking and drinking, this program is recommended to include the betel quid chewing. In another point, active endoscopy in surveillance of the mucosa over laryngo-pharynx, esophagus, and stomach among these patients is recommended at a 6-month interval if there is a precancer lesion noted by NBI before treatment.
Conclusions
The clinical characteristics of oral cancer among betel quid chewers exhibit highly unique presentations, either locally or systemically. Aside from the local problems of trismus, periodontitis, and submucosal fibrosis of the oral cavity—which would cause poorer quality of life after treatment—patients who are betel quid chewers would also experience much more tumor burden, either synchronous or metachronous cancer, and a higher incidence of medical conditions such as hypertension, cardiovascular disease, gastrointestinal diseases, type II diabetes mellitus, metabolic syndrome, obesity, and liver diseases than those without this habit would. In clinical implication, more attention should be paid to pretreatment evaluation, treatment strategy, and posttreatment follow-up among patients with betel quid-related oral cancer due to the local and systemic conditions from betel quid chewing.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Statement: Not Applicable. Informed consent was obtained by patients for image use, although no personal identifying information is visible in any images.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and Technology, ROC (grant number: MOST 108–2314-B-182A-112-MY3, MOST 107–2314-B-182A-085, and MOST 101–2314-B-182A-075-MY3).
Author's Contribution: Hui-Ching Chuang wrote this manuscript. Ming-Hsien Tsai, Yu-Tsai Lin, Ming-Huei Chou, and Kun-Lin Yang edited and reviewed this manuscript. Chih-Yen Chien designed and reviewed this manuscript. All authors gave final approval of this manuscript for submission.
ORCID iD: Chih-Yen Chien https://orcid.org/0000-0002-6690-6038
References
- 1.Health Promotion Administration, Ministry of Health and Welfare, ROC. Cancer Registry Report in Taiwan. https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=14913
- 2.Global Cancer Observatory - International Agency for Research on Cancer (IARC). https://gco.iarc.fr/. Accessed July 20, 2022.
- 3.Gorman CF. Excavations at spirit cave, north Thailand: SOME INTERIM INTERPRETATIONS. Asian Perspectives. 1970;13:79‐107. [Google Scholar]
- 4.Papke RL, Horenstein NA, Stokes C. Nicotinic activity of arecoline, the psychoactive element of "betel nuts", suggests a basis for habitual use and anti-inflammatory activity. PLoS One. 2015;10(10):e0140907. doi: 10.1371/journal.pone.0140907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YJ, Peng W, Hu MB, Xu M, Wu CJ. The pharmacology, toxicology and potential applications of arecoline: a review. Pharm Biol. 2016;54(11):2753‐2760. doi: 10.3109/13880209.2016.1160251 [DOI] [PubMed] [Google Scholar]
- 6.IARC Monographs Vol 128 group. Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncol. 2021;22(1):19‐20. doi: 10.1016/S1470-2045(20)30727-0 [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1‐538. [PMC free article] [PubMed] [Google Scholar]
- 8.Ren H, He G, Lu Z, et al. Arecoline induces epithelial-mesenchymal transformation and promotes metastasis of oral cancer by SAA1 expression. Cancer Sci. 2021;112(6):2173‐2184. doi: 10.1111/cas.14866 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.International Agency for Research on Cancer. Betel-quid and Areca-nut chewing and some Areca-nut-derived nitrosamines. IARC Monogr on the Eval of Carcinog Risks Hum. 2004;85:1‐349. [PMC free article] [PubMed] [Google Scholar]
- 10.Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24(10):450‐453. doi: 10.1111/j.1600-0714.1995.tb01132.x [DOI] [PubMed] [Google Scholar]
- 11.Chen SJ, Liu H, Liao CT, et al. Ultra-deep targeted sequencing of advanced oral squamous cell carcinoma identifies a mutation-based prognostic gene signature. Oncotarget. 2015;6(20):18066‐18080. doi: 10.18632/oncotarget.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576‐582. doi: 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.India Project Team of the International Cancer Genome Consortium. Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat Commun. 2013;4:2873. doi: 10.1038/ncomms3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao CT, Yang LY, Lee LY, et al. Whole-exome sequencing identifies biosignatures that predict adverse survival outcomes in surgically treated patients with oral cavity squamous cell carcinoma. Oral Oncol. 2021;122:105547. doi: 10.1016/j.oraloncology.2021.105547 [DOI] [PubMed] [Google Scholar]
- 15.Su SC, Lin CW, Liu YF, et al. Exome sequencing of oral squamous cell carcinoma reveals molecular subgroups and novel therapeutic opportunities. Theranostics. 2017;7(5):1088‐1099. doi: 10.7150/thno.18551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Guo Y, Chu H, Guan Y, Bi J, Wang B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci. 2013;14(5):10015‐10041. doi: 10.3390/ijms140510015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Descargues P, Sil AK, Karin M. IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer. EMBO J. 2008;27(20):2639‐2647. doi: 10.1038/emboj.2008.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak YT, Radaideh SM, Ding L, et al. Cells lacking IKKα show nuclear cyclin D1 overexpression and a neoplastic phenotype: role of IKKα as a tumor suppressor. Mol Cancer Res. 2011;9(3):341‐349. doi: 10.1158/1541-7786.MCR-10-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai AWY, Yee PS, Price S, et al. Genome-wide CRISPR screens of oral squamous cell carcinoma reveal fitness genes in the hippo pathway. Elife. 2020;9:e57761. doi: 10.7554/eLife.57761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang SL, Chen PH, Lee CH, et al. Up-regulation of inflammatory signalings by areca nut extract and role of cyclooxygenase-2 −1195G>a polymorphism reveal risk of oral cancer. Cancer Res. 2008;68(20):8489‐8498. doi: 10.1158/0008-5472.CAN-08-0823 [DOI] [PubMed] [Google Scholar]
- 21.Lin SC, Lu SY, Lee SY, Lin CY, Chen CH, Chang KW. Areca (betel) nut extract activates mitogen-activated protein kinases and NF-kappaB in oral keratinocytes. Int J Cancer. 2005;116(4):526‐535. doi: 10.1002/ijc.21104 [DOI] [PubMed] [Google Scholar]
- 22.Fadlullah MZ, Chiang IK, Dionne KR, et al. Genetically-defined novel oral squamous cell carcinoma cell lines for the development of molecular therapies. Oncotarget. 2016;7(19):27802‐27818. doi: 10.18632/oncotarget.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Wang M, Wu Q, et al. Mutational signatures and the genomic landscape of betel quid chewing-associated tongue carcinoma. Cancer Med. 2019;8(2):701‐711. doi: 10.1002/cam4.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su SC, Chang LC, Lin CW, et al. Mutational signatures and mutagenic impacts associated with betel quid chewing in oral squamous cell carcinoma. Hum Genet. 2019;138(11-12):1379‐1389. doi: 10.1007/s00439-019-02083-9.3 [DOI] [PubMed] [Google Scholar]
- 25.Adhikari BR, Yoshida K, Paudel D, et al. Aberrant expression of DUSP4 is a specific phenomenon in betel quid-related oral cancer. Med Mol Morphol. 2021;54(2):79‐86. doi: 10.1007/s00795-020-00265-3 [DOI] [PubMed] [Google Scholar]
- 26.Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade--an update (2000-2012). Asian Pac J Cancer Prev. 2013;14(10):5567‐5577. doi: 10.7314/apjcp.2013.14.10.5567 [DOI] [PubMed] [Google Scholar]
- 27.Ariyoshi Y, Shimahara M, Omura K, et al. Japanese Society of Oral and Maxillofacial Surgeons. Epidemiological study of malignant tumors in the oral and maxillofacial region: survey of member institutions of the Japanese Society of Oral and Maxillofacial Surgeons, 2002. Int J Clin Oncol. 2008;13(3):220‐228. doi: 10.1007/s10147-007-0756-9 [DOI] [PubMed] [Google Scholar]
- 28.Bajpai S, Zhang N, Lott DG. Tracking changes in age distribution of head and neck cancer in the United States from 1975 to2016. Clin Otolaryngol. 2021;46(6):1205‐1212. doi: 10.1111/coa.13817 [DOI] [PubMed] [Google Scholar]
- 29.Weatherspoon DJ, Chattopadhyay A, Boroumand S, Garcia I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000-2010. Cancer Epidemiol. 2015;39(4):497‐504. doi: 10.1016/j.canep.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nallasamy V, John A, Narasimhan S, Roselind FS. ICMR-NCDIR-NCRP investigator group. Cancer statistics, 2020: report from national cancer registry programme, India. JCO Glob Oncol. 2020;6:1063‐1075. doi: 10.1200/GO.20.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Mehta FS. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res. 1984;92(3):224‐229. doi: 10.1111/j.1600-0722.1984.tb00883.x [DOI] [PubMed] [Google Scholar]
- 32.Hsue SS, Wang WC, Chen CH, Lin CC, Chen YK, Lin LM. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: a follow-up study based in a Taiwanese hospital. J Oral Pathol Med. 2007;36(1):25‐29. doi: 10.1111/j.1600-0714.2006.00491.x [DOI] [PubMed] [Google Scholar]
- 33.Dijkstra P, Huisman P, Roodenburg J. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35(4):337‐342. doi: 10.1016/j.ijom.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 34.Kamstra JI, Dijkstra PU, van Leeuwen M, Roodenburg JL, Langendijk JA. Mouth opening in patients irradiated for head and neck cancer: a prospective repeated measures study. Oral Oncol. 2015;51(5):548‐555. doi: 10.1016/j.oraloncology.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 35.Kumar V, Mukharjee S, Akhtar N, et al. Tongue flap reconstruction in carcinoma of oral cavity: an institutional experience. J Maxillofac Oral Surg. 2019;18(3):428‐431. doi: 10.1007/s12663-018-1123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin AA, Sakkary MA, Khalil AA, Rifaat MA, Zayed SB. The submental flap for oral cavity reconstruction: extended indications and technical refinements. Head Neck Oncol. 2011;3(51). doi: 10.1186/1758-3284-3-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei FC, Chang YM, Kildal M, Tsang WS, Chen HC. Bilateral small radial forearm flaps for the reconstruction of buccal mucosa after surgical release of submucosal fibrosis: a new, reliable approach. Plast Reconstr Surg. 2001;107(7):1679‐1683. doi: 10.1097/00006534-200106000-00007 [DOI] [PubMed] [Google Scholar]
- 38.Chuang HC, Su CY, Jeng SF, Chien CY. Anterior lateral thigh flap for buccal mucosal defect after resection of buccal cancer. Otolaryngol Head Neck Surg. 2007;137(4):632‐635. doi: 10.1016/j.otohns.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 39.Balaji SM. Versatility of nasolabial flaps for the management of severe trismus in oral submucous fibrosis. Indian J Dent Res. 2016;27(5):492‐497. doi: 10.4103/0970-9290.195627 [DOI] [PubMed] [Google Scholar]
- 40.Idrees F, Patel P, Newaskar V, Agrawal D. Surgical defect coverage in oral submucous fibrosis patients with single-stage extended nasolabial flap. Oral Maxillofac Surg. 2016;20(4):411‐415. doi: 10.1007/s10006-016-0582-1 [DOI] [PubMed] [Google Scholar]
- 41.Chaturvedi P, Vaishampayan SS, Nair S, et al. Oral squamous cell carcinoma arising in background of oral submucous fibrosis: a clinicopathologically distinct disease. Head Neck. 2013;35(10):1404‐1409. doi: 10.1002/hed.23143 [DOI] [PubMed] [Google Scholar]
- 42.Chaturvedi P, Malik A, Nair D, et al. Oral squamous cell carcinoma associated with oral submucous fibrosis have better oncologic outcome than those without. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(3):225‐230. doi: 10.1016/j.oooo.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 43.Gadbail AR, Chaudhary M, Gawande M, et al. Oral squamous cell carcinoma in the background of oral submucous fibrosis is a distinct clinicopathological entity with better prognosis. J Oral Pathol Med. 2017;46(6):448‐453. doi: 10.1111/jop.12553 [DOI] [PubMed] [Google Scholar]
- 44.Zhou S, Qu X, Yu Z, et al. Survivin as a potential early marker in the carcinogenesis of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):575‐581. doi: 10.1016/j.tripleo.2009.10.054 [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Wang ZY, Huang Let al. et al. Do betel quid and areca nut chewing deteriorate prognosis of oral cancer? A systematic review, meta-analysis, and research agenda. Oral Dis. 2021;27(6):1366‐1375. doi: 10.1111/odi.13456 [DOI] [PubMed] [Google Scholar]
- 46.Ko CA, Lin YT, Tseng CH, et al. Intraoperative chemodetection for mucosal lesions among patients with head and neck cancer. Int J Head Neck Sci. 2018;2(3):124‐130. doi: 10.6696/IJHNS.201809_2(3).0005 [DOI] [Google Scholar]
- 47.Wellapuli N, Ekanayake L. Risk factors for chronic periodontitis in Sri Lankan adults: a population based case-control study. BMC Res Notes. 2017;10(1):460. doi: 10.1186/s13104-017-2778-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anand R, Dhingra C, Prasad S, Menon I. Betel nut chewing and its deleterious effects on oral cavity. J Cancer Res Ther. 2014;10(3):499‐505. doi: 10.4103/0973-1482.137958 [DOI] [PubMed] [Google Scholar]
- 49.Javed F, Tenenbaum HC, Nogueira-Filho G, et al. Severity of periodontal disease in individuals chewing betel quid with and without tobacco. Am J Med Sci. 2013;346(4):273‐278. doi: 10.1097/MAJ.0b013e31827333fb [DOI] [PubMed] [Google Scholar]
- 50.Sanz M, Del Castillo AM, Jepsen S, et al. Periodontitis and cardiovascular diseases. Consensus report. Glob Heart. 2020;15(1):1. doi: 10.5334/gh.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. 2013;40(Suppl 14):S70‐S84. doi: 10.1111/jcpe.12062 [DOI] [PubMed] [Google Scholar]
- 52.Karmakar S, Kar A, Thakur S, Rao VUS. Periodontitis and oral cancer-A striking link. Oral Oncol. 2020;106:104630. doi: 10.1016/j.oraloncology.2020.104630 [DOI] [PubMed] [Google Scholar]
- 53.Chen PT, Kuan FC, Huang CE, et al. Incidence and patterns of second primary malignancies following oral cavity cancers in a prevalent area of betel-nut chewing: a population-based cohort of 26,166 patients in Taiwan. Jpn J Clin Oncol. 2011;41(12):1336‐1343. doi: 10.1093/jjco/hyr152 [DOI] [PubMed] [Google Scholar]
- 54.Su YY, Fang FM, Chuang HC, Luo SD, Chien CY. Detection of metachronous esophageal squamous carcinoma in patients with head and neck cancer with use of transnasal esophagoscopy. Head Neck. 2010;32(6):780‐785. doi: 10.1002/hed.21252 [DOI] [PubMed] [Google Scholar]
- 55.Su YY, Chen WC, Chuang HC, et al. Effect of routine esophageal screening in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139(4):350‐354. doi: 10.1001/jamaoto.2013.46 [DOI] [PubMed] [Google Scholar]
- 56.Chitra S, Ashok L, Anand L, Srinivasan V, Jayanthi V. Risk factors for esophageal cancer in Coimbatore, Southern India: a hospital-based case-control study. Indian J Gastroenterol. 2004;23(1):19‐21. [PubMed] [Google Scholar]
- 57.Phukan RK, Ali MS, Chetia CK, Mahanta J. Betel nut and tobacco chewing; potential risk factors of cancer of oesophagus in Assam, India. Br J Cancer. 2001;85(5):661‐667. doi: 10.1054/bjoc.2001.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen PF, Chen IC, Chiang HC, Fang FM, Chien CY, Chuang HC. Occurrence of second primary tumor over digestive tract among patients with head and neck cancer in betel nut chewing area-A population-based case- control study in Taiwan. Int J Head Neck Sci. 2019;3(1):1‐11. doi: 10.6696/IJHNS.201903_3(1).0006 [DOI] [Google Scholar]
- 59.Liao CT, Kang CJ, Chang JT, et al. Survival of second and multiple primary tumors in patients with oral cavity squamous cell carcinoma in the betel quid chewing area. Oral Oncol. 2007;43(8):811‐819. doi: 10.1016/j.oraloncology.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 60.Tsai JF, Chuang LY, Jeng JE, et al. Betel quid chewing as a risk factor for hepatocellular carcinoma: a case-control study. Br J Cancer. 2001;84(5):709‐713. doi: 10.1054/bjoc.1999.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hung CR, Cheng JT. Betel quid chewing damaged gastric mucosa: protective effects of cimetidine and sodium bicarbonate. Chin J Physiol. 1994;37(4):213‐218. [PubMed] [Google Scholar]
- 62.Fernando N, Jayakumar G, Perera N, Amarasingha I, Meedin F, Holton J. Presence of Helicobacter pylori in betel chewers and non-betel chewers with and without oral cancers. BMC Oral Health. 2009;9:23. doi: 10.1186/1472-6831-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuang YS, Wu MC, Yu FJ, et al. Effects of alcohol consumption, cigarette smoking, and betel quid chewing on upper digestive diseases: a large cross-sectional study and meta-analysis. Oncotarget. 2017;8(44):78011‐78022. doi: 10.18632/oncotarget.20831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg A, Chaturvedi P, Gupta PC. A review of the systemic adverse effects of areca nut or betel nut. Indian J Med Paediatr Oncol. 2014;35(1):3‐9. doi: 10.4103/0971-5851.133702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heck JE, Marcotte EL, Argos M, et al. Betel quid chewing in rural Bangladesh: prevalence, predictors and relationship to blood pressure. Int J Epidemiol. 2012;41(2):462‐471. doi: 10.1093/ije/dyr191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tseng CH. Betel nut chewing is associated with hypertension in Taiwanese type 2 diabetic patients. Hypertens Res. 2008;31(3):417‐423. doi: 10.1291/hypres.31.417 [DOI] [PubMed] [Google Scholar]
- 67.Lin SH, Liao YS, Huang SH, Liao WH. Relationship between betel quid chewing and risks of cardiovascular disease in older adults: a cross-sectional study in Taiwan. Drug Alcohol Depend. 2014;141:132‐137. doi: 10.1016/j.drugalcdep.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 68.Lin WY, Chiu TY, Lee LT, Lin CC, Huang CY, Huang KC. Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am J Clin Nutr. 2008;87(5):1204‐1211. doi: 10.1093/ajcn/87.5.1204 [DOI] [PubMed] [Google Scholar]
- 69.Guh JY, Chen HC, Tsai JF, Chuang LY. Betel-quid use is associated with heart disease in women. Am J Clin Nutr. 2007;85(5):1229‐1235. doi: 10.1093/ajcn/85.5.1229 [DOI] [PubMed] [Google Scholar]
- 70.Tsai WC, Wu MT, Wang GJ, et al. Chewing areca nut increases the risk of coronary artery disease in Taiwanese men: a case-control study. BMC Public Health. 2012;12:162. doi: 10.1186/1471-2458-12-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yen AM, Chen LS, Chiu YH, Boucher BJ, Chen TH. A prospective community-population-registry based cohort study of the association between betel-quid chewing and cardiovascular disease in men in Taiwan (KCIS no. 19). Am J Clin Nutr. 2008;87(1):70‐78. doi: 10.1093/ajcn/87.1.70 [DOI] [PubMed] [Google Scholar]
- 72.Ho TJ Chi-Kang Tsai B, Kuo CH, et al. Arecoline induces cardiotoxicity by upregulating and activating cardiac hypertrophy-related pathways in Sprague-Dawley rats. Chem Biol Interact. 2022;354:109810. doi: 10.1016/j.cbi.2022.109810. [DOI] [PubMed] [Google Scholar]
- 73.Ku CW, Day CH, Ou HC, et al. The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats. Open Life Sci. 2021;16(1):1182‐1192. doi: 10.1515/biol-2021-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choudhury MD, Chetia P, Choudhury KD, Talukdar AD, Datta-Choudhari M. Atherogenic effect of arecoline: a computational study. Bioinformation. 2012;8(5):229‐232. doi: 10.6026/97320630008229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang WC, Hsiao CF, Chang HY, et al. Betel nut chewing and other risk factors associated with obesity among Taiwanese male adults. Int J Obes (Lond). 2006;30(2):359‐363. doi: 10.1038/sj.ijo.0803053 [DOI] [PubMed] [Google Scholar]
- 76.Lin WY, Pi-Sunyer FX, Liu CS, et al. Betel nut chewing is strongly associated with general and central obesity in Chinese male middle-aged adults. Obesity (Silver Spring). 2009;17(6):1247‐1254. doi: 10.1038/oby.2009.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung FM, Chang DM, Chen MP, et al. Areca nut chewing is associated with metabolic syndrome: role of tumor necrosis factor-alpha, leptin, and white blood cell count in betel nut chewing-related metabolic derangements. Diabetes Care. 2006;29(7):1714. doi: 10.2337/dc06-0628 [DOI] [PubMed] [Google Scholar]
- 78.Guh JY, Chuang LY, Chen HC. Betel-quid use is associated with the risk of the metabolic syndrome in adults. Am J Clin Nutr. 2006;83(6):1313‐1320. doi: 10.1093/ajcn/83.6.1313 [DOI] [PubMed] [Google Scholar]
- 79.Yen AM, Chiu YH, Chen LS, et al. A population-based study of the association between betel-quid chewing and the metabolic syndrome in men. Am J Clin Nutr. 2006;83(5):1153‐1160. doi: 10.1093/ajcn/83.5.1153 [DOI] [PubMed] [Google Scholar]
- 80.Hsu HF, Tsou TC, Chao HR, et al. Effects of arecoline on adipogenesis, lipolysis, and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. Toxicol Appl Pharmacol. 2010;245(3):370‐377. doi: 10.1016/j.taap.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 81.Tung TH, Chiu YH, Chen LS, Wu HM, Boucher BJ, Chen TH; Keelung Community-based integrated screening programme No. 2. A population-based study of the association between areca nut chewing and type 2 diabetes mellitus in men. Diabetologia. 2004;47(10):1776‐1781. doi: 10.1007/s00125-004-1532-2 [DOI] [PubMed] [Google Scholar]
- 82.Tseng CH. Betel nut chewing and incidence of newly diagnosed type 2 diabetes mellitus in Taiwan. BMC Res Notes. 2010;3:228. doi: 10.1186/1756-0500-3-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsiao TJ, Liao HW, Hsieh PS, Wong RH. Risk of betel quid chewing on the development of liver cirrhosis: a community-based case-control study. Ann Epidemiol. 2007;17(6):479‐485. doi: 10.1016/j.annepidem.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 84.Tsai JF, Jeng JE, Chuang LY, et al. Habitual betel quid chewing as a risk factor for cirrhosis: a case-control study. Medicine (Baltimore). 2003;82(5):365‐372. doi: 10.1097/01.md.0000090401.56130.59 [DOI] [PubMed] [Google Scholar]
- 85.Chu YH, Wang L, Ko PC, Lan SJ, Liaw YP. The risk of cirrhosis in non-alcohol drinkers is greater in female than male betel nut chewers. Oncotarget. 2018;9(9):8731‐8737. doi: 10.18632/oncotarget.23885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu GH, Boucher BJ, Chiu YH, Liao CS, Chen TH. Impact of chewing betel-nut (Areca catechu) on liver cirrhosis and hepatocellular carcinoma: a population-based study from an area with a high prevalence of hepatitis B and C infections. Public Health Nutr. 2009;12(1):129‐135. doi: 10.1017/S1368980008002073 [DOI] [PubMed] [Google Scholar]
- 87.Jeng JE, Tsai MF, Tsai HR, et al. Impact of chronic hepatitis B and hepatitis C on adverse hepatic fibrosis in hepatocellular carcinoma related to betel quid chewing. Asian Pac J Cancer Prev. 2014;15(2):637‐642. doi: 10.7314/apjcp.2014.15.2.637 [DOI] [PubMed] [Google Scholar]
- 88.Wang LY, You SL, Lu SN, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control. 2003;14(3):241‐250. doi: 10.1023/a:1023636619477 [DOI] [PubMed] [Google Scholar]
- 89.Sarma AB, Chakrabarti J, Chakrabarti A, et al. Evaluation of pan masala for toxic effects on liver and other organs. Food Chem Toxicol. 1992;30(2):161‐163. doi: 10.1016/0278-6915(92)90152-b [DOI] [PubMed] [Google Scholar]
- 90.Run-mei X, Jun-jun W, Jing-ya C, Li-juan S, Yong C. Effects of arecoline on hepatic cytochrome P450 activity and oxidative stress. J Toxicol Sci. 2014;39(4):609‐614. doi: 10.2131/jts.39.609 [DOI] [PubMed] [Google Scholar]
- 91.Dasgupta R, Saha I, Pal S, et al. Immunosuppression, hepatotoxicity and depression of antioxidant status by arecoline in albino mice. Toxicology. 2006;227(1-2):94‐104. doi: 10.1016/j.tox.2006.07.016 [DOI] [PubMed] [Google Scholar]
- 92.Wang TS, Lin CP, Chen YP, Chao MR, Li CC, Liu KL. CYP450-mediated Mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ Toxicol. 2018;33(10):1029‐1038. doi: 10.1002/tox.22588 [DOI] [PubMed] [Google Scholar]
- 93.Hsu SH, Wong YK, Wang CP, et al. Survival analysis of patients with oral squamous cell carcinoma with simultaneous second primary tumors. Head Neck. 2013;35(12):1801‐1807. doi: 10.1002/hed.23242 [DOI] [PubMed] [Google Scholar]
- 94.Adel M, Liao CT, Lee LY, et al. Incidence and outcomes of patients with oral cavity squamous cell carcinoma and fourth primary tumors: a long-term follow-up study in a betel quid chewing endemic area. Medicine (Baltimore). 2016;95(12):e2950. doi: 10.1097/MD.0000000000002950 [DOI] [PMC free article] [PubMed] [Google Scholar]