Abstract
Oxidative stress in skin cells can induce the formation of reactive oxygen species (ROS), which are critical for pathogenic processes such as immunosuppression, inflammation, and skin aging. In this study, we confirmed improvements from gamma-irradiated silk sericin (I-sericin) and gamma-irradiated silk fibroin (I-fibroin) to skin cells damaged by oxidative stress. We found that I-sericin and I-fibroin effectively attenuated oxidative stress-induced ROS generation and decreased oxidative stress-induced inflammatory factors COX-2, iNOS, tumor necrosis factor-α, and interleukin-1β compared to the use of non-irradiated sericin or fibroin. I-sericin and I-fibroin effects were balanced by competition with skin regenerative protein factors reacting to oxidative stress. Taken together, our results indicated that, compared to non-irradiated sericin or fibroin, I-sericin, and I-fibroin had anti-oxidation and anti-inflammation activity and protective effects against skin cell damage from oxidative stress. Therefore, gamma-irradiation may be useful in the development of cosmetics to maintain skin health.
Keywords: Gamma-irradiation, Silk fibroin, Silk sericin, Skin regeneration
INTRODUCTION
Skin primarily serves as a protective barrier against the external environment and is important for regulating moisture and body temperature. The skin consists largely of epidermis, dermis and subcutaneous tissue. Keratinocytes are the most important skin cell, constituting 90% to 95% of epidermal cells. Keratinocytes are stressed by reactive oxygen species (ROS) produced by external stimuli such as environmental toxins, ultraviolet radiation and heat [1,2]. Oxidative stress is involved in the formation of ROS and plays a key role in the pathogenesis of processes such as immunosuppression, skin aging and inflammation [3,4].
Recent evidence indicates that silkworms have anti-oxidant, anti-microbial, anti-cancer, and anti-viral activities [5,6]. Silk, the secreted fiber of silkworms, is a continuous strand of two proteins with greatly different properties, sericin and fibroin. Fibroin functions as a structural component and makes up 70% of the fiber strand weight [7,8]. Fibroin is a natural fibrous protein that is more biocompatible than some biological polymers such as collagen [9]. The second type of silk protein, sericin, is attached to fibroin threads to form the small, closed structure of cocoons [10]. Few studies have evaluated the anti-apoptotic, anti-oxidant, and anti-inflammatory effects of silk sericin [11-13].
Gamma-irradiation is a useful technique for safely storing and controlling the hygiene of food; it does not have detrimental effects on food quality and it reduces microbial load [14]. Several studies have reported on the effects of irradiation processing on total phenolic content and anti-oxidant activity in various plants and foods. Studies have reported that gamma-irradiation enhances anti-oxidant activity [15,16]. Therefore, gamma-irradiation is widely applied to food and used in the pharmaceutical, medical and beauty care industries [17]. However, the effects of gamma-irradiated sericin and fibroin on anti-oxidation, anti-inflammation and skin regeneration are not yet known.
We investigated the anti-oxidant and anti-inflammatory effects of gamma-irradiated sericin and fibroin in H2O2-treated HaCaT skin cells. In addition, we investigated how gamma-irradiated sericin and fibroin changed skin regeneration proteins in these cells.
METHODS
Chemicals
Cell culture medium was from Hyclone (Logan, UT, USA). Primary antibodies against cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) were from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies for pro-collagen type 1 (PCOL1) and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Matrix metalloprotease-1 (MMP-1) and elastin antibodies were from Abcam (Cambridge, MA, USA). Secondary antibodies (anti-rabbit, anti-goat and anti-mouse IgG antibody conjugated with horseradish peroxidase) were from Millipore (Temecula, CA, USA).
Preparation of I-sericin and I-fibroin
Based on the previous results [18], considering solubility (Table 1) and DPPH antioxidant effect (Table 2), sericin and fibroin were dissolved in distilled water to a concentration of 10 mg/ml and then irradiated at 5 kGy in a cobalt-60 irradiator (IR-79; Nordion International Ltd, Ottawa, Ont, Canada). Obtained gamma irradiated sericin and fibroin (I-sericin and I-fibroin) was diluted in distilled water to a concentration of 1.0 mg/ml and then filtered using a 0.2 μl filter and used in all subsequent experiments.
Table 1.
Changes in solubility of I-sericin and I-fibroin solutions under various concentrations
| Concentration (mg/ml) | Gamma-irradiation dose (kGy) | ||
|---|---|---|---|
| 0 | 5 | 10 | |
| 2.5 | 100/100 | 90.5*/98.7* | 87.2*/97.9* |
| 5 | 100/100 | 90.2*/98.8* | 86.9*/98.1* |
| 7.5 | 100/100 | 90.5*/98.5* | 87.2*/97.7* |
| 10 | 99.8/100 | 91.1*/98.4* | 87.0*/97.9* |
| 20 | 99.2/100 | 86.7*/99.1* | 76.8**/98.7* |
Data were expressed as I-sericin/I-fibroin (%). Asterisks mean a significant difference between gamma-irradiation with non-irradiation and each group by Student’s t-test. *p < 0.05, **p < 0.01, compared to non-gamma irradiation.
Table 2.
The effect of DPPH radical scavenging of I-sericin and I-fibroin
| Silk protein | Gamma-irradiation dose (kGy) | ||
|---|---|---|---|
| 0 | 5 | 10 | |
| I-sericin | 34.5 | 46.5* | 52.2* |
| I-fibroin | 8.1 | 83.6* | 79.8* |
Data were expressed as %. Asterisks mean a significant difference between gamma-irradiation with non-irradiation and each group by Student’s t-test. *p < 0.05, compared to non-gamma irradiation.
DPPH radical scavenging activity
The free radical-scavenging activity of sericin and fibroin after gamma-irradiation was measured as described by Furuno et al. [19]: 1 ml of 2 × 10-4 M DPPH solution in 99.5% ethanol was added to 2 ml sample (10 mg/ml) and mixed. After 30 min in the dark at room temperature, absorbance at 517 nm was recorded using a plate reader (BioTek, Winooski, VT, USA).
Cell culture
The human keratinocyte cell line HaCaT (ATCC, Rockville, MD, USA) was maintained in DMEM containing 10% FBS (Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in humid conditions at 37˚C under 5% CO2.
Cell viability assay
Cytotoxicity was measured via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kits (Sigma-Aldrich). Cells were plated at 2 × 104 cells/well on 48-well plates (Nunc, Rochester, NY, USA). After 24-h incubation, cells were treated with sericin and I-sericin (10–100 μg/ml) or fibroin and I-fibroin (10–100 μg/ml) for 24 h. We investigated if 1 h of pretreatment with sericin and I-sericin (10–100 μg/ml) or fibroin and I-fibroin (10–100 μg/ml) affected the viability of HaCaT cells treated with 1 mM H2O2 for 24 h. HaCaT cells were incubated with MTT for 2 h in a 37ºC, 5% CO2 incubator. Absorbance was determined at 540 nm using a microplate reader (PowerWave 2; Bio-Tek Instruments, Winooski, VT, USA).
Intracellular ROS assay
HaCaT cells were seeded in 48-well plates. We investigated if 1 h pretreatment with sericin (100 μg/ml) and I-sericin (25–100 μg/ml) or fibroin (100 μg/ml) and I-fibroin (25–100 μg/ml) affected ROS generation in cells treated with H2O2 (1 mM) for 30 min. After washing with PBS, cells were stained with 10 μM 2´7´-dichlorofluorescin diacetate (DCF-DA) (Invitrogen, Carlsbad, CA, USA) in PBS for 30 min in the dark at 37ºC. Fluorescence was measured with an excitation wavelength of 490 nm and an emission wavelength of 525 nm.
Anti-oxidant assay
HaCaT cells were seeded in 10-cm2dishes and cultured in a 5% CO2 incubator at 37ºC for 24 h. Cells were treated with sericin (100 μg/ml) and I-sericin (25–100 μg/ml) or fibroin (100 μg/ml) and I-fibroin (25–100 μg/ml) and 1 mM H2O2 for 24 h. Lysis buffer was added to homogenize cells and centrifuged. Superoxide dismutase (SOD) and glutathione (GSH) assays were measured using SOD and GSH assay kits (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Inflammatory cytokine analysis
Concentration of interleukin (IL)-1β and tumor necrosis factor (TNF)-α in supernatant samples were measured with ELISA kits (Abcam, London, UK). Cells were plated at 1 × 104 cells/well in 96-well plate. Cell were treated with sericin (100 μg/ml) and I-sericin (100 μg/ml) or fibroin (100 μg/ml) and I-fibroin (100 μg/ml) and 1 mM H2O2 for 24 h. Supernatants were used determine cytokines concentration according to the manufacturer’s instructions.
Western blot
Proteins in cells were subjected to SDS-polyacrylamide gel electrophoresis on 6%–10% gels and electrophoretically transferred to PVDF membranes (BioRad). After blocking in 5% skim milk in PBS and incubated overnight at 4ºC with each primary antibody diluted 1:1,000 in 1% skim milk in PBS. Membranes were incubated with secondary antibody diluted 1:10,000 at room temperature for 1 h. Immunoreactive bands were visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Rockford, IL, USA) on a chemiImager system (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
All sets of experiments were carried out in triplicate, at a minimum. Comparison between among the samples were analyzed using one-way ANOVA and student’s t-test. Data were presented as mean ± SEM. p < 0.05 were considered statistically significant.
RESULTS
I-sericin and I-fibroin prevent H2O2-induced cytotoxicity
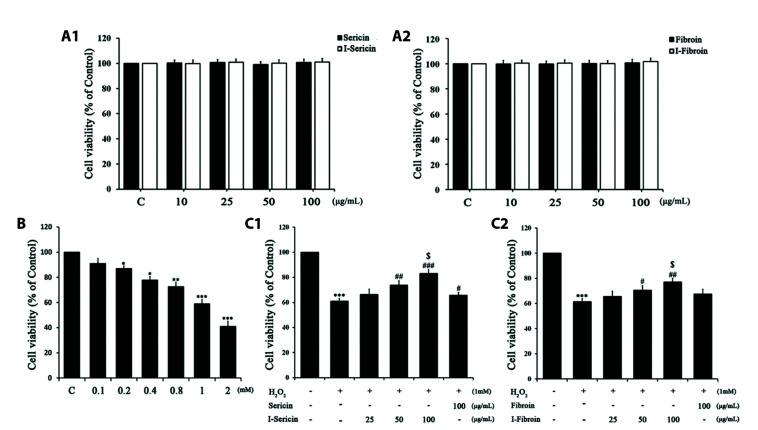
To assess the cytotoxicity and effects of I-sericin and I-fibroin on H2O2-treated HaCaT cells, we performed MTT assays. Cells treated with sericin and I-sericin or fibroin and I-fibroin did not exhibit significant cell death (Fig. 1A). Cell viability decreased by 60% with H2O2 1 mM for 24 h (Fig. 1B). Therefore, 1 mM H2O2 was used in all subsequent experiments. Pretreatment was with sericin and I-sericin or fibroin and I-fibroin for 1 h followed by incubation with 1 mM H2O2 for 24 h. I-sericin and I-fibroin at 50 and 100 μg/ml significantly increased cell viability compared to H2O2 alone. In addition, cells treated with I-sericin or I-fibroin showed higher cell viability than cells treated with sericin or fibroin at the same concentration (100 μg/ml) (Fig. 1C). These results suggested that gamma-irradiated samples gave higher protection against cytotoxicity induced by oxidative stress than non-gamma-irradiated samples.
Fig. 1. Effect of sericin and I-sericin or fibroin and I-fibroin on cell viability in H2O2-induced HaCaT cells.
Cell viability was measured with MTT assays. (A) HaCaT cells treated with indicated concentrations of sericin and I-sericin (10–100 μg/ml) or fibroin and I-fibroin (10–100 μg/ml) for 24 h. (B) HaCaT cells treated with indicated concentrations of H2O2 (0.1–2 mM) for 24 h. (C) HaCaT cells treated with indicated concentrations of sericin and I-sericin or fibroin and I-fibroin (25–100 μg/ml) and 1 mM H2O2 for 24 h. Data are mean ± SEM (n = 3). Different label means a significant difference between each group by one-way ANOVA. *p < 0.05, compared to control. **p < 0.01, compared to control. ***p < 0.001, compared to control. #p < 0.05, compared to H2O2-only group. ##p < 0.01, compared to H2O2-only group. ###p < 0.001, compared to H2O2-only group. $p < 0.05, compared to the sericin- or fibroin-treated group.
I-sericin and I-fibroin inhibit H2O2-induced oxidative stress
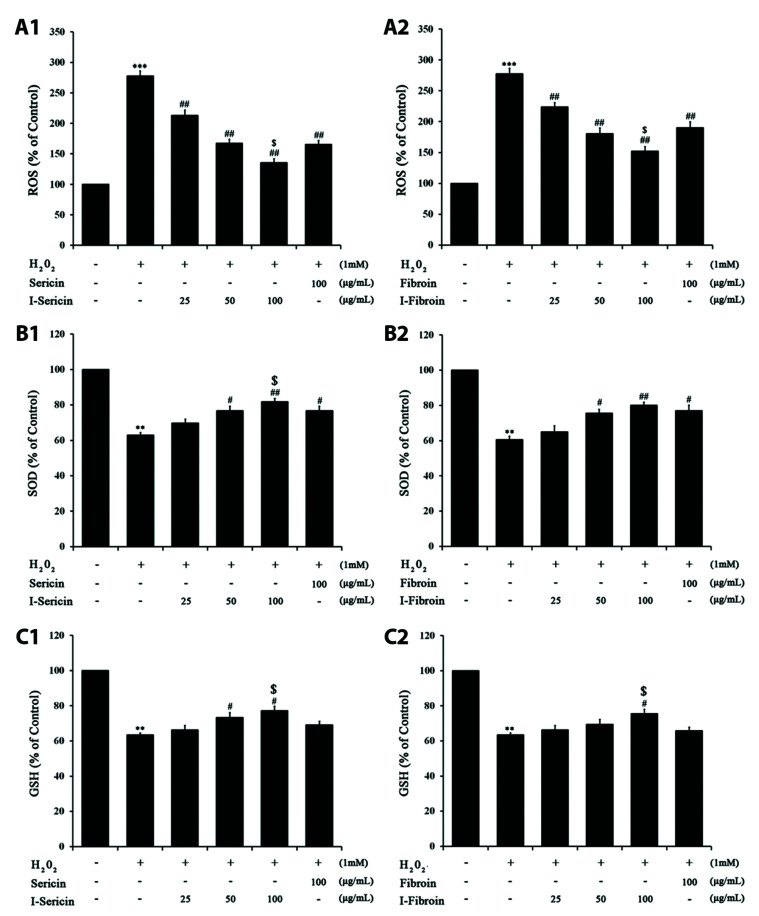
To determine the anti-oxidant ability of I-sericin and I-fibroin, we measured ROS, SOD and GSH in HaCaT cells after treatment with I-sericin or I-fibroin (25, 50, and 100 μg/ml) and 1 mM H2O2. ROS production of H2O2-treated cells was significantly increased compared to the control. I-sericin or I-fibroin treatment was significantly, dose-dependently attenuated compared to treatment with H2O2 alone. I-sericin or I-fibroin treatment resulted in a significant decrease in ROS production compared to treatment with sericin or fibroin at the same concentration (100 μg/ml) (Fig. 2A). SOD and GSH production after treatment with H2O2 alone was significantly attenuated compared to controls. I-sericin or I-fibroin treatment resulted in significant dose-dependent increases in production of these two enzymes compared to H2O2 alone treatment (Fig. 2B). Treatment with I-sericin or I-fibroin resulted in significantly increased GSH production compared to sericin or fibroin. Based on these results, 100 μg/ml I-sericin and I-fibroin were used in subsequent experiments. The results showed that gamma-irradiated proteins affected anti-oxidants in H2O2-treated HaCaT cells more than non-gamma-irradiated proteins.
Fig. 2. Effects of sericin and I-sericin or fibroin and I-fibroin on antioxidant enzymes in H2O2 treated HaCaT cells.
(A) ROS production of HaCaT cell with (A1) sericin or I-sericin and (A2) fibroin or I-fibroin pretreatment followed by H2O2; (B) SOD activity of HaCaT cell with (B1) sericin or I-sericin and (B2) fibroin or I-fibroin pretreatment followed by H2O2; (C) GSH content of HaCaT cell with (C1) sericin or I-sericin and (C2) fibroin or I-fibroin pretreatment followed by H2O2. ROS, reactive oxygen species; SOD, superoxide dismutase; GSH, glutathione. Different label means a significant difference between each group by one-way ANOVA. **p < 0.01, compared to control. ***p < 0.001, compared to control. #p < 0.05, ##p < 0.01 compared with H2O2-treated only. $p < 0.05, compared to the sericin or fibroin-treated group.
I-sericin and I-fibroin suppress H2O2-induced inflammation
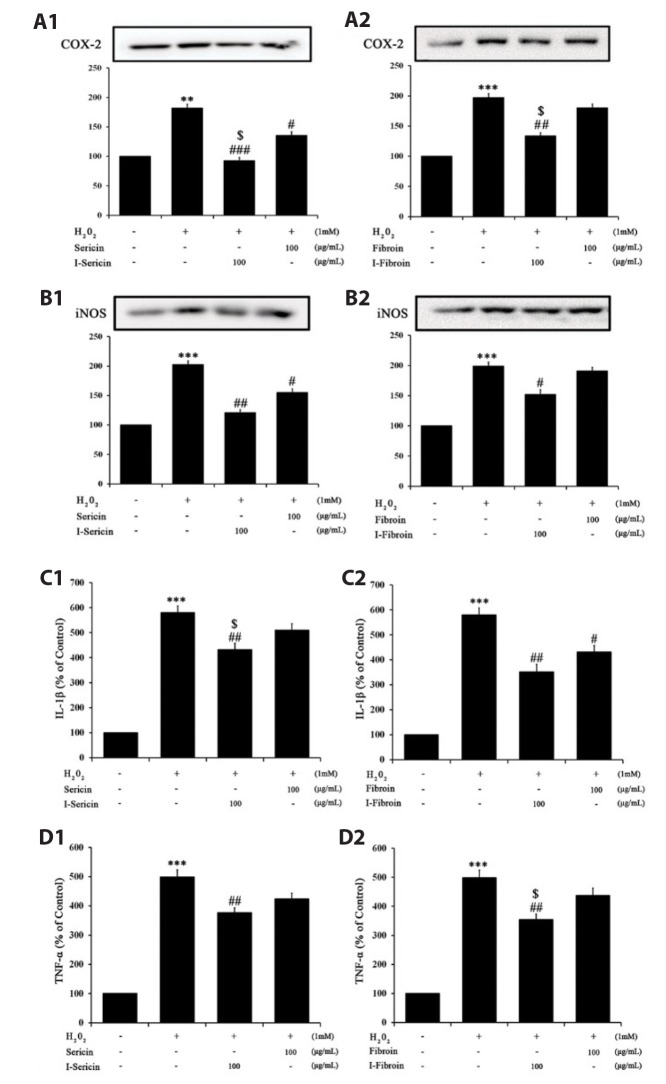
To confirm the anti-inflammation effects of I-sericin and I-fibroin, we measured pro-inflammatory factors in HaCaT cells treated with I-sericin or I-fibroin (100 μg/ml) and 1 mM H2O2. COX-2 and iNOS expression in cells treated with H2O2 alone was significantly increased, whereas cells co-treated with I-sericin or I-fibroin and H2O2 showed significantly attenuated expression of COX-2 and iNOS compared to treatment with H2O2 alone. I-sericin and I-fibroin treatment resulted in significantly attenuated COX-2 expression compared to sericin or fibroin (Fig. 3A, B). IL-1β and TNF-α generation in cells treated with H2O2 alone was significantly increased and cells co-treated with I-sericin or I-fibroin and H2O2 showed significantly decreased generation of IL-1β and TNF-α compared to cells treated with H2O2 alone (Fig. 3C, D). These results suggested that gamma-irradiated proteins had anti-inflammation effects in H2O2-treated HaCaT cells.
Fig. 3. Effects of sericin and I-sericin or fibroin and I-fibroin on inflammatory markers in H2O2-treated HaCaT cells.
(A) COX-2 expression of HaCaT cell with (A1) sericin or I-sericin and (A2) fibroin or I-fibroin pretreatment followed by H2O2; (B) iNOS expression of HaCaT cell with (B1) sericin or I-sericin and (B2) fibroin or I-fibroin pretreatment followed by H2O2; (C) IL-1β concentration of of HaCaT cell with (C1) sericin or I-sericin and (C2) fibroin or I-fibroin pretreatment followed by H2O2; (D) TNF-α concentration of HaCaT cell with (D1) sericin or I-sericin and (D2) fibroin or I-fibroin pretreatment followed by H2O2. COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; IL, interleukin; TNF, tumor necrosis factor. Different label means a significant difference between each group by one-way ANOVA. **p < 0.01, and ***p < 0.005 compared to control. #p < 0.05, ##p < 0.01, and ###p < 0.005 compared with H2O2-treated only. $p < 0.05, compared to the sericin or fibroin-treated group.
I-sericin and I-fibroin induce skin regeneration under oxidative stress
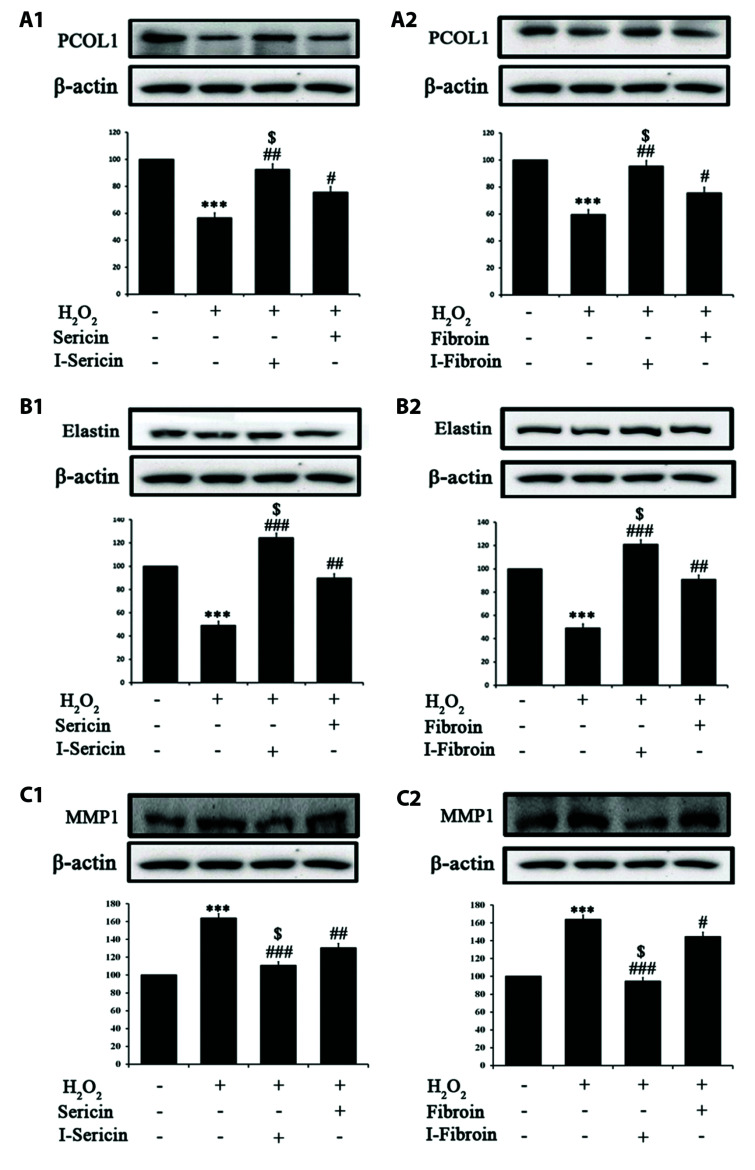
Collagen, the main structural protein of the dermis, provides strength and elasticity to the skin. Collagen is maintained in skin cells by a balance of PCOL1, a synthetic enzyme and MMP-1, a catabolic enzyme. The imbalance of MMP-1 and PCOL1 reduces collagen and elastin production and reduces skin regeneration [20,21]. We determined skin regeneration effects of I-sericin or I-fibroin (100 μg/ml) using MMP-1, PCOL1 and elastin proteins in HaCaT cells treated with 1 mM H2O2. PCOL1 and elastin expression in cells treated with H2O2 alone was significantly attenuated. Cells co-treated with I-sericin or I-fibroin and H2O2 showed significantly increased expression of PCOL1 and elastin compared to cells treated with H2O2 alone. I-sericin and I-fibroin treatment resulted in significantly increased PCOL1 and elastin expression compared to treatment with sericin or fibroin (Fig. 4A, B). MMP-1 expression in cells treated with H2O2 alone was significantly increased. Cells co-treated with I-sericin or I-fibroin and H2O2 showed significantly decreased expression of MMP-1 compared to cells treated with H2O2 alone. When compared to cells treated with sericin or fibroin, cells treated with I-sericin and I-fibroin showed significantly decreased MMP1 expression (Fig. 4C). These results showed that gamma-irradiated samples had a higher regenerative effect on skin damage due to oxidative stress than non-gamma-irradiated samples.
Fig. 4. Effect of of sericin and I-sericin or fibroin and I-fibroin on (A) procollagen type (PCOL1), (B) Elastin, and (C) MMP1 protein expression in in H2O2-treated HaCaT cells.
(A) PCOL1 expression of HaCaT cell with (A1) sericin or I-sericin and (A2) fibroin or I-fibroin pretreatment followed by H2O2; (B) Elastin expression of HaCaT cell with (B1) sericin or I-sericin and (B2) fibroin or I-fibroin pretreatment followed by H2O2; (C) MMP1 expression of of HaCaT cell with (C1) sericin or I-sericin and (C2) fibroin or I-fibroin pretreatment followed by H2O2. Different label means a significant difference between each group by one-way ANOVA. ***p < 0.005 compared to control. #p < 0.05, ##p < 0.01, and ###p < 0.005 compared with H2O2-treated only. $p < 0.05, compared to the sericin or fibroin-treated group.
DISCUSSION
ROS are highly active intermediates of oxygen molecules that diminish incompletely during breathing. ROS produced by oxidative damage induce processes linked to skin aging, increasing wrinkle formation and decreasing skin regeneration. In addition, excessive accumulation of ROS is implicated in skin disease and inflammation [22,23].
Gamma-irradiation is important in the medical and beauty care industries. It is a useful technology for the safe storage of food and food hygiene management [17]. In addition, gamma-irradiation reduces toxic substances such as biogenic amines and N-nitrosamines [24] and changes the structure and physiological characteristics of proteins [25]. Therefore, we investigated whether gamma-irradiation increases physiological activities such as anti-oxidation and anti-inflammatory effects of I-sericin and I-fibroin and affects skin regeneration.
We assessed the effect of gamma-irradiation on the skin cell-protective effects of I-sericin and I-fibroin by determining cell viability under oxidative stress. I-sericin and I-fibroin increased cell viability in H2O2-treated HaCaT cells more than non-irradiated sericin and fibroin (Fig. 1B, C). These results showed that gamma-irradiation prevented cytotoxicity from oxidative stress. We examined the effects of gamma-irradiation on the anti-oxidant activity of I-sericin and I-fibroin by measuring ROS, SOD, and GSH under oxidative stress. A balance between ROS production, SOD and GSH were maintained by I-sericin and I-fibroin competing against reactions to H2O2 (Fig. 2). In addition, increased COX-2, iNOS expression and IL-1β and TNF-α production after oxidative stress were reduced by I-sericin and I-fibroin. Interestingly, it reduced ROS production and COX-2 expression and increased GSH expression more than non-irradiated sericin and fibroin (Fig. 3). These results indicated that gamma-irradiation induced anti-oxidation and anti-inflammatory activity.
Collagen is the most important protein in the regulation of aged-skin cells [26]. MMP-1, a collagenase, recognizes substrates through domains such as hemopexin and can degrade fibrillar collagen [20]. We investigated the balance between expression of PCOL1, elastin and MMP1 maintained by I-sericin and I-fibroin competing against protein factors reacting to H2O2. I-sericin and I-fibroin were more effective for expressing skin regeneration-related proteins than non-irradiated sericin and fibroin (Fig. 4). These results indicated that gamma-irradiation promoted skin regeneration.
The results of this study suggested that gamma-irradiation increased physiological activities such as anti-oxidation and anti-inflammation of silk sericin and fibroin for a skin-improving effect. Although further studies are needed on the molecular structure and physiological activity of sericin and fibroin, our results demonstrated the potential of gamma-irradiation for development of beneficial cosmetics to enhance skin health to prevent skin aging.
ACKNOWLEDGEMENTS
None.
Footnotes
FUNDING
This research was supported by “Research Base Construction Fund Support Program” funded by Jeonbuk National University in 2020.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 3.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003;17:663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 4.Svobodová A, Walterová D, Psotová J. Influence of silymarin and its flavonolignans on H2O2-induced oxidative stress in human keratinocytes and mouse fibroblasts. Burns. 2006;32:973–979. doi: 10.1016/j.burns.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Li BC, Zhang SQ, Dan WB, Chen YQ, Cao P. Expression in Escherichia coli and purification of bioactive antibacterial peptide ABP-CM4 from the Chinese silk worm, Bombyx mori. Biotechnol Lett. 2007;29:1031–1036. doi: 10.1007/s10529-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 6.Manosroi A, Boonpisuttinant K, Winitchai S, Manosroi W, Manosroi J. Free radical scavenging and tyrosinase inhibition activity of oils and sericin extracted from Thai native silkworms (Bombyx mori) Pharm Biol. 2010;48:855–860. doi: 10.3109/13880200903300212. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Mora C, Mrowiec A, García-Vizcaíno EM, Alcaraz A, Cenis JL, Nicolás FJ. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS One. 2012;7:e42271. doi: 10.1371/journal.pone.0042271.b530bd87636d4b2eb98669ebc01af49d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meinel L, Kaplan DL. Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev. 2012;64:1111–1122. doi: 10.1016/j.addr.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YQ. Applications of natural silk protein sericin in biomaterials. Biotechnol Adv. 2002;20:91–100. doi: 10.1016/S0734-9750(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 11.Aramwit P, Towiwat P, ichana T., Sr Anti-inflammatory potential of silk sericin. Nat Prod Commun. 2013;8:501–504. doi: 10.1177/1934578X1300800424. [DOI] [PubMed] [Google Scholar]
- 12.Dash R, Acharya C, Bindu PC, Kundu SC. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008;41:236–241. doi: 10.5483/BMBRep.2008.41.3.236. [DOI] [PubMed] [Google Scholar]
- 13.Dash R, Mandal M, Ghosh SK, Kundu SC. Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol Cell Biochem. 2008;311:111–119. doi: 10.1007/s11010-008-9702-z. [DOI] [PubMed] [Google Scholar]
- 14.Leskovac A, Joksic G, Jankovic T, Savikin K, Menkovic N. Radioprotective properties of the phytochemically characterized extracts of Crataegus monogyna, Cornus mas and Gentianella austriaca on human lymphocytes in vitro. Planta Med. 2007;73:1169–1175. doi: 10.1055/s-2007-981586. [DOI] [PubMed] [Google Scholar]
- 15.Harrison K, Were LM. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 2007;102:932–937. doi: 10.1016/j.foodchem.2006.06.034. https://doi.org/10.1016/j.foodchem.2006.06.034. [DOI] [Google Scholar]
- 16.Mali AB, Khedkar K, Lele SS. Effect of gamma irradiation on total phenolic content and in vitro antioxidant activity of pomegranate (Punica granatum L.) peels. Food Nutr Sci. 2011;2:428–433. doi: 10.4236/fns.2011.25060. https://doi.org/10.4236/fns.2011.25060. [DOI] [Google Scholar]
- 17.Pricaz M, Uţă AC. Gamma radiation for improvements in food industry, environmental quality and healthcare. Rom J Biophys. 2015;2:143–162. https://www.rjb.ro/articles/423/2015-2_Pricaz.pdf. [Google Scholar]
- 18.Song IB, Han HJ, Kwon J. Immune-enhancing effects of gamma-irradiated sericin. Food Sci Biotechnol. 2020;29:969–976. doi: 10.1007/s10068-020-00734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuno K, Akasako T, Sugihara N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull. 2002;25:19–23. doi: 10.1248/bpb.25.19. [DOI] [PubMed] [Google Scholar]
- 20.Bae JY, Choi JS, Choi YJ, Shin SY, Kang SW, Han SJ, Kang YH. (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem Toxicol. 2008;46:1298–1307. doi: 10.1016/j.fct.2007.09.112. [DOI] [PubMed] [Google Scholar]
- 21.Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 22.Lu CY, Lee HC, Fahn HJ, Wei YH. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat Res. 1999;423:11–21. doi: 10.1016/S0027-5107(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 23.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 24.Ahn HJ, Kim JH, Kim JK, Kim DH, Yook HS, Byun MW. Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L.) Food Chem. 2005;89:589–597. doi: 10.1016/j.foodchem.2004.03.029. https://doi.org/10.1016/j.foodchem.2004.03.029. [DOI] [Google Scholar]
- 25.Lee SL, Lee MS, Song KB. Effect of gamma-irradiation on the physicochemical properties of gluten films. Food Chem. 2005;92:621–625. doi: 10.1016/j.foodchem.2004.08.023. https://doi.org/10.1016/j.foodchem.2004.08.023. [DOI] [Google Scholar]
- 26.el-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 2002;147:230–243. doi: 10.1046/j.1365-2133.2002.04871.x. [DOI] [PubMed] [Google Scholar]