Abstract
To clear a Cryptosporidium parvum infection, mice need CD4+ T cells, major histocompatibility complex class II, and an intact CD40-CD154 signaling pathway. CD40 is constitutively expressed on marrow-derived cells such as dendritic cells and B lymphocytes and is induced by gamma interferon (IFN-γ) on most somatic cells. To determine whether the CD40 needed to clear a C. parvum infection has to be on marrow-derived mononuclear cells or on the epithelial cells that normally harbor the parasite, we transplanted CD40−/− mice with CD40+/− bone marrow and then infected them with C. parvum. These chimeras cleared the C. parvum infection, while CD40+/− controls transplanted with CD40−/− marrow cells remained infected. CD40 expression on marrow-derived cells therefore suffices for a C. parvum infection to be cleared, while CD40 expression on intestinal epithelial cells is not sufficient. There was no difference between the acquisition of CD69 and CD154 by mesenteric lymph node T cells of C. parvum-infected animals with intact or disrupted CD40-CD154 pathways. CD4 T cells entered the intestinal laminae propriae of C. parvum-infected animals whether or not the CD40 genes of these recipients were intact. These results suggest that, for a C. parvum infection to be cleared, CD40 is not necessary for T-cell activation but may instead contribute to an effector pathway of marrow-derived cells.
Cryptosporidium parvum is an apicomplexan organism that infects intestinal epithelial cells and causes transient diarrhea in healthy individuals (11). C. parvum infections cause more severe problems, ranging from prolonged diarrhea to sclerosing cholangitis, in subjects with AIDS or X-linked immunodeficiency with hyper-immunoglobulin M (IgM) (6, 17). Experimental models in mice show that CD4+ T cells (26), major histocompatibility complex class II (MHC-II) (1), and an intact CD40-CD154 pathway (10) are all required for C. parvum to be cleared from the gut and biliary tree. Gamma interferon (IFN-γ) and interleukin-12 (IL-12) contribute to recovery from C. parvum infection (32, 33), although the ability of many IFN-γ knockout mice to recover from C. parvum (36, 30) indicates that this cytokine is not invariably necessary for the parasite to be cleared.
The nature of the interaction between the CD4+ T cell and an MHC-II-bearing cell that is required for the clearing of C. parvum from the gut is not understood. Cryptosporidial sporozoites invaginate into epithelial cells of the intestinal epithelium, but they remain separated from the cell cytoplasm by an intact cellular cytoplasmic membrane (24). Conventional pathways for antigen, and particularly class I antigen, presentation (15) may therefore not hold for the handling of C. parvum antigens. Intestinal epithelial cells can express MHC-II antigens and are thought capable of one or more pathways of antigen presentation (18). Populations of intraepithelial lymphocytes and lamina propria lymphocytes may have access to gut-derived antigens displayed in the context of class II antigens on the basolateral extensions of epithelial cells. It is therefore conceivable that T-cell responses that mediate immunity to C. parvum could occur without the participation of a marrow-derived antigen-presenting cell (APC) (19).
It was previously shown that CD4 T cells expressing a transgene for an ovalbumin-specific antigen receptor sufficed to clear C. parvum infection from SCID mice, provided that the transgenic T cells became activated and were able to express CD154 (23). This result suggested that the clearance of C. parvum required an interaction between CD154 (presumably expressed on an activated T cell) and CD40. A role for CD154-CD40 interactions has been described in immune responses to other intracellular parasites, albeit in the context of antigen-specific responses (25, 31, 35). These CD40-CD154 interactions may contribute a direct afferent signal to the T cell, or they may trigger the CD40-positive cell (for example, a B cell, dendritic cell, or macrophage) to make a mediator such as IL-12 (22, 14) or nitric oxide (29). Expression of CD40, however, can be induced on a wide range of cells by IFN-γ (12). If C. parvum-infected epithelial cells were to express CD40, then CD154 on T cells might act directly upon them to trigger their apoptosis (20) or perhaps affect their handling of an intracellular pathogen. For example, a soluble CD154 trimer sufficed to trigger the apoptosis of C. parvum-infected cells in vitro (17).
To determine whether the cellular target for CD154 binding that is required for C. parvum to be cleared is a marrow-derived cell or an epithelial cell (the cell type infected by C. parvum), we created chimeras by transplanting CD40+/− marrow into CD40−/− mice. The results presented here show that expression of CD40 on a marrow-derived cell is sufficient for C. parvum infection to be cleared from a mouse that cannot express CD40 on intestinal epithelial cells. In contrast, CD40+/− mice transplanted with CD40−/− marrow were unable to clear C. parvum. The induction of CD69 and CD154 on CD4 cells from the mesenteric lymph nodes of animals with disrupted and intact CD40-CD154 pathways argues against an essential role for CD40 in the activation phase of the T-cell response to C. parvum.
MATERIALS AND METHODS
Mice.
C57BL/6 wild-type, B6 RAG−/− and IgM(μ) heavy-chain knockout (JR2288) mice were purchased from Jackson Laboratory (details of these mice are given at www.jaxmice.jax.org). The sources, breeding, genotyping, and care of the C57BL/6 SCID, C57BL/6 CD154−/−, and C57BL/6 CD40−/− (knockout) mice are described in references 10 and 23. Animal conditions and experimentation were approved by the Institutional Animal Care and Use Committee of the University of Colorado School of Medicine.
Marrow transplant chimeras and adoptive transfer.
Mice were irradiated with 950 rads from a cesium source at 250 rads/min. Within 1 h they were injected with 107 spleen and marrow cells from donors of the same sex. These cells were prepared by flushing the cells from the marrow of the femurs and humeri of euthanized donors with Hanks' balanced salt solution (HBSS). These cells were counted and mixed with an equal number of spleen cells from the same donors, also suspended in HBSS. Clumps were removed by filtering through nylon mesh, and the cell suspension was adjusted to 108/ml. One hundred microliters (107 cells) was injected where indicated into the peritoneal cavity of the irradiated mice. Recipients were maintained on trimethoprim-sulfamethoxazole (Septra) for 2 weeks after transplantation. They were allowed to recover for 6 weeks before being used for experimentation in the expectation that this would allow sufficient time for reconstitution by donor-derived APCs.
Infection and testing for C. parvum.
Experimental groups of mice were always derived from more than one litter and included both males and females. Animals were transferred to a biohazard facility for infection with C. parvum strain GCH1, obtained from McKesson Bioservices (catalog no. 1372) through the AIDS Research and Reference Reagent Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, Md.), as previously described (23). Over 50% of these oocysts excyst after being washed in bleach and incubated for 4 h at 37°C. Animals were infected by gavage once with 106 oocysts in 0.1 ml of HBSS. This number of oocysts is several orders of magnitude greater than the mouse 50% infective dose for C. parvum. Mouse feces were collected weekly and stored at −20°C until the infection status was determined by fecal C. parvum antigen using a commercial C. parvum antigen enzyme-linked immunosorbent assay (ELISA) kit (LMD, catalog no. CP-35; Alexon, Ramsey, Minn.). Feces were resuspended in the homogenization buffer overnight at 4°C before testing according to the manufacturer's instructions. Positive and negative controls were included with each ELISA run. Animals were euthanized by CO2 inhalation (a) when they lost 15% of body weight or (b) at 6 to 13 weeks after infection or adoptive transfer of T cells. Tissues obtained at necropsy for routine histology were fixed in 10% buffered formalin and embedded in paraffin for sectioning and hematoxylin and eosin staining. Tissues to be used for detection of 5- (and 6-) carboxyfluorescein, succinimidyl ester (CFSE)-labeled cells were snap-frozen and sectioned on a cryostat.
Preparation and transfer of mesenteric lymph node suspensions.
For certain experiments, mesenteric lymph nodes were teased in HBSS and adjusted to 107/ml. These cells were fluorescence labeled by CFSE (catalog no. C-1311; Molecular Probes, Portland, Oreg.) as previously described (23) and resuspended at 108/ml in HBSS. Ten million cells were injected intraperitoneally into wild-type and knockout recipients as indicated below. The division of these cells was assessed by fluorescence-activated cell sorter analysis, and their appearance in the gut was detected by fluorescence microscopy of frozen sections. These were viewed on a Leitz microscope with incident UV light and transmitted phase-contrast optics. Images were captured with a Spot camera (model 1.3.0; Diagnostic Instruments, Sterling Heights, Mich.) and processed with Adobe Photoshop software.
Fluorescence staining.
For fluorescence-activated cell sorter analysis, 106 mesenteric lymph node or spleen cells were spun down and resuspended in 5 to 10 μl of fluorochrome-conjugated antibodies to B220, CD4, CD154, or CD69 (Caltag, Burlingame, Calif., or Pharmingen, San Diego, Calif.) and incubated on ice for 30 min. An aliquot of cells was stained with phycroerythrin and fluorescein isothiocyanate controls in parallel. The cells were washed twice and fixed in 1% paraformaldehyde before being viewed on an EPICS Elite cytofluorograph.
RESULTS
CD40 on a marrow-derived cell suffices for C. parvum to be cleared by C57BL/6 mice.
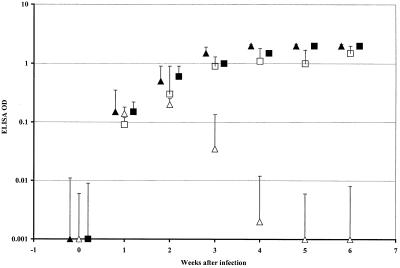
C57BL/6 CD40−/− mice, irradiated with 950 rads and reconstituted with 107 CD40+/− donor cells, were rested for 6 weeks to allow for the repopulation of APCs with donor-derived CD40+/− cells. Following infection by mouth with 106 C. parvum oocysts, these transplant chimeras cleared the infection as judged by C. parvum ELISAs (Fig. 1). The B220+ cells in blood and spleen samples obtained at necropsy from these animals stained for CD40 by two-color cytometry. Histology of necropsy sections of ileum also confirmed that the C. parvum infection had been cleared. CD40+/− animals transplanted with CD40−/− bone marrow remained infected by C. parvum for >8 weeks as shown by ELISA (Fig. 1), and this was confirmed by histology of the terminal ileum (not shown). B cells from these CD40+/− animals reconstituted by CD40−/− marrow were negative for cell surface CD40 (not shown). Positive-control transplant chimeras comprising CD40+/− mice reconstituted with wild-type marrow cleared the C. parvum infection (not shown), while negative controls (CD40−/− mice reconstituted with RAG knockout or CD40−/− marrow cells) remained infected for >8 weeks (Table 1).
FIG. 1.
C. parvum ELISA results (mean + 1 SD) for CD40−/− C57BL/6 mice infected with 106 C. parvum oocysts. ▴, CD40−/− mice, unmanipulated; ▵, CD40−/− mice transplanted with C57BL/6+/− bone marrow; ■, CD40−/− mice transplanted with CD40−/− marrow; □, CD40+/− mice transplanted with CD40−/− marrow. OD, optical density.
TABLE 1.
Outcome of C. parvum infection of control bone marrow transplant chimerasa
| Group no. | Donor marrow strain | No. of mice | Duration of infection (wk) | Final C. parvum ELISA ODb | Interpretation |
|---|---|---|---|---|---|
| 1 | C57BL/6 | 6 | <6 | 0.006 ± 0.004 | Cleared |
| 2 | μ−/−CD40+ | 8 | <6 | 0.008 ± 0.006 | Cleared |
| 3 | B6 RAG−/− | 4 | >12 | 0.13 ± 0.005 | Infected |
| 4 | CD40−/− | 3 | >8 | 1.00 ± 0.03 | Infected |
Chimeras were created by exposing CD40−/− mice to 950 rads of irradiation delivered over 4 min from a Cs source. They were then injected with 107 cells of the indicated bone marrow and spleen. All animals were infected 6 weeks later with 106 C. parvum oocysts, and the outcome of infection was determined by ELISA for C. parvum.
Results are the mean ELISA optical density (OD) ± 1 SD for the last fecal samples obtained before euthanasia. OD readings of >0.02 indicate infection. The difference in outcomes of infection after 6 weeks between the test (groups 1 and 2 combined) and control (groups 3 and 4 combined) mice is significant (P < 0.005 by the Fisher test).
To exclude a role for antibody in the clearing of C. parvum infection by the CD40−/− recipients of CD40+ marrow cells in the above experiment, we repeated the experiment using IgM (μ) chain knockout (B-cell deficient) bone marrow donors. Eight recipients of μ−/− marrow cells were infected with C. parvum 6 weeks after reconstitution, and all cleared their C. parvum infection in the ensuing 6 weeks (Table 1).
T-cell activation in CD40−/− mice infected with C. parvum.
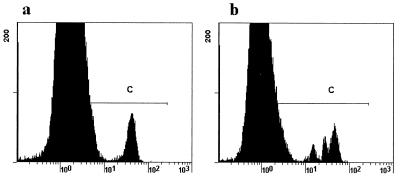
CD 40 transduces important signals into APC that stimulate the expression of B7 and MHC and the secretion of IL-12. These CD40-mediated functions are thought to increase the ability of an APC to stimulate a T cell. To determine whether CD40−/− mice would be unable to activate T cells in their mesenteric lymph nodes (and so fail to clear a C. parvum infection), we examined CD4 cells from infected and uninfected mice for expression of the early activation marker, CD69, and for CD154. A single time point was required to permit comparisons between groups, and because wild-type animals clear the infection promptly, 6 days after infection was the time point selected. This approximates the period of the 7 to 9 days after infection used in the study with calves reported by Pasquali and coworkers (27). Our results (Table 2) show in each case a greater percentage of activated CD69+ cells in the infected animals than in uninfected controls. Since there were no overlaps between the infected and uninfected mice within groups, these differences are significant by the Fisher exact test (P < 0.05). The percentages of CD4+ cells that expressed CD69+ approximately doubled in the wild-type, CD154−/−, or CD40−/− mice that were infected with C. parvum, compared with the uninfected controls. Expression levels of CD154 were similar in C. parvum-infected wild-type and CD40−/− mice. To determine whether the activation that occurred was followed by cell division, spleen and mesenteric lymph node cells from wild-type C57BL/6 mice were CFSE labeled and injected into control and C. parvum-infected CD40−/− and wild-type mice. Six days later, the recipients' mesenteric lymph nodes were recovered and examined for CFSE fluorescence. Cell division was detected only in the infected recipients (Fig. 2 illustrates cell division by CD4 cells in a C. parvum-infected but not an uninfected CD40−/− recipient). The mean + 1 standard deviation (SD) percentage of CD4 cells that divided in the CD40−/− mice was 48% + 8%, and in the C. parvum-infected wild-type recipients it was 38% + 12%. This difference does not reach statistical significance.
TABLE 2.
Activation markers on CD4 cells from mesenteric lymph nodes of control and C. parvum-infected micea
| Mouse examined | No. of mice | Infection status | % CD4 cells (mean ± 1 SD) staining for activation marker
|
|
|---|---|---|---|---|
| CD69 | CD154 | |||
| C57BL/6 | 6 | Uninfected control | 18 ± 5 | 5 ± 3 |
| C57BL/6 | 7 | C. parvum infected | 31 ± 3b | 12 ± 2b |
| CD154−/− C57BL/6 | 6 | Uninfected control | 14.6 ± 6.3 | <0.1 |
| CD154−/− C57BL/6 | 8 | C. parvum infected | 32 ± 2b | <0.1 |
| CD40−/− C57BL/6 | 5 | Uninfected control | 28 ± 4 | 7 ± 2 |
| CD40−/− C57BL/6 | 5 | C. parvum infected | 58 ± 3b | 15 ± 2b |
Mesenteric lymph nodes were recovered from the infected mice 6 days after infection. The cells were stained with p-conjugated CD4 antibody and fluorescein isothiocyanate antibodies to the activation markers, and 5,000 CD4 cells were examined. Results are the mean ± 1 SD of CD4 cells staining for the activation marker shown.
These results differ (P < 0.05 by Mann-Whitney U test) from the corresponding control.
FIG. 2.
Green CFSE fluorescence of CD4 cells from mesenteric lymph nodes of CD40−/− mice 6 days after injection of 107 CFSE-labeled spleen cells from wild-type donors. (a) Uninfected recipient; (b) C. parvum-infected recipient. The appearance of additional populations of cells with reduced green fluorescence in the region labeled C of the infected animal is indicative of cell division. This result is representative of five animals studied.
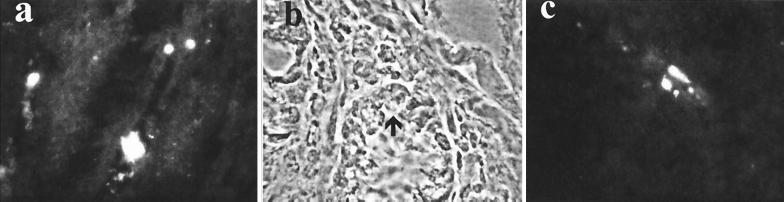
It was previously shown that T cells enter the laminae propriae of C. parvum-infected SCID mice prior to clearing of the infection (23). To determine whether CD40 was required for T cells to reach the lamina propria, we examined the guts of the CD40−/− and wild-type C. parvum-infected recipients of the CFSE-labeled cells described above. Donor-derived cells identified through CFSE fluorescence were found in 24% (SD, 7%) of high-power fields of laminae propriae from the guts of all five CD40−/− C. parvum-infected animals tested (an example is shown in Fig. 3). CFSE-labeled cells were found in 18% + 7% of high-power fields of laminae propriae from the guts of C. parvum-infected wild-type animals. The difference between CD40−/− and wild-type animals is not statistically significant. CFSE-labeled cells were not detected in the laminae propriae of CD40−/− animals that had not been infected by C. parvum (not shown).
FIG. 3.
(a) CFSE-labeled cells in the intestine of a C. parvum-infected CD40−/− mouse 6 days after injection of 107 CFSE-labeled wild-type spleen cells. Magnification, ×400. Higher-power (magnification, ×1,000) phase-contrast (b) and green fluorescence (c) views of ileum show that labeled cells lie adjacent to the base of a crypt (arrow). Comparable results were obtained with five more animals.
DISCUSSION
That T cells are necessary for a C. parvum infection to be cleared is well established (26) and is perhaps to be expected for an organism that principally resides in a parasitophorous vacuole in an epithelial cell. This location is likely to protect the intracellular sporozoite from antibody, but the C. parvum sporozoites do not penetrate the cell wall to enter the cytoplasm of the infected cell. Residence within a parasitophorous vacuole is therefore likely to prevent sporozoite antigens from entering a class I processing pathway. Certainly CD8 T cells and MHC-I antigens do not seem to be required for cryptosporidia to be cleared (1, 28). Even the CD4 T-cell response that can clear a C. parvum infection is unusual in that the specificity of the T-cell receptor seems less important than the activation of the T cells and their utilization of an intact CD154-CD40 signaling pathway (23). CD40 ligation can lead to apoptosis of fibroblasts (20) and, in vitro, of C. parvum-infected cells (17, 30), so the direct triggering of apoptosis in CD40+ infected cells by CD154 expressed on activated T cells was considered a potential mechanism that could account for the contribution of CD40 to C. parvum immunity. The results presented here clearly refute this interpretation because CD40 was not required on the surface of the infected intestinal epithelial cells for a C. parvum infection to be cleared. Instead, C. parvum infections were cleared only when CD40 was present on a marrow-derived cell. The marrow-derived populations that normally express CD40 are B lymphocytes and cells of the dendritic and mononuclear phagocyte series. Since CD40−/− recipients of B-cell-deficient marrow were able to clear C. parvum infections, it appears that the required CD40+ population is a member of the dendritic and mononuclear phagocyte series.
An implication from our finding that marrow-derived CD40+ cells are required for a C. parvum infection to be cleared is that antigen presentation to T cells through intestinal epithelial cells alone (19) is insufficient for immunity. This conclusion is consistent with the recent report from Blanas and colleagues (5) showing that a marrow-derived MHC-II- positive cell is necessary for ovalbumin-specific CD4 cells to proliferate in the mesenteric lymph nodes of mice fed ovalbumin.
Immune responses to other intracellular pathogens (particularly Leishmania, Toxoplasma, and Pneumocystis) have emphasized the role of CD40 in stimulating APCs (most probably dendritic cells) to make IL-12 (9). The production of IL-12 is relevant because it is known to bias T cells towards a IFN-γ-secreting (or Th1) immune response. In the response to Leishmania infection, IL-12 production by parasitized dendritic cells depends on a CD40 transduced signal (25). Since IL-12 is known to contribute to immunity to C. parvum (33), the principal contribution of CD40 ligation to the immune response to C. parvum might be to ensure T-cell activation and subsequent IFN-γ production. Experiments in transplant systems show that dendritic cells deficient in CD40 tend to make more of the down-regulatory cytokine IL-10 (13). Despite the relationship of CD40 to IL-12 production, our experimental results clearly show that CD4+ T cells in the mesenteric lymph nodes of CD40−/− C. parvum-infected mice activate at least to the extent of CD69 expression. About a third of the mesenteric lymph node CD4+ cells from C. parvum-infected animals also expressed CD154, whether these cells came from CD40-positive or -knockout mice. Studies with CFSE-labeled cells go further in showing that CD4 T cells divide in CD40−/− adoptive hosts when these are C. parvum infected. This result is consistent with the recent report of Howland et al. that CD4 T cells expressing the DO11.10 receptor for ovalbumin, but with disrupted CD154, divide when stimulated by ovalbumin—even though this response is not as well sustained as that by cells with intact CD154 (21). These results, and our own using CFSE labeling, are important because they suggest that the absence of a potential afferent signal through CD154 (7) does not limit T-cell responses in CD40−/− mice. Even IFN-γ production in response to intracellular pathogens can proceed when CD40-CD154 signaling is blocked (34), provided that the B7-CD28 pathway is intact. A recent report draws attention to TRANCE-TRANCE R signaling as a major pathway in the production of IFN-γ by activated T cells (2). Taken together, these data argue against the view that the principal role for CD40 in the response to C. parvum is for T-cell activation and subsequent IFN-γ production.
While the findings reported here do not define the contribution that CD40 makes to immunity to C. parvum, they do narrow the range of possibilities. The finding that educated CD40−/− T cells could not eliminate C. parvum from CD40−/− knockout mice suggests that CD40 acts to mediate an effector function that is not subserved by T cells and epithelial cells alone. Studies with other apicomplexans, such as Toxoplasma sp., implicate nitric oxide as an essential mediator of immunity, and nitric oxide production by mononuclear phagocytes is known to be stimulated through CD40. Even though nitric oxide would be a plausible candidate for the essential CD40-ligation-dependent mediator in C. parvum clearance, we show elsewhere that inducible nitric oxide synthetase and Fas/Fas ligand expression are not required to clear a C. parvum infection (16). Dendritic cells in the intestinal lamina propria are CD40+, and both their survival (8) and function (3) are affected by CD40 signaling. Mononuclear phagocytes of the dendritic cell and macrophage series have an important effector function against other intracellular pathogens, and in the human lamina propria, they are responsible for scavenging apoptotic epithelial cells (4). Our present data argue for a further study of their interactions with C. parvum-infected epithelial cells.
ACKNOWLEDGMENTS
We thank Gordon Macpherson, Ronald Gill, and Charles Dinarello for helpful discussions.
This research was supported by grants from the March of Dimes (6-FY99-427) and from the National Institutes of Health (AI40870).
REFERENCES
- 1.Aguirre S A, Mason P H, Perryman L E. Susceptibility of major histocompatibility complex (MHC) class I- and MHC class II-deficient mice to Cryptosporidium parvum infection. Infect Immun. 1994;62:697–699. doi: 10.1128/iai.62.2.697-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann M F, Wong B R, Josien R, Steinman R M, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Barkla D H, Gibson P R. The fate of epithelial cells in the human large intestine. Pathology. 1999;31:230–238. doi: 10.1080/003130299105043. [DOI] [PubMed] [Google Scholar]
- 5.Blanas E, Davey G M, Carbone F R, Heath W R. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J Immunol. 2000;164:2890–2896. doi: 10.4049/jimmunol.164.6.2890. [DOI] [PubMed] [Google Scholar]
- 6.Blanshard C, Jackson A M, Shanson D C, Francis N, Gazzard B G. Cryptosporidiosis in HIV-seropositive patients. Q J Med. 1992;85:813–823. [PubMed] [Google Scholar]
- 7.Brenner B, Koppenhoeffer U, Lepple-Wienhaues A, Grassme H, Muller C, Speer C-P, Lang F, Gulbins E. The CD40 ligand directly activates T-lymphocytes via tyrosine phosphorylation dependent PKC activation. Biochem Biophys Res Commun. 1997;239:11–17. doi: 10.1006/bbrc.1997.7415. [DOI] [PubMed] [Google Scholar]
- 8.Caux C, Vanbervliet B, Massactier C, Azuma M, Okumura K, Lanier L, Bancherau J. Activation of human dendritic cells through CD40 crosslinking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward A R. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 12.Fries K M, Sempowski G D, Gaspari A A, Blieden T, Looney R J, Phipps R P. CD40 expression by human fibroblasts. Clin Immunol Immunopathol. 1995;77:42–51. doi: 10.1016/0090-1229(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 13.Gao J-X, Madrenas J, Zeng W, Cameron M J, Zhang Z, Wang J-J, Zhong R, Grant D. CD40-deficient dendritic cells producing interleukin-10, but not interleukin-12, induce T cell hyporesponsiveness in vitro and prevent acute allograft rejection. Immunology. 1999;98:159–170. doi: 10.1046/j.1365-2567.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal I S, Xu J, Flavell R A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 15.Hammerling G J, Vogt A B, Kropshofer H. Antigen processing and presentation—towards the millennium. Immunol Rev. 1999;172:5–9. doi: 10.1111/j.1600-065x.1999.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 16.Hayward A R, Chmura K, Cosyns M. Interferon-γ required for innate immunity to Cryptosporidium parvum in mice. J Infect Dis. 2000;182:1001–1004. doi: 10.1086/315802. [DOI] [PubMed] [Google Scholar]
- 17.Hayward A R, Levy L, Facchetti F, Notarangelo L, Ochs H D, Etzioni A, Weinberg A. Cholangiopathy and tumors of the pancreas, liver and biliary tree in boys with X-linked immunodeficiency with hyper-IgM (XHIM) J Immunol. 1995;158:977–983. [PubMed] [Google Scholar]
- 18.Hershberg R M, Mayer L F. Antigen processing and presentation by intestinal epithelial cells—polarity and complexity. Immunol Today. 2000;21:123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 19.Hershberg R M, Framson P E, Cho D H, Lee L Y, Kovats S, Beitz J, Blum J S, Nepom G T. Intestinal epithelial cells utilize two distinct pathways for HLA class II antigen processing. J Clin Investig. 1997;100:204–215. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med. 1996;183:159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howland K C, Ausubel L J, London C A, Abbas A K. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 22.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukin K, Cosyns M, Mitchell T, Saffry M, Hayward A. Eradication of Cryptosporidium parvum infection by mice with ovalbumin-specific T cells. Infect Immun. 2000;68:2663–2670. doi: 10.1128/iai.68.5.2663-2670.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcial M A, Madura J L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90:583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- 25.Marovich M A, Dowell C D, Thomas E K, Nutman T B. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand dependent process. J Immunol. 2000;164:5858–5863. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 26.McDonald V, Bancroft G J. Mechanisms of innate and acquired resistance to Cryptosporidium parvum infection in SCID mice. Parasite Immunol. 1994;16:315–320. doi: 10.1111/j.1365-3024.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 27.Pasquali P, Fayer R, Almeria S, Trout J, Polidori G A, Gasbarre L C. Lymphocyte dynamic patterns in cattle during a primary infection with Cryptosporidium parvum. J Parasitol. 1997;83:247–250. [PubMed] [Google Scholar]
- 28.Perryman L E, Mason P H, Chrisp C E. Effect of spleen cell populations on resolution of Cryptosporidium parvum infection in SCID mice. Infect Immun. 1994;62:1474–1477. doi: 10.1128/iai.62.4.1474-1477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soong L, Xu J C, Grewal I S, Kima P, Sun J, Longley B J, Ruddle N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 30.Stephens J, Cosyns M, Jones M, Hayward A. Liver and bile duct pathology following Cryptosporidium parvum infection of immunodeficient mice. Hepatology. 1999;30:27–35. doi: 10.1002/hep.510300138. [DOI] [PubMed] [Google Scholar]
- 31.Subauste C S, Wessendarp M, Sorensen R U, Leiva L E. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–6700. [PubMed] [Google Scholar]
- 32.Theodos C M, Sullivan K L, Griffiths J K, Tzipori S. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect Immun. 1997;65:4761–4769. doi: 10.1128/iai.65.11.4761-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urban J F, Fayer R, Chen S J, Gause W C, Gately M K, Finkelman F D. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J Immunol. 1996;156:263–268. [PubMed] [Google Scholar]
- 34.Villegas E N, Wille U, Craig L, Linsley P S, Rennick D M, Peach R, Hunter C A. Blockade of costimulation prevents infection-induced immunopathology in interleukin-10-deficient mice. Infect Immun. 2000;68:2837–2844. doi: 10.1128/iai.68.5.2837-2844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiley J A, Harmsen A G. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 36.You X, Mead J R. Characterization of experimental Cryptosporidium parvum infection in IFN-gamma knockout mice. Parasitology. 1998;117:525–531. doi: 10.1017/s0031182098003424. [DOI] [PubMed] [Google Scholar]