Abstract
Background:
Substandard and falsified (SF) medical products are removed from circulation through a process called ‘product recall’ by medicines regulatory agencies. In Zambia, the Zambia Medicines Regulatory Authority (ZAMRA) is responsible for recalling SF medical products from the Zambian market through passive and active surveillance methods. This study aimed to describe the prevalence of recalls of SF medical products and to analyse the frequently recalled therapeutic categories, dosage forms, categories of defects that led to the recalls and their sources with respect to the country of the marketing authorisation holder (MAH) or manufacturer.
Methods:
We conducted a descriptive cross-sectional review of the product recalls issued by ZAMRA between January 2018 and December 2021. A search for all medical product alerts and recalls issued by ZAMRA was carried out by reviewing the internal post-marketing surveillance database kept at ZAMRA headquarters. Data were extracted using a structured Excel database and analysed using Microsoft Excel.
Results:
A total of 119 alerts were received during the review period, of which 83 (69.7%) were product recalls. Oral solid dosage forms were the most recalled dosage form (53%). Furthermore, the number of recalls increased in 2020 (44.6%) and 2021 (22.9%), with the majority (20.5%) of the recalled products being substandard products classified as antiseptics and disinfectants and were attributed to the high demand during the COVID-19 pandemic. Manufacturing laboratory control issues were the reason for product recall in almost half (47.4%) of the cases. Most of the products recalled originated from India (38.6%), followed by Zambia (25.3%). Only one suspected falsified product was recalled between 2018 and 2021. A total of 66 recalls of the 83 products were initiated by ZAMRA, with only 17 voluntarily by foreign MAHs. No product recall was initiated by the local representatives of foreign manufacturers or MAH.
Conclusion:
The majority of the pharmaceutical product recalls in Zambia were substandard products. Manufacturing laboratory control issues lead to most recalls and require investigation of the root causes, preventive action, and strict compliance with the good manufacturing practices guidelines by manufacturers.
Keywords: Pharmacovigilance, medical products, recall, substandard, falsified, ZAMRA, Zambia
Introduction
In May 2017, the World Health Organization (WHO) adopted the term substandard and falsified (SF) medical products to replace the previously used terms, spurious/falsely-labelled/falsified/counterfeit (SFFC). According to the WHO, substandard medical products, also known as ‘out of specification’, are ‘authorised medical products that fail to meet either their quality standards or specifications, or both’ and falsified medical products are products that are ‘deliberately or fraudulently misrepresent their identity, composition or source’.1 Furthermore, the WHO defines unregistered or unlicensed medicinal products as products that ‘have not undergone evaluation and/or approval by the national or regional regulatory authority for the market in which they are marketed/distributed or used, subject to permitted conditions under national or regional regulation and legislation’.1
The impact of SF medical products is devastating. They have been linked to causing thousands of deaths internationally,2,3 contributing to antimicrobial resistance, treatment failure, poisoning, and adverse drug reactions.4 –6 Moreover, SF medical products have been linked to causing enormous economic impact globally, estimated to be between US$10 and US$200 billion annually.6,7 In the Zambian context, SF medical products such as antimalarials have been estimated to cause an annual economic burden of US$141.5 million.8,9 This affects access to quality-assured medical products. Medicines access in Zambia is mainly through regulated public sector, that is, government central stores supplying all government health facilities across the country through provincial hubs. For the private sector, this is mainly through regulated private hospitals, clinics, retail pharmacies and healthshops.10,11
The spread of SF medical products is considered a ‘global pandemic’,12,13 as alarming reports have been previously published indicating that the global prevalence of SF medical products ranges from 1% to 50%.4,14 –17 However, prevalence studies are still a big obstacle even in high-income countries, and the true extent of the problem remains unknown.18,19 A meta-analysis in low- and middle-income countries (LMICs) revealed that 19.1% of antimalarials were either substandard or falsified.6 The WHO surveyed the quality of selected medicines from the list of 13 life-saving commodities identified by the United Nations Commission on life-saving commodities for women and children in 10 LMICs, including seven sub-Saharan African countries and excluding Zambia. The survey found that 23% of the samples (representing 40 products) were substandard.20 Within the Zambian context, a 2010 study found that 10.3% of registered and unregistered samples were substandard.21 In the recent past, various researchers have found and reported evidence of SF medical products in Zambia.22,23 These known examples of SF medical products are removed from the supply chain through a process called product recall by the national medicines regulatory authorities. Product recall results from meticulous pharmacovigilance and is an essential component of drug regulation to protect public health.24,25
Substandard medical products can be recalled due to several factors such as inappropriate good manufacturing practices (GMP), poor storage, stability failure, defectiveness in part of a medical device, and many others.25 –28 To provide accurate information, substandard medical products can be classified in several defect categories as reported in the literature.29,30 The European Medicines Agency (EMA) classifies substandard medical products in five high-level terms.31 These five high-level terms include manufacturing laboratory control issues, product contamination and sterility issues, product label issue, product packaging issues, and product physical issues. Examples of manufacturing laboratory control issues include out of specification test results for any of the specifications established for the finished product. Product contamination and sterility issues include chemical (cross contamination), microbial, physical (foreign material), and lack of sterility. Product label issues can be any defect in meeting the labelling requirements, such as damaged and loose labels, missing text, wrong labelling information and illegible information on both the primary and secondary packaging. Product packaging issues include defects to the container and closure system such as damage, leaking, incorrect package type and missing a component of the container closure system. Product physical issues are defects that lead to changes in the physical presentation of the medical product, such as precipitation of the product, crystallisation and product odour.31
In Zambia, the Zambia Medicines Regulatory Authority (ZAMRA) has the mandate to ensure that all medicines and allied substances in the country consistently meet the established quality, safety and efficacy requirements.32 Zambia has a robust and well-established surveillance system to monitor medical products. It is also part of the WHO Global Surveillance and Monitoring System (GSMS) member state mechanism on SF medical products and has adopted the mechanism’s strategy to prevent, detect and respond to SF medical products.33
ZAMRA uses active and passive surveillance methods to monitor the quality of medical products on the Zambian market. Active surveillance involves post-marketing surveillance inspections of medical products, including visual inspection, labelling assessment, rapid field screening of medicines using GHPF – Minilabs® and sampling of medicines and allied substances for analysis at the National Quality Control Laboratory (NQCL).9,11 Through the passive surveillance method, ZAMRA receives alerts about defective and suspected SF medical products from other regulatory authorities, WHO, patients and the pharmaceutical industry. Passive surveillance is important to receive alerts from poorly regulated border crossings, as eight countries surround Zambia.34,35
Like in most countries,36 –39 received and suspected SF medical product or possible safety issues are communicated by ZAMRA at three levels. These include the consumer or patient level meant to recall SF medical products up to patent level, and the retail/health facility level which is meant to recall products in hospitals, clinics and retail pharmacies. The third level is at the wholesale level which is meant to recall SF medical product from all public and private pharmaceutical wholesale points.40 Communications are made in the form of emails and letters when recalls are at the health facility and wholesale level. When communicating to the public, recalls are published in print media and official social media for ZAMRA. Safety alerts are usually communicated as a precaution to users to consider before using medical products, while quality alerts include situations that can compromise the quality of the medicine, such as the detection of SF medical products.41 –43
Well-resourced countries have introduced innovative systems such as track and trace in an effort to reduce the circulation of SF medical products and streamline the recall of SF medical products.44,45 However, such regulations have been contested due to the lack of reliable prevalence data to support balanced debate and decision-making related to SF medical product recalls by practitioners and policymakers.18 In the Zambian context, despite having a surveillance system to identify and withdraw SF medical products from the market, ZAMRA has not yet published any guidelines on product recall to guide the local industry. However, a draft guideline is in place, awaiting finalisation by the board once appointed by the minister of health. The available surveillance system has detected several medical products problems in recent years, leading to alerts and recalls of defective products. The authors are unaware of any published literature on the analysis of medical product recalls in Zambia. Therefore, there is a need to analyse SF medical product recalls made in Zambia to contribute to the global debate on the subject matter and provide data that stakeholders can find useful in decision-making. Information such as the prevalence of SF medical product recalls, types of defects which caused the recalls, the therapeutic category and recalled pharmaceutical dosage forms have not yet been studied in Zambia.
This study aimed to consolidate, characterise and assess information on the quality of medical products in Zambia by focusing on medical product recalls issued by ZAMRA from January 2018 to December 2021. Specifically, the study describes the prevalence of recalls of SF medical products recorded between 2018 and 2021, therapeutical categories and dosage forms, categories of defects that led to the recall, their sources with respect to the country of origin of the marketing authorisation holder (MAH) and the frequently recalled products. The findings inform relevant stakeholders about the prevalence of recalls of medical products and the implications on the quality surveillance system in Zambia.
Methods
Study design
A descriptive cross-sectional review was conducted to assess medical product alerts and recalls data from January 2018 to December 2021.
Data source
A search for all medical product alerts and recalls received and/or issued by ZAMRA was carried out by reviewing the internal post-marketing surveillance database kept at ZAMRA headquarters. Examples of two consumer/patient level recalls reviewed have been provided (see supplement data). ZAMRA actively started keeping records for medical product alerts/recall in 2017. To assess the documents, a search was done for documents recorded between 1 January 2018 and 31 December 2021.
Inclusion and exclusion criteria
In this study, a ‘recall’ was considered to be a circular or document issued by ZAMRA to the public, the manufacturer, distributors, and healthcare professionals as a regulatory action to control and reduce risk regarding critical quality issues, falsification or safety of a medical product for human use. All recalled medical products for human use recalled due to quality-related issues or falsification were included. Safety and quality alerts that did not lead to a recall were excluded, as were recalls made before 1 January 2018.
Data collection and analysis
To support the assessment and consolidation of the recalls, a structured Excel database was created to collect data. The following data were extracted from the alert/recall records: name of the product, dosage form, batch number(s), country of manufacture and number of manufacturers, the reason for the recall, recall initiator and year of the recall.
Data analysis
The recalled medical products were classified as substandard, falsified or unregistered according to the WHO definitions.5 To determine the most frequent therapeutic groups affected by recalls made by ZAMRA, the Anatomical Therapeutic Chemical (ATC) second level as per WHO classification was applied.46 The substandard medical products were further classified into the following five high-level terms using the EMA guidelines: manufacturing laboratory control issues, product contamination and sterility issues, product label issues, product packaging issues and product physical issues.31 Medical products that were misrepresented and met the WHO definition of a falsified medicinal product were classified as falsified medical products.5 To determine the prevalence of SF medical product recalls over the review period, the total number of confirmed alerts recorded (denominator) was divided by the actual recalls made/issued for SF medical products (numerator) multiplied by 100%. Microsoft Excel package was used for the data analysis.
Results
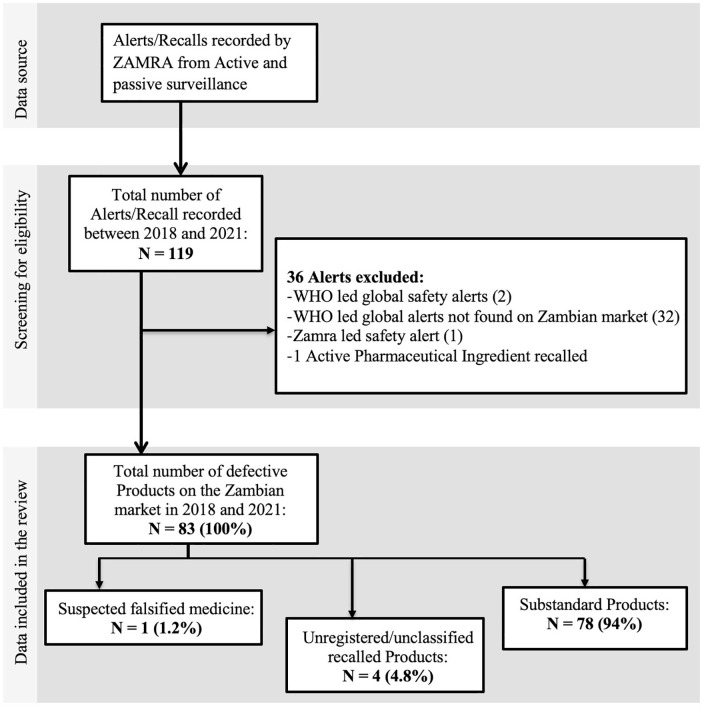
Between 1 January 2018 and 31 December 2021, ZAMRA recorded 119 alerts, of which 83 alerts were SF medical product recalls (Figure 1), giving a prevalence of 69.7% during the review period. Among these 83 recalls made, 78 (94%) were for suspected substandard products, 1 (1.2%) was a suspected falsified medicine, and 4 (4.8%) were unclassified and unregistered products (i.e. could not meet the WHO definition of SF medical products).
Figure 1.
Flow diagram depicting the search for medical products recalled incidents.
All 83 medical products recalled between 1 January 2018 and 31 December 2021 are presented in Table 1, showing the product name, batch numbers affected, country of manufacture, year of recall and recall initiator.
Table 1.
List of medical products recalled between 2018 and 2021.
| S/N | Name of product | Country of origin | Batches affected | Year of report | Initiator of recall/alert |
|---|---|---|---|---|---|
| 1. | Chlorine solution | Zambia | 2 | 2018 | ZAMRA |
| 2. | Chloramphenicol 5% + Beclomethasone Dipropionate 0.025% + Clotrimazole 1% + Lidocaine hydrochloride 2% ear/eye drops | India | 1 | 2018 | ZAMRA |
| 3. | Male condoms | India | 2 | 2018 | ZAMRA |
| 4. | Magnesium trisilicate tablets | Zambia | 2 | 2018 | ZAMRA |
| 5. | Co-trimoxazole tablets | Zambia | 1 | 2018 | ZAMRA |
| 6. | Vitamin B-complex injection | India. | 2 | 2018 | ZAMRA |
| 7. | Ringers lactate solution | Zambia | 3 | 2018 | ZAMRA |
| 8. | Dextrose 5% solution | Zambia | 1 | 2018 | ZAMRA |
| 9. | Chinese contraceptive pill (Levonorgestrel 6 mg + Quinestrol 3 mg) |
China | 1 | 2018 | ZAMRA |
| 10. | Valsartan 320 mg tablets | Germany | All batches | 2018 | MAH |
| 11. | Valsartan 160 mg tablets | Germany | All batches | 2018 | MAH |
| 12. | Valsartan 80 mg tablets | Germany | All batches | 2018 | MAH |
| 13. | Valsartan 40 mg tablets | Germany | All batches | 2018 | MAH |
| 14. | Valsartan 320 mg + Hydrochlorothiazide 12.5 mg tablets | Germany | All batches | 2018 | MAH |
| 15. | Valsartan 160 mg + Hydrochlorothiazide 12.5 mg tablets | Germany | All batches | 2018 | MAH |
| 16. | Oxytocin injection | China | 1 | 2018 | ZAMRA |
| 17. | Ringers lactate solution | Zambia | 1 | 2019 | ZAMRA |
| 18. | Amoxicillin 125 mg + clavulanic acid 31.25 mg powder for suspension | South Africa | 3 | 2019 | MAH |
| 19. | Amoxicillin 250 mg + clavulanic acid 62.5 mg powder for suspension | South Africa | 1 | 2019 | MAH |
| 20. | Atazanavir 300 mg + Ritonavir 100 mg tablets | India | 8 | 2019 | MAH |
| 21. | Isoniazid 100 mg tablets | Zambia | 1 | 2019 | ZAMRA |
| 22. | Ciprofloxacin 250 mg tablets | Zambia | 1 | 2019 | ZAMRA |
| 23. | Clotrimazole 1.0%w/w + Betamethasone 0.1%w/w cream | Kenya | 6 | 2019 | ZAMRA |
| 24. | Ranitidine 150 mg tablets | Not stated | All batches | 2019 | ZAMRA |
| 25. | Metronidazole 5 mg/mL intravenous infusion | India | 2 | 2019 | ZAMRA |
| 26. | Diclofenac 75 mg/5 mL injection | China | 1 | 2019 | ZAMRA |
| 27. | Amoxicillin 250 mg capsules | India | 1 | 2019 | ZAMRA |
| 28. | Dexamethasone 5 mg + Neomycin 1 mg eye/ear drops | India | 1 | 2020 | ZAMRA |
| 29. | Aspirin 453.6 mg + Caffeine 64.8 mg + Paracetamol 324 mg powder | South Africa | 13 | 2020 | ZAMRA |
| 30. | Aspirin 226.8 mg + Caffeine 32.4 mg + Paracetamol 162 mg tablets | South Africa | 13 | 2020 | ZAMRA |
| 31. | Aspirin 75 mg tablets | India | 3 | 2020 | ZAMRA |
| 32. | Co-trimoxazole 240 mg Oral Suspension BP | India | 6 | 2020 | ZAMRA |
| 33. | Amoxicillin 250 mg capsules | India | 1 | 2020 | ZAMRA |
| 34. | Amlodipine 5 mg tablets | India | 2 | 2020 | ZAMRA |
| 35. | Cefixime 50 mg suspension | India | 1 | 2020 | ZAMRA |
| 36. | Paracetamol 100 mg tablets | Zambia | 1 | 2020 | ZAMRA |
| 37. | Paracetamol 500 mg tablets | India | 18 | 2020 | ZAMRA |
| 38. | Paracetamol 100 mg tablets | India | 7 | 2020 | ZAMRA |
| 39. | Surgical gloves | India | 1 | 2020 | ZAMRA |
| 40. | Wonders hand sanitiser | Zambia | All batches | 2020 | ZAMRA |
| 41. | Avacare Instant hand sanitiser | Zambia | All batches | 2020 | ZAMRA |
| 42. | Bickmac Disinfectant 20 L | Zambia | All batches | 2020 | ZAMRA |
| 43. | Glitzcare hand sanitiser | Zambia | All batches | 2020 | ZAMRA |
| 44. | Flost antiseptic hand gel | Zambia | 1 | 2020 | ZAMRA |
| 45. | Classicmatch waterless hand sanitizer 100 mL | South Africa | All batches | 2020 | ZAMRA |
| 46. | Classicmatch waterless hand sanitizer 50 mL | South Africa | All batches | 2020 | ZAMRA |
| 47. | SoClean sanitizer germ killer | Zambia | All batches | 2020 | ZAMRA |
| 48. | SoClean ant-bacterial hand sanitizer | Zambia | All batches | 2020 | ZAMRA |
| 49. | 3X plus liquid sanitizer | Not stated | 1 | 2020 | ZAMRA |
| 50. | Vintage Instant hand sanitizer 50 mL | not stated | All batches | 2020 | ZAMRA |
| 51. | Plus hand sanitizer 750 mL | Zambia | All batches | 2020 | ZAMRA |
| 52. | Sterilix hand sanitizer | Zambia | 2 | 2020 | ZAMRA |
| 53. | Brooks disinfectant hand sanitizer | Zambia | 1 | 2020 | ZAMRA |
| 54. | Tasa’s hand sanitizer | Zambia | All batches | 2020 | ZAMRA |
| 55. | Lidocaine USP 2.0% solution | India | 1 | 2020 | ZAMRA |
| 56. | Clotrimazole USP 500 mg pessaries | India | 1 | 2020 | ZAMRA |
| 57. | Nystatin BP 100,000 IU/mL suspension | India | 1 | 2020 | ZAMRA |
| 58. | Metronidazole 200 mg tablets | India | 1 | 2020 | ZAMRA |
| 59. | Zinc sulphate USP 20 mg tablets | India | 1 | 2020 | ZAMRA |
| 60. | Nitrofurantoin BP 50 mg tablets | India | 1 | 2020 | ZAMRA |
| 61. | Chlorpheniramine BP 4 mg tablets | India | 1 | 2020 | ZAMRA |
| 62. | Examination Gloves, Medium | United Kingdom | 1 | 2020 | ZAMRA |
| 63. | Bio Claire Crème Corporelle Eclaircissante | Not stated | 1 | 2020 | ZAMRA |
| 64. | Black Opal Even True Tone Correct Fade Cream | Not stated | 1 | 2020 | ZAMRA |
| 65. | Folic Acid BP 5 mg tablets | India | 3 | 2021 | ZAMRA |
| 66. | Paracetamol1 25 mg/5 mL syrup | Zambia | 5 | 2021 | ZAMRA |
| 67. | Bupivacaine Hydrochloride USP 0.5%w/v solution | India | 1 | 2021 | ZAMRA |
| 68. | Clotrimazole 1% w/w cream | Kenya | 5 | 2021 | ZAMRA |
| 69. | Vitamin C 500 mg tablets | India | Various | 2021 | ZAMRA |
| 70. | Latex examination gloves Large | India | 1 | 2021 | ZAMRA |
| 71. | Male latex condoms | India | 2 | 2021 | ZAMRA |
| 72. | Glam & Glory Hand sanitizer 100 mL | India | 1 | 2021 | ZAMRA |
| 73. | Ascorbic acid 500 mg tablets | India | 1 | 2021 | MAH |
| 74. | Aspirin USP 75 mg tablets | India | 2 | 2021 | ZAMRA |
| 75. | Losartan 50 mg + hydrochlorothiazide 12.5 mg tablets | South Africa | All batches | 2021 | MAH |
| 76. | Losartan 100 mg + hydrochlorothiazide 12.5 mg tablets | South Africa | All batches | 2021 | MAH |
| 77. | Losartan 25 mg tablets | Germany | All batches | 2021 | MAH |
| 78. | Losartan 50 mg tablets | Germany | All batches | 2021 | MAH |
| 79. | Losartan 100 mg tablets | Germany | All batches | 2021 | MAH |
| 80. | Losartan 50 + hydrochlorothiazide 12.5 mg tablets | Germany | All batches | 2021 | MAH |
| 81. | Losartan 100 + hydrochlorothiazide 12.5 mg tablets | Germany | All batches | 2021 | MAH |
| 82. | Metronidazole 200 mg tablets | India | 1 | 2021 | ZAMRA |
| 83. | Ply Face masks | India | 1 | 2021 | ZAMRA |
ZAMRA: Zambia Medicines Regulatory Authority; MAH: marketing authorisation holder; BP: British Pharmacopoeia.
The year 2020 had the highest number of recalls, 44.6% (n = 37), followed by the year 2021, 22.9% (n = 19), then 2018 with 19.3% (n = 16) and 2019 with 13.3% (see Figure 2).
Figure 2.
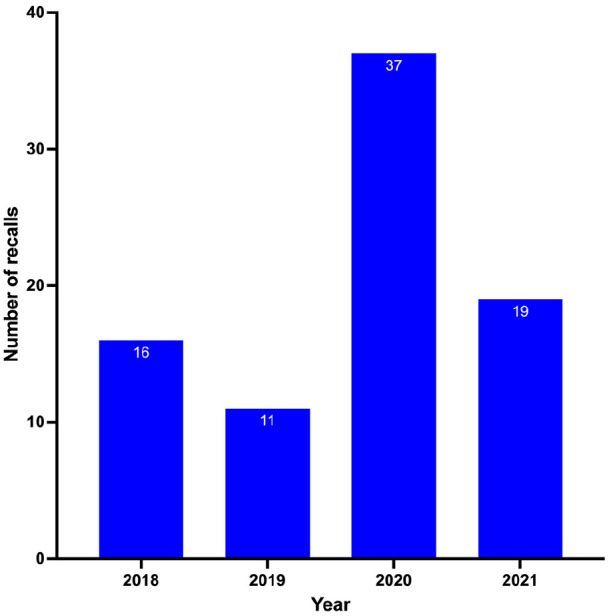
Number of recalled medical products in Zambia between 2018 and 2021.
Considering the pharmaceutical class of the recalled medical products (Table 2), 23 classes accounted for the incidence of product recalls. According to this classification of the medical products, the most affected class was the antiseptic and disinfectant (20.5%), followed by antihypertensives and antibiotics (16.9%). The lowest occurrences (1.2%) were for mineral supplement, surgical glove, contraceptives, antihistamine, systemic hormone, antacid, parenteral nutrition, facemask, anti-ulcer, antituberculosis and antiviral.
Table 2.
Therapeutic classification of recalled medical products (2018–2021).
| S/N | Classification | Number (%) of occurrences |
|---|---|---|
| 1. | Antiseptic and disinfectants | 17 (20.5) |
| 2. | Antihypertensive | 14 (16.9) |
| 3. | Antibiotics | 14 (16.9) |
| 4. | Analgesics | 7 (8.4) |
| 5. | Antifungals | 4 (4.8) |
| 6. | Vitamins | 4 (4.8) |
| 7. | Lightening body cream | 2 (2.4) |
| 8. | Male condoms | 2 (2.4) |
| 9. | Local anaesthetics | 2 (2.4) |
| 10. | Antithrombotic agents | 2 (2.4) |
| 11. | Examination gloves | 2 (2.4) |
| 12. | Electrolytes | 2 (2.4) |
| 13. | Mineral supplements | 1 (1.2) |
| 14. | Surgical gloves | 1 (1.2) |
| 15. | Contraceptives | 1 (1.2) |
| 16. | Antihistamines | 1 (1.2) |
| 17. | Systemic hormones | 1 (1.2) |
| 18. | Antacids | 1 (1.2) |
| 19. | Parenteral nutrition | 1 (1.2) |
| 20. | Facemask | 1 (1.2) |
| 21. | Anti-ulcer (systemic) | 1 (1.2) |
| 22. | Antituberculosis | 1 (1.2) |
| 23. | Antivirals | 1 (1.2) |
| Grand Total | 83 (100) |
Using the high-level terms classification of substandard medicine, the most common defect leading to the recall of the 78 substandard products was manufacturing laboratory controls issues, accounting for 47.4%, followed by product contamination and sterility issues (29.5%) and then product physical issues (15.4%). Less than a tenth (6.4%) of the recalls resulted from product packaging issues, while 1.3% were attributed to product label issues. The details of the reasons that led to the recall of these substandard medical products are presented in Table 3.
Table 3.
Recalled substandard pharmaceutical products classified using the adopted EMA defect categorisation terminology.
| High-level defect term | Number of products affected | Detail of the defect leading to recall | Number of products affected |
|---|---|---|---|
| Manufacturing laboratory control issue | 37 (47.4%) | Out of specification assay result Failed water leak test & Bursting pressure Out of specification impurities result after 24 months Failed uniformity of weight test Stability failures under high temperature and humidity conditions. Non-compliant to dissolution test Low alcohol content Failed pH test Failed disintegration test Failed hung and roll, bursting volume, length test |
9 4 1 4 2 1 13 1 1 1 |
| Product contamination and sterility issues | 23 (29.5%) | Lack of sterility Visible foreign particulates Identification of nitrosamine impurities Foreign matters on capsules Foreign materials in tablets Contained Mercury and a prescription only medication, Clobetasol Excessive amounts of Hydroxyquinone Detection of 4-chloro azido methyl tetrazole Failed bioburden test |
1 3 7 1 1 1 1 7 1 |
| Product label issue | 1 (1.3%) | Wrong labelling for the route of administration | 1 |
| Product packaging issues | 5 (6.4%) | Defective primary packaging | 5 |
| Product physical issue | 12 (15.4%) | Tablet discoloration Solution discolouration Suspension caking Failed appearance test Crystallisation of syrup |
4 2 1 4 1 |
EMA: European Medicines Agency.
Of the 83 medical product recalls analysed, more than half (53%) were oral dosage forms, followed by topical applications (24.1%). Parenteral dosage forms accounted for 10.8%, other dosage forms 8.4%, while 2.4% of recalls were ocular dosage forms and 1.2% were vaginal formulations, as depicted in Figure 3.
Figure 3.
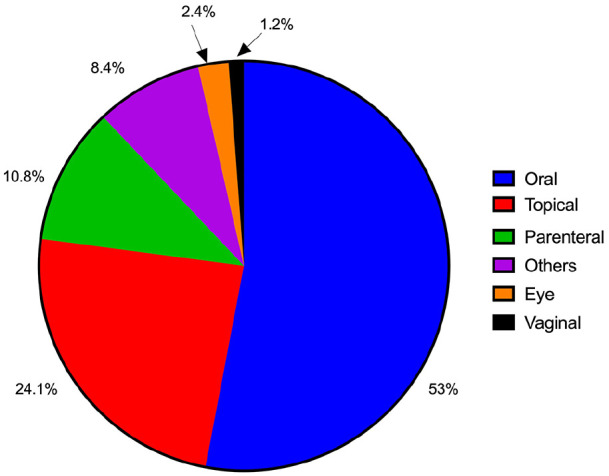
Affected dosage forms as a percentage of the number of recalls issued.
Most of the medical products recalled (n = 32, 38.6%) were from India, and 21 (25.3%) were locally manufactured in Zambia. The rest originated from Germany (n = 11, 14.1%), South Africa (n = 8, 9.6%), China (n = 3, 3.6%), Kenya (n = 2, 2.4%), the United Kingdom (n = 1, 1.2%) and 5 (6%) from an unknown country. Regarding the number of manufacturers whose products were recalled, the trend was somewhat similar, with India having 20 different manufacturers, 13 from Zambian manufacturers and 3 each for China and South Africa. Germany, the United Kingdom and Kenya each had 1 manufacturer, as shown in Figure 4.
Figure 4.
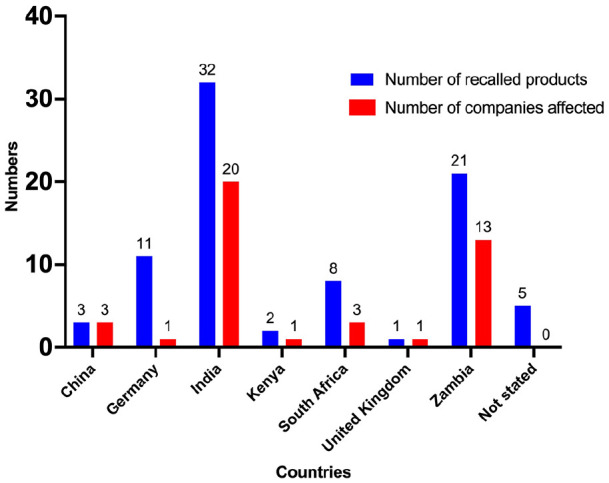
The number of recalled products and affected companies per country of origin.
An incident of a suspected falsified product was identified and recalled from the Zambian supply chain between 2018 and 2021 during the routine post-market surveillance activities by ZAMRA. The incident involved an ear drop containing Chloramphenicol 5% + Beclomethasone Dipropionate 0.025% + Clotrimazole 1% + Lidocaine hydrochloride 2%. Several labelling inconsistencies from the packaging of the falsely labelled product as compared with the original product meant for the Zambian market were identified and summarised in Table 4.
Table 4.
Summary of noted labelling inconsistencies for the suspected falsified medicine.
| Description | Comment/ observation as compared with genuine package |
|---|---|
| Primary label information | |
| Product name | ● The name was the same. |
| Active ingredients | ● The composition was the same but not in bold like on the genuine label. |
| Category of distribution | ● Stated as prescription medicines while the genuine print had Prescription only Medicine (POM). |
| Zambia Marketing Authorisation Number | ● The printed Marketing Authorisation number (i.e. Visa number) was not consistent with the Zambia Marketing Authorisation coding. |
| Storage condition | ● The storage condition was different. |
| Expiry date | ● The shelf life was different, that is, the suspected falsified product had a shelf life of 18 months compared with the 24 months on the genuine. |
| Name and manufacturing site address | ● Name of the manufacturer was the same but with incomplete manufacturing site address. |
| Others | ● Additional labelling, for example, instructions were provided
unlike in the genuine pack. ● The fill volume was less by over 1 mL compared with the original. ● Orientation of text ‘for external use only’ and barcode was changed. |
| Secondary label information | |
| Product name | ● The name was the same |
| Active ingredients | ● The Active Pharmaceutical Ingredient (API) labelled were the same; however, they were written in a different order and smaller font size. |
| Expiry date | ● The shelf life was different, that is, the suspected falsified product had a shelf life of 18 months compared with the 24 months on the genuine. |
| Storage condition | ● The storage condition was different |
| Name and manufacturing site address | ● Name of the manufacturer was the same but with incomplete manufacturing site address. |
| Zambia Marketing Authorisation Number | ● Not printed on the pack but put on the pack using a
sticker. The printed Marketing Authorisation number (i.e. Visa number) was not consistent with the Zambia Marketing Authorisation coding. |
| Colour of packaging | ● Slightly different packaging colours shade. |
| Others | ● The two batches of the suspected falsified products were
slightly heavier. ● A combination of English and another language was used. The font size for the ‘directions for use’, bar code, and other instructions were different and placed in different parts of the package compared with the genuine package. ● The package had colour lining on the edge of one face, while the original package did not have. |
Discussion
With the relatively high disease burden in LMICs such as Zambia, including the advent of global pandemics such as HIV and COVID-19, the demand for medical products is high, pushing the influx of SF medical products into the markets. Therefore, understanding the prevalence of SF medical products, different types of defects causing recalls, sources of SF medical products, pharmaceutical dosage forms recalled, and their therapeutic categories is important in shaping solutions aimed at curtailing the influx of SF medical products.
The results of the current study indicate a prevalence of 67.9% for recalls of SF medical products. Of the total of 83 product recalls made, 78 were substandard medical products. Similar studies have found varying numbers of recalled SF medical products. In Sri Lanka, 17 medical products were recalled between June 2018 and January 2022 due to multiple defects detected47 whereas in the United States, a total of 21,120 products were recalled during the 30-month study period.48
Our findings also showed that SF medical product recalls increased in 2020 and 2021, which can be attributable to the peak period of the COVID-19 pandemic in Zambia, with 20.5% of the recalls being hand sanitizers and disinfectants. We also found that three recalled hand sanitizers could not be considered as falsified products, as they did not misrepresent their source, identity or composition. They were adequately labelled as containing methanol by the correct manufacturers. The recall was because they contained the wrong active ingredient, methanol. Alcohol-based hand sanitizers contaminated with harmful impurities such as methanol, benzene and acetaldehyde have been documented to pose a risk and adverse effects.49 –52 However, the impact of these recalled methanol-containing hand sanitizers on the Zambian market has not been documented or reported. The increase in the medical product recalls can also be attributed to the increased surveillance by ZAMRA, the national regulatory agency. The trend is comparable with global observations, where more than 34,000 falsified COVID-19 products were seized in 2020.53 In a South African survey, for example, of the 94 hand sanitizer samples collected, three preparations contained no alcohol, while the rest contained either ethanol, 2-propanol or 1-propanol or a combination of two alcohols.54 The survey further revealed that of the remaining alcohol-containing hand sanitizers, 37 (41%) contained less than 60% alcohol. Similarly, we found out that 11 of the 14 recalled hand sanitizers were recalled for containing less than 60% alcohol.
In this study, nearly half (47.45%) of defects were due to manufacturing laboratory control issues, followed by product contamination and sterility issues (29.5%). The findings are consistent with the results of a study in Canada29 and contradict the findings of Janani et al.,47 Almuzaini et al.,30 Hall et al.,48 and AlQuadeib et al.,55 where the most frequently reported defect was product contamination and sterility issues. However, this indicates the need for continuous process improvement and corrective preventive action as errors occur even when stringent measures are implemented. In our study, product contamination and sterility issues was the second most common reason for the recall of substandard medical products. This also poses a serious threat to the end users of contaminated products. Therefore, it is of concern that ringers lactate locally manufactured by only one manufacturer, who is a major distributor of this product to government facilities, had different batches (4 in total over 2 years) affected, which could have caused a disruption in the supply chain.
Antiseptic and disinfectants 17 (20.5%) were found to be the most recalled therapeutic class of medical products, which is consistent with the results of a study conducted in Nepal.56 This can be attributed to the increased demand for this class of products in the face of the COVID-19 pandemic, as more suppliers imported these products to meet the demand. Antihypertensive and antibiotics (16.9%, n = 14) are the second most recalled class of drugs. Similar findings were reported by AlQuadeib et al.55 in Saudi Arabia. Poor quality antibiotics are particularly of concern because taking subtherapeutic doses of antibiotics can contribute to the global challenge of antimicrobial resistance.57 –59
We also found an unregistered product that was recalled due to misrepresentation. According to the report, the product was called a Chinese contraceptive pill and was misrepresented as a herbal contraceptive but contained high levels of Levonorgestrel and Quinestrol. The availability of unregistered medicines that have not had minimal regulatory oversight or import approval poses a great risk to public health in countries with weak border controls such as Zambia. Nyika et al.60 studied similarities and differences in the importation and distribution of unregistered medicines in the countries of the Southern African Development Community (SADC) and found that Zambia had a low relative implementation index level of 28% for minimum recommended standards for the importation of unregistered medicines. This increases the risk of exposure to SF medical products.60
Of the 42 manufacturers whose products were recalled, 29 (69%) were foreign-based manufacturers. The finding is similar to a study in Sri Lanka where the most recalled products involved imported products.47 According to the Global Economic Data, Indicators, Charts and Forecasts (CEIC) website,61 Zambia’s medicinal and pharmaceutical imports were US$65,591.083 by December 2021. The high number of foreign-based manufacturers with recalled SF medical products clearly indicates an economic burden on the Zambian health sector and poses a challenge to access to quality-assured medical products.
Several factors limited this study. First, the study only assessed documented recalls over 4 years. The number of years cannot provide a conclusive trend on SF medical products in Zambia, and more data need to be collected in the next few years. However, the study provides an important insight on the quality of medicines in Zambia. Second, an in-depth analysis was not possible due to the non-availability of data on quantities recalled, the number of actual quantities of recalled products received, regional distribution of recalls and the action taken following the recall to measure the impact of the recalls. Finally, the study did not attempt to establish the classification of the recalls issued. Therefore, future studies are needed to determine the classification of the recalls and an in-depth analysis of the recalls.
Recommendation
Medical products recall is an essential process in safeguarding public health. Therefore, this must be the responsibility of the regulators and the pharmaceutical industry. This study shows that 66 of 83 recalls made were initiated by ZAMRA, and the rest by foreign-based marketing authorisation. The lack of participation of local manufacturers and distributors can be attributed to the lack of specific published guidelines on product recall. Participation by foreign-based MAH could be attributed to the condition of their marketing authorisation which requires them to implement a product-specific pharmacovigilance system and report any detected problems to the regulatory authority. There is a need for urgent publication of recall guidelines in Zambia, like other countries in the region.62 –64 This will help guide the local industry on the timelines to make medical product recalls based on the class of recall and regulatory intervention needed.
Several African national medicines regulatory authorities publish product alerts and recalls in open source databases.39,65 –67 This allows stakeholders such as researchers to produce objective evidence that can inform regulatory decisions and protect public health, as illustrated in Malawi and Rwanda, where two extremely substandard brands of misoprostol tablets were found during a quality survey and led to the issuance of recalls by the regulatory authorities in the two countries and the WHO issued an alert to other countries.68,69 Therefore, it is recommended that all alerts be uploaded to a publicly available database (e.g. ZAMRA website and Med Safety mobile app). Information to be published on recalls should include, among others, the actual quantities recalled, quantities received, regional distribution, reason for recall and type of recall. Moreover, having evidence of documented communication with a feedback mechanism is one of the requirements for global benchmarking for the national regulatory system, which WHO has recently introduced,70 and this would be beneficial to ZAMRA during the benchmarking process in future. Continuous surveillance of SF medical products should be increased across the country.
Conclusion
This article has shown the presence of SF medical products on the Zambian market, which were eventually recalled. The manufacturing laboratory control issues were the most frequent cause of defective medicines, while oral solid dosage formulations were the most susceptible dosage form. Recall of defective medical products affected both locally and foreign manufactured products, with the majority being imported medical products. This poses a significant economic burden on the healthcare system and impedes access to quality-assured medical products. In future, a country-wide survey covering the entire supply chain is needed to indicate the trend of SF medical products on the Zambian market compared with other countries in the region and determine the class of recalls in Zambia.
Supplemental Material
Supplemental material, sj-pdf-1-map-10.1177_27550834221141767 for Substandard and falsified medical product recalls in Zambia from 2018 to 2021 and implications on the quality surveillance systems by Billy Chabalenge, Elimas Jere, Namuchindo Nanyangwe, Christabel Hikaambo, Steward Mudenda, Michelo Banda, Aubrey Kalungia and Scott Matafwali in The Journal of Medicine Access
Supplemental material, sj-pdf-2-map-10.1177_27550834221141767 for Substandard and falsified medical product recalls in Zambia from 2018 to 2021 and implications on the quality surveillance systems by Billy Chabalenge, Elimas Jere, Namuchindo Nanyangwe, Christabel Hikaambo, Steward Mudenda, Michelo Banda, Aubrey Kalungia and Scott Matafwali in The Journal of Medicine Access
Acknowledgments
The authors would like to thank the acting Director General and Senior Management at ZAMRA for providing permission to conduct this study.
Footnotes
ORCID iD: Billy Chabalenge  https://orcid.org/0000-0003-4864-887X
https://orcid.org/0000-0003-4864-887X
Declarations
Ethics approval and consent to participate: Ethics approval and informed consent to participate were not applicable due to the nature of the study. Permission was, however, sort from the ZAMRA acting Director General and senior management.
Consent for publication: Permission was sort from the ZAMRA acting Director General and Senior Management
Author contribution(s): Billy Chabalenge: Conceptualisation; Formal analysis; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Elimas Jere: Formal analysis; Validation; Writing – review & editing.
Namuchindo Nanyangwe: Formal analysis; Validation; Writing – review & editing.
Christabel Hikaambo: Data curation; Methodology; Writing – review & editing.
Steward Mudenda: Data curation; Supervision; Writing – review & editing.
Michelo Banda: Data curation; Supervision; Writing – review & editing.
Aubrey Kalungia: Conceptualisation; Data curation; Methodology; Writing – review & editing.
Scott Matafwali: Conceptualisation; Data curation; Methodology; Visualisation; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The first, second and third authors are Pharmacists currently working under Zambia Medicines Regulatory Authority, Department of Medicines Control and are involved in post-marketing surveillance activities. Zambia Medicines Regulatory Authority had no role in the design of this study and collection, analysis and interpretation of data. Any views and opinions expressed are personal and belong solely to the individuals and do not represent any individual institutions, or organisations that the individuals are associated with in a personal or professional capacity. The remaining authors declare that they have no conflict of interest.
Availability of data and materials: The data set generated and/or analysed during the current study is available from the corresponding author upon reasonable request.
References
- 1. World Health Organization. Definitions of substandard and falsified (SF) medical products, 2017, https://www.who.int/teams/regulation-prequalification/incidents-and-SF/background/definitions (accessed 22 September 2022).
- 2. Renschler JP, Walters KM, Newton PN, et al. Estimated under-five deaths associated with poor-quality antimalarials in sub-Saharan Africa. Am J Trop Med Hyg 2015; 92(Suppl. 6): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beargie SM, Higgins CR, Evans DR, et al. The economic impact of substandard and falsified antimalarial medications in Nigeria. PLoS One 2019; 14(8): e0217910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. A study on the public health and socioeconomic impact of substandard and falsified medical products, 2017, https://apps.who.int/iris/bitstream/handle/10665/331690/9789248513435-por.pdf (accessed 22 September 2022).
- 5. World Health Organization. WHO global surveillance and monitoring system for substandard and falsified medical products, 2017, https://apps.who.int/iris/bitstream/handle/10665/326708/9789241513425-eng.pdf (accessed 22 September 2022).
- 6. Ozawa S, Evans DR, Bessias S, et al. Prevalence and estimated economic burden of substandard and falsified medicines in low-and middle-income countries: a systematic review and meta-analysis. JAMA Netw Open 2018; 1: e181662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adepoju P. African nations to criminalise falsified medicine trafficking. Lancet 2020; 395: 324. [DOI] [PubMed] [Google Scholar]
- 8. Pisani E, Hasnida A, Rahmi M, et al. Substandard and falsified medicines: proposed methods for case finding and sentinel surveillance. JMIR Public Health Surveill 2021; 7: e29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson KD, Higgins CR, Laing SK, et al. Impact of substandard and falsified antimalarials in Zambia: application of the SAFARI model. BMC Public Health 2020; 20: 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaiser AH, Hehman L, Forsberg BC, et al. Availability, prices and affordability of essential medicines for treatment of diabetes and hypertension in private pharmacies in Zambia. PLoS One 2019; 14(12): e0226169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. USAID Global Health Supply Chain Program. Quality considerations of Zambian wholesalers and regulatory authorities to increase availability and access to quality maternal, newborn, and child health products in Zambia, 2020, https://www.ghsupplychain.org/sites/default/files/2020-05/Zambia_Wholesaler_Assessment_MNCH_Jan%202020.pdf (accessed 23 September 2022).
- 12. Nayyar GML, Breman JG, Herrington JE. The global pandemic of falsified medicines: laboratory and field innovations and policy perspectives. Am J Trop Med Hyg 2015; 92(Suppl. 6): 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCarthy M. Fake medicines are undermining global efforts to combat infectious disease, says US journal. BMJ 2015; 350: h2137. [DOI] [PubMed] [Google Scholar]
- 14. McManus D, Naughton BD. A systematic review of substandard, falsified, unlicensed and unregistered medicine sampling studies: a focus on context, prevalence, and quality. BMJ Glob Health 2020; 5(8): e002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nayyar GM, Breman JG, Newton PN, et al. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis 2012; 12(6): 488–496. [DOI] [PubMed] [Google Scholar]
- 16. Gautam CS, Utreja A, Singal GL. Spurious and counterfeit drugs: a growing industry in the developing world. Postgrad Med J 2009; 85(1003): 251–256. [DOI] [PubMed] [Google Scholar]
- 17. Newton PN, Green MD, Fernández FM, et al. Counterfeit anti-infective drugs. Lancet Infect Dis 2006; 6(9): 602–613. [DOI] [PubMed] [Google Scholar]
- 18. Naughton BD, Akgul E. Medicine quality in high-income countries: the obstacles to comparative prevalence studies. Med Access Point Care 2021; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet 2017; 389: 403–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization (WHO). Survey of the quality of medicines identified by the United Nations commission on life-saving commodities for women and children. WHO, 2016, https://apps.who.int/iris/bitstream/handle/10665/255550/9789241511117-eng.pdf (accessed 22 September 2022).
- 21. Bate R, Mooney L, Hess K. Medicine registration and medicine quality: a preliminary analysis of key cities in emerging markets. Res Rep Trop Med 2010; 1: 89–93. [Google Scholar]
- 22. Newton PN, Hanson K, Goodman C. Do anti-malarials in Africa meet quality standards? The market penetration of non quality-assured artemisinin combination therapy in eight African countries. Malar J 2017; 16: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mumphansha H, Nickerson JW, Attaran A, et al. An analysis of substandard propofol detected in use in Zambian anesthesia. Anesth Analg 2017; 125: 616–619. [DOI] [PubMed] [Google Scholar]
- 24. Ro hit VVSS, Veluchuri JP, Adhikari S, et al. An overview on pharmaceutical drug recalls. Pharm Chem J 2020; 7: 16–22. [Google Scholar]
- 25. Nagaich U, Sadhna D. Drug recall: an incubus for pharmaceutical companies and most serious drug recall of history. Int J Pharm Investig 2015; 5(1): 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eissa ME. Drug recall monitoring and trend analysis: a multidimensional study. Glob J Qual Saf Health Care 2019; 2: 34–39. [Google Scholar]
- 27. Committee on Understanding the Global Public Health Implications of Substandard, Falsified, and Counterfeit Medical Products, Board on Global Health and Institute of Medicine. Countering the problem of falsified and substandard drugs (eds Buckley GJ, Gostin LO.). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 28. Kaur H, Clarke S, Lalani M, et al. Fake anti-malarials: start with the facts. Malar J 2016; 15: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Almuzaini T, Sammons H, Choonara I. Quality of medicines in Canada: a retrospective review of risk communication documents (2005–2013). BMJ Open 2014; 4: e006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Almuzaini T, Sammons H, Choonara I. Substandard and falsified medicines in the UK: a retrospective review of drug alerts (2001–2011). BMJ Open 2013; 3(7): e002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. European Medicines Agency. How to use the defective product report to notify a quality defect to European Medicines Agency, 2018, https://www.ema.europa.eu/en/human-regulatory/post-authorisation/compliance/quality-defects-recalls/reporting-quality-defect-ema (2018, accessed 28 March 2022).
- 32. Kabali E. Review of the effectiveness of the medicines regulatory systems in Zambia over the period 1995 to 2015. MSc Thesis, University of the Western Cape, Cape Town, South Africa. [Google Scholar]
- 33. World Health Organization (WHO). The WHO member state mechanism on substandard and falsified medical products. WHO, Geneva, 2020. [Google Scholar]
- 34. Stratton KR, Howe CJ, Johnston RB., Jr. Case reports and passive surveillance. In: Stratton KR, Howe CJ, Johnston RB., Jr (eds) Research strategies for assessing adverse events associated with vaccines: a workshop summary. Washington, DC: The National Academies Press, 1994. [PubMed] [Google Scholar]
- 35. Gwatidzo SD, Murambinda PK, Makoni Z. Medicines counterfeiting in Africa: a view from Zimbabwe. Med Access Point Care 2017; 1. [Google Scholar]
- 36. Therapeutic Goods Administration. Recall actions database, https://www.tga.gov.au/recall-actions-database (accessed 23 September 2022).
- 37. Health Products Regulatory Authority. Medicines safety notices, https://www.hpra.ie/homepage/medicines/safety-notices?type=3&orderby=DATE_NEWEST (accessed 23 September 2022).
- 38. Rasheed H, Hoellein L, Bukhari KS, et al. Regulatory framework in Pakistan: situation analysis of medicine quality and future recommendations. J Pharm Policy Pract 2019; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rwanda Food and Drugs Authority. Publications – product recalls, https://www.rwandafda.gov.rw/publications?tx_filelist_filelist%5Baction%5D=list&tx_filelist_filelist%5Bcontroller%5D=File&tx_filelist_filelist%5Bpath%5D=%2Fuser_upload%2FRwandaFDA%2FPublications%2FSafety_Alerts%2FProduct_Recalls%2F&cHash=367ab27c8b861d7ab4f25b89285c6c79 (accessed 23 September 2022).
- 40. Kiguba R, Olsson S, Waitt C. Pharmacovigilance in low-and middle-income countries: a review with particular focus on Africa. Br J Clin Pharmacol. Epub ahead of print 22 December 2021. DOI: 10.1111/bcp.15193. [DOI] [PubMed] [Google Scholar]
- 41. Jere J. 13 hand sanitizer brands face recall. Zambia National Broadcasting Cooperation, 19 June 2020, https://www.znbc.co.zm/news/13-hand-sanitizer-brands-face-recall/ (accessed 23 September 2022).
- 42. Malunga J. International Drug Company recalls toxic aspirin. News Diggers, 14 January 2021, https://diggers.news/local/2021/01/14/intl-drug-company-recalls-toxic-aspirin/ (accessed 23 September 2022).
- 43. Zambia Medicines Regulatory Authority. Public notice – product recall update, https://www.zamra.co.zm/wp-content/uploads/2022/03/Public-Notice_Losartan-Recall_Azido-Impurities_02032022-1.pdf (accessed 23 September 2022).
- 44. Merks P, Swieczkowski D, Byliniak M, et al. The European Falsified Medicines Directive in Poland: background, implementation and potential recommendations for pharmacists. Eur J Hosp Pharm 2018; 25(1): 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parmaksiz K, Pisani E, Kok MO. What makes a national pharmaceutical track and trace system succeed? Lessons from Turkey. Glob Health Sci Pract 2020; 8: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization. The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD), https://www.who.int/standards/classifications/other-classifications/the-anatomical-therapeutic-chemical-classification-system-with-defined-daily-doses
- 47. Janani TSJ, Ketharam M, Herath KB, et al. Quality of medicines in Sri Lanka: a retrospective review of safety alerts, 2022, https://assets.researchsquare.com/files/rs-1897516/v1/c413828d-45a4-4c14-bd6d-a595a281f701.pdf?c=1659980667 (accessed 23 September 2022). [DOI] [PMC free article] [PubMed]
- 48. Hall K, Stewart T, Chang J, et al. Characteristics of FDA drug recalls: a 30-month analysis. Am J Health Syst Pharm 2016; 73: 235–240. [DOI] [PubMed] [Google Scholar]
- 49. Chan APL, Chan TYK. Methanol as an unlisted ingredient in supposedly alcohol-based hand rub can pose serious health risk. Int J Environ Res Public Health 2018; 15: 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicol A-M. Recalls highlight importance of hand sanitiser safety. Environ Health Rev 2021; 64: 6–10. [Google Scholar]
- 51. Matatiele P, Southon B, Dabula B, et al. Monitoring quality of alcohol-based hand sanitizers used in Johannesburg area during the covid-19 pandemic, 2021, https://www.researchsquare.com/article/rs-612413/v1 [DOI] [PMC free article] [PubMed]
- 52. Abuga K, Nyamwey N, King’ondu O. Quality of alcohol based hand sanitizers marketed in the Nairobi metropolitan area. East Centr Afr J Pharm Sci 2021; 24: 29–37. [Google Scholar]
- 53. Ahmed J, Modica De Mohac L, Mackey TK, et al. A critical review on the availability of substandard and falsified medicines online: incidence, challenges and perspectives. J Med Access 2022; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matatiele P, Southon B, Dabula B, et al. Assessment of quality of alcohol-based hand sanitisers used in Johannesburg area during the CoViD-19 pandemic. Sci Rep 2022; 12: 4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. AlQuadeib BT, Alfagih IM, Alnahdi AH, et al. Medicine recalls in Saudi Arabia: a retrospective review of drug alerts (January 2010–January 2019). Futur J Pharm Sci 2020; 6: 91. [Google Scholar]
- 56. Neupane A, Bastakoti M, Tamang S, et al. Review of drug recalls and quality of pharmaceutical products in Nepal. BMJ Open 2022; 12: e053479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zabala GA, Bellingham K, Vidhamaly V, et al. Substandard and falsified antibiotics: neglected drivers of antimicrobial resistance? BMJ Glob Health 2022; 7(8): e008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Orubu ESF, Ching C, Zaman MH, et al. Tackling the blind spot of poor-quality medicines in universal health coverage. J Pharm Policy Pract 2020; 13: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kelesidis T, Falagas ME. Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev 2015; 28(2): 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nyika A, Ngara B, Mutingwende I, et al. Importation and distribution of unregistered medicines in the public sector: similarities, differences, and shared challenges among Southern African Development Community (SADC) countries. BMC Health Serv Res 2022; 22: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. CEIC. Zambia imports: medicinal and pharmaceutical product 1995 – 2021, https://www.ceicdata.com/en/indicator/zambia/imports-medicinal-and-pharmaceutical-product (accessed 23 September 2022).
- 62. Medicines Control Authority of Zimbabwe. Guidelines for the notification of medicinal product problem or defect and recall procedure, https://www.mcaz.co.zw/licensing-and-enforcement/product-defects-recalls/ (accessed 23 September 2022).
- 63. Tanzania Medicines and Medical Devices Authority. Guidelines for recall, handling and disposal of unfit medicines, https://www.tmda.go.tz/pages/recall-of-medicines (accessed 23 September 2022).
- 64. Botswana Medicines Regulatory Authority. Product recall and withdrawal guideline, 2020, https://bomra.co.bw/bomra-downloads/#132-311-wpfd-product-recalls-notifications (accessed 23 September 2022).
- 65. South African Health Products Regulatory Authority. Product recalls, https://www.sahpra.org.za/product-recalls/ (accessed 23 September 2022).
- 66. Uganda National Drug Authority. Drug recalls, https://www.nda.or.ug/drug-recalls-2018-2020/ (accessed 23 September 2022).
- 67. Kenya Pharmacy and Poisons Board. Safety and rapid alerts, https://web.pharmacyboardkenya.org/safety-and-rapid-alerts/ (accessed 23 September 2022).
- 68. Hagen N, Khuluza F, Heide L. Quality, availability and storage conditions of oxytocin and misoprostol in Malawi. BMC Pregnancy Childbirth 2020; 20: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bizimana T, Hagen N, Gnegel G, et al. Quality of oxytocin and misoprostol in health facilities of Rwanda. PLoS One 2021; 16(1): e0245054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. World Health Organization. WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory systems of medical products: revision VI, 2021, https://apps.who.int/iris/bitstream/handle/10665/341243/9789240020245-eng.pdf?sequence=1 (accessed 23 September 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-map-10.1177_27550834221141767 for Substandard and falsified medical product recalls in Zambia from 2018 to 2021 and implications on the quality surveillance systems by Billy Chabalenge, Elimas Jere, Namuchindo Nanyangwe, Christabel Hikaambo, Steward Mudenda, Michelo Banda, Aubrey Kalungia and Scott Matafwali in The Journal of Medicine Access
Supplemental material, sj-pdf-2-map-10.1177_27550834221141767 for Substandard and falsified medical product recalls in Zambia from 2018 to 2021 and implications on the quality surveillance systems by Billy Chabalenge, Elimas Jere, Namuchindo Nanyangwe, Christabel Hikaambo, Steward Mudenda, Michelo Banda, Aubrey Kalungia and Scott Matafwali in The Journal of Medicine Access