Abstract
Drought stress is an increasing concern because of climate change and increasing demands on water for agriculture. There are still many unknowns about how plants sense and respond to water limitation, including which genes and cellular mechanisms are impactful for ecology and crop improvement in drought-prone environments. A better understanding of plant drought resistance will require integration of several research disciplines. A common set of parameters to describe plant water status and quantify drought severity can enhance data interpretation and research integration across the research disciplines involved in understanding drought resistance and would be especially useful in integrating the flood of genomic data being generated in drought studies. Water potential (ψw) is a physical measure of the free energy status of water that, along with related physiological measurements, allows unambiguous description of plant water status that can apply across various soil types and environmental conditions. ψw and related physiological parameters can be measured with relatively modest investment in equipment and effort. Thus, we propose that increased use of ψw as a fundamental descriptor of plant water status can enhance the insight gained from many drought-related experiments and facilitate data integration and sharing across laboratories and research disciplines.
Drought stress experiments are difficult to conduct and interpret. We highlight how integrating measurements of water status, particularly water potential, with genetic and genomic assays would benefit many types of plant stress research.
Introduction
Drought is a topic of strong interest across plant biology from agronomy to ecology and to molecular and cell biology. The prospect that climate change will cause more frequent drought episodes will only add to this interest in the coming years. From an agronomic perspective, the goal of many studies is to identify and study factors that influence crop yield and how these factors are modified by drought. From the ecological perspective, there are strong interests to understand how plants adapt to water-limited environments and how that adaptation scales to community and ecosystem function. Molecular genetics research aims to find the genes and cellular mechanisms plants use to detect and respond to drought stress and thereby develop fundamental knowledge of plant function. A common thread of these types of plant drought research is that they seek to understand the genotype by environment interactions that determine the distribution and productivity of different plant species. All these research fields also have a strong interest in being able to make predictions and interventions: predictions of how climate change will alter ecosystems or crop productivity; and, interventions, such as genetic modification of crop plants or ecosystem management strategies, that can mitigate the effects of drought.
These objectives require scaling across different disciplines and types of experiments. Multiple types of scientists need to be able to compare their studies of drought stress and learn from each other’s results. This in turn requires a common terminology and core methods to quantitate the severity of drought stress and report the most relevant plant responses. Then researchers can get the most insights from their data and have confidence in comparing the results of different studies. But, what are the best methods to quantify drought stress severity? Salt stress can be reported as salt concentration or changes in the conductivity of soil water. Temperature stresses (freezing, chilling, and heat stress) can be standardized based on the timing and extent of the temperature change (and whether ice nucleation is provided in the case of freezing) (Verslues et al., 2006). In this article, we describe equivalent measures for “drought” stress and show how incorporating such measurements can increase the insight gained from many types of experiments, including ‘omics approaches, and also allow us to avoid common pitfalls in data interpretation.
The above sentence refers to “drought” in quotations because some readers may point out, correctly, that the core definition of drought is meteorological (a period of below-average precipitation). However, for much of plant biology, and the majority of papers in this journal, the term drought is instead used to refer to the plant’s relationship with water (i.e. plant water status). Thus, “drought” responses as commonly discussed in most molecular and cellular studies are really responses to an altered water status where water has become less available to the plant because it is at a lower free energy state compared with unstressed conditions. For plant biology, the energy state of water can be quantified and reported in terms of the water potential (ψw).
ψw and water movement through the SPAC: A brief primer on plant water relations
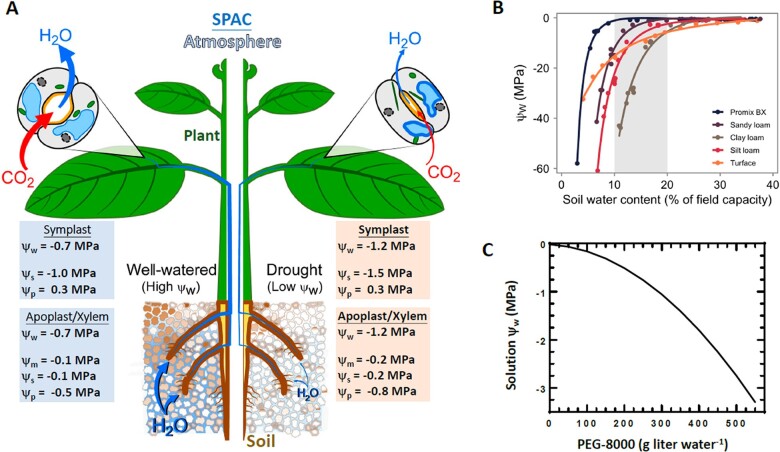
Land plants move copious quantities of water through the soil–plant–atmosphere continuum (SPAC) whereby water taken up from the soil moves upward through the plant vascular system (Figure 1A). Most of this water is lost from the plant by transpiration through stomata. The movement of water through the SPAC is driven by differences in ψw. The ψw is defined as the amount by which the chemical potential of water is reduced below that of pure water (pure water being zero, thus ψw is always negative) and is expressed in units of pressure (Kramer and Boyer, 1995). A higher concentration of dissolved solutes will decrease osmotic potential (ψs) to lower (more negative) values. Adhesion of water molecules to surfaces also decreases their free energy; this is referred to as the matric potential (ψm) and is an important determinant of soil ψw. However, as most techniques for measuring ψw cannot separate the effects of solutes versus adherence of water molecules along surfaces (e.g. the surface of soil particles), ψm is often considered to be part of ψs for practical purposes. A positive pressure (ψp), in turgid cells, for example, will increase ψw (make it less negative) while a negative pressure (e.g. in xylem as water is pulled up by transpiration) will reduce ψw. A higher position in gravity (ψg) will also make ψw more negative; but, the effect of gravity is negligible for those not studying large trees and is also typically omitted for practical purposes. Thus, for purposes of most plant biology research, ψw is essentially determined by two major components, osmotic potential ψs (which is denoted as π in some cases, also referred to as “solute potential”) and ψp. Thus, the full set of ψw components (ψw = ψs + ψm + ψp + ψg) can be reduced to the basic ψw equation of ψw = ψs + ψp (Kramer and Boyer, 1995).
Figure 1.
Water movement through the SPAC and water status of soils, solutions and plant tissue can be described in a unified manner by ψw. A, Diagram of water movement through the SPAC. A continuous column of water exists from the soil, through the root tissue and into the xylem, and into capillary spaces around leaf cells. Evaporation of water from leaf tissue and adhesion of water molecules pulls water up through the SPAC. The rate of water evaporation from leaf tissue is controlled primarily by the opening and closing of stomata. The boxes give typical values of ψw and its components inside plant cells (symplast) or just outside these cells (apoplast/xylem) for well-watered conditions or drought conditions. The low ψw conditions shown would be within the range experienced by crop plants (or other mesophytic plants) and would be at or just above the permanent wilting point (i.e. the ψw below which the plant cannot regain turgor even overnight when stomata close and transpiration is minimal) for most such species (depending on light, temperature and air humidity conditions). B, Example soil moisture retention curves for five different soils highlighting the potential variability in soil ψw (x-axis; ΨW) at various soil–water contents (y-axis; expressed as a percentage of field capacity). The banded area (10–20% of field capacity) highlights the range of soil–water content typically utilized in studies imposing drought stress on plants. Data were collected using the Decagon WP4 Dewpoint Potentiometer. Curves are presented for two common horticultural mixes (Promix BX, Turface) along with several field soils spanning a gradient of texture and composition (sandy loam, clay loam, and silt loam). For plotting purposes, the best fitting model (second-order polynomial of the reciprocal term and soil interaction) was selected by Akaike Information Criterion and showed an adjusted R2 of 0.9893 (P <1e−10) and significant differences among soils. All statistical analyses were performed with the stats package in the R software environment (R Development Core Team). C, Relationship between PEG-8000 concentration and ψw. Note that the values are for a given amount of PEG-8000 added to 1 L of water without adjustment of the final volume (a significant increase in volume will occur for higher PEG concentrations). The ψw of PEG solutions was measured using a Wescor Psypro system with C52 sample chambers following the manufacturer’s instructions and using ψw standards provided by the manufacturer (Verslues, 2010) or by using a Wescor vapor pressure osmometer. For the osmometer, osmolality readings in mmol kg−1 were converted to ψw using the van’t Hoff calculation (Verslues, 2010; Banks and Hirons, 2019). Both instruments gave essentially identical results. Note that because PEG-8000 is a large hygroscopic molecule, which affects ψw primarily by adherence to water molecules, there is a nonlinear relationship between PEG content and ψw, similar to the nonlinear relationship between soil ψw and water content. Data are replotted from Verslues et al. (2006) and from previously unpublished data of the Verslues laboratory.
At different points in the SPAC, the components of ψw differ. In the soil, matric forces of water adhering to soil particles are the dominant component of ψw (except in highly saline soils where dissolved salts also decrease ψs). Inside the plasma membrane of plant cells, the active accumulation of solutes decreases ψs to drive water uptake. This osmotically driven uptake of water produces a positive turgor pressure inside plant cells because they have rigid cell walls which restrict cellular volume. As solutes accumulate, turgor pressure will increase until the cell reaches ψw equilibrium with its immediate surroundings (Figure 1A). In wall-less cells, the accumulation of solutes determines the cell volume. Thus, all cells must osmoregulate (regulate their solute content) at all times, to maintain cell volume or regulate turgor. In the apoplast and xylem, tension (negative pressure) generated by adhesion of water molecules moving up through the plant (and some matric and solute effects) is the major component of ψw. This upward movement of water is driven by water evaporation from capillary surfaces in the inner air spaces of the leaf. As water vapor, even at near saturation humidities, has a much lower ψw than liquid water, evaporation can always occur at appreciable rates. Thus, the rate of water loss from the leaf is controlled primarily by stomata. The regulation of stomatal opening and closing, as well as control of stomatal size and density, is key to balancing the competing priorities of controlling water loss while allowing CO2 uptake.
For readers from a plant physiology background, the above information is well known. For those approaching plant stress biology from other backgrounds who are interested in a more thorough introduction to plant water relations, we recommend the Taiz and Zeiger Plant Physiology textbook (renamed as Plant Physiology and Development for later editions) (Taiz and Zeiger, 2006) as well as the comprehensive book of Kramer and Boyer (1995) or its methods focused companion (Boyer, 1995) or, the following web pages provided by makers of instruments for ψw measurement (Ψw versus water content).
Advantages of monitoring ψw in plant biology research
A key advantage of ψw for plant research, compared with other metrics such as soil water content, is that it allows laboratory or field-based stress treatments to be described in a manner that is unambiguous and thus able to be replicated across experimental systems while also giving a clearer picture of the stress severity experienced by the plant. The different distribution of particle sizes among soil types means that the relationship between soil water content versus ψw varies greatly among soil types (Figure 1B). Clay soils (smaller average particle size) have much higher water content at even very low ψw than sandy soils which have larger average particle size and thus less surface area for water adhesion (Kramer and Boyer, 1995); see also Soil water content versus ψw). Thus, a drought stress severity reported in terms of soil water content could not be reproduced in another laboratory by subjecting plants to the same soil water content unless both laboratories used exactly the same soil. Many soils, including the peat-based horticultural soil mixes often used in plant research, exhibit a dramatic decline in soil ψw once soil water content passes a threshold level (Figure 1B; Walczak et al., 2002; Fields et al., 2014; Dowd et al., 2019). Thus, a seemingly small difference in soil water content between different replicates or different genotypes can actually indicate a substantial difference in stress severity. This is most acute for sandy soils (soils comprised mostly of large particles with lower portion of smaller silt and clay-type particles), as these soils have low water-holding capacity.
In some studies, calculation of the fraction of transpirable soil water (FTSW) has been used to estimate the degree of water limitation experienced by plants. FTSW utilizes soil moisture data along with the threshold soil water content (or threshold ψw) below which the plant under study can no longer extract water (i.e. transpiration decreases to near zero) to calculate the relative amount of plant available water that remains at various bulk soil water contents (Serraj and Sinclair, 1997). Since the threshold for water extraction is determined largely by ψw (along with soil hydraulic conductivity), FTSW is strongly correlated with soil ψw or leaf ψw (see e.g. Lacape et al., 1998; Yan et al., 2017; Devi and Reddy, 2020). FTSW can be useful for field studies and irrigation scheduling; however, for laboratory or greenhouse studies, measurement of ψw still offers a more straightforward measure of stress severity that is physically grounded and not dependent upon the properties of the growth media or properties of the plants under study. Use of ψw also facilitates comparison of results between soil experiments and experiments conducted in other types of media where FTSW is not applicable, such as agar plates or liquid culture. It should also be noted that in studies of plant stress acclimation, it has been observed that many important physiological parameters, such as abscisic acid (ABA) accumulation, proline accumulation, and growth show a linear or near-linear relationship with ψw (see e.g. van der Weele et al., 2000; Verslues and Bray, 2004; Liu et al., 2005) . Use of ψw to scale data is further discussed later in this article. Thus, for genetic studies of drought response, FTSW may be a supplement to, but not a replacement for, measurement of ψw.
For such laboratory- or greenhouse-based studies of controlled soil drying, where the type of growth media used can be deliberately selected, the approach described by Dowd et al. (2019) may be applicable. They analyzed several types of growth media and found dramatic differences in water-holding capacity. They then selected the type of growth media that had a high water-holding capacity over the range of low-to-moderate ψw treatments they wished to impose (−0.25 to −0.4 MPa; the unstressed control was −0.1 MPa). By using this selection of appropriate growth media, along with monitoring of soil weight and maintaining a high humidity around the soil surface, they were able to expose maize seedlings to stable ψw treatments over 9 days to quantify the effects of ψw on maize lateral root development (Dowd et al., 2019). Thus, they could assay effects of reduced ψw on root development that would not be apparent if they used a media with low water holding capacity over the target range of ψw and not apparent if they had simply allowed the soil to dry rapidly over the experimental period.
It is also important to note that declines in soil ψw lead to a dramatic decline in soil hydraulic conductivity (i.e. the water that remains in the soil is more tightly bound to soil particles). This decline in hydraulic conductivity varies greatly among soils and determines how much water the soil can supply to the plant at a given ψw. Soil hydraulic conductivity can be a key factor in determining stomatal opening (and perhaps other drought-responsive traits) as the plant seeks to match the water supply available from the soil with transpirational demand (Carminati and Javaux, 2020).
For other types of experiments, high molecular weight polyethylene glycol (PEG; such as PEG-8000) is a useful agent to impose low ψw upon plants, especially when the PEG is incorporated into a solid matrix such as agar plates (van der Weele et al., 2000; Verslues et al., 2006). High molecular weight PEG is useful because large PEG molecules reduce ψw primarily by matric forces rather than osmotic forces and because large PEG molecules are not able to penetrate the pores of plant cell walls and thus cause cytorrhysis (withdrawal of water from and shrinkage of both cell wall and protoplast) rather than plasmolysis where only the protoplast loses water and may separate from the cell wall (Oertli, 1985). For these reasons, treatment with high molecular weight PEG is more similar to soil drying than osmotic stress imposed with low molecular weight solutes. However, high molecular weight PEG does not behave as an ideal solute and there is a nonlinear relationship between PEG concentration and ψw (Figure 1C) which needs to be accounted for when determining the amount of PEG needed to impose a moderate versus more severe low ψw treatment (van der Weele et al., 2000; Verslues et al., 2006). When using PEG for liquid cultures, it should also be kept in mind that solutions of high molecular weight PEG are viscous and can cause root hypoxia unless the solutions are well aerated (Verslues et al., 1998). At the same time, root damage should be avoided as this may allow PEG molecules to enter the plant tissue. Use of PEG-infused agar plates avoids these problems and is a good experimental system for subjecting small plants/seedlings to a constant and defined severity of low ψw stress (Van der Weele et al., 2000; Verslues et al., 2006) while avoiding complications that may arise from using low molecular weight solutes such as mannitol which, in addition to causing plasmolysis, may elicit specific responses unrelated to changes in ψw (Trontin et al., 2014).
Plants respond to restricted water supply by avoiding water loss and tolerating reduced ψw
The key factor in our plant physiological definition of drought is the decline in external ψw. This is primarily caused by reduced soil water content such that the remaining soil water is held by stronger matric forces. Increased solute content of soil water can also be a factor, as well as a high vapor pressure deficit resulting from drying atmospheric conditions (low humidity and high temperature, perhaps accompanied by rapid air movement) which may cause drying of the plant tissue even when soil moisture is still available. The most basic effect of decreased external ψw is to collapse, or reverse, the ψw gradient that had allowed the plant tissue to take up water. Thus, when the external ψw decreases, ψw of the plant tissue will also decline. If the plant does nothing, this will occur passively by water efflux from cells leading to loss of turgor and decrease in cell volume until equilibrium with external ψw is reached. Because water flux through the SPAC is rapid, the most immediate need of the plant is to stop the bleeding (of water) by closing stomata. Stomatal closure, along with other drought responses that aim to reduce water loss (e.g. rolling or shedding of leaves, thicker cuticle, increased trichome density, or reduced stomatal density on newly formed leaves) are classically referred to as avoidance responses because they aim to avoid depletion of the available water. For mesophytic plants which genetically prioritize high photosynthesis rates and rapid growth (such as most crop plants), or in cases where soil drying is rapid, avoidance is a key component of the drought response. It is also the dominating factor in many laboratory experiments where plants are grown in a small volume of soil such that terminal drying rapidly occurs once water is withheld from the plant. For these reasons, avoidance responses are the aspects of drought response that we are most familiar with at the molecular genetic level. The stomatal closure responses described above are regulated through relatively well-characterized signaling pathways involving ABA and other plant hormones (see e.g. Vaidya et al., 2019; Yang et al., 2019; Berrio et al., 2022).
While avoidance responses are essential to conserve water and may help slow the rate of soil ψw decline, this avoidance of water loss cannot itself restore water uptake and turgor. To do this, the plant must decrease its internal ψw to a value below the external soil ψw by osmotic adjustment, the active accumulation of additional solutes inside cells (Figure 1A). Osmotic adjustment to maintain turgor is a prerequisite for longer term developmental responses such as increasing the root-to-shoot ratio and changing root growth patterns through maintenance of root elongation to reach deeper in the soil profile, hydrotrophic responses, or altered lateral root initiation. Active osmotic adjustment, as opposed to passive increase in solute content by tissue dehydration, is an adaptive tolerance response as it allows the plant to maintain function at reduced ψw. A simple example of osmotic adjustment in Arabidopsis thaliana is shown in Figure 2A where seedlings were transferred to agar plates which had varying amounts of PEG added to generate a range of ψw from mild stress (small decrease of ψw) that had no apparent detrimental effect to severe stress levels. Seedling ψs and relative water content (RWC; water content of the tissue relative to its water content when fully hydrated) were measured three days after transfer (Verslues, 2010). The plants were able to fully osmotically adjust and maintain high RWC after transfer to ψw as low as −1.0 MPa (and because ψs of the plant tissue is less than ψw of the agar media, it can be inferred that turgor was also maintained over this range). The extent of osmotic adjustment, and its role in maintaining high RWC even at substantially reduced ψw, can be easily observed in this experimental system because transpiration is minimal and thus avoidance responses do not dominate the phenotype observations in the way that often occurs in pot-based soil drying experiments.
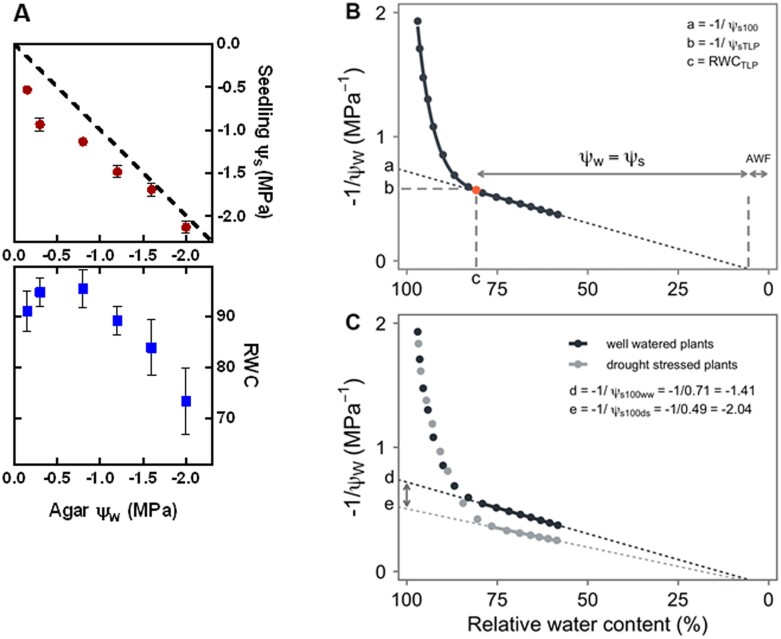
Figure 2.
Quantifying solute content, turgor, and plant tissue water content. A, Seedling osmotic potential (ψs) and RWC of A. thaliana (Col-0 accession) seedlings provide a simple illustration of osmotic adjustment and the TLP concept (replotted from Verslues, 2010). Seven-day-old seedlings (ecotype Col-0) were transferred to PEG-agar plates of the indicated ψw and whole seedlings (10–30 per sample, depending on treatment) collected 3 days after transfer. As transpiration is minimal in this system, one can assume that the ψw of the plant tissue is in near equilibrium with the agar ψw. Thus, a seedling ψs below the dashed line indicates a positive turgor pressure. When osmotic adjustment can no longer maintain turgor (at ∼−1.2 MPa), further declines in seedling ψw occur via water loss (indicated by reduced RWC) and passive concentration of solutes. B, A theoretical PV curve representing the expected relationship between −1/ψw and RWC for a drying leaf. At high RWC, ψw-leaf is a function of both turgor pressure ψp and osmotic potential ψs and exhibits an exponential decline with drying. The TLP (the red dot) occurs when dehydration proceeds until ψp = 0. The linear decline in −1/ψw past TLP is driven by the passive concentration of solutes with water loss. The linear extension of the relationship to the y-axis reveals the osmotic potential at full hydration (a: −1/ψs100). The osmotic potential at zero turgor (b: −1/ψsTLP) can be determined by extending a horizontal line from TLP to the y-axis while the RWC at TLP (c: RWCTLP) can be determined by a perpendicular line from TLP to the x-axis. Symplastic and apoplastic (AWF) fractions can be inferred from the linear intersection of the fit line with the x-axis. The modulus of elasticity (ε) can be inferred from the slope of the ψp from full hydration to the TLP. C, Example PV curves derived from well-water and drought-stressed plants depicting osmotic adjustment and a shift in TLP. The osmotic potential at full turgor for the drought-stressed plant material (e: −1/ψs100ds) can be subtracted from the osmotic potential at full turgor for the well-water watered plant material (d: −1/ψs100ww) to obtain an estimate of osmotic adjustment. C, Redrawn from Sanders and Arndt (2012), adapted by permission from Springer Nature.
While osmotic adjustment is a fundamental aspect of plant responses to low ψw, whether or not increased osmotic adjustment is of value for improving crop productivity during drought has been controversial. It has been argued that at relatively low ψw near the permanent wilting point (around −1.5 MPa for most crop plants), the amount of water that could be extracted by osmotic adjustment is likely too small to affect productivity (Morgan, 1995, 2000; Serraj and Sinclair, 2002). At higher soil ψw, increased osmotic adjustment may lead to more rapid water depletion and thus may not be beneficial under prolonged drought where water conservation and increased water use efficiency (WUE) would be more valuable. However, others have strongly disagreed with this assessment, and have argued that the capacity for osmotic adjustment is associated with crop productivity during drought and that root osmotic adjustment in particular can facilitate further growth to reach water in deeper soil layers (Blum, 2017). It seems likely that whether or not increased osmotic adjustment will allow improved plant productivity depends on the timing and duration of water limitation during the plant life cycle as well as the distribution of water among deep versus shallow soil layers. As we discuss below, a better understanding of how osmotic adjustment is regulated would help both to answer these agronomic questions and to answer fundamental biological questions of how plants detect and respond to changes in water availability.
It must also be kept in mind that plant growth is not determined solely by physical limitations on turgor and water uptake. For example, when Arabidopsis seedlings are exposed to a moderately reduced ψw (−0.7 MPa) on PEG-agar plates we routinely observe that growth (quantified by fresh and dry weight or root elongation) is reduced by two-thirds compared with the unstressed high ψw control (Bhaskara et al., 2017; Longkumer et al., 2022). The data in Figure 2A make it clear that there is no sustained loss of turgor or tissue dehydration at −0.7 MPa that could explain such reduced growth. Rather the growth reduction observed is the result of active growth restriction in response to low ψw (Verslues and Longkumer, 2022). We can also hypothesize from these observations that a moderate low ψw treatment (such as −0.7 MPa, Figure 2A) would be ideal for identifying genotypes that either fail to restrict growth at low ψw, and thus have increased growth maintenance compared with the wild-type, or genotypes that are more sensitive, perhaps because of a failure to osmotically adjust, and thus exhibit more severe dehydration and growth restriction compared with the wild-type. Indeed, our research has identified both negative and positive effectors of growth and osmotic adjustment (Verslues and Bray, 2004; Bhaskara et al., 2012; Longkumer et al., 2022).
A related, but more extensive, method of examining plant water relations often used by ecophysiologists is the generation of pressure–volume (PV) curves (Figure 2B; Koide et al., 1989). In this approach, a sample (typically a leaf, but could be a branch for larger plants or the entire shoot for smaller plants) is detached from the rest of the plant and is subjected to repeated bulk ψw and fresh weight measurements while being allowed to dehydrate. Fully hydrated weight is also measured to allow the RWC of the sample to be calculated at each of the ψw measurement points. This is referred to as a PV curve because traditionally the ψw was measured using a pressure chamber. However, recent refinements have shown that more rapid methods using a vapor pressure osmometer can also be effective (Bartlett et al., 2012a, 2012b; Banks and Hirons, 2019). After performing a series of such measurements, a plot of −1/ψw versus RWC is constructed (Figure 2B). PV curves generally exhibit a steep initial nonlinear decline driven by a rapid drop in turgor (ψp) until at a certain RWC the turgor pressure is lost (turgor loss point [TLP]). The linear decline in ψw past TLP is subsequently driven by the passive concentration of solutes with water loss. During the linear phase of the PV curve, the ψw will equal the osmotic potential (ψw = ψs). Linear extension of this portion of the function can be used to estimate several parameters including the ψs at full hydration (−1/ψs100), ψs at the point of turgor loss (−1/ψsTLP) and RWC at the TLP (RWCTLP) (Figure 2B). Information about cell wall elasticity (ε, modulus of elasticity) can be derived from the slope of ψp between full hydration to the TLP: a steep slope (high ε) results from rigid cell walls while a shallow slope indicates elastic walls (low ε). Finally, estimates of the apoplastic water fraction (AWF) can be derived from the RWC at which ψw approaches infinity. The PV curve highlights how RWC can be difficult to interpret in the absence of other water relations data, especially whether ψw has decreased past the TLP. Without such data, it can be ambiguous whether a decreased RWC is associated with a reduction in turgor; or, whether turgor has already been lost and decreased RWC indicates dehydration of the tissue that is likely to damage cellular structure.
It has been proposed that ψs at the point of turgor loss (ψsTLP) is a key determinant of plant adaptation to water-limited environments, as more negative values of ψsTLP extend the range of ψw over which the leaf can remain turgid and functional (Bartlett et al., 2012a, 2012b). Theoretically, plants may improve their drought tolerance by accumulating intracellular solutes (decrease ψs) to decrease their TLP, decreasing intracellular volume while maintaining a relatively high amount of apoplastic water (increasing AWF), and increasing cell wall flexibility (decreasing ε) so that cell volume can decrease without a loss of turgor. Bartlett et al. (2012a, 2012b) provide a detailed discussion and examples of how various PV parameters may impact TLP. Also, the physiological literature contains reports of the water relations characteristics of most model or crop species. For example, a number of papers have reported water relations parameters for A. thaliana (Des Marais et al., 2012; Scoffoni et al., 2018), information that may be valuable for designing and interpreting Arabidopsis drought stress experiments.
Similar to the ψs and RWC data in Figure 2A, PV curve analysis (Figure 2B) can provide valuable baseline information for experimental design. For example, what is the range of ψw over which a plant is likely to retain the capacity to generate turgor and preserve cellular function? Studies employing ‘omics analyses, or other techniques, to understand how the plant acclimates and continues to function at low ψw would need to collect samples from tissue at ψw above the TLP. Conversely, we may expect that at ψw below the TLP where extensive cell shrinkage and cytorrhysis occur, many changes in gene expression or protein accumulation are likely to be involved in cellular damage control and can be interpreted in that light (Lang et al., 2014). A limitation of PV curve analysis has been that it is laborious and requires specialized pressure chamber equipment, although, as mentioned above, this limitation may no longer apply. Also, because a detached sample is used, PV curve analysis may not capture plastic responses of osmotic adjustment or cell wall properties as the plant acclimates to different moisture conditions over time. In this case, a series of samples would need to be analyzed from plants exposed to different moisture conditions to see if ψs100 or ψsTLP are shifted as the plant acclimates to different environmental conditions (Bartlett et al., 2012a, 2012b). For instance, osmotic adjustment can be calculated with PV curves by subtracting the ψs100 of drought-stressed plants from ψs100 of well-watered plants (Figure 2C).
Knowledge of plant water status can add a new level of insight to many types of physiological and molecular data
Interpretation of experimental data, including ‘omics data and various genetic analyses, can be greatly enhanced by knowledge of plant water status and ψw of the soil or growth media. Perhaps the most fundamental use of ψw and plant water relations data is to allow the experimenter to unambiguously tell the difference between low ψw avoidance versus tolerance. Many molecular genetic studies involve comparison of genetically modified plants (e.g. a mutant or transgenic line) to a wild-type control. A common experimental design is to grow the different genotypes in separate pots and subject them to a fixed duration of water withholding before phenotypic assay (often plant survival after re-watering; Figure 3). In this case, the severity of stress experienced by the plant is not controlled by the experimenter. Rather it is determined by the plant itself through the amount of water removed from the soil by transpiration. Plants that have less water loss through transpiration will deplete the soil water more slowly and thus be exposed to a less severe stress (higher ψw) at the end of the water withholding period than plants with more rapid transpiration. Not surprisingly, the plants that were exposed to a less severe stress (higher ψw) will typically have a higher survival rate at the end of the experiment. In the absence of measurements of soil ψw or water content, it is difficult, or impossible, to conclusively determine whether differences in survival are the result of differences in avoidance of water loss or differences in tolerance-related parameters. While both can be important, the underlying mechanisms are different.
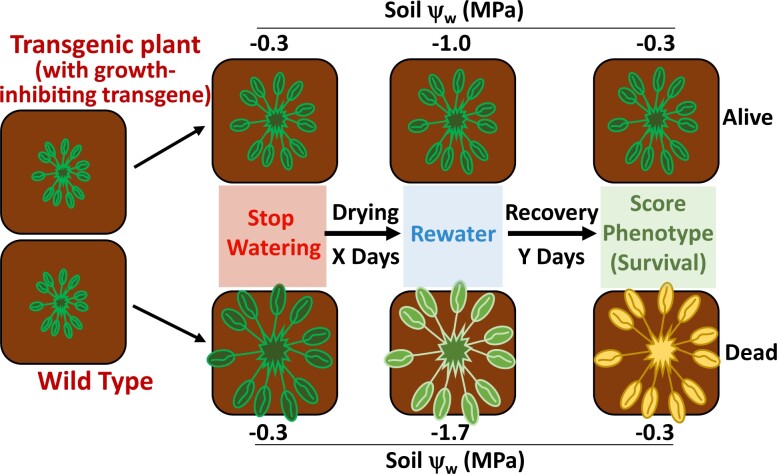
Figure 3.
Potential pitfalls in uncontrolled soil drying experiments. The diagram depicts a scenario in which a reference genotype (wild-type) is compared with another genotype that is similar but has a slower growth rate resulting in a smaller plant size (less leaf area) at the start of the soil drying period (e.g. a mutant or transgenic line that has reduced growth compared with its wild-type background). If each genotype is grown in separate pots and subjected to a set period of soil drying, the smaller genotype will deplete the soil water more slowly by virtue of having less transpiring leaf area. In this case, the smaller genotype will likely have better recovery and less tissue damage after this set period of soil drying; however, this may not indicate a difference in drought resistance as the two genotypes were never exposed to the same severity of stress (the smaller genotype remained at higher ψw). A more robust comparison of drought resistance between these two genotypes could be achieved by modifying the experiment design such that the soil water content (pot weight) is monitored and adjusted through the experiment to ensure that both genotypes experience the same ψw. Alternatively (or in addition), both genotypes can be grown close together in the same symmetrical container such that they fully inter-root and thus experience the same soil ψw regardless of which genotype transpires more rapidly.
A further complication is that mutants or transgenic lines that constitutively grow more slowly, often for reasons unrelated to stress response, can better survive the water withholding period solely by virtue of their relatively small transpiring leaf area. Such a difference is of uncertain value in terms of increasing plant productivity during drought. Similarly, unequal rates of soil water depletion can also complicate phenotypic analysis of genotypes that have altered ABA levels or altered sensitivity to ABA in stomatal closure. In uncontrolled soil drying experiments, these stomatal-dependent differences in soil water depletion will dominate the experimental results. If one wants to examine other, nonstomatal-related effects, steps need to be taken to ensure that all genotypes are exposed to the same soil ψw. Similar concerns exist for comparisons among genotypes with altered stomal size or stomatal density. From a practical point of view for plant improvement, both avoidance of water depletion to conserve soil water and improve WUE (without sacrificing productivity in biomass gain or seed yield) as well as improved response to reduced ψw can all be of value. Which type of response may be most useful for plant improvement is a matter of debate and depends upon the timing, duration, and severity of drought in different environments. If genetic and molecular studies can do a better job of disentangling these two types of drought responses, we can provide more relevant information to develop new germplasm for use by agronomists who study crop productivity in the field.
The limitations of uncontrolled soil drying experiments and endpoint survival measurements have been highlighted in several studies. Skirycz et al. (2011) found that mutants reported to have increased survival after water withholding did not differ from the wild-type in growth responses to water limitation when they were exposed to an equal and moderate severity of soil drying using an automated pot weighing and watering system. Similarly, the dwarf mutant chiquita1-1 (chiq1-1), also referred to as constitutively stressed 1, was originally described as drought “tolerant” based on uncontrolled soil drying survival assays (Bao et al., 2020). Later experiments where soil water content was monitored and controlled found that chiq1-1 had reduced water usage because of its small size but did not differ in tolerance compared with the wild-type when both were exposed to the same severity of soil drying (Ginzburg et al., 2022). Another example of the avoidance of water loss phenomenon is provided in this issue by Wang et al. (2023), who describe a component in ABA signaling, SPIRAL1 (SPR1), that mediates microtubule disassembly during ABA-induced stomatal closure in Arabidopsis. When subjected to water withholding experiments, spr1 mutant plants failed to close their stomata and therefore exhibited significantly greater water loss and lower survivability than the wild-type after a fixed time of water withholding. This indicates that SPR1 primarily affects drought avoidance. Such experiments do not themselves completely rule out additional effects of SPR1 on drought tolerance. However, determining whether or not SPR1 also affects microtubules in other cell types leading to differences in low ψw tolerance would require further experiments where spr1 is exposed to the same external ψw and parameters, such as osmotic adjustment and RWC, and growth maintenance quantified.
Similarly, in cases where transcriptome or proteome data are collected at the end of uncontrolled soil drying experiments, it is difficult (or impossible) to deconvolute the effect of unequal soil drying from true genotype-dependent differences. This pitfall can be avoided by weighing pots and doing a partial re-watering to adjust all genotypes to the same soil water content or by growing the different genotypes together in a container that is sufficiently small and symmetrical to allow the plants to fully inter-root throughout the soil volume and thus be exposed to the same degree of drying even if different plants have differing rates of water usage (Verslues et al., 2006). At the same time, plants must be grown in a sufficient volume of soil to allow adequate root growth and prevent rapid drying that can obscure drought acclimation responses that occur over longer time scales as several days or longer are often needed for differences in growth, metabolism, or proteome remodeling to become apparent. As discussed above, consideration of the water holding capacity of the growth media can help in designing experimental conditions that allow the rate of soil drying and stability of the desired ψw treatment to be optimized (Dowd et al., 2019).
Many molecular stress studies focus on comparing the responses of well-watered control plants with plants that experience a single severity of drought stress. Unfortunately, stress treatments are often poorly controlled and therefore measurements collected from the stressed plants usually exhibit increased variability relative to measurements from control plants. It is simply easier to target a homogenous and benign soil ψw in a control treatment compared with consistently maintaining a specific soil ψw in treatment pots experiencing dynamic drying. This complicates the statistical analyses of stress experiments, often violating assumptions of homoscedasticity, and can reduce the power to observe real treatment impacts when they occur. Moreover, a single stress level may not capture the important range of response to the stress gradient plants would experience in nature. Many eco-physiological studies focus on exploring stress responses across a more dynamic and natural gradient of stress frequency and amplitude (Beier et al., 2012). These types of experiments are important as we anticipate that many stress responses result in nonlinear physiological or performance impacts. Measures of plant water status can also be used to evaluate relationships between physiological responses and the severity of water stress. For example, ψw measurements from leaves during the day can be a strong indicator of plant water stress as a function of soil water availability and atmospheric demand. This is because midday ψw reflects the balance of the amount of water supplied by the root system and transported through the xylem, and the strong demand caused by transpiration. Measures of plant ψw-predawn are also valuable as a picture of water status in the absence of transpiration as stomates are generally closed at night. Predawn leaf ψw is expected to be in equilibrium with the “wettest” soil ψw accessed by roots (Richter, 1997) and should generally reflect the ψw of the soil prospected by the root system, although a number of factors can complicate this relationship (Donovan et al., 2001). Predawn ψw measures can therefore provide a whole-plant metric of the stress severity imposed by declining soil ψw. Moreover, the difference in predawn ψw and ψw measured midday can give insight into the degree of maximum stress that plants experience due to the entire SPAC continuum, including transpiration loss from leaves (Martínez-Vilalta et al., 2014). Thus, measurements of plant ψw can be used to scale data to facilitate comparisons across different types of experiments and experimental conditions including plants grown in growth chambers versus greenhouse or field; or experiments using different types of growing media with differing soil water holding capacity; or comparisons among different species, cultivars, and genotypes.
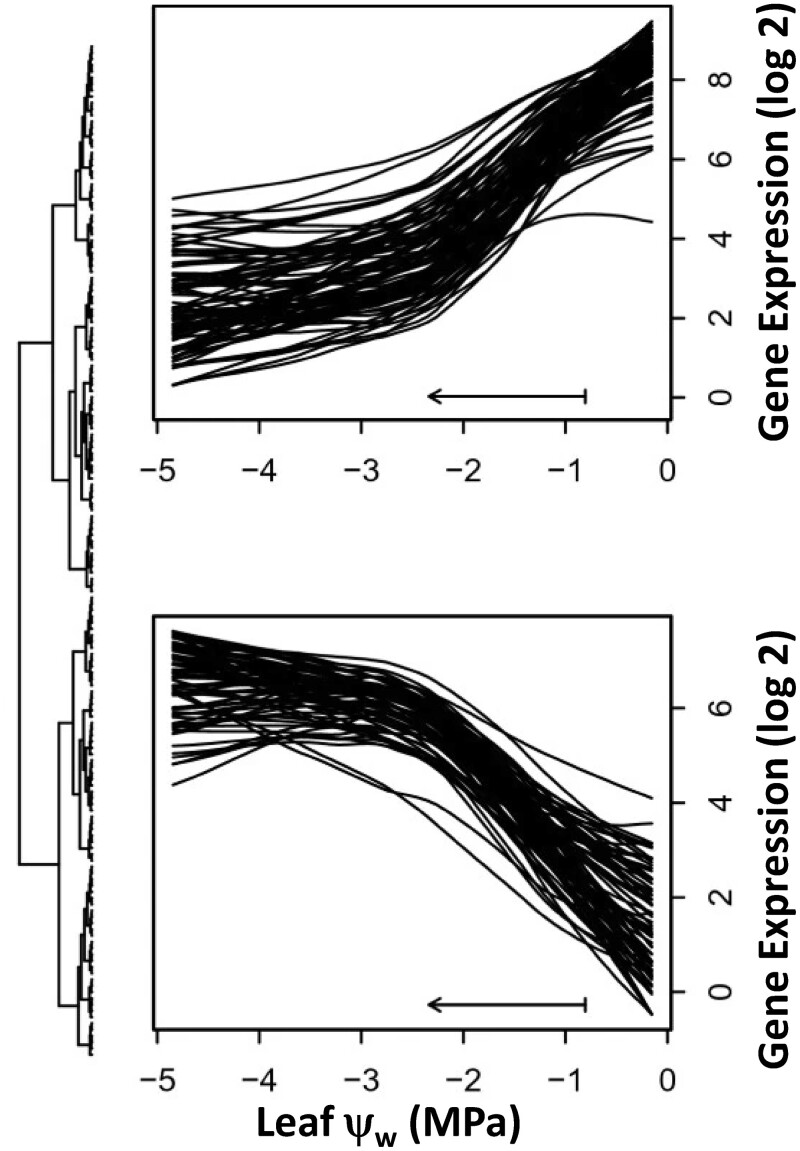
From a biological perspective, using ψw measurements to identify genes that are differentially expressed can be more powerful and may give greater insight than only considering a contrast of a single stress treatment with a control. On a practical level, using actual ψw measurements to scale data can obviate the need to always “hit” a certain target level of stress when imposing the water limitation treatment. For example, Meyer et al. (2014) studied stress responses of switchgrass using a progressive dry-down experiment. The experiment generated predawn ψw measurements ranging from −4.8 to −0.6 MPa in the drought treatment and from −1.5 to −0.2 MPa in the controls. Analyses of covariation revealed nonlinear relationships between gene expression, predawn ψw, and paired physiological traits (Figure 4) suggesting critical thresholds in drought stress responses that are likely associated with turgor loss. Similarly, Lovell et al. (2016) used ψw-predawn and ψw-midday to compare gene expression responses of switchgrass to soil drying in pots, field cylinders, and field rainout shelters to identify a core set of drought-responsive genes. Measuring ψw-predawn not only allowed the three distinct experimental designs to be incorporated into a single analysis framework, it also allowed variability in stress generated from dry down rates (leading to differences in ψw-predawn) to be incorporated in the analysis, thus increasing statistical power. This type of meta-analysis may allow more general discoveries of key biological processes involved in adaptive stress responses and recovery from water deficit.
Figure 4.
Nonlinear relationship between gene expression and predawn leaf ψw in a switchgrass drought experiment. Each line represents paired gene expression and physiological data from a progressive dry-down experiment with switchgrass. The sets of genes correspond to transcripts with significant nonlinear relationships with leaf ψw. The fit lines for two clusters of stress-responsive genes indicate critical thresholds in expression that are likely related to turgor loss. Reprinted from Meyer et al. (2014).
We note that in this case, the use of ψw to scale the data is more robust than using RWC. This is because RWC does not directly measure the severity of the stress but rather represents a composite of the stress severity along with the plant response to stress in avoiding water loss and osmotic adjustment to retain water and turgor. Defining stress severity in terms of ψw allows the severity measurement to be independent of the plant’s stress response so that convolution of severity and response does not hamper data interpretation. That said, RWC measurements can be valuable in experiments conducted below the ψw-TLP as they give an indication of the extent of dehydration and the extent of cellular damage the plant has experienced.
Genetic and genomic analyses are crucial to answer long-standing questions in plant water relations
Despite the well-developed methodology of plant water relations measurements illustrated above, and in contrast to drought avoidance responses, little is known about the genetic and cellular mechanisms that determine the capacity for osmotic adjustment or that determine cell wall properties that influence PV relationships in vegetative tissues (as opposed to guard cells which are distinct and not symplastically connected to other cells). Such mechanisms are not only important for drought research but also are a critical part of cell biology. For example, when external ψw does not change, cellular ψs remains constant even as cells transition from expansion (where rapid solute deposition is needed to drive water uptake) to cell maturation where cell expansion has ceased, and thus intracellular solute and water amounts are constant. The constancy of ψs and turgor during cell expansion and transition to elongation was demonstrated by Sharp et al. (1990) and Spollen and Sharp (1991) who found that even though low ψw increased solute content and decreased turgor overall, there was no change in these parameters as root cells exited the root elongation zone and ceased to expand.
In response to reduced external ψw, the mechanisms that control cellular solute content must be altered to allow more solutes to accumulate. A decrease of cellular ψs by −0.5 MPa, which is within the capability of most plants, requires a 200 mM increase in solute content (assuming they act as ideal solutes). There is information about the regulation of individual proteins potentially related to water status, for example, aquaporins that control membrane water permeability (Ehlert et al., 2009; Sutka et al., 2011; Chaumont and Tyerman, 2014). However, the integrative mechanisms by which these molecular responses are coordinated to couple solute deposition with external ψw and solute dilution by cell expansion to maintain an appropriate ψs and turgor remain unknown. Interpretation of mutant or overexpression phenotypes is sometimes limited because effects on osmotic adjustment and plant water status were inferred rather than directly measured and avoidance versus tolerance effects may be convoluted (Osakabe et al., 2013; Um et al., 2018; Ren et al., 2021). Also, the solutes that accumulate differ between different compartments and these processes must somehow be coordinated (Wilson et al., 2014). This also illustrates how it is perhaps unlikely (although sometimes assumed) that changing the production or transport of a single solute is sufficient to change overall osmotic adjustment and water relations. For example, the stress signaling protein phosphatase highly ABA-induced1 (HAI1) has a greater effect on ψs than the closely related phosphatases HAI2 (also known as AIP1), ABA-insenstive1 (ABI1), or ABI2, even though mutants of all four phosphatases have increased proline accumulation compared with wild-type (Bhaskara et al., 2012). Similarly, cell wall responses to drought that can influence growth and PV relationships are varied and incompletely understood. There has been recent interest in how cell wall integrity affects cellular drought responses (see e.g. Bacete et al., 2022). Despite some recent progress, investigation of the genetic and cellular underpinnings of true tolerance of low ψw remains an underexplored area of drought research.
At least part of the reason for our limited understanding of the cellular basis of water relations and true drought tolerance mechanisms such as osmotic adjustment is that these phenotypes have seldom been the focus of molecular genetic studies. The studies mentioned above all started from the study of specific genes or metabolic pathways which may, or may not, affect core water relations parameters such as osmotic adjustment. Forward genetic or reverse genetic screening for drought tolerance traits has been surprisingly limited. In part, this is due to the difficulty in measuring such traits rapidly enough and in a nondestructive manner as well as by lack of reporters that directly respond to differences in solute content or ψw. New types of sensors and techniques may help alleviate this bottleneck (see below). We anticipate that joining new high-throughput tools for measuring water status and ‘omics responses to stress will drive many new discoveries. For example, Condorelli et al. (2022) recently used genome wide association (GWA) studies of a Durum wheat diversity panel to identify candidate genes underlying osmotic adjustment.
Measurement of plant water status and new methods used to scale up analysis of water status and physiological responses to drought
Plant water relations measurements are sometimes seen as laborious and require specialized equipment only available in a few laboratories. However, basic measurements of soil ψw or plant tissue ψw can be performed using readily available instruments (e.g. the WP4C) or several types of soil probes) for costs that are reasonable compared with the level of investment that is often required for ‘omic analyses whose interpretation would be enhanced by use of soil or plant ψw data. We think that the above examples convincingly show how much additional insight can be gained when water relations measurements are incorporated into the experimental design. Recent advances in techniques and instrumentation have made water relations measurements easier and more accessible. In one example, Sack and co-workers have described how ψsTLP can be determined more rapidly by using a vapor pressure osmometer (such as the widely available Wescor Vapro model) for direct estimation of ψs at full hydration (also referred to as π0; Bartlett et al., 2012a, 2012b; Banks and Hirons, 2019).
Perhaps the biggest single change in instrumentation for drought research is the availability of automated weighing and watering systems. These systems allow individual pots to be weighed and rewatered up to predetermined soil water content to maintain plants under well-watered conditions or under a set severity of soil drying from mild stress to more severe stress. This can allow many plants to be exposed to the same severity of stress. Controlled soil drying can also be scaled to field studies using large soil monoliths and weighing lysimeters that allow gravimetric measures of evapotranspiration of plants from field soil (Schmidt et al., 2013). However, as described above, soil water content is not a parameter that can be used to report and reproduce the level of stress across laboratories because of different soil water holding capacities. Thus, the automated weighing and water approach can be coupled with the generation of soil moisture curves to relate soil water content to ψw for the soil type used (Figure 1B) and also potentially to PV curve analysis (Figure 2, B and C) to determine whether the stress imposed would be expected to push plants past the ψw-TLP. As long as the same soil is consistently used, the soil moisture curves would only need to be generated once and could then be used to calibrate soil water content versus ψw for many subsequent experiments. A similar approach can be taken to incorporate the information in PV curves into the design of high-throughput experiments. This would allow the severity of stress imposed to be selected more precisely and reported in a manner that could be repeated in other laboratories, even if they are using a different type of soil. Thus, measurements of ψw are helpful at the experimental design stage both for reporting stress severity and for choosing the soil water content that imposes the desired severity of stress and also at later stages to improve interpretation of the resulting data.
Typically, automated weighing and watering combined with automated imaging to track plant growth parameters and hyperspectral cameras are increasingly being used to extract more data from such imaging analysis. Interestingly, data from hyperspectral imaging have been used to predict plant water relations parameters once proper calibration models were developed (Cotrozzi et al., 2020). Weighing and watering systems which track the amount of water added to each pot may also be used in calculations of gravimetric WUE, provided that nontranspirational soil drying is minimized. For those researchers without access to automated phenotyping systems, relatively simple procedures such as growing several genotypes together in one pot combined with manual pot weighing and watering along with checks of soil ψw can still allow robust measurements of growth responses to low ψw (see e.g. Bhaskara et al., 2017) and relatively simple procedures are available for medium throughput gravimetric WUE assays (Bhaskara et al., 2022). Agar plates incorporating high molecular weight PEG, when prepared properly, also allow medium throughput analysis of seedling low ψw responses while better mimicking the cytorrhytic type of water loss that plants experience during soil drying (Verslues et al., 2006).
Even more rapid detection of plant ψw, perhaps even rapid enough for evaluations of large plant populations or to enable forward genetic screening for altered water relations, may become possible using new sensing technology. Jain et al. (2021) have described a hydrogel (which they named “AquaDust”) that reports leaf ψw based on changes in Förster Resonance Energy Transfer (FRET) between two fluorophores as the gel expands or contracts due to changes in hydration state. After infiltration into maize leaves, AquaDust FRET emission could be calibrated by using a pressure chamber to impose defined ψw onto the leaf. Postcalibration, AquaDust had sufficient resolution to detect ψw gradients along maize leaves. Cuevas-Velazquez et al. (2021) developed a genetically encoded FRET sensor which may detect osmotic changes inside living cells. They hypothesized that intrinsically disordered proteins, in their case an Arabidopsis late embryogenesis abundant (LEA) protein, may change conformation in response to changes in cellular osmolarity and this conformation change could be reported by the FRET signal between fluorophores attached to each of the protein. Changes in FRET were observed in response to large osmotic shifts in yeast, plant, or mammalian cells, but interestingly not in Arabidopsis which was the source of the LEA protein used to construct the sensor. Further testing and development of this technology will be of interest. In addition, several studies have reported the use of terahertz radiation to analyze tissue water content and construct PV curves (Baldacci et al., 2017; Browne et al., 2020; Li et al., 2020). Further development of all these tools is promising both to enable more extensive field measurements of plant water status and also to facilitate higher throughput laboratory screening.
Conclusions
Plant biology is fundamentally intertwined with the study of plant–water relations, and yet the various fields of plant biology have historically taken disparate approaches to the analysis and reporting of plant water relations. Plant physiologists have traditionally studied parameters defining the relationship between the environment and plant–water status in exhaustive detail, but have yet to uncover many of the molecular or genetic processes that explain the diversity of traits and responses to water-deficit we see in nature. Ecologists often focus on larger temporal and spatial scales, evaluating how precipitation and water availability impact plant population or community dynamics, but these studies are often divorced from the physiological functions driving outcomes. Molecular and cell biologists usually simplify their experimental systems to afford greater control and precision, but in doing so handicap their ability to interpret or understand nature as it actually exists (Bergelson et al., 2021). Each of these perspectives has made valuable contributions to our understanding of plant function. Nevertheless, we argue that an integration of water relations data into cell and molecular studies is needed to truly gain an understanding of plant function and ultimately, to address the many impacts of climate change and ongoing threats to food security.
In this article, we have tried to show that converging on common and fundamentally sound ways of defining and reporting the severity of water limitation is not only advantageous for all types of drought researchers, it is also increasingly possible as water relations measurements continue to be refined and streamlined by new technologies and techniques, while genomic technologies also become ever more widely used. As a baseline for designing drought experiments, we would recommend that experiments seeking to compare the responses of multiple genotypes include sufficient data of plant or soil ψw to determine whether all the genotypes experienced the same decline in ψw during the stress period. This will allow a clear distinction of whether any differences in phenotype can be attributed to altered response to low ψw or altered water use such that some genotypes avoided water depletion and thus were not exposed to the same ψw as other genotypes. Also, as discussed above, using ψw to directly scale data can also enhance data interpretation, especially when combined with knowledge of related parameters such as the TLP. In this case, one can unambiguously determine if a loss of turgor, cellular dehydration, and damage have occurred versus moderate stresses where the plants can successfully acclimate to the reduced ψw and maintain cellular turgor and, at least partially, maintain growth. Applying these distinctions to large transcriptome or proteome data sets will help clarify damage responses versus acclimation responses to reduced ψw. For higher throughput laboratory experiments with model plants such as Arabidopsis, there are well-established protocols for making plates of defined ψw severities that cover the range from mild stress to more severe low ψw (van der Weele et al., 2000; Verslues et al., 2006). As long as the protocols are followed (e.g. do not autoclave high molecular weight PEG), these experimental systems can apply stable ψw treatments (such that repeated checking of media ψw can be minimized) that mimic many of the key aspects of soil drying.
We also recommend a certain degree of circumspection in interpreting laboratory results. Drought is a complex phenomenon and the record of basic research in model organisms having an impact on improving drought resistance of crop plants or understanding the cellular basis for differences in the ecophysiology of drought-prone environments is not especially good. This is not because model organisms commonly studied are somehow flawed or lack drought resistance mechanisms. Rather, a key limitation is how we design experiments using these model organisms and how we interpret the data. There is much to learn as we still do not know either the genes and molecular mechanisms of how plants detect changes in water status, osmoregulate, and control turgor pressure, nor do we know what genetic factors will be most important to improve crop productivity (Nuccio et al., 2018) or understand ecosystem transformations as climate changes (Novick et al., 2022). Knowing your plants’ water status is fundamental to all these efforts.
Acknowledgments
We apologize to the authors of many relevant studies that were not mentioned. Mention of specific instruments or instrument manufacturers does not constitute endorsement over other manufacturers or products. We thank Patrice Salomé (ASPB) for assistance with the artwork in Figure 1A, Ashutosh Tiwari (Academia Sinica) for assistance with the artwork in Figure 3, and Caio G. Pereira (UT Austin) for help with artwork in Figure 2, B and C. We also thank Jason Bonnette for help collecting soil moisture release curve data. Finally, we thank Robert Heckman and Bhaskara Badiger (University of Texas-Austin) and two anonymous reviewers for their useful comments on the manuscript.
Funding
This research was supported by the Office of Science (BER), U.S. Department of Energy, Grant No DE-SC0021126, and by a research grant from the Human Frontier Science Program RGP0011/2019 to T.E.J. and by an Academia Sinica Investigator Award (AS-IA-108-L04) to P.E.V.
Conflict of interest statement. The authors declare no conflicts of interest.
Contributor Information
Thomas E Juenger, Department of Integrative Biology, University of Texas, Austin, Texas 78712, USA.
Paul E Verslues, Institute of Plant and Microbial Biology, Academia Sinica, Taipei 11528, Taiwan.
T.E.J and P.E.V designed the research, analyzed data, and both wrote the manuscript.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) are: Thomas E. Juenger (tjuenger@austin.utexas.edu) and Paul E. Verslues (paulv@gate.sinica.edu.tw).
References
- Bacete L, Schulz J, Engelsdorf T, Bartosova Z, Vaahtera L, Yan G, Gerhold JM, Tichá T, Øvstebø C, Gigli-Bisceglia N, et al. (2022) THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc Natl Acad Sci USA 119: e2119258119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacci L, Pagano M, Masini L, Toncelli A, Carelli G, Storchi P, Tredicucci A (2017) Non-invasive absolute measurement of leaf water content using terahertz quantum cascade lasers. Plant Methods 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JM, , Hirons AD (2019) Alternative methods of estimating the water potential at turgor loss point in Acer genotypes. Plant Methods 15: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Song W-M, Wang P, Yu X, Li B, Jiang C, Shiu S-H, Zhang H, Bassham DC (2020) COST1 regulates autophagy to control plant drought tolerance. Proc Natl Acad Sci USA 117: 7482–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett MK, Scoffoni C, Ardy R, Zhang Y, Sun S, Cao K, Sack L (2012a) Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods Ecol Evol 3: 10–23. [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L (2012b) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett 15: 393–405 [DOI] [PubMed] [Google Scholar]
- Beier C, Bieerkuhnlein C, Wohlgemuth T, Penuelas J, Emmett B, Körner C, de Boeck H, Christensen JH, Leuzinger S, Janssens IA, et al. (2012) Precipitation manipulation experiments—challenges and recommendations for the future. Ecol Lett 15: 899–911 [DOI] [PubMed] [Google Scholar]
- Bergelson J, Kreitman M, Petrov DA, Sanchez A, Tikhonov M (2021) Functional biology in its natural context: a search for emergent simplicity. eLife 10: e67646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrio RT, Nelissen H, Inze D, Dubois M (2022) Increasing yield on dry fields: molecular pathways with growing potential. Plant J 109: 323–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara GB, Nguyen TT, Verslues PE (2012) Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol 160: 379–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara GB, Wen TN, Nguyen TT, Verslues PE (2017) Protein phosphatase 2Cs and microtubule-associated stress protein 1 control microtubule stability, plant growth, and drought response. Plant Cell 29: 169–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara GB, Lasky JR, Razzaque S, Zhang L, Haque T, Bonnette JE, Civelek GZ, Verslues PE, Juenger T (2022) Natural variation identifies new effectors of water use efficiency in Arabidopsis. Proc Natl Acad Sci USA 119: e2205305119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40: 4–10 [DOI] [PubMed] [Google Scholar]
- Boyer JS (1995) Measuring the Water Status of Plants and Soils. Academic Press, San Diego, CA [Google Scholar]
- Browne M, Yardimci NT, Scoffoni C, Jarrahi M, Sack L (2020) Prediction of leaf water potential and relative water content using terahertz radiation spectroscopy. Plant Direct 4: e00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati A, Javaux M (2020) Soil rather than xylem vulnerability controls stomatal response to drought. Trend Plant Sci 25: 868–880 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164: 1600–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli GE, Newcomb M, Groli EL, Maccaferri M, Forestan C, Babaeian E, Tuller M, White JW, Ward R, Mockler T, et al. (2022) Genome wide association study uncovers QTLome for osmotic adjustment and related drought adaptive traits in durum wheat. Genes 13: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrozzi L, Peron R, Tuinstra MR, Mickelbart MV, Couture JJ (2020) Spectral phenotyping of physiological and anatomical leaf traits related with maize water status Plant Physiol 184: 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Velazquez CL, Vellosillo T, Guadalupe K, Schmidt HB, Yu F, Moses D, Brophy JA, Cosio-Acosta D, Das A, Wang LX, et al. (2021) Intrinsically disordered protein biosensor tracks the physical–chemical effects of osmotic stress on cells. Nat Commun 12: 5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DL, McKay JK, Richards J, Sen S, Wayne R, Juenger T (2012) Physiological genomics of responses to soil drying in diverse Arabidopsis accessions. Plant Cell 24: 893–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi MJ, Reddy V (2020) Cotton genotypic variability for transpiration decrease with progressive soil drying. Agronomy-Basel 10: 1290 [Google Scholar]
- Donovan LA, Linton M, Richards JH (2001) Predawn plant water potential does not necessarily equilibriate with soil water potential under well-watered conditions. Oecologia 129: 328–335 [DOI] [PubMed] [Google Scholar]
- Dowd TG, Braun DM, Sharp RE (2019) Maize lateral root developmental plasticity induced by mild water stress. I: genotypic variation across a high-resolution series of water potentials. Plant Cell Environ 42: 2259–2273 [DOI] [PubMed] [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T (2009) Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol 150: 1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JS, Fonteno WC, Jackson BE (2014) Hydrophysical properties, moisture retention, and drainage profiles of wood and traditional components for greenhouse substrates. HortScience 49: 827–832 [Google Scholar]
- Ginzburg DN, Bossi F, Rhee SY (2022) Uncoupling differential water usage from drought resistance in a dwarf Arabidopsis mutant. Plant Physiol 190: 2115–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Liu W, Zhu S, Chang CYY, Melkonian J, Rockwell FE, Pauli D, Sun Y, Zipfel WR, Holbrook NM, et al. (2021) A minimally disruptive method for measuring water potential in planta using hydrogel nanoreporters. Proc Natl Acad Sci USA 118: e2008276118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide RT, Robichaux RH, Morese SR, Smith CM (1989) Plant water status, hydraulic resistance and capacitance. InPearcy RW, Ehleringer JR, Mooney HA, Rundel PW, eds, Plant Physiological Ecology: Field Methods and Instrumentation. Kluwer Dordrecht, The Netherlands, pp 1161–1831 [Google Scholar]
- Kramer PJ, Boyer JS (1995) Water Relations of Plants and Soils. Academic Press, San Diego, CA [Google Scholar]
- Lacape MJ, Wery J, Annerose DJM (1998) Relationships between plant and soil water status in five field-grown cotton (Gossypium hirsutum L.) cultivars. Field Crops Res 57: 29–43 [Google Scholar]
- Lang I, Sassmann S, Schmidt B, Komis G (2014) Plasmolysis: loss of turgor and beyond. Plants 3: 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Lu Y, Peters JMR, Choat B, Lee AJ (2020) Non-invasive measurement of leaf water content and pressure–volume curves using terahertz radiation. Sci Rep 10: 21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F,, Andersen MN,, Jacobsen S-E,, Jensen CR (2005) Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ Exp Bot 54: 33–40 [Google Scholar]
- Longkumer T, Chen CY, Biancucci M, Bhaskara GB, Verslues PE (2022) Spatial differences in stoichiometry of EGR phosphatase and microtubule-associated stress protein 1 control root meristem activity during drought stress. Plant Cell 34: 742–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JT, Shakirov EV, Schwartz X, Lowry DB, Aspinwall MJ, Taylor SH, Bonnette J, Palacio-Mejía JD, Hawkes CV, Fay PA, et al (2016) Promises and challenges of eco-physiological genomics in the field: tests of drought responses in switchgrass. Plant Physiol 172: 734–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Poyatos R, Aguadé D, Retana J, Mencuccini M (2014) A new look at water transport regulation in plants. New Phytol 204: 105–115 [DOI] [PubMed] [Google Scholar]
- Meyer E, Aspinwall MJ, Lowry DB, Palacio-Mejía JD, Logan TL, Fay PA, Juenger TE (2014) Integrating transcriptional, metabolomic, and physiological responses to drought stress and recovery in switchgrass (Panicum virgatum L.). BMC Genomics 15: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JM (1995) Growth and yield of wheat lines with differing osmoregulative capacity at high soil–water deficit in seasons of varying evaporative demand. Field Crop Res 40: 143–152 [Google Scholar]
- Morgan JM (2000) Increases in grain yield of wheat by breeding for an osmoregulation gene: relationship to water supply and evaporative demand. Austral J Agric Res 51: 971–978 [Google Scholar]
- Novick KA, Ficklin DL, Baldocchi D, David KJ, Ghezzehei TA, Konings AG, MacBean N, Raolt N, Scott RL, Shi Y, et al. (2022) Confronting the water potential information gap. Nat Geosci 15: 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio ML, Paul M, Bate NJ, Cohn J, Cutler SR (2018) Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnological effort in crop improvement. Plant Sci 273: 110–119 [DOI] [PubMed] [Google Scholar]
- Oertli JJ (1985) The response of plant cells to different forms of moisture stress. J Plant Physiol 121: 295–300 [Google Scholar]
- Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Ohiraki H, Yamada K, Seo SU, Abo M, et al. (2013) Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25: 609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JH, Yang XX, Ma CY, Wang YL, Zhao J, Kang L (2021) Meta-analysis of the effect of the overexpression of aquaporin family genes on the drought stress response. Plant Biotechnol Rep 15: 139–150 [Google Scholar]
- Richter H (1997) Water relations of plants in the field: some comments on the measurement of selected parameters. J Exp Bot 48: 1–7 [Google Scholar]
- Sanders G, Arndt S (2012) Osmotic adjustment under drought conditions. InAroca R, ed, Plant Responses to Drought Stress. Springer-Verlag, Berlin, Germany, pp. 199–229 [Google Scholar]
- Schmidt R, Pereira F, Oliveira A, Junior J, Vellame L (2013) Design, installation and calibration of a weighing lysimeter for crop evapotranspiration studies. Water Resource Irrigation Manag 2: 77–85 [Google Scholar]
- Scoffoni C, Albuquerque C, Cochard H, Buckley TN, Fletcher LR, Caringella MA, Bartlett M, Broderson CR, Jansen S, McElrone AJ, et al. (2018) The causes of lead hydraulic vulnerability and its influence on gas exchange in Arabidopsis thaliana. Plant Physiol 178: 1584–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R,, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions?. Plant Cell Environ 25: 333–341 [DOI] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR (1997) Variation among soybean cultivars in dinitrogen fixation response to drought. Agron J 89: 963–969 [Google Scholar]
- Sharp RE, Hsiao TC, Silk WK (1990) Growth of the maize primary root at low water potentials. 2. Role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol 93: 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Vandenbroucke K, Clauw P, Maleux K, De Meyer B, Dhondt S, Pucci A, Gonzalez N, Hoeberichts F, Tognetti VB, et al (2011) Survival and growth of Arabidopsis plants given limited water are not equal. Nat Biotechnol 29: 212–214 [DOI] [PubMed] [Google Scholar]
- Spollen WG, Sharp RE (1991) Spatial-distribution of turgor and root-growth at low water potentials. Plant Physiol 96: 438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutka M, Li GW, Boudet J, Boursiac Y, Doumas P, Maurel C (2011) Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol 155: 1264–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2006) Plant Physiology. 4th edn.Sinauer Associates Inc., Sunderland, MA [Google Scholar]
- Trontin C, Kiani S, Corwin JA, Hematy K, Yansouni J, Kliebenstein DJ, Loudet O (2014) A pair of receptor-like kinases is responsible for natural variation in shoot growth response to mannitol treatment in Arabidopsis thaliana. Plant J 78: 121–133 [DOI] [PubMed] [Google Scholar]
- Um TY, Lee S, Kim JK, Jang G, Choi YD (2018) Chloride channel 1 promotes drought tolerance in rice, leading to increased grain yield. Plant Biotechnol Rep 12: 283–293 [Google Scholar]
- Vaidya AS, Helander JDM, Peterson FC, Elzinga D, Dejonghe W, Kaundal A, Park SY, Xing ZN, Mega R, Takeuchi J, et al. (2019) Dynamic control of plant water use using designed ABA receptor agonists. Science 366: 446. [DOI] [PubMed] [Google Scholar]
- van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Verslues PE (2010) Quantification of water stress-induced osmotic adjustment and pro line accumulation for Arabidopsis thaliana molecular genetic studies. InSunkar R, ed, Plant Stress Tolerance: Methods and Protocols. Vol 639. Humana Press, Totowa, NJ, pp 301–315 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu JH, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45: 523–539 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Bray EA (2004) LWR1 and LWR2 are required for osmoregulation and osmotic adjustment in Arabidopsis. Plant Physiol 136: 2831–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Longkumer T (2022) Size and activity of the root meristem: a key for drought resistance and a key model of drought-related signaling. Physiol Plant 174: e13622. [DOI] [PubMed] [Google Scholar]
- Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol 116: 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak R, Rovdan E, Witkowska-Walczak B (2002) Water retention characteristics of peat and sand mixtures. Int Agrophys 16: 161–165 [Google Scholar]
- Wang P, Qi S, Wang S, Dou L, Jia M, Mao T, Guo Y, Wang X (2023) The OPEN STOMATA1–SPIRAL1 module regulates microtubule stability during abscisic acid-induced stomatal closure in Arabidopsis. Plant Cell 35: 260–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Basu MR, Bhaskara GB, Verslues PE, Haswell ES (2014) Plastid osmotic stress activates cellular stress responses in Arabidopsis. Plant Physiol 165: 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Li XN, Liu FL (2017) ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ Exp Bot 139: 99–104 [Google Scholar]
- Yang ZY, Liu JH, Poree F, Schaeufele R, Helmke H, Frackenpohl J, Lehr S, von Koskull-Doring P, Christmann A, Schnyder H, et al. (2019) Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiol 180: 1066–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]