Abstract
Objective:
Aim of the study was to assess the clinical outcome and associated factors of respiratory distress syndrome among preterm neonates admitted to the neonatal intensive care unit of Adama Hospital and Medical College.
Methods:
Hospital-based cross-sectional study was conducted using 242 randomly selected medical records of preterm neonates admitted to Adama comprehensive specialized hospital. Clinical outcome was categorized as poor if the neonate died or left against advice and good if discharged after improvement. Data were coded, entered into Epidata v.7.4.2 and exported to SPSS v.27 for analysis. After initial bi-variable logistic regression analysis, predictor variables with p-value of <0.2 were included in multivariable analysis. Significant association of factors with clinical outcome was claimed at p-value <0.05 and calculated 95% adjusted odds ratio.
Results:
Majority of admissions were male (63.2%), mean birth weight of 1440.3 g (+321.2 SD) and sepsis (82%), hypothermia (73%), and apnea (21.5%) were leading comorbidities. One hundred fifty-two (62.8%) of preterm neonates had poor outcomes. Neonates born singleton were 47% less likely to develop poor clinical outcomes (adjusted odds ratio 0.53 (0.48–0.94). The odds of poor clinical outcomes were higher during the first 3 days of admission (adjusted odds ratio 3.83 (3.28–14.77). Extremely preterm neonates (adjusted odds ratio 4.16 (4.01–12.97), extremely low birth weight preterm neonates had higher odds of poor clinical outcome.
Conclusion:
The study found higher poor clinical outcome among preterm neonates admitted with respiratory distress syndrome. Poor outcome was higher in lower gestational age, lower birth weight, twins and majority of it happened during 3 days of their life. Effective preventive care and initiation of low-cost, life-saving interventions including heated humidified high-flow nasal cannula and surfactant administration could significantly improve the clinical outcome of the neonates.
Keywords: Preterm neonates, respiratory distress syndrome, neonatal mortality, clinical outcome
Introduction
World Health Organization defined preterm birth as all births before 37 weeks of gestation or <259 days from the last normal menstrual period of the women.1 Preterm births are further classified as extreme preterm (<28 weeks), very preterm (28–316/7 weeks), moderate (32–336/7 weeks), and late preterm (34–366/7 weeks).2,3
Every year, an estimated 15 million infants are born preterm, and it is increasing globally.4 The rate of survival and morbidities of preterm babies indicate the quality of care at the health facilities. Despite progress in the advancement of care and survival rate of preterm neonates, there is still a long way to go for lower- and middle-income countries (LMICs),5,6 For instance, 90% of preterm neonates born before 28weeks of gestational age die in LMICs, compared to <10% in developed nations.7 In Ethiopia, prematurity is the leading (34%) cause of neonatal deaths and the fourth (11%) cause of under-five mortality.8,9
Respiratory distress syndrome (RDS) is a neonatal respiratory disorder that appears shortly after birth.10 It is one of the most common causes of admission to the neonatal intensive care unit (NICU) and respiratory failure in neonates. Neonatal mortality attributable to RDS is evident in all complications of prematurity.11 RDS accounted for a preterm newborn death rate of 12.8% in Poland, 46.9% in Nigeria, and 45% in Ethiopia.12–14 Another study reported that the preterm newborn death rate in hospitals in low-resource countries ranged from 40% to 60% and the majority have only oxygen therapy.15 However, a case fatality rate of 100% in a community-based setting was also reported.16 In Ethiopia, RDS is the leading cause of morbidity and mortality in preterm neonates.17,18
Access to early screening and adequate care could save a three-quarter of preterm neonates with RDS.19 Caring for the critically ill neonate in developing countries is a challenge, where health needs often surpass available resources including infrastructures. Despite WHO recommendation of surfactant replacement therapy for preterm neonates with RDS, high flow nasal oxygen administration and corticosteroid therapy for the at-risk mother, the trend in its morbidity and mortality is increasing.3,20,21 Respiratory distress syndrome in premature neonates typically manifests as a double burden due to comorbidity with major neonatal killers. For instance, various studies reported leading comorbidity as neonatal sepsis (26.1%–71%), hypothermia (63%), and apnea of prematurity (28.6%).22–25 Beyond the impact of RDS on the neonate, the family and health system itself suffer repercussions.26
Despite significant reduction in under-five mortality in Sub-Saharan Africa, neonatal mortality is still stagnant.27–30 In Ethiopia, few studies reported varying levels of mortality and identified compound fetal presentation during delivery, longer duration of hospitalization, male sex as the factors associated with RDS mortality in preterm neonates.31,32 There is a dearth of evidence on its clinical outcomes and examining broader associated factors; specifically among preterm newborns. As RDS often manifests alongside co-morbidities, a more thorough identification of the predictors of good or poor clinical outcome is essential to filling a crucial void in medical care. The findings highlight the contributions of RDS-related preterm newborn mortality to the slow decline in neonatal mortality in Ethiopia. Therefore, this study aimed to assess the admission outcome of RDS and its associated factors among preterm neonates admitted to the NICU of Adama comprehensive specialized hospital.
Methods and materials
Study area and period
The study was conducted in the preterm neonates’ unit of Adama comprehensive and specialized hospital medical college’s NICU. It is found in Adama town, 99 km South-East of the capital, Addis Ababa, Ethiopia. The town is found in East Shewa zone of Oromia region situated between the western foot of an escarpment and the Eastern Great Rift Valley. National census of 2007 reported total residents of the town as 388,925 of which 196,407 are males.33 There are 8 public health facilities in the town (one hospital, the rest health centers), 9 private hospitals, 6 non-governmental health centers, and 104 private clinics. The hospital serves an estimated five million catchment and surrounding population, and also serves as a teaching center for specialty programs (Pediatrics, Internal Medicine, general surgery, and Gynecology and obstetrics), and other general medical fields. It has a 212-bed capacity of which 77 beds are allocated for NICU. The hospital has admitted 659 preterm neonates with RDS during the past 3 years (1 January 2019–31 December 2021), with an average of 18 cases per month. The study period was from 7 February to 7 March 2022.
Study design
A retrospective, hospital-based cross-sectional study design was used.
Population and eligibility criteria
The source population was all preterm babies who were admitted to the NICU of Adama Hospital and Medical College with RDS. The total preterm admitted for RDS during the 3 years retrieved from the register was 659 admissions. The study population was randomly selected preterm neonates admitted to a preterm unit with RDS for 3 years (1 January 2019–31 December 2021). All preterm babies admitted with RDS regardless of the place of delivery were included while those whose medical record was not found and were incomplete were excluded. Preterm neonates having any form of congenital anomaly and birth weight >2500 g were excluded.
Sample size and sampling procedure
The sample size was calculated by single population proportion formula using assumptions of 95% confidence level, 5% margin of error and estimated good clinical outcome from RDS was 50.5%.34 However, after adjustment using the correction formula the final sample size was 242. Systematic random sampling with a sampling interval of three was used to select preterm neonates’ records using their medical record numbers.
Data collection method and tool
A pre-designed structured checklist was used to collect data on the variables of the study. The checklist was adapted from two studies.9,26 and includes socio-demography, neonatal, intrapartum and maternal obstetric factors. Four medical interns collected data and one public health expert supervised the task. One of them retrieved the medical record number of preterm neonates treated for RDS from the NICU registration book and three filled the tool queries using the record checklist.
Data quality management
The three data collectors and a supervisor were trained for a day on the objective of the study, sampling procedures and ethical issues. The principal investigator monitored data completeness daily. Before actual data collection, the checklist was pretested on 5% (13 records of preterm babies with RDS) in a similar unit, but in a different period. After checking for clarity, sensitivity, and consistency, necessary amendments were adopted. Two data clerks entered data separately and were cross-checked for its consistency.
Statistical analysis
Data were coded and entered into Epi-info v7.2.4.0, exported and analyzed using IBM SPSS statistics Version.27. The study used assumptions of 95% confidence interval, 5% margin of error and significance level of p-value of <0.05 in computing crude odds ratio (COR) and adjusted odds ratio. The initial association of variables with the outcome variable was analyzed using bivariate logistic regression and those with a p-value of <0.2 was included for multiple variable logistic regression. Multi-collinearity was checked by using variance inflation factors and tolerance test. Finally, multiple binary logistic regression was used to determine the independent association of each predictor variable to the outcome variable at a significance level of p-value <0.05. Model fitness was assessed using Hosmer-Lemeshow tests and Omnibus tests of coefficients.35,36 Descriptive statistics including frequencies, mean, standard deviation, and percentage were used to summarize the findings and presented using tables and charts.
Study variables and operational definitions
Discharge outcome of the preterm neonate was outcome variable dichotomized as good if discharged alive after improvement and poor if dead or left against medical advice. Independent variables were sex, age on admission, gestational age, birth weight, appearance, pulse, grimace, activity and respiration (APGAR) score (at the first and fifth minute), length of hospital stay (from admission up to occurrence of either of clinical outcomes), place of delivery, mode of delivery and also maternal factors including the number of gestations, parity, history of obstetric complication, preventive corticosteroid therapy, and full antenatal care. Range of fifth minute APGAR score used high for poor outcome (4–6), normal (7–10), and unknown for those outborn babies or lacking record.37
❖ RDS: formerly known as “hyaline membrane disease,” is chiefly in premature neonates characterized by the deficiency of surfactant coating the inner surface of the lungs resulting in labored breathing and lung collapse. In this study, any clinically or radiographically diagnosed preterm neonates treated with RDS were included. Clinical diagnosis referred to signs of respiratory distress (grunting, nasal flaring, chest indrawing, inter/sub-costal retractions, and cyanosis), decreased oxygen saturation, and decreased breath sound on auscultation. Chest X-rays conducted were seldom suggestive of low lung volume, diffuse, bilateral and symmetrical granular opacities and air-bronchograms.
❖ Obstetric complications: is the presence of at least one maternal obstetric illness including placental abruption, Chorio-amnitis, PROM, antepartum hemorrhage, pre-eclampsia and others listed in national guideline.
Ethical clearance
The ethical review committee of the Adama Hospital and Medical College granted ethical approval and waived informed consent. In addition, a formal letter of cooperation from the college aided in accessing the patient medical record.
Result
Socio-demographic and clinical characteristics of preterm neonates
The majority (63.2%) of preterm neonates admitted with RDS were male with a male to female ratio of 1.6:1 and more than half of them 140 (57.9% ) were born to mothers residing in the rural area. Two hundred thirty-four (96.7%) preterm neonates were admitted with RDS during the first 3 days of their life and had a mean birth weight of 1440.3g (±321.2 SD). The most common gestational age at birth was very preterm category (n = 114; Table 1).
Table 1.
Socio-demographic, clinical characteristics and outcomes of preterm neonates admitted with RDS at Adama Hospital and Medical College, Ethiopia (n = 242).
| Variables | Categories | Frequency |
|---|---|---|
| No (%) | ||
| Sex of neonate | Male | 153 (63.2) |
| Female | 98 (36.8) | |
| Age at admission | Birth-24 h | 234 (96.7) |
| 24.1–72 h | 4 (1.7) | |
| >72 years | 4 (1.7) | |
| Gestational age at birth(weeks) Mean 31.12 weeks (+ 2.3 SD) | Late preterm (34 to <37) | 38 (15.7) |
| Moderate preterm (32 to <34 weeks) | 84 (34.7) | |
| Very preterm (28 to <32 weeks) | 114 (47.1) | |
| Extremely preterm (<28 weeks) | 6 (2.5) | |
| Birth weight Mean = 1440.3 g(+321.2 SD) | LBW (1500 to <2500 g) | 113 (46.7) |
| VLBW (1000 to <1500 g) | 120 (49.6) | |
| ELBW(<1000 g) | 9 (3.7) | |
| APGAR score At fifth minute | 4–6 | 59 (24.4) |
| 7–10 | 168 (69.4) | |
| Unknown | 15 (6.2) | |
| Length of stay (7.13 days + 8.9 SD) | 0–3 | 100 (41.3) |
| 4–7 | 72 (29.8) | |
| >7 | 70 (28.9) |
APGAR: appearance, pulse, grimace, activity and respiration; LBW: LBW; VLBW: very low-birth weight; ELBW: extremely low-birth weight.
Clinical outcome of preterms admitted with RDS
Of the overall preterm admissions with RDS, the majority (62.8%) had poor clinical outcomes and 90 (37.2%) were discharged with a good outcome or after clinical improvement (Figure 1).
Figure 1.
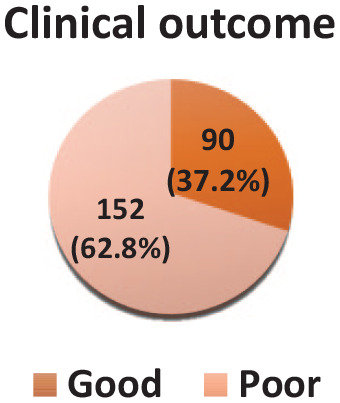
A pie chart showing clinical outcomes of preterm neonates admitted with RDS.
Comorbidity pattern among preterm neonates admitted with RDS
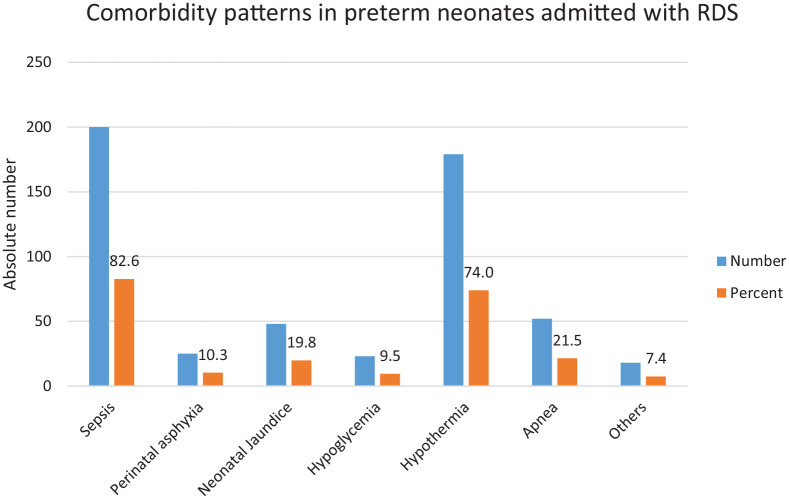
Sepsis was the most common comorbidity presenting concomitantly in 82.6% of preterm babies with RDS, followed by hypothermia 179 (73.9%) and apnea 52 (21.5%) respectively (Figure 2).
Figure 2.
Bar graph showing common comorbidity patterns of preterm neonates admitted with RDS.
Sum is more than 100% because of more than one comorbidity per case.
Maternal obstetric and Intra-partum factors
In this study, the mean age of mothers was 33.22 ± 4.93(SD) years and the majority (45%) of them were in the age group of 18–24 years. More than three-fold of them (78.1%) gave birth to singleton, 137 (56.6%) were para-I and only a few (2.9%) lacked any antenatal care visit. The majority of mothers 154 (63.6%) had no previous history of obstetric complications and 198 (81.8%) received prenatal corticosteroid therapy. Two hundred thirty-two (95.9%) women delivered their newborn at a health institution, of which 195 (80.6%) were through spontaneous vertex delivery (Table 2).
Table 2.
Obstetric and intrapartum characteristics of mothers of preterm neonates admitted with RDS at dama Hospital and Medical College, Ethiopia (n = 242).
| Variables | Categories | Frequency No (%) |
|---|---|---|
| Age of mother | <18 | 18 (7.4) |
| Mean age 24.79(SD ± 4.93) | 18–24 | 109 (45.0) |
| 25–34 | 104 (43.0) | |
| >35 | 11 (4.5) | |
| Number of gestations | Single | 189 (78.1) |
| Multiple | 53 (21.9) | |
| Parity | Para-I | 137 (56.6) |
| Para-II–IV | 93 (38.4) | |
| Para-V and more | 12 (5.0) | |
| Maternal hx of obstetric complications | Yes | 154 (63.6) |
| No | 88 (36.4) | |
| Corticosteroid therapy | Yes | 198 (81.8) |
| No | 44 (18.2) | |
| ANC follow-up | Yes | 235 (97.1) |
| No | 7 (2.9) | |
| Place of delivery | Health institution | 232 (95.9) |
| Home | 10 (4.1) | |
| Mode of delivery | SVD | 195 (80.6) |
| Assisted (breech, instrumental) | 4 (1.7) | |
| C/S | 43 (17.8) |
Abbreviations: ANC: antenatal care; Hx: history; SVD: spontaneous vertex delivery; C/S: cesarean section.
Predictors of clinical outcome of preterm newborns admitted with RDS
After bi-variable logistic regression 10 variables from neonatal clinical and socio-demographic characteristics, maternal obstetric history, and intrapartum factors having a p-value ⩽0.2 were fitted into the final model. Then, the number of gestations, APGAR score at the fifth minute after birth, hospital stay, gestational age, and birth weight were significantly associated with clinical outcome, whereas the age of the mother, parity, maternal history of antenatal and corticosteroid therapy, and mode of delivery were not significantly associated with clinical outcome (Table 3).
Table 3.
Bi-variable and multivariable logistic regression of predictors of clinical outcome among preterm babies admitted with RDS at Adama Hospital and Medical College, Ethiopia (n = 242).
| Variables | Response category | Clinical outcome | COR 95%CI | AOR 95%CI | p-Value | |
|---|---|---|---|---|---|---|
| Poor | Good | |||||
| Number of gestation | Single | 74 | 115 | 0.79 (0.16–0.88) | 0.53 (0.48–0.94) | 0.002 |
| Multiple | 16 | 37 | 1 | 1 | ||
| APGAR score at five minutes after birth | Unknown | 12 | 3 | 2.21 (1.54–12.36) | 1.87 (1.24–6.91) | 0.04 |
| 4–6 | 43 | 16 | 3.42 (3.22–10.48) | 4.62 (4.19–9.26) | 0.03 | |
| 7–10 | 97 | 71 | 1 | 1 | ||
| Length of hospital stay (days) | <3 | 96 | 4 | 2.71 (2.11–8.25) | 3.83 (3.28–14.77) | 0.016 |
| 3–7 | 47 | 25 | 3.49 (2.93–13.49) | 1.65 (1.09–9.48) | 0.039 | |
| >7 | 9 | 61 | 1 | 1 | ||
| Gestational age (weeks) | <28 | 5 | 1 | 3.75 (3.10–8.81) | 4.16 (4.01–12.97) | 0.001 |
| 28–31.9 | 84 | 30 | 3.51 (3.06–14.26) | 2.29 (2.13–9.74) | 0.019 | |
| 32–33.9 | 45 | 39 | 4.21 (2.97–16.21) | 1.61 (0.33–6.81) | 0.054 | |
| 34–37 | 17 | 21 | 1 | 1 | ||
| Birth weight | <1000 | 9 | 0 | 4.29 (2.97–11.49) | 5.771 (4.67–12.95) | 0.022 |
| 1000 to <1500 | 95 | 25 | 1.66 (1.19–12.31) | 2.81 (1.94–7.86) | 0.006 | |
| 1500 to <2500 | 48 | 65 | 1 | 1 | ||
Abbreviations: APGAR: appearance, pulse, grimace, activity, and respiration; COR: crude odds ratio; AOR: adjusted odds ratio.
Discussion
This study aimed to assess the admission outcome of RDS and its associated factors among preterm neonates admitted at the NICU of Adama comprehensive specialized hospital from 1 January 2019, to 31 December 2021. Preterm neonates born twin, lower APGAR score at fifth minute after birth, lower extremes of birth and gestational ages and length of hospital stay were significantly associated with poor clinical outcomes. This study showed that 62.8% of preterm neonates admitted with RDS had poor clinical outcome.
This finding is higher compared to other countries including Nepal (6.3%), Sudan (41%), Tanzania (20.8%), and Ghana (39.27%).38–41 However, this finding is in line with reports from other studies.7,42,43. The similarities and differences could be partly due to varying healthcare systems, quality of perinatal care, and advancement of technologies in the prevention and management of RDS in preterm neonates. In addition, delayed presentation of the outborn preterm neonates to health facility, inadequate dose coverage of corticosteroid therapy for pregnant mothers at risk of preterm birth, and non-invasive respiratory support (unavailable heated humidified high-flow nasal cannula (HHHFNC) and irregular use of nasal continuous positive airway pressure (nCPAP)) had contributions.
The study revealed that RDS was higher (63.2%) in male preterm neonates. This is consistent with studies from Egypt and Poland.44,45 This might be because, during gestation, the female fetal lung produces surfactant earlier than the male as a result of androgen delaying lung fibroblasts secretion of fibroblast-pneumocyte factor and estrogen mediation of fetal lung development by increasing alveolar type II cells.
Poor outcomes were more likely in preterm neonates born with <7 APGAR score at fifth minute after birth compared to those with higher score. This is consistent with studies conducted in Brazil and USA.46,47 This is attributable to asphyxia causing reduced cardiopulmonary faction and its association to sympatho-adrenal activity at birth.48,49
Preterm babies born singleton were less likely to develop poor outcome compared to twins. This is similar with findings of other studies.50,51.This could be due to higher risk of second twin to asphyxia, growth discrepancy, and genetic dispositions at the earlier gestations.52,53
The mean length of hospital duration until the occurrence of clinical outcome was 7.13 days ± 8.9 SD. The greater majority (94%) of poor outcomes from RDS occurred during the first 7 days of admission and 63% in the first 72 h of hospital admission. This is consistent with another study that reported 85% of poor outcome during a week of admission, of which 43% was within the first 3 days of hospital admission with RDS.54 This implies that initiating care within the first week of neonatal age and strengthening community-based referral strategies for outborn babies will aid in saving the lives of the newborn.55
Preterm mortality was four-fold higher among extremely preterm neonates and twice in very preterm neonates compared to moderate preterm neonates. Likewise, similar findings were observed in Orotta Pediatric Hospital of Eritrea and New Haven Hospital United States.53,56,57 Convergence of the findings could be because the lower their gestational age the weaker their body sustains the sequela of RDS.
In this study, the odds of poor clinical outcome was six-fold in extremely LBW and thrice in very LBW infants compared to moderate LBW. This is higher compared to findings from Ghana and Gitwe district hospital, Rwanda; which reported a 14.3% survival rate in ELBW and 20% in the VLBW category.41,51,58 The divergence might be due to geographical differences and varying levels of care quality.
Strength and limitation
This study used previous 3 years’ data which helps endure seasonal variability of cases attributed to delivery season. The findings can be generalizable to similar settings in the country with the same levels of facilities. The study included three records of newborns who left against medical advice as poor outcome; although their status was untraceable. Unlike other studies, the study did not reveal a significant association between mode of delivery, previous history of corticosteroid therapy, and maternal age. Further longitudinal studies starting from the perinatal period might illustrate further real-time inquiries.
Conclusion
This higher poor clinical outcome of RDS among preterm neonates indicates the major proportion of overall neonatal mortality which is the hallmark of quality of care provided in saving the life of newborns. The identified comorbidities are among the top killers of the neonate, urging for prompt follow-up, care, and intervention. Major factors associated with the clinical outcome are calling to attention the concerted efforts of stakeholders in availing advanced, life-saving services ranging from initiation of surfactant administration to provision of HHHFNC and liaising network of referral among facilities for screening of at-risk mothers for reduction of RDS incidence and complications.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221146068 for Clinical outcome and associated factors of respiratory distress syndrome among preterm neonates admitted to the neonatal intensive care unit of Adama Hospital and Medical College by Lensa Tamiru Bacha, Wase Benti Hailu and Edosa Tesfaye Geta in SAGE Open Medicine
Acknowledgments
Authors are grateful for the support of medical record officers in facilitating access to patient records and appreciation goes to data collectors and supervisor.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was obtained from the ethical review committee of Adama Hospital and Medical College (Approval No. AHMC/ER13/13). Informed consent was waived by the committee.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was waived by the ethics committee for the present study. However, confidentiality of their records has been maintained.
ORCID iDs: Wase Benti Hailu  https://orcid.org/0000-0002-7945-2696
https://orcid.org/0000-0002-7945-2696
Edosa Tesfaye Geta  https://orcid.org/0000-0002-7050-6846
https://orcid.org/0000-0002-7050-6846
Supplemental material: Supplemental material for this article is available online.
References
- 1. WHO. Newborns: improving survival and well-being. https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality (2020, accessed 2 August 2022).
- 2. WHO. Newborn health. https://www.who.int/health-topics/newborn-health (2019, accessed 31 December 2020).
- 3. Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants – 2010 Update. Neonatology (Basel, Switzerland) 2010; 97(4): 402–417. [DOI] [PubMed] [Google Scholar]
- 4. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388(10063): 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bamat N, Fierro J, Wang Y, et al. Positive end-expiratory pressure for preterm infants requiring conventional mechanical ventilation for respiratory distress syndrome or bronchopulmonary dysplasia. Cochrane Database Syst Rev 2019; 2019(2): CD004500–CD004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sivanandan S, Agarwal R, Sethi A. Respiratory distress in term neonates in low-resource settings. Semin Fetal Neonatal Med 2017; 22(4): 260–266. [DOI] [PubMed] [Google Scholar]
- 7. Costil J, Cloup M, Leclerc F, et al. Acute respiratory distress syndrome (ARDS) in children: multicenter collaborative study of the French group of pediatric intensive care. Pediatr Pulmonol 1995; 19(S11): 106–107. [DOI] [PubMed] [Google Scholar]
- 8. Assefa N, Lakew Y, Belay B, et al. Neonatal mortality and causes of death in Kersa health and demographic surveillance system (Kersa HDSS), Ethiopia, 2008–2013. Matern Health Neonatol Perinatol 2016; 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tekleab M, Amaru GM, Tefera YA. Reasons for admission and neonatal outcome in the neonatal care unit of a tertiary care hospital in Addis Ababa: a prospective study. Res Rep Neonatol 2016; 6: 17–23. [Google Scholar]
- 10. Rao Y, Sun X, Yang N, et al. Neonatal respiratory distress syndrome and underlying mechanisms in cloned cattle. Mol Reprod Dev 2018; 85(3): 227–235. [DOI] [PubMed] [Google Scholar]
- 11. Chen A, Shi L, Zheng J, et al. Clinical characteristics and outcomes of respiratory distress syndrome in term and late-preterm neonates. Zhonghua er ke za zhi 2008; 46(9): 654. [PubMed] [Google Scholar]
- 12. Sahoo T, Anand P, Verma A, et al. Outcome of extremely low birth weight (ELBW) infants from a birth cohort (2013-2018) in a tertiary care unit in North India. J Perinatol 2020; 40(5): 743–749. [DOI] [PubMed] [Google Scholar]
- 13. Adebami O, et al. Determinants of outcome in newborns with respiratory distress in Osogbo, Nigeria. Int J Res Med Sci 2017; 5: 1487. [Google Scholar]
- 14. Kebede ZT, et al. Hematologic profiles of Ethiopian preterm infants with clinical diagnoses of early-onset sepsis, perinatal asphyxia, and respiratory distress syndrome. Glob Pediatr Health 2020; 7: 2333794X20960264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muhe LM, McClure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health 2019; 7(8): e1130–e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bang T, Bang RA, Baitule S, et al. Burden of morbidities and the unmet need for health care in rural neonates–a prospective observational study in Gadchiroli, India. Indian Pediatr 2001; 38(9): 952–965. [PubMed] [Google Scholar]
- 17. Mitiku HD. Neonatal mortality and associated factors in Ethiopia: a cross-sectional population-based study. BMC Women’s Health 2021; 21(1): 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tadesse W, Negussie YM, Aychiluhm SB. Neonatal mortality and its associated factors among neonates admitted at public hospitals, pastoral region, Ethiopia: a health facility based study. PLoS One 2021; 16(3): e0242481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manandhar SR. Outcome of surfactant replacement therapy in preterm babies with hyaline membrane disease at neonatal intensive care unit of a tertiary hospital. Birat J Health Sci 2018; 3(3): 3. [Google Scholar]
- 20. Requejo J, Merialdi M, Althabe F, et al. Born too soon: care during pregnancy and childbirth to reduce preterm deliveries and improve health outcomes of the preterm baby. Reprod Health 2013; 10(1), S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdelazim I, Farghali MMM, Elbiaa AAM, et al. Impact of antenatal oxytocin infusion on neonatal respiratory morbidity associated with elective cesarean section. Arch Med Sci 2017; 13(3): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen C, Ebbesen F, Petersen JP, et al. PO-0742 hypothermia a risk factor for respiratory distress syndrome in premature infants? Arch Dis Child 2014; 99(Suppl 2), A498–A498. [Google Scholar]
- 23. Olivier F, Nadeau S, Caouette G, et al. Association between Apnea of prematurity and respiratory distress syndrome in late preterm infants: an observational study Front Pediatr 2016; 4: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salama K, Gad A, Tatawy SEI. Sepsis profile and outcome of preterm neonates admitted to neonatal intensive care unit of Cairo University Hospital. Egypt Pediatr Assoc Gaz 2021; 69(1): 8. [Google Scholar]
- 25. Carns J, Kawaza K, Quinn MK, et al., Impact of hypothermia on implementation of CPAP for neonatal respiratory distress syndrome in a low-resource setting. PLoS One 2018; 13(3): e0194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herridge S, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 2016; 42(5): 725–738. [DOI] [PubMed] [Google Scholar]
- 27. Masaba BB, Mmusi-Phetoe RM. Neonatal survival in sub-Sahara: a review of Kenya and South Africa. JMDH 2020; 13: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alamneh TS, Teshale AB, Worku MG, et al. Preterm birth and its associated factors among reproductive aged women in sub-Saharan Africa: evidence from the recent demographic and health surveys of sub-Sharan African countries. BMC Pregnancy Childbirth 2021; 21(1): 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olack B, Santos N, Inziani M, et al. Causes of preterm and low birth weight neonatal mortality in a rural community in Kenya: evidence from verbal and social autopsy. BMC Pregnancy Childbirth 2021; 21(1): 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gebreheat G, Teame H. Survival and mortality of preterm neonates in a neonatal intensive care unit in Northern Ethiopia: a retrospective cohort study. Sci Rep 2022; 12(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chanie ES, Alemu AY, Mekonen DK, et al. Impact of respiratory distress syndrome and birth asphyxia exposure on the survival of preterm neonates in East Africa continent: systematic review and meta-analysis. Heliyon 2021; 7(6): e07256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gebreheat G, Tadesse B, Teame H. Predictors of respiratory distress syndrome, sepsis and mortality among preterm neonates admitted to neonatal intensive care unit in northern Ethiopia. J Pediatr Nurs 2022; 63: e113–e120. [DOI] [PubMed] [Google Scholar]
- 33. Central Statistical Agency (CSA). Summary and statistical report of the 2007 population and housing census: population size by age and sex. Ethiopia. Office of the population. https://www.ethiopianreview.com/pdf/001/Cen2007_firstdraft(1).pdf (2008, accessed 2 October 2022). [Google Scholar]
- 34. Birihane BM, Bayih WA, Alemu AY, et al. The burden of hyaline membrane disease, mortality and its determinant factors among preterm neonates admitted at Debre tabor general hospital, North Central Ethiopia: a retrospective follow up study. PLoS One 2021; 16(3): e0249365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hosmer DW, Hosmer T, Le Cessie S, et al. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997; 16(9): 965–980. [DOI] [PubMed] [Google Scholar]
- 36. Doornik JA, Hansen H. An omnibus test for univariate and multivariate normality. Oxf Bull Econ Stat 2008; 70(s1): 927–939. [Google Scholar]
- 37. Iliodromiti S, Mackay DF, Smith GCS, et al. Apgar score and the risk of cause-specific infant mortality: a population-based cohort study. Lancet 2014; 384(9956): 1749–1755. [DOI] [PubMed] [Google Scholar]
- 38. Rijal P, Shrestha M. Analysis of neonatal respiratory distress in neonatal intensive care unit at Nepal medical college. J Nepal Health Res Counc 2018; 16(2): 2. [PubMed] [Google Scholar]
- 39. Salih SA, A/Gadir YS. Early outcome of pre-term neonates delivered at Soba University Hospital. Sudan J Paediatr 2013; 13(2): 37–44. [PMC free article] [PubMed] [Google Scholar]
- 40. Moshiro R, Perlman JM, Mdoe P, et al. Potential causes of early death among admitted newborns in a rural Tanzanian hospital. PLoS One 2019; 14(10): e0222935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abdul-Mumin A, Owusu SA, Abubakari A. Factors associated with treatment outcome of preterm babies at discharge from the neonatal intensive care unit (NICU) of the tamale teaching hospital, Ghana. Int J Pediatr 2020; 2020: e5696427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong JJM, Loh TF, Testoni D, et al. Epidemiology of pediatric acute respiratory distress syndrome in Singapore: risk factors and predictive respiratory indices for mortality. Front Pediatr 2014; 2: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goh Y, Chan PW, Lum LC, et al. Incidence of acute respiratory distress syndrome: a comparison of two definitions. Arch Dis Child 1998; 79(3): 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niesłuchowska-Hoxha A, Cnota W, Czuba B, et al. A retrospective study on the risk of respiratory distress syndrome in singleton pregnancies with preterm premature rupture of membranes between 24+0 and 36+6 weeks, using regression analysis for various factors. BioMed Res Int 2018; 2018: e7162478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qari SA, Alsufyani AA, Muathin SH, et al. Prevalence of respiratory distress syndrome in neonates. Egypt J Hosp Med 2018; 70(2): 257–264. [Google Scholar]
- 46. Kalikkot Thekkeveedu R, Dankhara N, Desai J, et al. Outcomes of multiple gestation births compared to singleton: analysis of multicenter KID database. Matern Health Neonatol Perinatol 2021; 7(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marinonio SS, Costa-Nobre DT, Miyoshi MH, et al. Clusters of preterm live births and respiratory distress syndrome-associated neonatal deaths: spatial distribution and cooccurrence patterns. BMC Public Health 2022; 22(1): 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luerti M, Parazzini F, Agarossi A, et al. Risk factors for respiratory distress syndrome in the newborn: a multicenter Italian survey. Acta Obstet Gynecol Scand; 1993; 72(5): 359–364. [DOI] [PubMed] [Google Scholar]
- 49. Wennergen M, Krantz M, Hjalmarson O, et al. Low Apgar score as a risk factor for respiratory disturbances in the newborn infant. J Perinat Med 1987; 15(2): 153–160. [DOI] [PubMed] [Google Scholar]
- 50. Marttila R, Kaprio J, Hallman M. Respiratory distress syndrome in twin infants compared with singletons. Am J Obstet Gynecol 2004; 191(1): 271–276. [DOI] [PubMed] [Google Scholar]
- 51. Ma X, Huang C, Lou S, et al., The clinical outcomes of late preterm infants: a multi-center survey of Zhejiang, China. J Perinat Med 2009; 37(6): 695–699. [DOI] [PubMed] [Google Scholar]
- 52. Hacking D, Watkins A, Fraser S, et al. Respiratory distress syndrome and birth order in premature twins. Arch Dis Child-Fetal Neonatal Ed 2001; 84(2): F117–F121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marttila R, Kaprio J, Hallman M. Respiratory distress syndrome in twin infants compared with singletons. Am J Obstet Gynecol 2004; 191(1): 271–276. [DOI] [PubMed] [Google Scholar]
- 54. Tamene A, Abeje G, Addis Z. Survival and associated factors of mortality of preterm neonates admitted to Felege Hiwot specialized hospital, Bahir Dar, Ethiopia. SAGE Open Med 2020; 8: 2050312120953646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hailu WB, Salgedo WB, Walle AA. Factors affecting implementation of integrated community case management of childhood illnesses in South West Shoa Zone, Central Ethiopia. JPHE 2018; 10(5): 132–138. [Google Scholar]
- 56. Tibaijuka L, Bawakanya SM, Owaraganise A, et al. , Incidence and predictors of preterm neonatal mortality at Mbarara regional referral hospital in South Western Uganda. PLoS One 2021; 16(11), e0259310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. St. Clair C, Norwitz ER, Woensdregt K, et al. The probability of neonatal respiratory distress syndrome as a function of gestational age and lecithin/sphingomyelin ratio. Am J Perinatol 2008; 25(8): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Di Mauro, Capozza M, Cotugno S, et al. Nasal high flow therapy in very low birth weight infants with mild respiratory distress syndrome: a single center experience. Ital J Pediatr 2017; 43(1): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221146068 for Clinical outcome and associated factors of respiratory distress syndrome among preterm neonates admitted to the neonatal intensive care unit of Adama Hospital and Medical College by Lensa Tamiru Bacha, Wase Benti Hailu and Edosa Tesfaye Geta in SAGE Open Medicine