Abstract
Background
In the face of the Covid-19 pandemic and the need for social distancing new therapeutic tools like mobile health applications might gain in importance for outpatient care. Objective of the present study was to assess if and to what extent the implementation of a free available transplant application in a cohort of liver transplant recipients was possible.
Methods
Patients of the aftercare program at the Department of Transplant Surgery Graz in June 2016 were first asked to complete a survey concerning knowledge about mobile health and their management of everyday life. After using the application for 2 months a second survey evaluated whether the implementation of the application in the daily routine was achievable.
Results
Among 135 patients, 124 (91.9%) agreed to participate. Seventy-one (57.3%) owned a mobile device with which they could use the application, 42 patients (33.8%) decided to try it out for 2 months. The majority stated that the application supported them for therapy management and surveillance of vital parameters. Successful implementation of the application has been reached in 57.1% of patients after 2 months testing period.
Conclusion
The technical prerequisites are only partially met and should be improved. Older patients need extensive support and motivation.
Keywords: mHealth, transplantation, long-term survival, quality of live, social distancing
Introduction
The global pandemic of severe acute respiratory coronavirus 2 (SARS-CoV-2) caused new challenges for health care systems. There was an uncompromising priorization of resources towards critically ill patients with Covid-19 infection. However, treatment was restricted for all other patient cohorts but emergencies. Additionally, social distancing including suspension of outpatient care units for elective check-ups was recommended. As patients after liver transplantation (LT) require regular surveillance and care, the use of alternative and secure means available at home gains more importance. The possibility of implementation of mobile Health (mHealth) tools in our valuable patient cohort is necessary and should be evaluated in order not to jeopardize the quality of the good long-term results.
The use of mobile technology is already an integral part of our daily life gaining more and more importance. The rising impact and future potential is best expressed by the fact that in 2014, for the first time ever, the access and use of the internet was rather performed through mobile devices than desktop computers.1 The green paper on mHealth (2012–2020) introduced by the European Union (EU) highlights the potential of mHealth services and discusses their advantages in maintaining and evaluating health. The EU estimated that healthcare applications (apps) in Europe could save 99 billion Euros per year by enabling early diagnosis and promoting disease prevention.2,3 Already 40% of the 300.000 currently available mobile apps worldwide focus on health topics.4
One basic requirement for the successful implementation of mHealth in the population is the increasing availability of smartphones. In correlation with the global trend, the spread of smartphones has also increased in Austria from 43% up to 77% between 2013 and 2019.5 While the technical framework for the useful implementation of mHealth seems given in many parts of the world today, there is still a lot to evaluate on the patient side until it will finally establish itself as a reliable tool in the long-term post-transplant management.6,7,8 In recent years, many aspects at the patient and app development level have been highlighted by surveys in order to find the optimal basic requirements to make mHealth accessible, interesting and as user-friendly as possible for long-term use.9
So far there have only been a few efforts to use mHealth in transplanted patients. The feasibility, acceptability and outcome of a prototype mHealth medication and blood pressure self-management system for patients after renal transplantation was evaluated by McGillicuddy et al.10 In this randomized, controlled study the participation rate was 75%. The results showed that patients who were randomized to the mHealth intervention group gained significant improvements in medication adherence compared to patients in the standard care control group. Controverse results were demonstrated in another randomized, controlled study with the Adhere4U mobile medication manager application in 138 patients after kidney transplantation,11 who were randomized to a control group (n = 67) and a mobile group (n = 71). Non-adherence to immunosuppressive therapy occurred in 65% of the mobile group and 62.1% of the standard group. There was no difference in the improvement of adherence to immunosuppressive therapy in both groups. Remarkable was the low rate of patient engagement with the app in the mobile group. The app use was 47.6% at 28 days. Therefore, successful implementation is the first and most essential step on the way to ensure effectivity and sustainability of a new therapeutic tool.
Concerning the liver, a recently published study with liver transplant candidates described successful exercise training with the help of the self-created smartphone application EL-FIT (Exercise and Liver FITness).12 The results showed an increase of 35% in the physical performance of participants and therefore this smartphone app might become a precious tool for home-based preoperative training of liver transplant candidates.
Data about mHealth in adult patients after LT are still scarce. However, the rate of non-adherence in these patients is high and reducing it has the potential for improved medium and long-term results after organ transplantation.13 Therefore, objective of the present study was to assess if and to what extent the implementation of a free available transplant app in a cohort of patients after LT was possible. In addition, information on drug management after LT, organization of monitoring of vital functions, knowledge of mHealth, experience with mHealth and assessments of the transplant app and factors, which had an influence on the use of the app were evaluated.
Methods
Study design and setting
The aim of this single-centre cohort study was to evaluate the implementation of a free available transplant app to support follow-up care in liver transplant patients. In the context of our study, implementation was defined as a process to promote behaviour change in terms of the regular (daily) use of a mhealth tool.14 The success of this process was measured by the statement of our patients, whether they used the app in their everyday life or not. Patients who agreed to participate filled out a questionnaire with general questions about their knowledge about mHealth, but also about their management of everyday life as a transplant patient. Then the app and its features were explained in detail to every patient and they were asked to use it for 2 months, the time which is necessary to form a habit in everyday life.15 This was followed by a second survey to evaluate the experiences and opinions on the app and whether implementation of the app in the daily routine was possible.
Participants
Patients after LT who were in the aftercare program at the Department of Transplant Surgery of the Medical University of Graz, Austria in June 2016, were asked to participate in the study (June 2016 to June 2017). Approval was given by the local ethical committee (protocol number: 26–534 ex 13/14). All persons gave their informed consent prior to their inclusion in the study.
Transplant App
The transplant app is a health app making it easier to collect, manage and monitor health information. It can be downloaded for free from the app store. The transplant app combined features of medication tracking apps and apps for maintaining a healthy lifestyle. In this function it encourages patients to stick to their therapeutic regimens by tracking their doses and time intervals. Furthermore, it helps to educate patients for a healthy and well-being lifestyle following transplantation. The goals of the app are to support patients with medication management and to provide helpful and vital information. Users of the app can easily generate relevant health data themselves at any time, and make them available to their doctor if necessary. The app includes lists of relevant drugs, a medication/dose converter (e.g. switching from Prograf to Advagraf), a doctor visits – alert and infection alerts – via Google Alert. There is information on the complete follow-up care, which answers questions about the immune system as well as very practical questions, for example about infections, sexuality, smoking, alcohol, diet, travel, vaccinations and skin cancer screening. Information about sports and nutrition is available. Important addresses, for example, social institutions, self-help groups are deposited. Furthermore, a patient diary (for blood pressure & pulse, body temperature weight, fluid intake and discharge, results of prescribed self-controls, e.g. sugar levels) is part of the app and enables a precise observation and documentation of the condition of one's own body. Finally a question check before the doctor's visit can be performed.
Questionnaires
The two questionnaires (added as supplements) were created at the Department of Transplant Surgery of the Medical University Graz, Austria. The surveys consisted of questions that were easy to understand, pragmatic, concise and without double negation or hypothetical connotations. The questionnaires contained both, open and closed questions. Pre-testing was conducted in a neutral test group of the same age to gain data on the duration of completion, feedback on comprehensibility and plausibility.16 In order to achieve test quality, objectivity and reliability were considered. To check objectivity, 12 neutral persons fulfilled the survey. To raise reliability the first survey was repeated in 12 patients after 2 weeks. The results of the first and second measurement were correlated. In the test-retest procedure, it is checked whether a repetition of the measurement delivers the same measured values while the property to be measured remains constant. The retest reliability indicates the degree of agreement. The reliability coefficient for the first questionnaire was 0.96. The reliability coefficient for the second questionnaire was collected in the same way as for the first and was 0.94.
The first survey consisted of 10 closed and 5 open (in total 15) questions. The first part of it was intended to provide an overview of the patient's level of knowledge with regard to mHealth and health apps and to evaluate how many liver transplant recipients would have the opportunity to use an app. In the further part of the questionnaire, methods of the patients to manage medication intake are queried, and how the adherence is assessed and the monitoring of vital parameters is handled. Following these questions, the transplant app was demonstrated and explained to the patient. The aim of the last questions was to find out how the patient thought the app could help him (e.g. for medication intake, for observation of vital parameter, to gain useful information). This should clarify the question of possible advantages and expectations. The second survey was performed 2 months after the use of the app, consisted of 14 questions (10 closed and 4 open ones) and aimed to find out the usability of the specific transplant application. The majority of the remaining questions evaluated the patient's satisfaction with the application and to what extent they thought that the application could support them in taking medication regularly and to what extent they could obtain useful information. Patients were asked whether they will continue to use the transplant application and whether they would recommend it to other transplanted patients. Furthermore, concerns about data protection were queried. Both questionnaires were fulfilled together with the patients in an interview during a routine outpatient appointment.
Variables
Variables of the first questionnaire comprise habits and knowledge of cell phones and apps, management of medication intake and the management of changes of vital parameters. The second questionnaire primarily evaluates the frequency of use of the transplant app, extent of use of the transplant app, on-time medication intake and patient satisfaction. Furthermore, age, gender, indication for LT, time-point after LT and level of education were investigated.
Data management and statistical methods
The variables are collected by interviewing the patients and then evaluating the questionnaire. The data are entered in an excel table and displayed with descriptive statistics.
Results
Participants
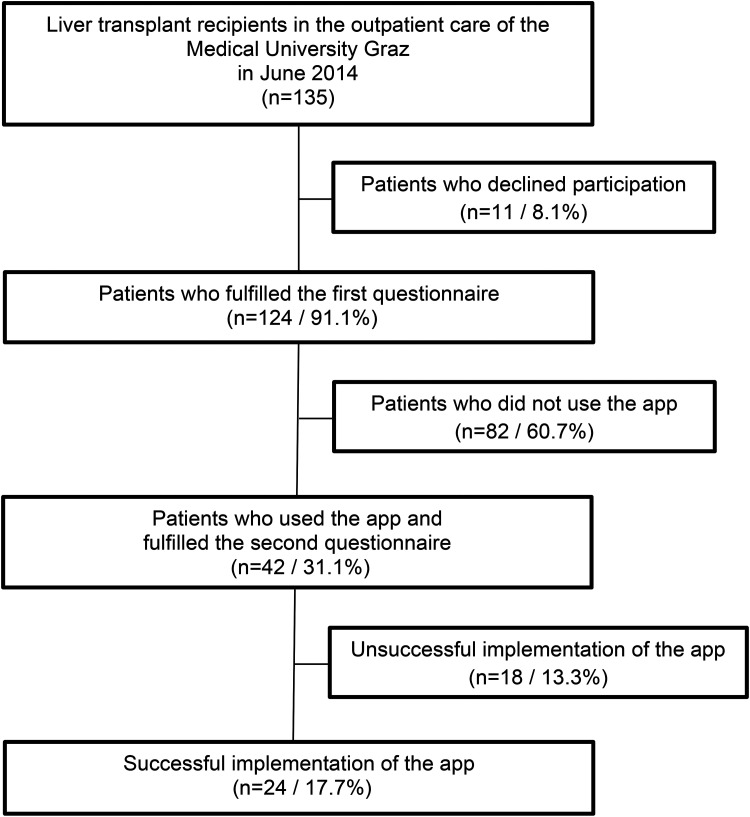
Among 135 liver transplant recipients who were in the outpatient care of our Department at this time point, 124 (91.9%; male 74.1%) agreed to participate (the flow diagram is displayed in Figure 1). The mean age of the liver transplant patients was 63.2 years old (19–76 years), the mean time after LT was 6.5 years (3 months to 21.3 years). Indications for LT are hepatitis (20.8%), alcoholic liver disease (38.6%), hepatocellular carcinoma (25.2%) and others (15.4%). Those patients who declined participation (8.1%) were older compared to those who participated (mean age 66.5 years and 60.1 years, respectively). Regarding to gender, education and indication for transplantation no significant differences were found between patients who participated and who did not.
Figure 1.
Flow of patients through the study.
First questionnaire
The first questionnaire was completed by 124 patients. The reliability coefficient for the first survey was 0.96. Concerning basic requirements and knowledge about mobile apps the following results were found. Seventy-one patients (57.3%) owned a mobile device with which they could use the app. Although 90 patients (72.6%) maintained to know what an app was, only 18 (14.5%) had used a health care app before. Patients’ age who had already used health apps was significantly lower than the mean age of the study cohort (53.5 years and 63.2 years, respectively). Regarding medication adherence, 80 patients (64.5%) explained that they think automatically about their medication intake and do not need any remainder. Among those needing reminders, 20 (45.5%) set an alarm clock, 14 (31.8%) were reminded by their spouse and only 10 (22.7%) used the alarm function of their cell phone. Patients who were reminded by their spouse were significantly older than those, using their electronic device (63.8 years and 53.6 years, respectively). While 88 patients (71%) stated that they would never forget the immunosuppressive medication, 36 (29%) admitted that in rare occasions they forget to take it. Recording of vital parameters was an important feature for 48 patients (38.7%). While 121 stated that they immediately recognize changes of their health status by themselves, only a few patients relied on their relatives or the routinely performed medical follow-ups (97.6% and 2.4%, respectively). Concerning questions about the usability of the app, more than half of the patients stated that this app could be helpful for the management of medication intake (54.8%) and surveillance of vital parameters (56.5%). The majority of patients (86.3%) expected to gain helpful information about the challenges of daily life after solid organ transplantation by using the app. After full evaluation and explanation of the app, 42 patients (33.8%) had the technical framework and wanted to try out the app for 2 months. The mean age of these patients was 55.4 years, which is significantly below the average age of the entire group. All results of questionnaire 1 are displayed in Tables 1 and 2.
Table 1.
Results of survey 1, yes/no questions.
| Number of question | Yes | No | Remarks |
|---|---|---|---|
| 1 | 57.3% | 42.7% | |
| 2 | 50.0% | 50.0% | Used 1 or more than 1 feature |
| 3 | 72.6% | 27.4% | |
| 4 | 14.5% | 85.5% | |
| 5 | 41.1% | 58.9% | |
| 8 | 38.7% | 61.3% | |
| 10 | 24.2% | 75.8% | |
| 11 | 54.8% | 45.2% | |
| 12 | 65.5% | 34.5% | |
| 13 | 54.0% | 46.0% | |
| 14 | 86.3% | 13.7% | |
| 15 | 33,8% | 66,2% |
Table 2.
Results of survey 1, multiple answers.
| Number of question | Answer 1 | Answer 2 | Answer 3 | Answer 4 |
|---|---|---|---|---|
| 6 | 16.1% | 64.5% | 11.3% | 8.1% |
| 7 | 71% | 29% | 0 | 0 |
| 9 | 97.6% | 0.8% | 1.6% |
Second questionnaire
After using the transplant app for 2 months, all 42 patients completed the second questionnaire. The reliability coefficient for the second survey was 0.94. Regarding applicability, usability and patient satisfaction the following data resulted. Almost half of the patients (47.6%) fully used the app, 17 patients (40.5%) used it partially and only 5 patients (11.9%) did not use the app at all. Out of those who used the app on a regular basis, 61.9% stated that they used three to five different features, 23.8% used more than five features and only 16.7% used just one tool. Particular importance was given to the functions information, reminder, medication management and service apps. The majority of the patients (66.7%) stated that the app supported them sufficiently for the therapy management and the surveillance of vital parameters. Successful implementation of the app and the intention to continue using the app every day was achieved in 57.1% of patients who tried it for 2 months. Also 69% would recommend this app to other transplant patients. Referring to personal data protection 59.5% (25 patients) were concerned about this, 17 patients (40.5%) had no concerns. The whole results of questionnaire 2 are shown in Tables 3 and 4.
Table 3.
Results of survey 2, yes/no questions.
| Number of question | Yes | No | Remarks |
|---|---|---|---|
| 1 | 57.1% | 42.9% | |
| 3 | 66.7% | 33.3% | |
| 4 | 28.6% | 71.4% | |
| 5 | 50.0% | 50.0% | |
| 6 | 71.4% | 28.6% | |
| 8 | 57.1% | 42.9% | |
| 9 | 52.4% | 47.6% | |
| 10 | 69.0% | 31.0% | |
| 11 | 59.5% | 40.5% | |
| 12 | 52.4% | 47.6% | |
| 13 | 52.4% | 47.6% | Used 1 or more than 1 feature |
| 14 | 52.4% | 47.6% |
Table 4.
Results of survey 2, multiple answers.
| Number of question | Answer 1 | Answer 2 | Answer 3 |
|---|---|---|---|
| 2 | 47.6% | 40.5% | 11.9% |
| 7 | 14.7% | 61.9% | 23.4% |
Level of education
Our results showed that of the 124 patients who participated in the study, the distribution of graduation was as follows: elementary school (14.5%), secondary school (72.6%), high school graduation (10.5%) and university (2.4%). Concerning the educational level of the patients who tried the app for 2 months, none had only the graduation of elementary school, 34 patients (27.4%) had graduated at a secondary school, 7 patients (5.6%) had a high school graduation and only 1 patient (0.8%) showed a university graduation (Table 5). Of the total of three patients with a university degree, only one wanted to use the transplant app. However, these three patients are among the oldest in this group.
Table 5.
Level of graduation of all patients and of those who used the app.
| Level of graduation | Patients in general | Patients who used the app |
|---|---|---|
| Elementary school | 18 (14.5%) | 0 |
| Secondary school | 90 (72.6%) | 34 (27.4%) |
| High School | 13 (10.5%) | 7 (5.6%) |
| University | 3 (2.4%) | 1 (0.8%) |
Discussion
Key-results
The present study reflects a realistic picture of the feasibility to implement mHealth in patients after LT. Seventy-one patients of our liver recipient cohort (57.3%) owned a mobile device with which they could use an app. Forty-two patients (33.8%) had the technical framework and were motivated to try out the app for 2 months. The mean age of this group was well below the average age of the entire group. Patients who denied to participate stated that they did not have a suitable mobile phone or iPad or that they were not familiar with them and with the use of health apps. However, 91.9% of our patients were interested in the subject. More than half of the patients assumed that this app could be helpful for the management of medication intake and surveillance of vital parameters and confirmed that after using the app. The majority of patients claimed to gain helpful information about the challenges of daily life after solid organ transplantation by using the app. In summary, the interest in mHealth among liver transplant recipients is high and there are chances for support in aftercare. However, the technical prerequisites are only partially met and should be significantly improved, for example by providing suitable devices. Particularly, older patients should receive extensive training, support in the initial phase and special motivation for the long term.
Influencing factors for implementation
A relevant factor might be the age of the patients. It was found that younger patients tend to have better access to mHealth and are more willing to deal with it than older patients. This is also reflected in a study of adolescent transplant patients17 which evaluated a mHealth app to improve adherence. Additionally, a lot is done to make cell phones and smartphones more usable for older people. Another important factor for the implementation might be the duration of the trial period with the app. For younger patients, it was no problem to familiarize themselves with the app in a relatively short period of time. Older patients took longer to get to know their way around and used fewer functions of the app compared to younger patients. Perhaps a longer trial period for the app could increase the level of implementation and, under certain circumstances, repeated training on the app would be helpful for many patients, especially older people. The level of education does not seem to have any impact on the use of the app.
Distribution of the information about the app
As part of the first survey, it was evaluated how extensive the patients’ knowledge about health apps is. It was shown that 72.6% of our liver transplant cohort know what an app is, but only 14.5% have used a health app so far. So far, 41.1% of our patients had heard of the transplant app – they were below average in terms of age. Placing information brochures in the appropriate places could certainly be a good approach to bring patients closer to such an option. Targeted information events and training courses might also be helpful.
Support with long-term management of therapy and health status
In order to get an overview of how patients have managed their therapy, corresponding questions have been included in the surveys. The older the patients are, the more likely they are to have comorbidities or to have limited ability to take care of their medication themselves. Here the partners often help out accordingly. In contrast, it was found that younger patients already have a different access to information and communication technologies and have already partially implemented them for themselves. In connection with taking medication, adherence was also queried. In the literature, the results concerning improvement of adherence of transplant recipients using mHealth are controversy.18–22 In the present study, adherence was queried asking how often it happens that patients forget to take immunosuppression. Possible answers were ‘never, rarely, regularly, often’. The result of this question was surprising because only the answer options ‘never’ and ‘seldom’ were chosen, but not a single patient ticked ‘regularly’ or ‘often’. This does not agree with recent studies,23, 24 in which it was stated that 20–50% of transplant patients regularly forget their medication. Maybe the possible answers to the adherence question were not formulated clearly enough or that terms such as regular, often or seldom should have been more precisely defined. On the other hand, our particularly patient-oriented and personal care of liver transplant patients could be reflected in the high adherence rate. In addition, the patients showed no abnormalities in their checks of immunosuppressive levels.
So far, mhealth tools have been used rather cautiously in transplanted patients, whereas good successes in long-term management have been shown in non-transplanted patients. For example, it was shown that patients with type 2 diabetes and a higher app engagement had a greater weight loss and reduction of HbA1C.25 Controverse data were demonstrated concerning mhealth apps and cardiovascular disease risk factors.26 Most of the studies of this review assessed the relationship between user engagement and reduction in weight, BMI, body fat percentage and waist circumference. There were statistically different results between patients with greater app user engagement and patients with lower engagement. Thus, motivation of the patients to use mhealth apps is crucial. mHealth is also used successfully in gynaecology for the care of pregnant women or in orthopaedics to optimize physiotherapy.27,28 Furthermore, there are some published study protocols that have good concepts for the use of mhealth in different areas in the future.29,30,31 In order to evaluate the effectiveness of mHealth, future research should focus on studies with longer follow-up periods and randomized trials. The motivation of patients for the use of mHealth tools will be an important concern.
Expectations of the patients to the app
More than half of the participating patients expect advantages in using the app. They see support of therapy management and in the possibility of receiving information. By using the app the patients gain more personal responsibility. Since the app also offers numerous information options regarding lifestyle, patients are motivated to deal with it and receive good tips on how to implement a healthy lifestyle for transplant patients. This in turn promotes the patient education, which is associated with improved drug adherence.32
Limitations
A limitation of the study arose from the fact that patients had to have their own smartphone and none could be provided. As a result, some patients could not participate because they did not have a suitable mobile phone, although they would have liked to. Another limitation concerns the app: it is a valuable tool for monitoring and information gain, but it has no feature to remember patients to their medication intake and there is no direct connection to the treating physicians. The further development of the app with the addition of these features is the content of our next project. The last limitation comprises the fact, that the study was performed in the time before pandemic, when lockdown, social distancing and virtual patient care were unknown. In the meantime, many patients have had to become familiar with virtual healthcare. However, the patient collective for LT has remained the same and should be supported according to their needs for the better implementation of these concepts.
Conclusion
In the face of the COVID-19 pandemic, new remote care options should be implemented, which could emerge as an important and reliable tool for outpatient follow-up and monitoring. Before we ask ourselves whether our transplant patients will benefit from mHealth tools, we have to question critically which circumstances lead to broad acceptance and successful implementation. In addition, we need to filter out the circumstances that motivate and support our patients to use mHealth at all. However, we should invest in innovative models of care like mHealth as supportive tool for the follow-up care of liver transplant patients. Future research should investigate how to achieve higher levels of successful implementation and after that evaluate the effects and costs of mHealth on the long-term aftercare in liver transplant recipients.
Supplemental Material
Supplemental material, sj-doc-1-dhj-10.1177_20552076221145855 for Implementation of a mobile application for outpatient care after liver transplantation by Michaela Andrä, Milan Sibinovic, Karl Pfeiffer and Daniela Kniepeiss in Digital Health
Supplemental material, sj-doc-2-dhj-10.1177_20552076221145855 for Implementation of a mobile application for outpatient care after liver transplantation by Michaela Andrä, Milan Sibinovic, Karl Pfeiffer and Daniela Kniepeiss in Digital Health
Footnotes
Contributorship: MA wrote the manuscript and performed accurate search of the recent literature. MS supported the literature search. KP was involved in the conception of the questionnaire and the data analysis and supervised the study procedure. DK designed the questionnaire, analysed the data and was responsible for coordination and publication of data and revised the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: The ethics committee of the Medical University Graz approved this study (26–534 ex 13/14).
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Guarantor: DK.
ORCID iD: Daniela Kniepeiss https://orcid.org/0000-0003-1097-467X
Supplemental material: Supplemental material for this article is available online.
References
- 1.Eurostat. Mobile connection to internet. https://tinyurl.com/rkqxoo5. 2016.
- 2. https://ec.europa.eu/digital-agenda/en/news/ehealth-action-plan-2012-2020-innovative-healthcare-21st-century .
- 3. https://ec.europa.eu/digital-agenda/en/news/green-paper-mobile-helath-mhealth .
- 4.Business Insider. IQVIA Institute for Human Data Science Study: Impact of digital health grows as innovation, evidence and adoption of mobile health apps accelerate. https://tinyurl.com/y7qamjat. 2017.
- 5. https://de.statista.com/statistik/daten/studie/568185/umfrage/smartphone besitz-und-smartphone-nutzung-in-oesterreich/
- 6.Lim D, Norman R, Robinson S. Consumer preference to utilise a mobile health app: A stated preference experiment. Plos One 2020; 15: e0229546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming JN, Taber DJ, Mc Elligott J, et al. Mobile health in solid organ transplant: The time is now. Am J Transplant 2017; 17: 2263–2276. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Lames L, Howell M, et al. Ehealth interventions for solid organ transplant recipients: A systematic review and meta-analysis of randomized controlled trials. Transplantation 2020; 104: e224–e235. [DOI] [PubMed] [Google Scholar]
- 9.Mogul DB, Fredericks EM, Brady TM, et al. Digital wings: Innovations in transplant readiness for adolescent and young adult transplant recipients. Transplantation 2019; 103: 1970–1974. [DOI] [PubMed] [Google Scholar]
- 10.McGillicuddy J, Gregoski MJ, Weiland AK, et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: A proof-of-concept randomized controlled trial. JMIR Res Protoc 2013; 2: e32 p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han A, Min S, Ahn S, et al. Mobile medication manager application to improve adherence with immunosuppressive therapy in renal transplant recipients: A randomized controlled trial. PLOS ONE 2019; 14(11): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte-Rojo A, Bloomer PM, Rogers RJ, et al. Introducing EL-FIT (exercise and liver FITness): A smartphone app to prehabilitate and monitor liver transplant candidates. Liver Transpl 2021 Apr; 27: 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuberger JM, Bechstein WO, Kuypers DRJ, et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: A guidance report and clinical checklist by the consensus on managing modifiable risk in transplantation (COMMIT) group. Transplantation 2017; 101: 1–56. [DOI] [PubMed] [Google Scholar]
- 14.Selbmann HK, Kopp I. Implementing guidelines in daily treatment practice. Die Psychiatrie 2005; 2: 33–38. [Google Scholar]
- 15.Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: Modelling habit formation in the real world. Eur J Soc Psychol 2010; 40: 998–1009. [Google Scholar]
- 16.Rust J, Golombok S. Modern Psychometrics. London: Routledge Verlag, 1999. [Google Scholar]
- 17.Shellmer DA, Dew MA, Mazariegos G, et al. Development and field testing of Teen Pocket PATH (®), a mobile health application to improve medication adherence in adolescent solid organ recipients. Pediatr Transplant 2016; 20: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanetti-Yabur A, Rizzo A, Hayde N, et al. Exploring the usage of a mobile phone application in transplanted patients to encourage medication compliance and education. Am J Surgery 2017; 214: 743–747. [DOI] [PubMed] [Google Scholar]
- 19.Geramita E, DeVito Dabbs AJ, DiMartini AF, et al. Impact of a mobile health intervention on long-term nonadherence after lung transplantation: Follow-up after a randomized controlled trial. Transplantation 2020; 104: 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha A, Henderson M. Developing mobile health tools for long-term medication adherence in transplant patients? Transplantation 2020; 104: 456–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO: Adherence to long-term therapies: evidence for action. (2003) Reference Number: ISBN 92 4 154599 2.
- 22.Butler JA, Roderick P, Mullee Met al. et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: A systematic review. Transplantation 2004; 77: 769–776. [DOI] [PubMed] [Google Scholar]
- 23.Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients using text messaging. Pediatrics 2009; 124: e844–e850. [DOI] [PubMed] [Google Scholar]
- 24.Levine D, Torabi J, Choinski K, et al. Transplant surgery enters a new era: Increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg 2019; 218: 18–20. [DOI] [PubMed] [Google Scholar]
- 25.Lim SL, Tay MHJ, Ong KW, et al. Association between mobile health app engagement and weight loss and glycemic control in adults with type 2 diabetes and prediabetes (D´Lite study): Prospective cohort study. JMIR Diabetes 2022 Sep 30; 7: e35039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaulding EM, Marvel FA, Piasecki RJ, et al. User engagement with smartphone apps and cardiovascular disease risk factor outcomes: Systematic review. JMIR Cardio 2021, Feb 3; 5: e18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhury A, Choudhury M. Mobile for mothers mHealth intervention to augment maternal health awareness and behaviour of pregnant woman in tribal societies: Randomized quasi-controlled study. JMIR Mhealth Uhealth 2022 Seg 21; 10: e38368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arensman R, Kloek C, Pisters M, et al. Patient perspectives on using a smartphone app to support home-based exercise during physical therapy treatment: Qualitative study. JMIR Hum Factors 2022 Sep 13; 9: e35316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinsen L, Osteras N, Monseng T, et al. Effect of a mHealth exercise intervention compared with supervised exercise therapy in osteoarthritis management: Protocol of the DigiOA trial. BMJ Open 2022 Sep23; 12: e066248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazemi S, Zarei F, Heidarnia A, et al. Improve the cervical cancer prevention behaviors through mobile-based educational intervention based on I-CHANGE model: Study protocol for a randomized controlled trial. Trials 2022 Sep 24; 23: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinman RS, Nelligan RK, Campbell PK, et al. Exercise adherence mobile app for knee osteoarthritis: Protocol for the MappKO randomised controlled trial. BMC Musculoskelet Disord 2022 Sep 20; 23: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant 2009; 9: 35–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-dhj-10.1177_20552076221145855 for Implementation of a mobile application for outpatient care after liver transplantation by Michaela Andrä, Milan Sibinovic, Karl Pfeiffer and Daniela Kniepeiss in Digital Health
Supplemental material, sj-doc-2-dhj-10.1177_20552076221145855 for Implementation of a mobile application for outpatient care after liver transplantation by Michaela Andrä, Milan Sibinovic, Karl Pfeiffer and Daniela Kniepeiss in Digital Health