Abstract
Previous studies suggest that autism spectrum disorders are characterized by alterations in the microbiota–gut–brain axis. Probiotics may modify the composition and the functionality of the gut microbiota of autism spectrum disorder individuals, with possible cascading effects on brain function. In this study, we analyzed possible brain modifications induced by the administration of probiotics in 46 children with autism spectrum disorder using electroencephalography. A randomized 6-month controlled trial was performed. In subjects treated with probiotics, we observed a decrease of power in frontopolar regions in beta and gamma bands, and increased coherence in the same bands together with a shift in frontal asymmetry, which suggests a modification toward a typical brain activity. Electroencephalography measures were significantly correlated with clinical and biochemical measures. These findings support the importance of further investigations on probiotics’ benefits in autism spectrum disorder to better elucidate mechanistic links between probiotics supplementation and changes in brain activity.
Lay abstract
This study investigates the effects of a probiotic on preschoolers’ brain electrical activity with autism spectrum disorder. Autism is a disorder with an increasing prevalence characterized by an enormous individual, family, and social cost. Although the etiology of autism spectrum disorder is unknown, an interaction between genetic and environmental factors is implicated, converging in altered brain synaptogenesis and, therefore, connectivity. Besides deepening the knowledge on the resting brain electrical activity that characterizes this disorder, this study allows analyzing the positive central effects of a 6-month therapy with a probiotic through a randomized, double-blind placebo-controlled study and the correlations between electroencephalography activity and biochemical and clinical parameters. In subjects treated with probiotics, we observed a decrease of power in frontopolar regions in beta and gamma bands, and increased coherence in the same bands together with a shift in frontal asymmetry, which suggests a modification toward a typical brain activity. Electroencephalography measures were significantly correlated with clinical and biochemical measures. These findings support the importance of further investigations on probiotics’ benefits in autism spectrum disorder to better elucidate mechanistic links between probiotics supplementation and changes in brain activity.
Keywords: autism spectrum disorder, clinical trial, EEG, preschoolers, probiotics
Introduction
Autism spectrum disorders (ASD) affect about 1% of the population worldwide (Elsabbagh et al., 2012; Narzisi et al., 2018). Although the exact etiopathogenesis of idiopathic ASD is not fully elucidated, compelling evidence suggests an interaction between genetic liability and environmental factors in producing early alterations in brain development, which in turn underlie atypical neuropsychological functioning and core ASD symptoms (Bai et al., 2019).
In recent decades, several studies have highlighted an association between physiological and metabolic abnormalities in ASD and immune dysregulation/inflammation (Ashwood et al., 2011; Wei et al., 2012). Interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and macrophage chemoattractant protein-1 (CCL2) have been proposed as potentially involved in brain inflammation at least in a subgroup of subjects with ASD (Burnette et al., 2011). Evidence from other studies had highlighted the role of pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, as main factors in the generation of atypical behavioral and electroencephalography (EEG) patterns occurring in ASD (Meltzer & Van de Water, 2017).
Besides, an increasing body of preclinical and clinical evidence has revealed that alterations in the microbiota–gut–brain (MGB) axis (i.e. the bidirectional communication between the intestinal microbiota and the brain) may contribute to the development and/or maintenance of ASD (Iannone et al., 2019). In this framework, recent studies reported a different gut microbiota composition (which normally consists mostly of bacteria, and also other microorganisms, such as archaea, fungi, parasites, and bacteriophages resident in the gastrointestinal (GI) tract) in individuals with ASD compared with age-matched typically developing (TD) controls (Kelly, Minuto, et al., 2017), and a positive correlation between the reduced diversity of gut microbiota and the severity of autistic symptoms (Kang et al., 2013). The gut dysbiosis of ASD subjects may, in turn, be related to the high prevalence of co-morbid GI symptoms in these subjects (Holingue et al., 2018).
Based on this knowledge, there has been an increasing rationale and interest in using probiotics, that is, “live microorganisms which, when administered in adequate amounts confer a health benefit on the host” (Hotel & Cordoba, 2001), to modify the composition and the functionality of the gut microbiota of ASD individuals, with the final aim of improving both GI and ASD features. A mixture of Bifidobacteria, Streptococci, and Lactobacilli is thought to be the most promising treatment for GI problems and behavioral symptoms in ASD subjects (Fattorusso et al., 2019). Previous research conducted on children with ASD showed that probiotic supplementation (PS) with the strains mentioned above: (a) improves GI dysfunction (West et al., 2013), (b) positively influences the gut microbiota composition through the normalization of Bacteroides/Firmicutes ratio and the decrease of Desulfovibrio spp. (Tomova et al., 2015), and (c) reduces ASD severity (Shaaban et al., 2018). However, the studies conducted so far in humans investigating the impact of PS on GI dysfunction and ASD symptoms are generally affected by several methodological limitations, including the limited sample size, the absence of rigorous assessment criteria for ASD diagnosis, the short duration of treatments (usually less than 1 month) and the low-quality design, being mostly open-label trials or case-control studies (Patusco & Ziegler, 2018). In the last few years, some PS randomized controlled trials (RCTs), the design that best protects against bias, have been conducted in ASD subjects (Grimaldi et al., 2018; Liu et al., 2019; Parracho, 2010; Santocchi et al., 2020).
In particular, Santocchi et al. (2020) recently showed that preschoolers with a confirmed diagnosis of ASD could benefit from a multistrain probiotic mixture (Vivomixx®). In more detail, the supplementation with the PS Vivomixx® resulted in no statistically significant differences in autism severity of the whole sample over 6 months as compared with placebo. An exploratory secondary analysis on subgroups of children with or without GI symptoms revealed a significantly greater improvement in autism severity in the group without GI symptoms treated with probiotics, and greater improvements in some GI symptoms, adaptive functioning, and sensory profiles in the group with GI symptoms treated with probiotics. To provide an objective evaluation of PS response on brain function (Willyard, 2016), we examined EEG power spectra during resting before and after PS compared to placebo in ASD children enrolled in that RCT.
Previous data provided evidence that rehabilitative intervention for subjects with ASD could enhance neuroplasticity, that is, the cerebral neurons and neural circuits’ capacity to structurally and functionally change in response to external stimuli or environmental modifications (Pascual-Leone et al., 2005). In this framework, thanks to longitudinal studies that include pre- and post-treatment acquisition, advanced neuroimaging techniques, such as magnetic resonance imaging (MRI), have been recently used to investigate brain plasticity by monitoring the effects of therapy in ASD subjects (Calderoni et al., 2016).
Other studies have used EEG to evaluate brain changes during interventions. EEG is a non-invasive, flexible technique that can provide a precise millisecond-timescale to examine physiologic and pathologic temporal dynamics. Some studies have applied different EEG analysis methods, showing altered neural networks in ASD during rest and specific task conditions (Billeci et al., 2013; Schwartz et al., 2017). However, there is a lack of studies analyzing brain function connectivity changes before and after specific interventions for people with ASD. Only a few studies have been performed in this direction showing that EEG is a powerful tool to detect brain modifications induced by a rehabilitative (Billeci et al., 2017; Portnova et al., 2020; Van Hecke et al., 2015) or pharmacological (Larsson et al., 2012; Raz et al., 1987) intervention.
Although up to now, there are no studies evaluating the effects of PS on the autistic brain using EEG, previous investigations have shown that the administration of probiotics can induce changes at the brain level in healthy humans. The administration of Bifidobacterium Longum 1714 in healthy volunteers determined an enhanced frontal midline electroencephalographic mobility together with an improvement in hippocampus-dependent visuospatial memory performance (Allen et al., 2016). Conversely, no statistically significant changes in memory and sustained attention and associated EEG measures (brain activity in frontal, parietal, and central regions) emerged after the administration of the Lactobacillus rhamnosus (JB-1) for 4 weeks in healthy male subjects (Kelly, Allen, et al., 2017). Moreover, in a study (Takada et al., 2017) evaluating the effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults, the authors showed the beneficial effects of probiotics on sleep through EEG measures (decreased sleep latency, maintenance of the percentage of stage 3 non-rapid eye movement (REM) sleep, increased delta power).
In the field of ASD, some animal studies showed brain modifications using probiotics. For instance, a study showed that Lactobacillus Reuteri (L. Reuteri), a species relatively scarce in the animal model of ASD Shank3 KO mice, positively correlated with the expression of ɣ-aminobutyric acid (GABA) receptor subunits in the brain (Tabouy et al., 2018). In particular, Shank3 KO mice’s treatment with L. Reuteri induced attenuation of unsocial behavior, limited to male Shank3 mice, and a decrease in repetitive behaviors in both male and female Shank3 KO mice. Besides, L. Reuteri treatment affected GABA receptor gene expression and protein levels in multiple brain regions. Thus, a possible relationship between Lactobacillus, autism-related behaviors, and GABAergic function emerges.
Since the few encouraging proof-of-principle studies on healthy volunteers and animal models suggested that multistrain probiotics can alter resting brain activity, the central hypothesis of this study examined whether neurophysiological changes would be evident in children with ASD treated with probiotics. Given the paucity of studies in this sense and the absence of studies specifically in ASD, we did not make a priori hypothesis on the brain regions or frequency bands involved. Thus, the first and primary aim of this study was to examine whether neurophysiological characteristics (power, coherence and asymmetry) changed specifically in children treated with probiotics. The second aim of this study was to examine relations between these neurophysiological modifications and clinical and inflammatory measures after PS. To date, immune and gut alterations in ASD have mostly been studied separately, considering the immune system as one of the routes for gut-brain communication. In this work, we hypothesized possible common mechanisms of action for the gut microbiota and inflammation on the neural basis of ASD evaluable by EEG. In particular, looking for a mechanism that underlies the possible brain modifications induced by the administration of probiotics measured, we hypothesized that intestinal dysbiosis could, altering intestinal permeability, increase systemic inflammation and therefore induce neuroinflammation. In turn, neuroinflammation could induce an alteration of brain function that could be affected through the use of the PS improving systemic and central inflammation.
Materials and methods
Experimental protocol
The experimental study protocol is already published (Santocchi et al., 2016). The study is a 6-month double-blind, randomized parallel, factorial, efficacy-controlled trial with probiotics, and an allocation ratio of 1:1. The patients’ parents/guardians provided their written informed consent to participate in the study in accordance with the declaration of Helsinki.
Subjects were examined before treatment (T0) and after 6 months of probiotic/placebo treatment (T1). The probiotic supplement was the De Simone Formulation (marked as Vivomixx® in the EU and Visbiome® in the United States). The bacterial strains included in the De Simone Formulation are: Streptococcus thermophilus, Bifidobacterium breve, Bi-fidobacterium longum, Bifidobacterium infantis, Lacto-bacillus acidophilus, Lactobacillus plantarum, Lactobacillus para-casei, and Lactobacillus delbrueckii subsp. Bulgaricus.
The two groups were randomly assigned 1:1 to supplementation with probiotics or placebo for 6 months, the randomization was made independently in children with and without GI problems (GI and non-gastrointestinal (NGI) groups, respectively, based on the gastrointestinal severity index (GSI) score, see Santocchi et al., 2016) to obtain four parallel arms. At both T0 and T1, clinical/behavioral measures and blood samples were collected, and electroencephalographic recordings were performed. For each measure of interest (power, coherence, and asymmetry), we analyzed longitudinal differences and correlations with clinical and biochemical measures.
Participants
Sixty-three children aged 18–72 months diagnosed with ASD completed the RCT. Forty-six of these subjects (35 males and 11 females; mean age 46.56 months ± 13.92, range 26.64–73.32 months) had good quality EEG signal at T0 and T1, and were included in the present study.
The diagnosis of ASD was based on the Diagnostic and Statistical Manual of Mental Disorders—5th edition (DSM-5) diagnostic criteria (American Psychiatric Association (APA), 2013), and confirmed with the Autism Diagnostic Observation Schedule—second version (ADOS-2) (Lord et al., 2012) and the Autism Diagnostic Interview—Revised (ADI-R) (Rutter, Le Couteur, et al., 2003). ASD subjects were enrolled in a tertiary care university hospital. We excluded subjects with neurological syndromes, focal neurological signs, history of asphyxia at birth, severe premature birth, perinatal injuries, epilepsy, significant sensory impairment, diagnosis of not functional GI disorder or coeliac disease, special diets already underway, known brain anomalies.
Table 1 shows the main clinical characteristics of the sample at T0 and T1 (see Table S1 in Supplementary Materials for all the clinical features).
Table 1.
The main clinical characteristics of the sample at T0 and T1. Ordinal variables were compared using independent sample t-tests, while categorical variables were compared using chi-square tests.
| Characteristics | Groups (n, %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo T0 (20, 43) |
Probiotics T0 (26, 57) |
p-value | Placebo T1 (20, 43) |
Probiotics T1 (26, 57) |
|||||
| Age, mean (SD), y [range] |
3.78 (0.86) [2.57–5.58] |
4.40 (1.29) [2.20–6.10] |
0.06 | 4.33 (0.88) [3.08–6.10] |
4.95 (1.32) [2.72–6.70] |
||||
| Boys, No. (%) | 15 (75.0) | 20 (76.9) | 0.57 | ||||||
| ADOS CSSa, No. | 20 | 26 | 20 | 26 | |||||
| Score, mean (SD) | |||||||||
| Total | 6.8 (2.0) | 7.0 (1.1) | 0.54 | 6.9 (1.9) | 6.3 (1.6) | ||||
| DQb, standardized test, No. | 15 | 22 | 18 | 23 | |||||
| Mean (SD) | |||||||||
| General quotient, mean (SD) | 66.9 (21.4) 13 out of 15 |
66.1 (17.8) 19 out of 22 |
0.91 | 63.8 (19.6) 16 out of 18 |
62.3 (21.9) 22 out of 23 |
||||
| VABS-IIc, No. | 20 | 26 | 20 | 26 | |||||
| Composite score, mean (SD) | 58.1 (17.0) | 65.1 (21.0) | 0.23 | 61.9 (16.3) | 67.5 (21.4) | ||||
| GSI Severity Indexd, No. | 20 | 26 | 20 | 26 | |||||
| Score, mean (SD) | |||||||||
| Total 6-GSI | 1.7 (1.6) | 2.1 (2.2) | 0.46 | 1.5 (1.1) | 1.2 (2.5) | ||||
Abbreviations: ADI-R: Autism Diagnostic Interview—Revised; ADOS: Autism Diagnostic Observation Schedule; CARS: Childhood Autism Rating Scale; CBCL 1.5–5: Child Behavior Checklist 1.5–5; CSS: Calibrated Severity Score; DQ: Developmental Quotient; GI: gastrointestinal; GSI: gastrointestinal severity index; No.: number; NGI: non-gastrointestinal; PSI: parental stress index; RBS-R: Repetitive Behaviors Scale—Revised; SCQ: Social Communication Questionnaire; SD: standard deviation; VABS-II: Vineland Adaptive Behavior Scales-II; y: years.
Higher scores indicate greater severity (range of possible scores for total, social affect and restricted and repetitive behavior is 1–10).
Higher scores indicate greater cognitive ability. Scores around 100 indicate normal intelligence; scores below 70 indicate a developmental delay.
Higher scores indicate greater adaptive competences. Scores around 100 indicate normal adaptive capacities; scores below 70 indicate a delay with respect to age.
Higher scores indicate greater severity of gastrointestinal symptoms; Total 6-GSI has a range of 0 to 12, Total GSI has a range of 0 to 17.
Clinical and biochemical data
Clinical evaluation included neurological and psychiatric examination along with a standardized assessment of GI symptoms investigated through the GSI (Schneider et al., 2006); autism severity through ADOS-2 (Lord et al., 2012), Childhood Autism Rating Scale (CARS) (Schopler et al., 1980), and Social Communication Questionnaire (SCQ) (Rutter, Bailey, et al., 2003); restricted and repetitive behaviors through the Repetitive Behavior Scale—Revised (RBS-R; Bodfish et al., 1999); emotional, behavioral, and social problems screening through the Child Behavior Checklist (CBCL; Frigerio et al., 2006); cognitive development through the Griffiths Mental Development Scales—Extended Revised (GMDS-R; Griffiths, 2006); adaptive functioning through the Vineland Adaptive Behavior Scales-II (VABS-II) (Balboni et al., 2016; Sparrow et al., 2005); language abilities through the McArthur-Bates Communicative Development Inventories (CDI) (Fenson, 2007).
We also collected demographics (i.e. age, sex, parental education and employment, family, and residential information), physical parameters (i.e. weight, height, and head circumference), medical history, and detailed treatment data.
For biochemical analysis, blood samples were collected at T0 and at T1 by venipuncture in the morning after overnight fasting, rapidly separated by centrifugation for 15 min at 4°C, and plasma samples were stored frozen at −80°C until assay.
Plasma levels of leptin, TNF-α, IL-6, PAI-1, CCL2 were measured by a specific assay (MILLIPLEX MAP Millipore corporation, Billerica, MA, USA), using an integrated multi-analyte detection platform (high-throughput technology MagPix system, Luminex xMAP technology) with combined Analyst software (MILLIPLEX®) for the biomarker quantification developing new curve fitting algorithms and optimizing mathematical methods to minimize fitting errors. Biochemical data of the sample are reported in Table S1 in Supplementary Materials.
EEG acquisition set-up
The EEG signal was recorded with a 128-channel HydroCel Sensor Net (HCGSN 128, Electrical Geodesics Inc., USA) system. Data were acquired at a sampling rate (SR) of 500 Hz, setting impedances below 50 kΩ, and using a band-pass filter between 0.1 and 100 Hz, and a notch filter at 50 Hz for a visualization purpose. The signals were acquired during 8 min long passive attention resting-states, where children were looking at a video without audio. Recordings were performed in an isolated, quiet room. All the children watched the same video.
The use of a high-density system for signals recording allows to have a clearer distinction between signal and noise components, such as those deriving from eye movements (Klug & Gramann, 2021) using appropriate processing methods like independent component analysis (ICA; Pion-Tonachini et al., 2019).
EEG data processing
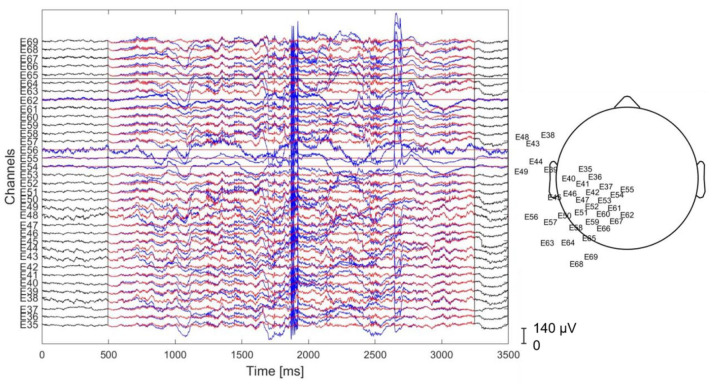
The EEG signal was processed with EEGLAB (Delorme et al., 2011) and custom MATLAB functions (MATLAB 2019a, The MathWorks, Natick, 2019). First, the signal was high-pass filtered above 1 Hz with a zero-phase filter. Then, our preprocessing pipeline relied on two complementary techniques for EEG cleaning (Loo et al., 2019): the artifact subspace reconstruction (Mullen et al., 2015) and ICA. The former removes high-amplitude time-varying artifacts (e.g. sensor motion, muscle) with a sliding-window optimized principal component analysis (PCA)-based spatial filter (Mullen et al., 2015). The latter allows to decompose the signal into stationary brain and nonbrain sources of activity. Here, we used the artifact subspace reconstruction (ASR) with optimal values according to the results presented in the work of Chang et al. (2020), to avoid data overcleaning. In Figure 1, we report an example of the raw and corrected data. Afterward, the preprocessed data signal was visually inspected, and those parts of the signal that were not properly cleaned by the ASR were removed (Urigüen & Garcia-Zapirain, 2015). Specifically, we excluded those time windows in which artifactual activity was clearly evident from the EEG tracing (e.g. high-amplitude distortions of the signal).
Figure 1.
Example of signal cleaning for a subset of channels applying the preprocessing procedure. Blue: raw signal, red: clean signal. On the right side, the scalp map of the selected electrodes is reported.
Then, the preprocessed EEG signals were average-referenced and decomposed into sets of temporal-maximally independent components (ICs) with the AMICA algorithm (Palmer et al., 2012). These components represented both brain sources and different types of artifacts (muscular, ocular, and other sources of noise). Artifactual components were removed using the ADJUST EEGLAB plugin (Mognon et al., 2011) and by visual inspection and then the EEG signal was reconstructed without these components’ contribution. Specifically, we took special care of identifying and removing eye-movement related components as children were watching a video during the acquisition. Finally, a 5-min long artifact-free window was extracted for each participant centered at half of the recording for which we performed the subsequent power, asymmetry, and coherence analyses (Billeci et al., 2017). Each measure was evaluated within the following frequency bands: delta (1–3) Hz, theta (4–7) Hz, alpha (8–12) Hz, beta (13–24) Hz, and low-gamma (30–45) Hz. Details about the number of electrodes included in each region can be found in the Supplementary Material (Figure 1S).
EEG power analysis
We evaluated the EEG power spectral density (PSD) for each participant using the Welch’s method. Specifically, we applied a sliding Hamming window with a length of 125 sampling points (250 ms) and an overlap of 50%. For each participant, we estimated the regional power according to the following subdivision of the scalp: left frontopolar (LFP), right frontopolar (RFP), left frontal (LF), right frontal (RF), left parietal (LP), right parietal (RP), left temporal (LT), right temporal (RT), left occipital (LO), and right occipital (RO). Relative powers were used since they are more reliable than absolute powers in terms of less variability among different subjects, as well as they are less affected by artifacts (Sheikhani et al., 2012). To compute relative powers, the PSD results of each frequency band were normalized to the whole frequency band
| (1) |
where P(·) indicates the power, RP(·) indicates the relative power, f1, f2 indicate the low and high frequency of the band, and L, H indicate the low and high frequencies of the signal (i.e. 1 and 45 Hz). The RP for each frequency band was averaged in each region.
EEG coherence analysis
The coherence of two discrete-time signals and is defined as follows (Piersol & Bendat, 2000)
| (2) |
where is the PSD of the time-series, is the PSD of the time-series and is the cross-power spectral density (CSPD) between and .
We evaluated the coherence between regions of the left and right hemispheres: frontopolar coherence, frontal coherence, parietal coherence, temporal coherence, and occipital coherence. Furthermore, we calculated intrahemispheric coherence: frontopolar–occipital, fronto–occipital, fronto–temporal, and occipital–temporal. Coherence values were estimated for the delta, theta, alpha, beta, and low-gamma frequency bands.
EEG asymmetry analysis
Interhemispheric asymmetry value represents the balance between left and right brain activities. The asymmetry index (AI) was calculated as follows (Pivik et al., 1993)
| (3) |
where PL and PR were the power obtained from left and right regions of a homologous region pair (frontopolar, frontal, parietal, temporal, and occipital).
Community Involvement: there is no community involved in this study.
Statistical analysis
Statistical analysis was performed using SPSS 20.0. First, EEG measures computed at T0 were analyzed t-test to evaluate whether between-groups (placebo/probiotic) differences exist at T0.
For the first aim of this study, that is, evaluate longitudinal changes (T1–T0) in neurophysiological measures, we considered three outcome measures: power, coherence, and asymmetry. These measures were analyzed through a repeated-measures analysis of variance (ANOVA) with treatment (placebo/probiotic) as between-subject factors and time (T0 and T1) and frequency bands (delta, theta, alpha, beta, and gamma) as within-subject factors. A separate repeated-measure ANOVA was performed for each measure. Greenhouse–Geisser corrections were applied when necessary to correct violations of the sphericity assumption. Post hoc tests were performed using paired t-test. As an exploratory investigation, the above analysis is presented without multiple comparison correction. The false discovery rate (FDR) function in the post hoc test was briefly explored. For the second aim of the study, correlation analysis between neurophysiological and clinical or biochemical measures was performed using bivariate correlations (Pearson) for the EEG and behavioral or biochemical measures calculated at T1. The level of significance for all tests was set at p < 0.05.
Results
As a preliminary analysis we examined the EEG parameters between T0 and T1 in GI and NGI groups. We did not find significant differences between groups, for this reason, we considered two parallel arms (probiotic and placebo groups) instead of the four arms planned in the study protocol.
Changes in EEG measures after probiotics administration
Power analysis
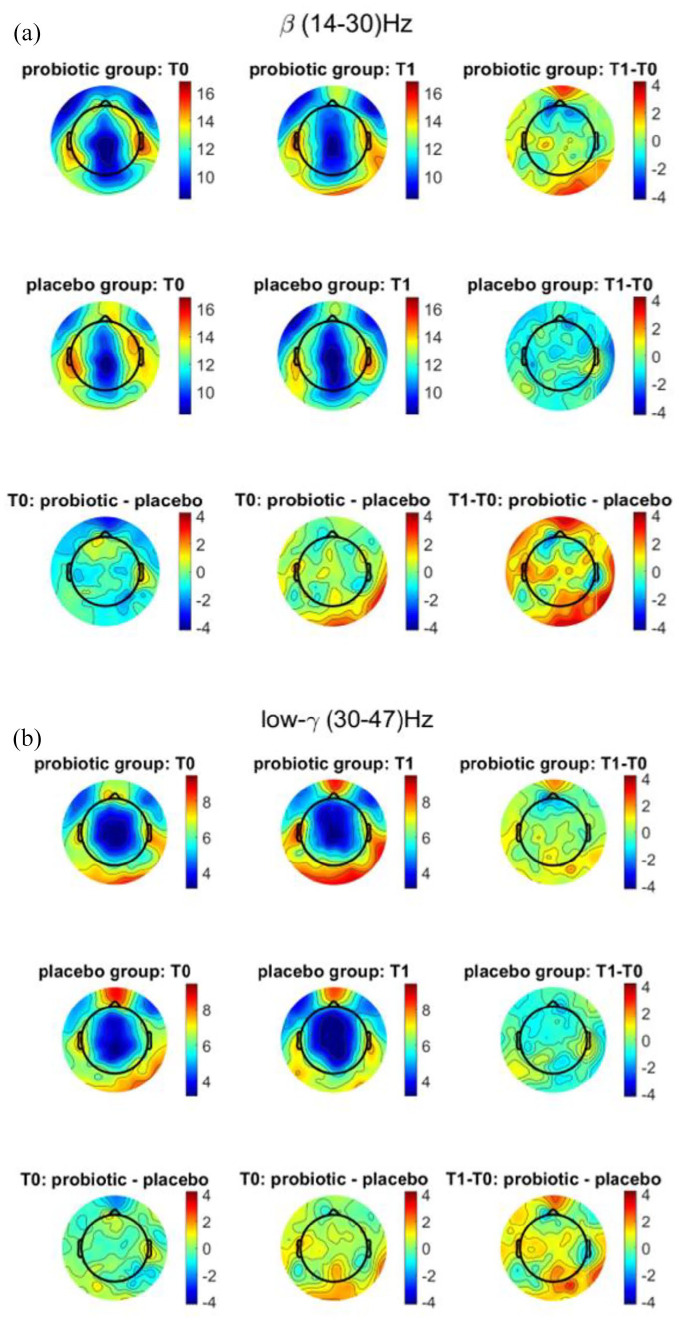
Power analysis showed a significant time × bands × treatment effect for RFP (F = 3.62180, p = 0.03814, η2 = 0.0761) and LFP (F = .3.75, p = 0.04, η2 = 0.079). Notably, the effect time × bands was not significant (F = 0.536, p = 0.710, η2 = 0.012 and F = 0.526, p = 0.545, η2 = 0.012), meaning that changes in EEG measures are not influenced by age difference from T0 to T1. No significant effect of treatment. We further performed post hoc analysis by means of paired t-test. We observed that in the subjects treated with probiotic RFP and LFP power decreased from T0 to T1 both in beta (T0: 13.09 ± 3.46, T1: 11.43 ± 2.76, t = 2.629, p = 0.014; T0: 11.97 ± 3.11, T1: 10.75 ± 2.42, t = 2.132, p = 0.043, respectively) and gamma (T0: 5.80 ± 2.42, T1: 4.89 ± 1.82, t = 2.097, p = 0.046; T0: 5.50 ± 2.30, T1: 4.63 ± 1.39, t = 2.525, p = 0.033, respectively) bands (Figure 2). The modification in RFP power in beta band approaches significance after FDR correction (p-corrected = 0.05). No significant change in RFP power was observed in subjects treated with placebo. No other significant effects were found.
Figure 2.
Topographic mapping of mean EEG spectral power at T0 and T1 and difference between the two timepoints for probiotic and placebo groups in (a) beta and (b) low-gamma bands.
Coherence analysis
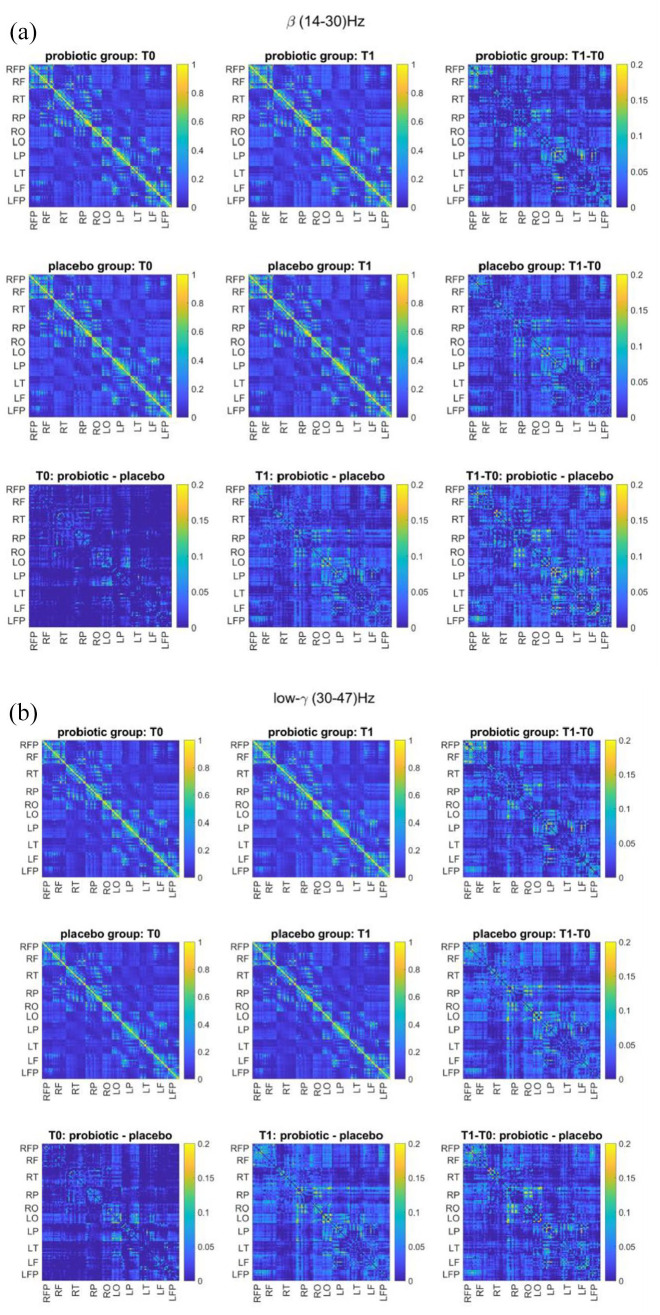
Repeated measures ANOVA showed a significant time × bands × treatment effect for frontopolar coherence (F = 2.481, p = 0.04, η2 = 0.56). Also in this case, the time × bands effect was not significant (F = 0.677, p = 0.415, η2 = 0.065). Paired t-test post hoc comparison showed that in the subjects treated with probiotic frontopolar coherence increased from T0 to T1 both in beta (T0: 0.099 ± 0.046, T1: 0.130 ± 0.046, t =−2.396, p = 0.024) and gamma (T0: 0.094 ± 0.059, T1: 0.139 ± 0.089, t =−2.563, p = 0.017) bands (Figure 3). The modification in frontopolar coherence in gamma band approaches significance after FDR correction (p-corrected = 0.06). On the contrary, no significant change in frontopolar coherence was observed in subjects treated with placebo. No significant effect of treatment or group was found for the other coherence measures.
Figure 3.
Mean coherence spectrum matrix at T0 and T1 and difference between the two timepoints for probiotic and placebo groups in (a) beta and (b) low-gamma bands.
Asymmetry analysis
As far as asymmetry, there was a significant time × bands × treatment effect on frontopolar asymmetry (F = 2.695, p = 0.045, η2 = 0.217) and on frontal asymmetry (F = 3.119, p = 0.026, η2 = 0.242) while the effect time × bands was not significant (F = 0.208, p = 0.934, η2 = 0.005 and F = 0.840, p = 0.502, η2 = 0.019, respectively). Post hoc analysis showed that frontal asymmetry in ASD subjects treated with probiotic decreased in delta band (T0: 0.029 ± 0.053, T1: –0.024 ± 0.047, t = 2.791, p = 0.032) while frontopolar asymmetry increased in alpha band in ASD subjects treated with placebo (T0: 0.022 ± 0.043, T1: 0.077 ± 0.043, t = –2.991, p = 0.03).
Correlation between EEG, clinical, and biochemical measures
We performed a correlation analysis between neurophysiological and clinical or biochemical measures using bivariate correlations (Pearson) for the EEG and behavioral or biochemical measures calculated at T1. In particular, EEG measures were selected for inclusion in correlational analyses based on outcomes from the longitudinal analysis, to preserve power. Since we did not have a priori hypothesis on which clinical or biochemical variables could be related to EEG modifications after probiotics administration, EEG measures were correlated with all the collected clinical and biochemical data. For a purpose of brevity, only significant results are reported.
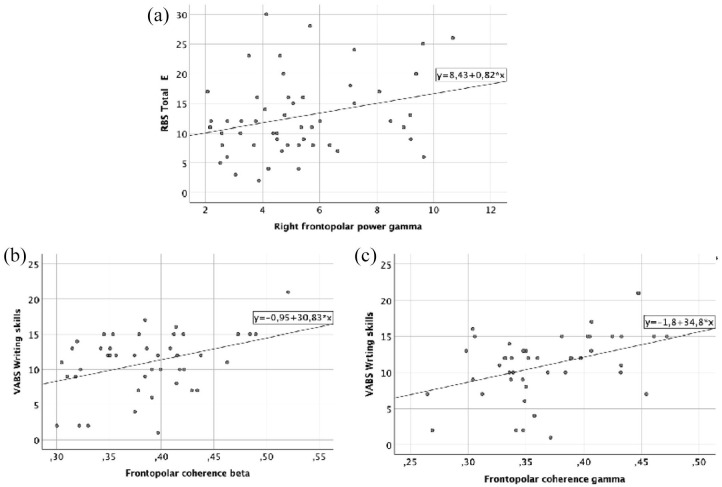
There was a significant positive correlation between RFP power in gamma band and RBS-R total number endorsed (r = 0.28, p = 0.04), meaning that children with ASD who showed lower RBS-R total number endorsed score at post-test also showed lower frontopolar power in gamma band (Figure 4(a)).
Figure 4.
Correlations between neurophysiological and clinical measures. (a) Correlation between RFP power in gamma and RBS-R total number endorsed (number of positive responses to RBS-R questionnaire), (b) correlation between frontopolar coherence in beta and the item “Writing skills” of the VABS-II, and (c) correlation between frontopolar coherence in gamma and the item “Writing skills” of the VABS-II.
There was a significant positive association between frontopolar coherence in the beta and gamma band and the item “Writing skills” of the VABS-II (r = 0.37, p = 0.012 and r = 0.40, p = 0.007, respectively), meaning that children with ASD who showed higher “writing skills” at post-test also showed higher frontopolar coherence in beta and gamma bands (Figure 4(b) and (c)).
No significant correlations between asymmetry and clinical measures were found.
Correlation between EEG and biochemical measures
A significant negative correlation was found between frontopolar coherence in the gamma band and TNF-α (r = –0.30, p = 0.04), meaning that children with ASD with lower levels of TNF-α at post-test showed higher frontopolar coherence in gamma band (Figure 5).
Figure 5.
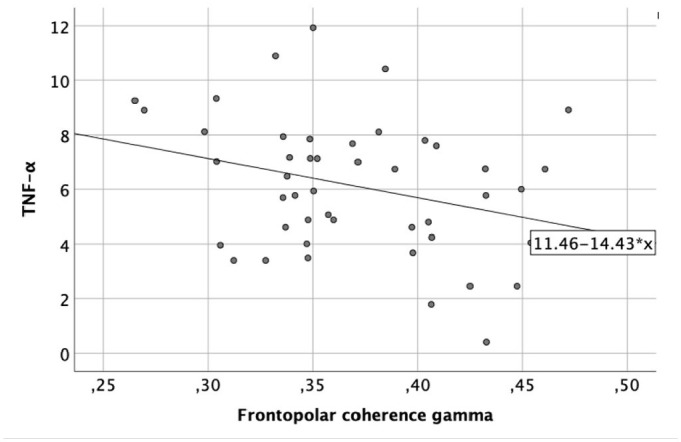
Correlation between frontopolar coherence in gamma and TNF-α (pg/ml).
No significant correlations between power or asymmetry and biochemical measures were found.
Discussion
To our knowledge, this is the first exploratory study that analyzes the effects of multiple probiotic strains in combination on neurophysiological characteristics in children with ASD using EEG measures. Results showed that children who received probiotics showed decreased frontopolar power, with a concurrent increase in frontopolar coherence in beta and gamma bands compared to ASD children who received placebo. In addition, changes in the frontal and frontopolar asymmetry in delta and alpha were observed.
The modifications in brain power and coherence after probiotic administration mainly occur in the beta and gamma band. Beta waves occur in subjects with open eyes and are associated with physiological activation, attention, concentration, analytical thinking, and in states of particular mental commitment or motor tasks (Tallon-Baudry, 2003). Gamma waves are associated with working-memory tasks and with a variety of early sensory responses (Tallon-Baudry, 2003).
Literature reports that the resting EEG of ASD subjects commonly shows increased activity of delta, theta, beta, and gamma spectral bands, with reduced medium alpha frequencies compared to TD individuals (Nicotera et al., 2019). This atypical power pattern describes a U-shaped profile in which the activity of the extreme frequencies (low and high) is significantly increased compared with TD subjects, while that of medium frequencies appears reduced.
According to a recent review (Gurau et al., 2017), gamma power increase in ASD compared to non-ASD controls is one of the most consistent findings across studies. Indeed, several investigations performing EEG in ASD during a resting-state condition reported gamma power increase in ASD, particularly in the frontal regions (Chan et al., 2007, 2011; Lushchekina et al., 2010, 2012; Sheikhani et al., 2009; Stroganova et al., 2007; van Diessen et al., 2015).
Although the results are less consistent, some studies also showed an increase in beta band power of ASD subjects compared to controls (Chan et al., 2007, 2011; Chan & Leung, 2006; Coben et al., 2008; Murias et al., 2007; Pop-Jordanova et al., 2010). Interestingly, the beta power was one of the best index for differentiating autistic from TD children, with an accuracy rate of 95.2% (Chan & Leung, 2006).
The atypical increase in high-frequency bands in ASD could be partly attributed to atypical functioning of the GABAergic tone in the inhibitory circuit, which influences the development of plasticity and brain function and is thought to impact on the modulation of EEG frequency bands (Baumgarten et al., 2016). GABA is the main inhibitory neurotransmitter in the brain. It has been observed that an altered GABA pattern is a key characteristic of the neurophysiology of ASD. Specifically, the impairment in inhibitory GABAergic that characterizes ASD subjects may result in an atypical balance of brain excitation/inhibition, alteration of neural signaling, processing of information and responding behavior (Foss-Feig et al., 2017).
Taken into consideration, the above-mentioned differences in frontal gamma and beta activity in ASD subjects compared to controls, as well as the decrease in beta and gamma power after PS, we can suggest that probiotics promote a change in brain activity in ASD children toward a pattern that resemble that of typical controls. Such a modification in brain activity in the children who received probiotics may reflect an improvement of the imbalance between excitatory and inhibitory neurons. Indeed, studies on animal models of ASD indicated that treatment with probiotics affects GABA receptor gene expression and protein levels in multiple brain regions (Kouser et al., 2013; Lee et al., 2015).
As regards coherence, an opposite pattern has been suggested in the literature: that is, decreased coherence in the higher frequency bands (alpha, beta, and gamma) compared with controls (Mehdizadefar et al., 2019; Schwartz et al., 2017). In particular, researchers detected a reduction in alpha, beta, and gamma in short- and medium-range connections in children with ASD relative to TD peers. Several studies reported reduced coherence in gamma and beta bands in ASD (Coben et al., 2008; Duffy & Als, 2012; Lazarev et al., 2010; Léveillé et al., 2010; Sheikhani et al., 2012). In particular, gamma bands reflect long-range neural synchronization and connectivity (Engel et al., 2001; Varela et al., 2001), which have been described to be impaired in ASD (Just et al., 2012). The increase in frontopolar gamma and beta coherence after probiotics administration suggests that this treatment fosters a change in brain connectivity toward a typical pattern.
As regards asymmetry, we found a shift from LF to RF asymmetry in delta band in subjects treated with probiotics, while children treated with placebo showed increased LFP asymmetry in the alpha band. Previous investigations on resting EEG showed greater activity in all frequency bands in the left compared to the right hemisphere in ASD (Burnette et al., 2011; Cantor et al., 1986; Stroganova et al., 2007; Sutton et al., 2005). In particular, Cantor et al. (1986) reported that subjects with ASD had enhanced power in the delta band of the posterior-temporal, midline, and occipital regions of the left hemisphere. Similarly, Stroganova et al. (2007) found enhanced delta power in the frontal, temporal, and parietal regions of the left hemisphere in individuals with ASD. Left-hemisphere dominance in the alpha band of mid-frontal regions has been repeatedly reported in ASD individuals (Burnette et al., 2011; Sutton et al., 2005). Considering these results, we can suppose a strong interconnection between the GI system and brain activity: the treatment with probiotics directly affects the central nervous system (CNS) contrasting the natural tendency to the atypical lateralization to the left observed in ASD.
The correlation analysis showed some interesting relationships between neurophysiological and clinical or biochemical measures. In particular, the decrease in power was related to a reduction in the RBS-R total score, which measures the breadth of repetitive behavior in subjects with ASD (Fulceri et al., 2016), while the increase in coherence was related to an improvement in the “Writing skills” subdomain of the VABS-II scale. This subdomain is applicable for children 3 years of age and older, and has been used as a parent-report measure of literacy-related abilities (Davidson & Weismer, 2014), since examples of items include “Identifies at least 10 printed letters of the alphabet” or “Copies own first name.” Therefore, the relevance of the “Written skills” subdomain of the VABS-II is not to be underestimated since it significantly correlated with the direct assessment of emergent literacy skills, which in turn is strongly associated to broader language ability in 5-year olds with ASD (Davidson & Weismer, 2014).
The correlation analysis between EEG and biochemical measures showed that an increase in coherence was associated with a decrease in TNF-α cytokine level. TNF-α levels have been positively correlated with ASD severity (Inga Jácome et al., 2016) having a critical role in regulating synaptic strength and plasticity (Steinmetz & Turrigiano, 2010), thus affecting the EEG patterns. Hoban et al. (2016) observed that the gut microbiota regulates the expression of genes linked to myelination and myelin plasticity in prefrontal cortex. Although our understanding of the influence of gut microbiota on the brain is mainly based on rodent studies, initial evidence in humans seem to support a similar relationship between our gut microbes and our brain (Bagga et al., 2019). Thus, it can be suggested that the changes in brain connectivity, we described can be mediated by chemicals, cytokines, hormones released by gut microbiota, which were manipulated with probiotic administration (Stroganova et al., 2007). A recent study (Yamanashi et al., 2021) described the set-up of a mouse model of delirium induced by systemic inflammation via lipopolysaccharides injection and quantified the cognitive disturbances by EEG. This study proves that the EEG method can quantify the level of neuroinflammation induced by systemic inflammation due to intestinal microbiota alteration. Within the gut–brain axis theory, an impaired intestinal barrier has been linked to a “permissive” blood–brain barrier (BBB), allowing the passage of antigens and immune-activated complexes at first into the bloodstream and subsequently at a distance in the brain through a leaky BBB (Fiorentino et al., 2016). Therefore, we can hypothesize a central neuroprotective effect of the probiotics through this pathway, as suggested by animal models (Yang et al., 2020).
Although this exploratory study provides new information on neural plasticity in response to probiotics administration in children with ASD, there are several important limitations that deserve mention.
First of all, the study’s main limitation is the small sample of subjects wide-ranging in age, even considering the difficulty of conducting EEG acquisition in a sample of young children with ASD.
Given the small sample size as well as the exploratory nature of the study, we decided to present results without multiple comparison correction to avoid invalidating any interesting effect of probiotics on EEG measures. Nevertheless, we acknowledge findings of clinical trials without multiple without adjustment of the p-value should be taken with caution because of the increased risk of type I errors (false positive findings). Notably, since as a side effect, p-value adjustments that reduce the chance of making a type I error (the chance of introducing ineffective treatments), inevitably increase the chance of making a type II error (the chance that effective treatments are not discovered). In this study, we originally assumed that type II error was a more relevant issue than observing potential false positives, but larger samples will be needed to confirm these preliminary results.
Moreover, the limited sample size did not allow us to perform separate analyses by sex, which could be of particular significance since preclinical studies observed sex differences not only in gut microbiota composition (Coretti et al., 2017), but also in its modulatory effects on CNS (Clarke et al., 2013). Similarly, the relatively small numerosity of the sample prevents a homogeneous stratification of ASD children based on their clinical profile (e.g. IQ and language level, ASD symptom severity, GI problems, adaptive functioning) to identify the impact of patient baseline characteristics on the outcome. Furthermore, to facilitate the subject’s collaboration during the EEG recording, we exposed children to an animated video without audio (the same for all subjects), which may have impacted on findings involving the visual areas. Despite the same time-lapse considered between T0 and T1 for all the participants, it is worth mentioning that changes in brain connectivity are age-dependent, and more evident at the earlier ages (Gao et al., 2017): therefore, a possible bias due to different baseline ages of recruited children cannot be ruled out. However, results showed that the effect of time, in the ANOVA, was not significant but only the time × group was significant, suggesting that the modifications we observed are indeed due to PS and not merely to the effect of age.
In conclusion, confirmatory studies should then be undertaken to provide support for the efficacy of probiotics in a large ASD patient population. Moreover, results of this exploratory study pave the way for the use of EEG activity as an objective and quantitative measure of treatment response in children with ASD.
Supplemental Material
Supplemental material, sj-docx-1-aut-10.1177_13623613221082710 for A randomized controlled trial into the effects of probiotics on electroencephalography in preschoolers with autism by Lucia Billeci, Alejandro Luis Callara, Letizia Guiducci, Margherita Prosperi, Maria Aurora Morales, Sara Calderoni, Filippo Muratori and Elisa Santocchi in Autism
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial is funded by the Italian Ministry of Health and by Tuscany Region with the grant ‘GR-2011-02348280’. This work was also partially supported by grant from the IRCCS Stella Maris Foundation (Ricerca Corrente and the “5 × 1000” voluntary contributions, Italian Ministry of Health n. 2763768)
ORCID iD: Margherita Prosperi  https://orcid.org/0000-0002-9917-788X
https://orcid.org/0000-0002-9917-788X
Supplemental material: Supplemental material for this article is available online.
References
- Allen A. P., Hutch W., Borre Y. E., Kennedy P. J., Temko A., Boylan G., Murphy E., Cryan J. F., Dinan T. G., Clarke G. (2016). Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Translational Psychiatry, 6(11), e939–e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). [DOI] [PubMed] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. (2011, Jan). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity, 25(1), 40–45. 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga D., Aigner C. S., Reichert J. L., Cecchetto C., Fischmeister F. P. S., Holzer P., Moissl-Eichinger C., Schöpf V. (2019). Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. European Journal of Nutrition, 58(5), 1821–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D., Yip B. H. K., Windham G. C., Sourander A., Francis R., Yoffe R., Glasson E., Mahjani B., Suominen A., Leonard H., Gissler M., Buxbaum J. D., Wong K., Schendel D., Kodesh A., Breshnahan M., Levine S. Z., Parner E. T., Hansen S. N., Sandin S. (2019, Jul 17). Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry, 76(10), 1035–1043. 10.1001/jamapsychiatry.2019.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni G., Belacchi C., Bonichini S., Coscarelli A. (2016). Vineland-II, Survey Interview Form. Standardizzazione Italiana [Vineland-II, Survey Interview Form. Italian standardization]. Giunti OS. [Google Scholar]
- Baumgarten T. J., Oeltzschner G., Hoogenboom N., Wittsack H.-J., Schnitzler A., Lange J. (2016). Beta peak frequencies at rest correlate with endogenous GABA+/Cr concentrations in sensorimotor cortex areas. PLOS ONE, 11(6), Article e0156829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeci L., Narzisi A., Tonacci A., Sbriscia-Fioretti B., Serasini L., Fulceri F., Apicella F., Sicca F., Calderoni S., Muratori F. (2017). An integrated EEG and eye-tracking approach for the study of responding and initiating joint attention in Autism Spectrum Disorders. Scientific Reports, 7(1), Article 13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeci L., Sicca F., Maharatna K., Apicella F., Narzisi A., Campatelli G., Calderoni S., Pioggia G., Muratori F. (2013). On the application of quantitative EEG for characterizing autistic brain: A systematic review. Frontiers in Human Neuroscience, 7, Article 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish J., Symons F., Lewis M. (1999). The Repetitive Behavior Scale (Western Carolina Center Research Reports).
- Burnette C. P., Henderson H. A., Inge A. P., Zahka N. E., Schwartz C. B., Mundy P. C. (2011). Anterior EEG asymmetry and the modifier model of autism. Journal of Autism and Developmental Disorders, 41(8), 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderoni S., Billeci L., Narzisi A., Brambilla P., Retico A., Muratori F. (2016). Rehabilitative interventions and brain plasticity in autism spectrum disorders: Focus on MRI-based studies. Frontiers in Neuroscience, 10, Article 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor D. S., Thatcher R. W., Hrybyk M., Kaye H. (1986). Computerized EEG analyses of autistic children. Journal of Autism and Developmental Disorders, 16(2), 169–187. [DOI] [PubMed] [Google Scholar]
- Chan A. S., Han Y. M., Sze S. L., Cheung M.-c., Leung W. W.-m., Chan R. C., To C. Y. (2011). Disordered connectivity associated with memory deficits in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 5(1), 237–245. [Google Scholar]
- Chan A. S., Leung W. W. (2006). Differentiating autistic children with quantitative encephalography: A 3-month longitudinal study. Journal of Child Neurology, 21(5), 391–399. [DOI] [PubMed] [Google Scholar]
- Chan A. S., Sze S. L., Cheung M.-c. (2007). Quantitative electroencephalographic profiles for children with autistic spectrum disorder. Neuropsychology, 21(1), 74–81. [DOI] [PubMed] [Google Scholar]
- Chang C. Y., Hsu S. H., Pion-Tonachini L., Jung T. P. (2020, Apr). Evaluation of artifact subspace reconstruction for automatic artifact components removal in multi-channel EEG recordings. IEEE Transactions on Biomedical Engineering, 67(4), 1114–1121. 10.1109/tbme.2019.2930186 [DOI] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R. D., Shanahan F., Dinan T. G., Cryan J. F. (2013, Jun). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry, 18(6), 666–673. 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- Coben R., Clarke A. R., Hudspeth W., Barry R. J. (2008). EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology, 119(5), 1002–1009. [DOI] [PubMed] [Google Scholar]
- Coretti L., Cristiano C., Florio E., Scala G., Lama A., Keller S., Cuomo M., Russo R., Pero R., Paciello O., Mattace Raso G., Meli R., Cocozza S., Calignano A., Chiariotti L., Lembo F. (2017, Mar 28). Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Scientific Reports, 7, Article 45356. 10.1038/srep45356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. M., Weismer S. E. (2014). Characterization and prediction of early reading abilities in children on the autism spectrum. Journal of Autism and Developmental Disorders, 44(4), 828–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Mullen T., Kothe C., Akalin Acar Z., Bigdely-Shamlo N., Vankov A., Makeig S. (2011). EEGLAB, SIFT, NFT, BCILAB, and ERICA: New tools for advanced EEG processing. Computational Intelligence and Neuroscience, 2011, Article 130714. 10.1155/2011/130714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy F. H., Als H. (2012). A stable pattern of EEG spectral coherence distinguishes children with autism from neuro—typical controls—A large case control study. BMC Medicine, 10(1), Article 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Divan G., Koh Y. J., Kim Y. S., Kauchali S., Marcín C., Montiel-Nava C., Patel V., Paula C. S., Wang C., Yasamy M. T., Fombonne E. (2012, Jun). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3), 160–179. 10.1002/aur.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. K., Fries P., Singer W. (2001). Dynamic predictions: Oscillations and synchrony in top–down processing. Nature Reviews Neuroscience, 2(10), 704–716. [DOI] [PubMed] [Google Scholar]
- Fattorusso A., Di Genova L., Dell’Isola G. B., Mencaroni E., Esposito S. (2019, Feb 28). Autism spectrum disorders and the gut microbiota. Nutrients, 11(3), Article 521. 10.3390/nu11030521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L. (2007). MacArthur-Bates communicative development inventories. Paul H. Brookes. [Google Scholar]
- Fiorentino M., Sapone A., Senger S., Camhi S. S., Kadzielski S. M., Buie T. M., Kelly D. L., Cascella N., Fasano A. (2016). Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Molecular Autism, 7, Article 49. 10.1186/s13229-016-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig J. H., Adkinson B. D., Ji J. L., Yang G., Srihari V. H., McPartland J. C., Krystal J. H., Murray J. D., Anticevic A. (2017). Searching for cross-diagnostic convergence: Neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biological Psychiatry, 81(10), 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio A., Cozzi P., Pastore V., Molteni M., Borgatti R., Montirosso R. (2006). La valutazione dei problemi emotivo comportamentali in un campione italiano di bambini in eta prescolare attraverso la Child Behavior Checklist e il Caregiver Teacher Report Form. Infanzia e Adolescenza, 5(1), 24–37. [Google Scholar]
- Fulceri F., Narzisi A., Apicella F., Balboni G., Baldini S., Brocchini J., . . . Calderoni S. (2016). Application of the Repetitive Behavior Scale-Revised —Italian version —In preschoolers with autism spectrum disorder. Research in Developmental Disabilities, 48, 43–52. [DOI] [PubMed] [Google Scholar]
- Gao W., Lin W., Grewen K., Gilmore J. H. (2017, Apr). Functional connectivity of the infant human brain: Plastic and modifiable. Neuroscientist, 23(2), 169–184. 10.1177/1073858416635986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. (2006). The Griffiths Mental Developmental Scales, Extended Revised. Association for Research in Infant and Child Development, The Test Agency. [Google Scholar]
- Grimaldi R., Gibson G. R., Vulevic J., Giallourou N., Castro-Mejía J. L., Hansen L. H., Leigh Gibson E., Nielsen D. S., Costabile A. (2018, Aug 2). A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome, 6(1), Article 133. 10.1186/s40168-018-0523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurau O., Bosl W. J., Newton C. R. (2017). How useful is electroencephalography in the diagnosis of autism spectrum disorders and the delineation of subtypes: A systematic review. Frontiers in Psychiatry, 8, Article 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban A. E., Stilling R. M., Ryan F. J., Shanahan F., Dinan T. G., Claesson M. J., Clarke G., Cryan J. F. (2016). Regulation of prefrontal cortex myelination by the microbiota. Translational Psychiatry, 6(4), e774–e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holingue C., Newill C., Lee L. C., Pasricha P. J., Daniele Fallin M. (2018, Jan). Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Research, 11(1), 24–36. 10.1002/aur.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotel A. C. P., Cordoba A. (2001). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention, 5(1), 1–10. [Google Scholar]
- Iannone L. F., Preda A., Blottiere H. M., Clarke G., Albani D., Belcastro V., Carotenuto M., Cattaneo A., Citraro R., Ferraris C., Ronchi F., Luongo G., Santocchi E., Guiducci L., Baldelli P., Iannetti P., Pedersen S., Petretto A., Provasi S., . . . Striano P. (2019, Oct). Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Review of Neurotherapeutics, 19(10), 1037–1050. 10.1080/14737175.2019.1638763 [DOI] [PubMed] [Google Scholar]
- Inga Jácome M. C., Morales Chacòn L. M., Vera Cuesta H., Maragoto Rizo C., Whilby Santiesteban M., Ramos Hernandez L., Noris García E., González Fraguela M. E., Fernandez Verdecia C. I., Vegas Hurtado Y. (2016). Peripheral inflammatory markers contributing to comorbidities in autism. Behavioral Sciences, 6(4), Article 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M. A., Keller T. A., Malave V. L., Kana R. K., Varma S. (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews, 36(4), 1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. W., Park J. G., Ilhan Z. E., Wallstrom G., Labaer J., Adams J. B., Krajmalnik-Brown R. (2013). Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLOS ONE, 8(7), Article e68322. 10.1371/journal.pone.0068322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. R., Allen A. P., Temko A., Hutch W., Kennedy P. J., Farid N., Murphy E., Boylan G., Bienenstock J., Cryan J. F., Clarke G., Dinan T. G. (2017, Mar). Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain, Behavior, and Immunity, 61, 50–59. 10.1016/j.bbi.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Kelly J. R., Minuto C., Cryan J. F., Clarke G., Dinan T. G. (2017). Cross talk: The microbiota and neurodevelopmental disorders. Frontiers in Neuroscience, 11, Article 490. 10.3389/fnins.2017.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M., Gramann K. (2021). Identifying key factors for improving ICA-based decomposition of EEG data in mobile and stationary experiments. European Journal of Neuroscience, 54(12), 8406–8420. [DOI] [PubMed] [Google Scholar]
- Kouser M., Speed H. E., Dewey C. M., Reimers J. M., Widman A. J., Gupta N., Liu S., Jaramillo T. C., Bangash M., Xiao B. (2013). Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. Journal of Neuroscience, 33(47), 18448–18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P. G., Bakke K. A., Bjørnæs H., Heminghyt E., Rytter E., Brager-Larsen L., Eriksson A.-S. (2012). The effect of levetiracetam on focal nocturnal epileptiform activity during sleep—A placebo-controlled double-blind cross-over study. Epilepsy & Behavior, 24(1), 44–48. [DOI] [PubMed] [Google Scholar]
- Lazarev V., Pontes A., Mitrofanov A., DeAzevedo L. (2010). Interhemispheric asymmetry in EEG photic driving coherence in childhood autism. Clinical Neurophysiology, 121(2), 145–152. [DOI] [PubMed] [Google Scholar]
- Lee J., Chung C., Ha S., Lee D., Kim D.-Y., Kim H., Kim E. (2015). Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Frontiers in Cellular Neuroscience, 9, Article 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillé C., Barbeau E. B., Bolduc C., Limoges É., Berthiaume C., Chevrier É., Mottron L., Godbout R. (2010). Enhanced connectivity between visual cortex and other regions of the brain in autism: A REM sleep EEG coherence study. Autism Research, 3(5), 280–285. [DOI] [PubMed] [Google Scholar]
- Liu Y.-W., Liong M. T., Chung Y.-C. E., Huang H.-Y., Peng W.-S., Cheng Y.-F., Lin Y.-S., Wu Y.-Y., Tsai Y.-C. (2019). Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: A randomized, double-blind, placebo-controlled trial. Nutrients, 11(4), Article 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S. K., Miyakoshi M., Tung K., Lloyd E., Salgari G., Dillon A., . . . Makeig S. (2019). Neural activation and connectivity during cued eye blinks in Chronic Tic Disorders. NeuroImage: Clinical, 24, Article 101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. (2012). Autism diagnostic observation schedule, (ADOS-2) modules 1–4. Western Psychological Services. [Google Scholar]
- Lushchekina E., Podreznaia E., Lushchekin V., Strelets V. (2010). Comparative EEG study in normal and autistic children. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni I P Pavlova, 60(6), 657–666. [PubMed] [Google Scholar]
- Lushchekina E., Podreznaya E., Lushchekin V., Strelets V. (2012). A comparative EEG study in normal and autistic children. Neuroscience and Behavioral Physiology, 42(3), 236–243. [Google Scholar]
- Mehdizadefar V., Ghassemi F., Fallah A. (2019). Brain connectivity reflected in electroencephalogram coherence in individuals with autism: A meta-analysis. Basic and Clinical Neuroscience, 10(5), 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer A., Van de Water J. (2017). The role of the immune system in autism spectrum disorder. Neuropsychopharmacology, 42(1), 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. (2011, Feb). ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology, 48(2), 229–240. 10.1111/j.1469-8986.2010.01061.x [DOI] [PubMed] [Google Scholar]
- Mullen T. R., Kothe C. A., Chi Y. M., Ojeda A., Kerth T., Makeig S., Jung T. P., Cauwenberghs G. (2015, Nov). Real-time neuroimaging and cognitive monitoring using wearable dry EEG. IEEE Transactions on Biomedical Engineering, 62(11), 2553–2567. 10.1109/tbme.2015.2481482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M., Webb S. J., Greenson J., Dawson G. (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62(3), 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narzisi A., Posada M., Barbieri F., Chericoni N., Ciuffolini D., Pinzino M., Romano R., Scattoni M. L., Tancredi R., Calderoni S., Muratori F. (2018, Sep 6). Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiology and Psychiatric Sciences, 29, E5. 10.1017/s2045796018000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera A. G., Hagerman R. J., Catania M. V., Buono S., Di Nuovo S., Liprino E. M., Stracuzzi E., Giusto S., Di Vita G., Musumeci S. A. (2019). EEG abnormalities as a neurophysiological biomarker of severity in autism spectrum disorder: A pilot cohort study. Journal of Autism and Developmental Disorders, 49(6), 2337–2347. [DOI] [PubMed] [Google Scholar]
- Palmer J., Kreutz-Delgado K., Makeig S. (2011). AMICA: An adaptive mixture of independent component analyzers with shared components. Swartz Center for Computatonal Neursoscience, University of California San Diego, Tech. Rep. [Google Scholar]
- Parracho H. M. R. T., Gibson G. R., Knott F. J., Bosscher D., Kleerebezem M., McCartney A. L. (2010). A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. International Journal of Probiotics and Prebiotics, 2, 69–74. [Google Scholar]
- Pascual-Leone A., Amedi A., Fregni F., Merabet L. B. (2005). The plastic human brain cortex. The Annual Review of Neuroscience, 28, 377–401. [DOI] [PubMed] [Google Scholar]
- Patusco R., Ziegler J. (2018, Sep 1). Role of probiotics in managing gastrointestinal dysfunction in children with autism spectrum disorder: An update for practitioners. Advances in Nutrition, 9(5), 637–650. 10.1093/advances/nmy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersol A. G., Bendat J. S. (2000). Random data: Analysis and measurement procedures. Wiley. [Google Scholar]
- Pion-Tonachini L., Kreutz-Delgado K., Makeig S. (2019). ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage, 198, 181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivik R. T., Broughton R. J., Coppola R., Davidson R. J., Fox N., Nuwer M. R. (1993, Nov). Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology, 30(6), 547–558. 10.1111/j.1469-8986.1993.tb02081.x [DOI] [PubMed] [Google Scholar]
- Pop-Jordanova N., Zorcec T., Demerdzieva A., Gucev Z. (2010). QEEG characteristics and spectrum weighted frequency for children diagnosed as autistic spectrum disorder. Nonlinear Biomedical Physics, 4(1), Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnova G. V., Ivanova O., Proskurnina E. V. (2020). Effects of EEG examination and ABA-therapy on resting-state EEG in children with low-functioning autism. AIMS Neuroscience, 7(2), 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Pritchard W. S., August G. J. (1987). Effects of fenfluramine on EEG and brainstem average evoked response in infantile autism. Neuropsychobiology, 18(2), 105–109. [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. (2003). The Social Communication Questionnaire: Manual. Western Psychological Services. [Google Scholar]
- Rutter M., Le Couteur A., Lord C. (2003). Autism Diagnostic Interview—Revised. Western Psychological Services. [Google Scholar]
- Santocchi E., Guiducci L., Fulceri F., Billeci L., Buzzigoli E., Apicella F., Calderoni S., Grossi E., Morales M. A., Muratori F. (2016). Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry, 16, Article 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santocchi E., Guiducci L., Prosperi M., Calderoni S., Gaggini M., Apicella F., Tancredi R., Billeci L., Mastromarino P., Grossi E., Gastaldelli A., Morales M. A., Muratori F. (2020). Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: A randomized controlled trial. Frontiers in Psychiatry, 11, Article 550593. 10.3389/fpsyt.2020.550593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. K., Melmed R. D., Barstow L. E., Enriquez F. J., Ranger-Moore J., Ostrem J. A. (2006, Nov). Oral human immunoglobulin for children with autism and gastrointestinal dysfunction: A prospective, open-label study. The Journal of Autism and Developmental Disorders, 36(8), 1053–1064. 10.1007/s10803-006-0141-y [DOI] [PubMed] [Google Scholar]
- Schopler E., Reichler R. J., DeVellis R. F., Daly K. (1980, Mar). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). The Journal of Autism and Developmental Disorders, 10(1), 91–103. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Kessler R., Gaughan T., Buckley A. W. (2017). Electroencephalogram coherence patterns in autism: An updated review. Pediatric Neurology, 67, 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban S. Y., El Gendy Y. G., Mehanna N. S., El-Senousy W. M., El-Feki H. S. A., Saad K., El-Asheer O. M. (2018, Nov). The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutritional Neuroscience, 21(9), 676–681. 10.1080/1028415x.2017.1347746 [DOI] [PubMed] [Google Scholar]
- Sheikhani A., Behnam H., Mohammadi M. R., Noroozian M., Mohammadi M. (2012). Detection of abnormalities for diagnosing of children with autism disorders using of quantitative electroencephalography analysis. Journal of Medical Systems, 36(2), 957–963. [DOI] [PubMed] [Google Scholar]
- Sheikhani A., Behnam H., Noroozian M., Mohammadi M. R., Mohammadi M. (2009). Abnormalities of quantitative electroencephalography in children with Asperger disorder in various conditions. Research in Autism Spectrum Disorders, 3(2), 538–546. [Google Scholar]
- Sparrow S. S., Cicchetti D. V., Balla D. A., Doll E. A. (2005). Vineland Adaptive Behavior Scales: Survey Forms Manual. American Guidance Service. [Google Scholar]
- Steinmetz C. C., Turrigiano G. G. (2010). Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. Journal of Neuroscience, 30(44), 14685–14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova T. A., Nygren G., Tsetlin M. M., Posikera I. N., Gillberg C., Elam M., Orekhova E. V. (2007). Abnormal EEG lateralization in boys with autism. Clinical Neurophysiology, 118(8), 1842–1854. [DOI] [PubMed] [Google Scholar]
- Sutton S. K., Burnette C. P., Mundy P. C., Meyer J., Vaughan A., Sanders C., Yale M. (2005). Resting cortical brain activity and social behavior in higher functioning children with autism. Journal of Child Psychology and Psychiatry, 46(2), 211–222. [DOI] [PubMed] [Google Scholar]
- Tabouy L., Getselter D., Ziv O., Karpuj M., Tabouy T., Lukic I., Maayouf R., Werbner N., Ben-Amram H., Nuriel-Ohayon M. (2018). Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain, Behavior, and Immunity, 73, 310–319. [DOI] [PubMed] [Google Scholar]
- Takada M., Nishida K., Gondo Y., Kikuchi-Hayakawa H., Ishikawa H., Suda K., Kawai M., Hoshi R., Kuwano Y., Miyazaki K. (2017). Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Beneficial Microbes, 8(2), 153–162. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C. (2003). Oscillatory synchrony and human visual cognition. Journal of Physiology, 97(2–3), 355–363. [DOI] [PubMed] [Google Scholar]
- Tomova A., Husarova V., Lakatosova S., Bakos J., Vlkova B., Babinska K., Ostatnikova D. (2015, Jan). Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior, 138, 179–187. 10.1016/j.physbeh.2014.10.033 [DOI] [PubMed] [Google Scholar]
- Urigüen J. A., Garcia-Zapirain B. (2015). EEG artifact removal—State-of-the-art and guidelines. Journal of Neural Engineering, 12(3), Article 031001. [DOI] [PubMed] [Google Scholar]
- van Diessen E., Senders J., Jansen F. E., Boersma M., Bruining H. (2015). Increased power of resting-state gamma oscillations in autism spectrum disorder detected by routine electroencephalography. European Archives of Psychiatry and Clinical Neuroscience, 265(6), 537–540. [DOI] [PubMed] [Google Scholar]
- Van Hecke A. V., Stevens S., Carson A. M., Karst J. S., Dolan B., Schohl K., McKindles R. J., Remmel R., Brockman S. (2015). Measuring the plasticity of social approach: A randomized controlled trial of the effects of the PEERS intervention on EEG asymmetry in adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 316–335. [DOI] [PubMed] [Google Scholar]
- Varela F., Lachaux J.-P., Rodriguez E., Martinerie J. (2001). The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience, 2(4), 229–239. [DOI] [PubMed] [Google Scholar]
- Wei H., Chadman K. K., McCloskey D. P., Sheikh A. M., Malik M., Brown W. T., Li X. (2012). Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1822(6), 831–842. [DOI] [PubMed] [Google Scholar]
- West R., Roberts E., Sichel L., Sichel J. (2013). Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the Delpro® probiotic and immunomodulator formulation. Journal of Probiotics & Health, 1(2), 1–6. [Google Scholar]
- Willyard C. (2016). New efforts to design better tools to track autism therapy response. Nature Medicine, 22(6), 570–571. 10.1038/nm0616-570 [DOI] [PubMed] [Google Scholar]
- Yamanashi T., Malicoat J. R., Steffen K. T., Zarei K., Li R., Purnell B. S., . . . Shinozaki G. (2021). Bispectral EEG (BSEEG) quantifying neuro-inflammation in mice induced by systemic inflammation: A potential mouse model of delirium. Journal of Psychiatric Research, 133, 205–211. [DOI] [PubMed] [Google Scholar]
- Yang X., Yu D., Xue L., Li H., Du J. (2020). Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharmaceutica Sinica B, 10(3), 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-aut-10.1177_13623613221082710 for A randomized controlled trial into the effects of probiotics on electroencephalography in preschoolers with autism by Lucia Billeci, Alejandro Luis Callara, Letizia Guiducci, Margherita Prosperi, Maria Aurora Morales, Sara Calderoni, Filippo Muratori and Elisa Santocchi in Autism