Abstract
Background/Aim: Peritoneal metastasis (PM) of gastric cancer (GC) leads to poor clinical outcomes. Tumor-derived exosomes promote metastasis via communication between tumor cells and host cells. In this study, we investigated the effect of Rab27, which is required for exosome secretion, on the PM of GC.
Materials and Methods: We established a stable knockdown of two Rab27 homologs, Rab27a and Rab27b, in human GC cells (58As9) with a high potential of PM. We examined the level of exosome secretion from Rab27-knockdown 58As9 cells by Western blotting and the ability of Rab27b knockdown to suppress PM in 58As9 cells using a mouse xenograft model. In vitro proliferation and invasion assays were performed in the Rab27b-knockdown cells. Next, Rab27b expression was evaluated in human GC tissues by immunohistochemistry. Finally, we assessed the clinicopathological and prognostic significance of Rab27b expression by RT-qPCR in both our and other TCGA datasets of GC.
Results: Rab27a and Rab27b knockdown in 58As9 cells decreased the secretion of exosomes, characterized by the endocytic marker CD63. Rab27b knockdown decreased PM in vivo without affecting the in vitro proliferation or invasion ability of 58As9 cells. In human GC tissues, Rab27b was overexpressed in tumor cells. The overall and recurrence-free survival rates were significantly lower in GC patients with high compared to low Rab27b mRNA expression in our and other TCGA datasets.
Conclusion: Rab27b expression potentially serves as a poor prognostic biomarker, possibly affecting PM via exosome secretion from GC cells.
Keywords: Exosome, Rab27b, gastric cancer, peritoneal metastasis
Gastric cancer (GC) remains one of the most common causes of cancer-related deaths worldwide (1,2). Peritoneal metastasis (PM) is the most frequent type of GC metastasis or recurrence and is associated with poor prognosis (3). Therefore, developing a novel therapy for PM would improve the clinical outcomes of GC patients.
PM development consists of a highly complex series of mechanisms including cancer cell detachment from the primary tumor, survival in the free abdominal cavity, attachment to distant peritoneal sites, invasion into the subperitoneal space, and proliferation with angiogenesis (4). However, the details of these steps remain largely unknown.
A large body of evidence has accumulated in recent years regarding the association between exosomes and metastasis (5,6). We previously reported that exosomal microRNA-203 is associated with metastasis, possibly by activating tumor-associated macrophages in colorectal cancer (7). Exosomes are small membrane vesicles derived from the luminal membranes of multivesicular endosomes and are constitutively released upon fusion with the cell membrane. Exosomes are secreted from various cell types and mediate the transfer of mRNAs, microRNAs, and proteins to distant tissue cells. Thus, exosomes play important roles in intercellular communication (8,9). In fact, it has been reported that exosomes can convey potential biological information to nearby or distant sites and create a premetastatic niche to establish organotypic metastasis (10-12). For example, pancreatic cancer-derived exosomes can induce a fibrotic environment in the liver and promote liver metastasis from pancreatic cancer (13). In addition, breast cancer-derived exosomes can destroy the vascular endothelial barrier and promote tumor metastasis to the liver, lung, and brain (14). Therefore, exosomes derived from GC cells could play essential roles in the crosstalk between tumor cells and peritoneal host cells, followed by PM development (15,16).
Ras-related proteins in brain (Rabs) are small GTPases that regulate vesicle trafficking and cellular functions such as proliferation (17,18). Rab27, a member of the Rab family, consists of two homologs, Rab27a and Rab27b, and is essential for exosome secretion (14,19,20). Accumulating evidence suggests that Rab27 facilitates tumor progression by increasing exosome secretion and is associated with poor prognosis in some solid cancers (19,21). Thus, we hypothesized that Rab27 contributes to PM via exosome secretion from GC cells. In this study, we examined whether Rab27 affected PM of GC using a mouse xenograft model established with Rab27-knockdown GC cells and observed the clinical significance of Rab27 expression in GC patients.
Materials and Methods
Cell lines and cell culture. The highly disseminated peritoneal human GC cell line (58As9) was provided by Kazuyoshi Yanagihara, National Cancer Center Research Institute, Japan (22,23). Cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS, Life Technologies, Grand Island, NY, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin. Cells were cultured in a humidified 5% CO2 incubator at 37˚C. The cells were homogenized, and the lysates were stored at −80˚C until RNA or protein extraction.
Exosome purification from cultured cell supernatants and transmission electron microscopy. Exosome purification was performed by ultracentrifugation as described previously (24). Briefly, supernatant from 58As9 cell cultures was collected after incubation with exosome-depleted FBS for 3 days. The supernatant was collected and centrifuged at 2,000 g for 10 min at room temperature and at 12,000 g for 30 min, followed by filtration through a 0.22-mm filter to remove cell debris. Then, we purified the exosomes by ultracentrifugation at 100,000 g for 70 min at 4˚C. Exosomes were detected by transmission electron microscopy, as described previously (24), and immunoblotting of exosome marker CD63.
Total RNA extraction and reverse transcription–quantitative polymerase chain reaction (RT-qPCR). Total RNA was extracted from tissues and cell lines using ISOGEN (Nippon Gene, Tokyo, Japan). RT was performed using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. qPCR was performed using the LightCycler 480 SYBR Green I Master Mix (Roche, Basel, Switzerland) as described previously (25). The mRNA levels of Rab27a and Rab27b were normalized to those of GAPDH as an internal control. Gene expression values are presented relative to the level of cDNA derived from Human Universal Reference Total RNA (Takara Bio Inc. Shiga, Japan). The primer sequences used for qPCR were as follows: Rab27a, forward 5’-GTGCCAGCCAAAGACAGCAG-3’ and reverse 5’-TGGCCATTGCACGAGTGAGA-3’; Rab27b, forward 5’-TGGACCCTACTCTGTCTGTGGA-3’ and reverse 5’-CCCACTGCATTACAGCGAGAT-3’; GAPDH, forward 5’-TTGGTATCGTGGAAGGACTCTA-3’ and reverse 5’-TGTCATATTTGGCAGGTT-3’.
Immunohistochemical analysis. Immunohistochemical analysis of Rab27b in GC cases was performed on formalin-fixed, paraffin-embedded surgical sections obtained from patients with GC, as described previously (26). The primary antibody against Rab27b (ab103418, Abcam, Cambridge, UK) was used at a dilution of 1:100. All sections were counterstained with hematoxylin. Histological analysis was performed by an experienced pathologist at Kyushu University Beppu Hospital.
Protein extraction and immunoblotting. For total protein extraction, cells were lysed in lysis buffer [25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.2 mM ethylenediaminetetraacetic acid, 0.1% NP40, 5% glycerol] supplemented with proteinase inhibitor cocktail as described previously (27).
Immunoblotting was performed as described previously (28). Briefly, equal amounts of protein (35 μg) were electrophoresed on 4-20% Tris-glycine gels and then electroblotted onto Immobilon-P transfer membranes (Merck Millipore, Billerica, MA, USA) at 70 V for 4 h at room temperature. Nonspecific binding sites were blocked using blocking buffer (Tris-buffered saline, 0.1% Tween-20, and 5% nonfat milk powder) for 1 h at room temperature, and the blots were incubated with the specific primary antibodies anti-Rab27a (ab55667) and Rab27b (ab103418) (Abcam) at a 1:250 dilution, anti-CD63 antibody (ab134045, Abcam) at a 1:5,000 dilution, and anti-vimentin (ab196602, Abcam) and anti-β-actin antibodies (SC-47778, Santa Cruz Biotechnology, Dallas, TX, USA) at a 1:1000 dilution, in blocking buffer at 4˚C overnight. After washing, the blots were incubated with an appropriate secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. After washing, signals were detected using SuperSignal (Pierce Inc, Rockford, IL, USA).
Construction of Rab27a- and Rab27b-targeted short hairpin (sh) RNA stable expression plasmid vectors. The expression plasmids pcDNA6.2-GW/EmGFP-miR-Rab27a or Rab27b shRNA, containing shRNA sequences targeting the Rab27a or Rab27b gene, and pcDNA6.2-GW/EmGFP-miR-neg, containing an unrelated insert, were constructed using the Block-iT Pol II miR RNAi Expression Vector Kit (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Rab27a and Rab27b shRNA sequences were designed using online software (http://www.invitrogen.com/rnai).
MTT proliferation assay and in vitro invasion assay. In vitro cell proliferation was assessed using an MTT assay kit (Roche Applied Science) according to the manufacturer’s instructions as described previously (29,30).
Cell invasion capacity was assessed using the BD BioCoat Tumor Invasion System, 24 Multiwell (BD Bioscience, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions as described previously (29,30). In brief, cells were placed in the upper chamber, and the lower chamber was filled with 750 μl of RPMI 1640 with 10% FBS as a chemoattractant and incubated in a humidified atmosphere (37˚C and 5% CO2). Invasive cells that migrated through the membrane were evaluated in a fluorescence plate reader at excitation/emission wavelengths of 485/535 nm. Invasiveness was measured as the percentage of fluorescence of an invasive fibrosarcoma cell line (HT-1080) that served as a control.
Xenograft mouse model. Five-week-old female BALB/c nu/nu mice were obtained from Japan SLC, Inc (Hamamatsu, Japan) and maintained under specific pathogen-free conditions. A total of 1×106 cancer cells were injected into the peritoneal cavity of each mouse subcutaneously as described previously (31). Mice were euthanized for analysis at 28 days after injection and PM nodules were examined. The number of metastatic nodules larger than 5 mm in diameter was determined. All animal procedures were performed in compliance with the Guidelines for the Care and Use of Experimental Animals established by the Committee for Animal Experimentation of Kyushu University.
Patients with GC and clinical sample collection. Primary GC samples and paired normal tissues were obtained from 178 patients who underwent surgery at Kyushu University Beppu Hospital and the affiliated hospitals between 1993 and 2010. All patients had a histological diagnosis of GC and were closely followed at 3-month intervals. All patients were treated following the Japanese Society of Cancer of the Stomach Guidelines for the Treatment of Gastric Cancer. Written informed consent was obtained from all patients, and the institutional review board of our university approved this study. All tumor tissues were freshly frozen and stored at –80˚C until RNA extraction, as described previously (32,33). Clinical data on patient age, sex, histology, depth of tumor invasion, lymph node metastasis, lymphatic invasion, venous invasion, peritoneal metastasis, and UICC TNM stage were obtained from medical records. This study was approved by the Ethics and Indications Committee of Kyushu University (#2020-302).
TCGA data analysis. The mRNA expression datasets and survival data from 406 available patients with GC were obtained from TCGA (http://cancergenome.nih.gov/), as described previously (29,34). The TCGA data were normalized by quantile normalization (35).
Statistical analysis. For continuous variables, data are expressed as mean±standard deviation, and statistical analyses were performed using Student’s t-test. Categorical variables were compared using χ2 tests. Overall survival and recurrence-free survival were estimated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Based on the Rab27b mRNA levels in our and TCGA datasets of GC, the cases were divided into two groups using the minimum p-value approach, a comprehensive method used to determine the optimal cutoff points among continuous gene expression measurements (36). The statistical analyses were performed using JMP 16 software (SAS Institute, Cary, NC, USA) and R software version 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria) (29,34). Clinicopathological factors and clinical stages were classified using the 7th edition of the UICC TNM classification (37).
Results
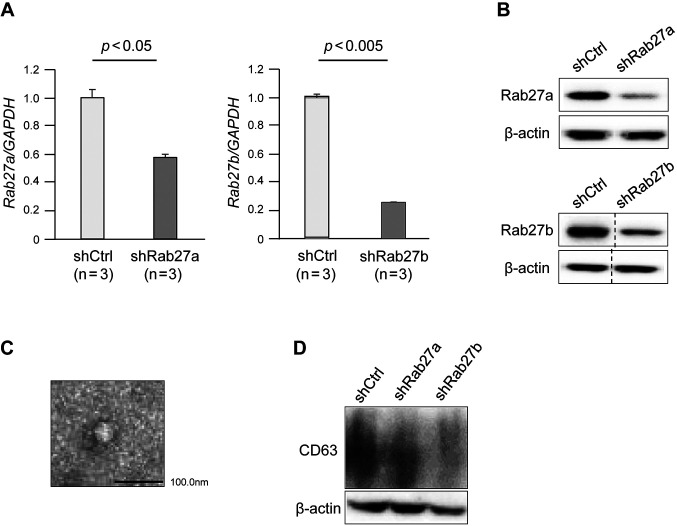
Rab27a and Rab27b were successfully knocked down in GC cells by shRNAs. To examine the functional role of Rab27 in PM of GC, we established stable knockdown of Rab27a or Rab27b in GC cells (58As9) using shRNAs. shRNAs targeting Rab27a or Rab27b induced significant downregulation of the mRNA and protein levels of Rab27a or Rab27b, respectively, in 58As9 cells (Figure 1A and B).
Figure 1. Rab27a and Rab27b knockdown suppressed exosome secretion in gastric cancer cells. (A) RT-qPCR of Rab27a and Rab27b in 58As9 cells transfected with Rab27a or Rab27b shRNAs. (B) Immunoblotting of Rab27a and Rab27b in 58As9 cells transfected with Rab27a or Rab27b shRNAs. (C) Transmission electron microscopy image of exosomes obtained from the cell supernatants. (D) Immunoblotting of CD63 in the culture supernatant from 58As9 cells transfected with Rab27a or Rab27b shRNAs. shCtrl: shControl 58As9 cells; shRab27a: Rab27a shRNA-transfected 58As9 cells; shRab27b: Rab27b shRNA-transfected 58As9 cells.
Rab27a and Rab27b knockdown suppressed exosome secretion from GC cells. We isolated exosomes from the culture supernatant of Rab27a- or Rab27b-knockdown 58As9 cells. Transmission electron microscopy showed that the obtained exosomes had a characteristic ovoid or round shape delimited by a bilayer membrane and had a size distribution of mainly 30-100 nm (Figure 1C). Immunoblotting confirmed lower expression of CD63, an exosomal marker (38), in the Rab27a- or Rab27b-knockdown 58As9 cells compared with control cells, suggesting that the number of exosomes in the culture supernatant was decreased by Rab27a or Rab27b knockdown (Figure 1D).
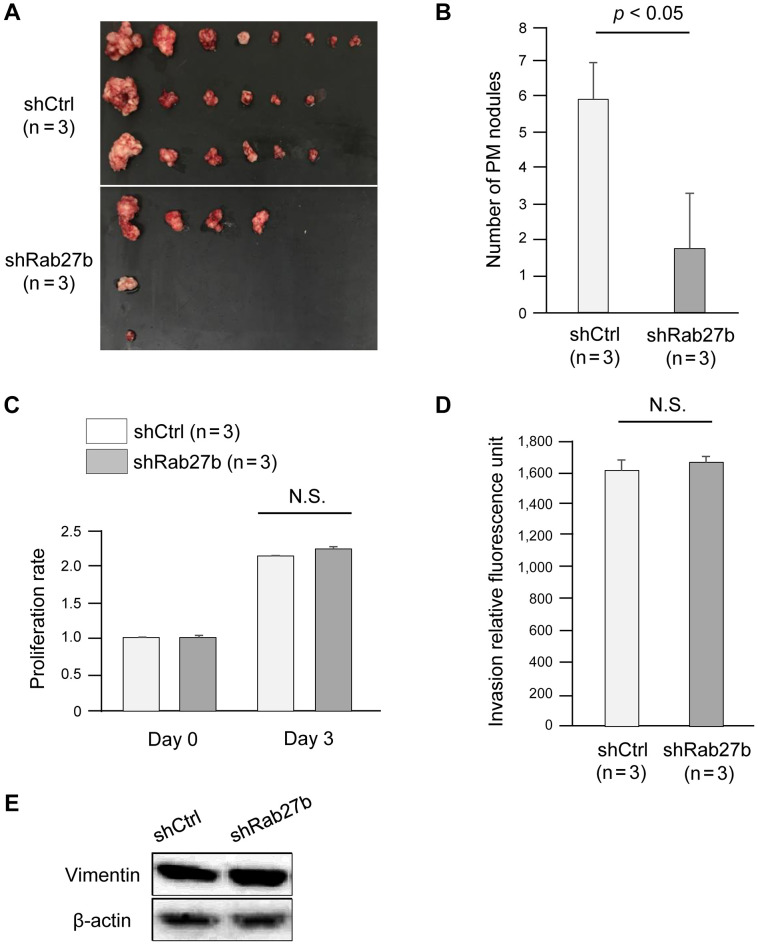
Rab27b knockdown suppressed PM of GC cells in a xenograft mouse model. To evaluate the effects of Rab27 knockdown on PM of GC cells in vivo, we established a xenograft mouse model by subcutaneously injecting Rab27-knockdown 58As9 cells into the peritoneal cavity of nude mice (n=6). We used Rab27b-knockdown 58As9 cells because immunoblotting showed greater downregulation of the exosomal marker CD63 in Rab27b- than Rab27a-knockdown cells (Figure 1D). Figure 2A shows the PM nodules derived from Rab27b-knockdown cells and control cells. As expected, there were significantly fewer PM nodules derived from Rab27b-knockdown cells than control cells (p<0.05, Figure 2B).
Figure 2. Rab27b knockdown suppressed peritoneal metastasis of gastric cancer without affecting the proliferation or invasion ability of tumor cells. (A) Images of peritoneal metastasis nodules derived from Rab27b-knockdown 58As9 cells. (B) The number of peritoneal metastasis nodules derived from Rab27b-knockdown 58As9 cells. shCtrl: shControl 58As9 cells; shRab27b: Rab27b shRNA-transfected 58As9 cells. (C) MTT proliferation assays. The proliferation of Rab27b-knockdown 58As9 cells. (D) In vitro invasion assays. Invasion ability of Rab27b-knockdown 58As9 cells. (E) Immunoblotting of vimentin in Rab27b-knockdown gastric cancer cells. shCtrl: shControl 58As9 cells; shRab27b: Rab27b shRNAtransfected 58As9 cells. N.S.: not significant.
Rab27b knockdown did not affect the proliferation or invasion of GC cells. We examined whether Rab27b affected the proliferation or invasion of 58As9 cells by MTT and in vitro invasion assays, respectively. Notably, Rab27b did not affect proliferation or invasion potential (Figure 2C and D). In addition, there was no difference in the expression of vimentin, an EMT marker, between Rab27b knockdown and control cells (Figure 2E).
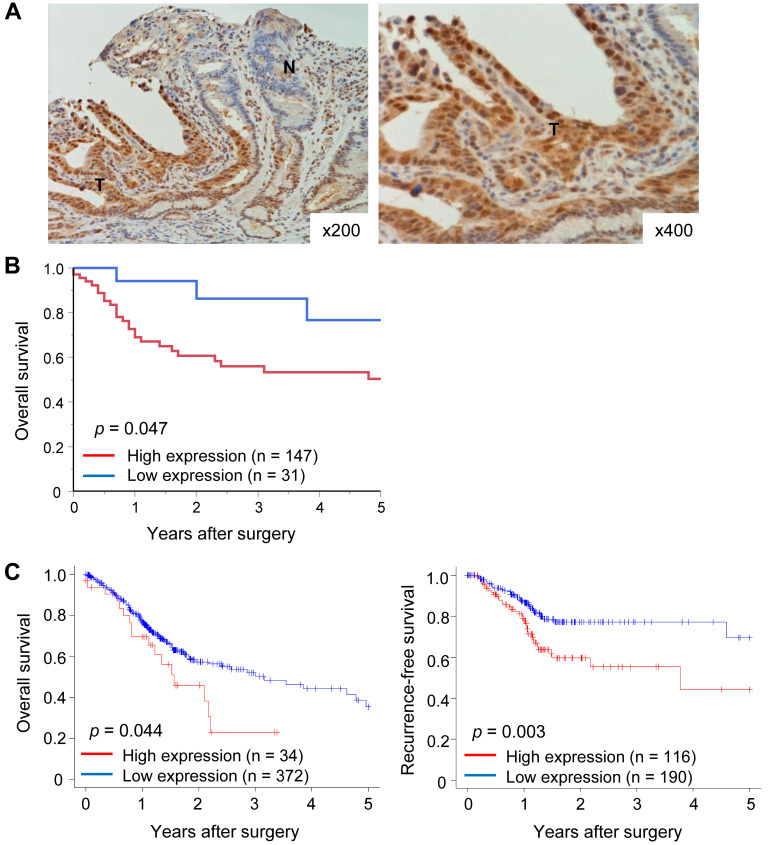
High expression of Rab27b mRNA in tumor tissues predicted a poor prognosis in GC patients. Finally, we assessed the clinical significance of Rab27b mRNA expression in GC patients. First, we performed immunohistochemical analysis to evaluate Rab27b expression in GC cells. Rab27b staining was more intense in the nuclei and cytoplasm of tumor cells compared to normal cells (Figure 3A). Next, we evaluated survival rates according to Rab27b mRNA expression in tumor tissues from GC patients. The overall survival rate was significantly lower in patients in the high Rab27b mRNA expression group than in patients in the low expression group in our dataset (p<0.05, Figure 3B). In the GC dataset from TCGA, the overall and recurrence-free survival rates were significantly lower in the high than in the low Rab27b mRNA expression group (p<0.05, Figure 3C).
Figure 3. Prognostic significance of Rab27b mRNA expression in gastric cancer. (A) Immunohistochemical staining of Rab27b in representative samples of gastric cancer. Original magnification: ×200 (left) and ×400 (right). (B) The overall survival rate in gastric cancer patients according to Rab27b mRNA expression in tumor tissues in our dataset. (C) Overall (left) and recurrence-free (right) survival rates in gastric cancer patients according to Rab27b mRNA expression in tumor tissues in the TCGA dataset.
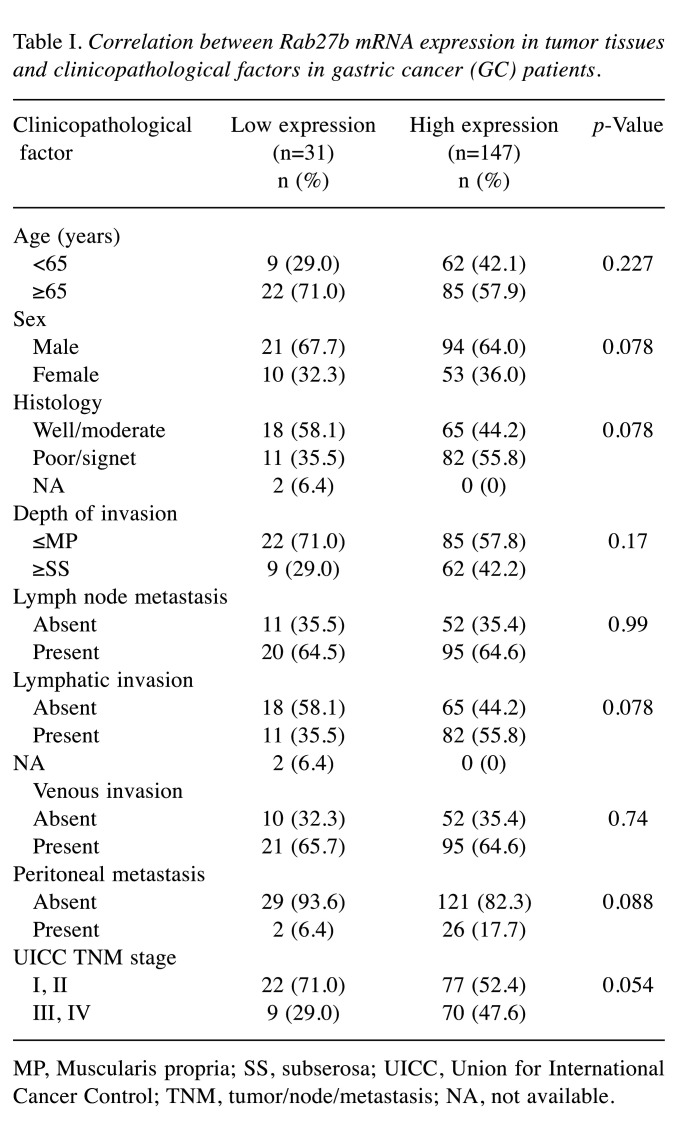
Clinicopathological significance of Rab27b mRNA expression in GC. We examined the associations between Rab27b mRNA expression in tumor tissues and clinicopathological factors in GC patients from our hospital (Table I). There was no significant association between Rab27b mRNA expression and age, histology, depth of invasion, lymph node metastasis, venous invasion, lymphatic invasion, PM, or TNM stage. However, high Rab27b mRNA expression tended to be associated with poor histological differentiation, lymphatic invasion, PM, and a higher TNM stage (p=0.078, 0.078, 0.088, and 0.054, respectively; Table I).
Table I. Correlation between Rab27b mRNA expression in tumor tissues and clinicopathological factors in gastric cancer (GC) patients.
MP, Muscularis propria; SS, subserosa; UICC, Union for International Cancer Control; TNM, tumor/node/metastasis; NA, not available.
Discussion
In this study, we showed that Rab27b, a regulator of exosome secretion, was associated with PM from GC using a xenograft mice model and was predictive of a poor prognosis in GC patients. To the best of our knowledge, this is the first study to provide evidence that Rab27b acts as a key player in PM development from GC.
Exosomes play important roles in metastasis in various malignant tumors. Hood et al. reported that exosomes released by melanoma cells induce an environment suitable for lymph node metastasis (10). Bobrie et al. demonstrated that Rab27a blockade decreased lung dissemination of breast metastatic carcinoma cells in mouse models (14). In GC, tumor-derived exosomes were reported to promote PM by destroying the mesothelial barrier or increasing the expression of adhesion molecules in mesothelial cells (39,40). Also, Che et al. has reported that the exosomal mesenchymal-epithelial transition factor could educate tumor-associated macrophages to promote GC progression (41). Our xenograft mouse model showed that Rab27b knockdown in GC cells decreased exosome secretion, leading to suppressed PM without affecting the proliferation or invasion ability of tumor cells. These results suggest that exosomes play a crucial role in the formation of PM as a communicator between tumor cells and host peritoneal cells in the tumor microenvironment in GC.
Our clinical study showed that Rab27b was overexpressed in GC cells. Furthermore, high expression of Rab27b was associated with poor overall and recurrence-free survival in GC patients. High expression of Rab27b has been correlated with poor survival in patients with solid cancers, such as colorectal, pancreatic, and esophageal squamous cell cancer (21,42-44). An et al. also reported that Rab27b expression predicts lymph node metastases and survival in GC patients (45). These findings suggest that Rab27b expression in tumor tissues can serve as a useful biomarker of poor prognosis in various malignancies.
Rab27a and Rab27b regulate exocytosis of multivesicular endosomes and control different steps of the exosome secretion pathway (46-48). Rab27a likely has a function in membrane fusion; on the other hand, Rab27b interacts with a motor protein that regulates the motility of a subset of multivesicular endosomes. In HeLa cells, Bobrie et al. showed that secretion of exosomes from CD63-containing intracellular compartments was strongly decreased after knockdown of either Rab27a or Rab27b by shRNAs (14). Interestingly, in two mammary carcinoma cell lines, those authors also showed that Rab27a, but not Rab27b, is required for exosome secretion (14). These findings imply that the contribution of Rab27 to exosome secretion depends on the cell type (20). In this study, we focused on the clinical and biological significance of Rab27b (versus Rab27a) expression in GC because Rab27b knockdown reduced exosome secretion better than Rab27a did. Rab27b might play a critical role in exosome secretion, followed by cancer development in GC. Further study will be required to clarify this.
Some limitations exist in this study. First, the reason that Rab27b is overexpressed in GC remains unknown (19). We examined the DNA copy number of Rab27b in GC tissues, but we found no amplification of the DNA copy number (data not shown). Further studies are needed to clarify the mechanism of Rab27b overexpression in tumor cells. Second, we did not evaluate the cargo molecules (i.e., miRNAs, mRNAs, and proteins) carried by exosomes and their role in promoting PM. A previous study found that exosomal miR-21-5p derived from GC cells promoted PM via EMT (49). Regardless of these limitations, we provide important evidence for Rab27b as an effective therapeutic target for PM of GC.
Conclusion
In conclusion, we demonstrated that Rab27b is overexpressed in GC cells and potentially facilitates PM via exosome secretion. Furthermore, we showed that Rab27b expression in tumor tissues serves as a prognostic biomarker of GC. Rab27b may be a promising therapeutic target to inhibit PM in GC patients.
Conflicts of Interest
All Authors have no conflicts of interest to disclose.
Authors’ Contributions
SN and TM wrote the manuscript. SN and TM conducted the experiments. SN, TM, KH, QH, TT, YO, JK, YH, YH, TI, KS, EO, TY and KM designed the study and interpreted the data. SN and TM prepared the figures and conducted the statistical analysis.
Acknowledgements
This research used the super-computing resource provided by the Human Genome Center, The Institute of Medical Science, The University of Tokyo (http://sc.hgc.jp/shirokane.html). We thank K. Oda, M. Kasagi, M. Sakuma, T Fukuda, M Uto, N. Mishima, and T. Kawano for their technical assistance. This work was supported by the following grants and foundations: Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Science Research (grant numbers 19K09176, 19H03715, 20H05039, 20K08930, 20K17556 and 22K09006); OITA Cancer Research Foundation; AMED under Grant Number (22ama221501h0001, 21ck0106690s0201, 20ck0106547h0001 and 20ck0106541h0001); Takeda Science Foundation; The Princess Takamatsu Cancer Research Fund.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.You L, Lv Z, Li C, Ye W, Zhou Y, Jin J, Han Q. Worldwide cancer statistics of adolescents and young adults in 2019: a systematic analysis of the Global Burden of Disease Study 2019. ESMO Open. 2021;6(5):100255. doi: 10.1016/j.esmoop.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20(Suppl 1):111–121. doi: 10.1007/s10120-016-0662-9. [DOI] [PubMed] [Google Scholar]
- 4.Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22(30):6829–6840. doi: 10.3748/wjg.v22.i30.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J, Wang X, Yuan X, Liu G, Chu Q. Emerging roles of exosome-derived biomarkers in cancer theranostics: messages from novel protein targets. Am J Cancer Res. 2022;12(5):2226–2248. [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell MI, Ma J, Carter CL, Loudig O. Circulating exosome cargoes contain functionally diverse cancer biomarkers: from biogenesis and function to purification and potential translational utility. Cancers (Basel) 2022;14(14):3350. doi: 10.3390/cancers14143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takano Y, Masuda T, Iinuma H, Yamaguchi R, Sato K, Tobo T, Hirata H, Kuroda Y, Nambara S, Hayashi N, Iguchi T, Ito S, Eguchi H, Ochiya T, Yanaga K, Miyano S, Mimori K. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8(45):78598–78613. doi: 10.18632/oncotarget.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, Mochizuki T. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5(10):e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 15.Ohzawa H, Kumagai Y, Yamaguchi H, Miyato H, Sakuma Y, Horie H, Hosoya Y, Kawarai Lefor A, Sata N, Kitayama J. Exosomal microRNA in peritoneal fluid as a biomarker of peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2019;4(1):84–93. doi: 10.1002/ags3.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Wang H, Huang Y, Chen Y, Chen C, Zhuo W, Teng L. Comprehensive roles and future perspectives of exosomes in peritoneal metastasis of gastric cancer. Front Oncol. 2021;11:684871. doi: 10.3389/fonc.2021.684871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120(Pt 22):3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 18.Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res. 2014;328(1):1–19. doi: 10.1016/j.yexcr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Fang R, Fang J, He S, Liu T. Functional implications of Rab27 GTPases in Cancer. Cell Commun Signal. 2018;16(1):44. doi: 10.1186/s12964-018-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaé N, McEwan DG, Manavski Y, Boon RA, Dimmeler S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 2015;589(20 Pt B):3182–3188. doi: 10.1016/j.febslet.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Koh HM, Jang BG, Kim DC. Prognostic significance of Rab27 expression in solid cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):14136. doi: 10.1038/s41598-020-71104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagihara K, Takigahira M, Tanaka H, Komatsu T, Fukumoto H, Koizumi F, Nishio K, Ochiya T, Ino Y, Hirohashi S. Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci. 2005;96(6):323–332. doi: 10.1111/j.1349-7006.2005.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagihara K, Takigahira M, Takeshita F, Komatsu T, Nishio K, Hasegawa F, Ochiya T. A photon counting technique for quantitatively evaluating progression of peritoneal tumor dissemination. Cancer Res. 2006;66(15):7532–7539. doi: 10.1158/0008-5472.CAN-05-3259. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano Y, Shinden Y, Eguchi H, Yamamoto H, Doki Y, Mori M, Ochiya T, Mimori K. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113(2):275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda TA, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y, Utsunomiya T, Mori M. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res. 2003;9(15):5693–5698. [PubMed] [Google Scholar]
- 26.Shimizu D, Saito T, Ito S, Masuda T, Kurashige J, Kuroda Y, Eguchi H, Kodera Y, Mimori K. Overexpression of FGFR1 promotes peritoneal dissemination via epithelial-to-mesenchymal transition in gastric cancer. Cancer Genomics Proteomics. 2018;15(4):313–320. doi: 10.21873/cgp.20089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nambara S, Masuda T, Kobayashi Y, Sato K, Tobo T, Koike K, Noda M, Ogawa Y, Kuroda Y, Ito S, Eguchi H, Sugimachi K, Mimori K. GTF2IRD1 on chromosome 7 is a novel oncogene regulating the tumor-suppressor gene TGFβR2 in colorectal cancer. Cancer Sci. 2020;111(2):343–355. doi: 10.1111/cas.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda T, Xu X, Dimitriadis EK, Lahusen T, Deng CX. “DNA Binding Region” of BRCA1 affects genetic stability through modulating the intra-S-phase checkpoint. Int J Biol Sci. 2016;12(2):133–143. doi: 10.7150/ijbs.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato K, Masuda T, Hu Q, Tobo T, Gillaspie S, Niida A, Thornton M, Kuroda Y, Eguchi H, Nakagawa T, Asano K, Mimori K. Novel oncogene 5MP1 reprograms c-Myc translation initiation to drive malignant phenotypes in colorectal cancer. EBioMedicine. 2019;44:387–402. doi: 10.1016/j.ebiom.2019.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitagawa A, Masuda T, Takahashi J, Tobo T, Noda M, Kuroda Y, Hu Q, Kouyama Y, Kobayashi Y, Kuramitsu S, Sato K, Fujii A, Yoshikawa Y, Wakiyama H, Shimizu D, Tsuruda Y, Eguchi H, Doki Y, Mori M, Mimori K. KIF15 expression in tumor-associated monocytes is a prognostic biomarker in hepatocellular carcinoma. Cancer Genomics Proteomics. 2020;17(2):141–149. doi: 10.21873/cgp.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurashige J, Hasegawa T, Niida A, Sugimachi K, Deng N, Mima K, Uchi R, Sawada G, Takahashi Y, Eguchi H, Inomata M, Kitano S, Fukagawa T, Sasako M, Sasaki H, Sasaki S, Mori M, Yanagihara K, Baba H, Miyano S, Tan P, Mimori K. Integrated molecular profiling of human gastric cancer identifies DDR2 as a potential regulator of peritoneal dissemination. Sci Rep. 2016;6:22371. doi: 10.1038/srep22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata H, Sugimachi K, Komatsu H, Ueda M, Masuda T, Uchi R, Sakimura S, Nambara S, Saito T, Shinden Y, Iguchi T, Eguchi H, Ito S, Terashima K, Sakamoto K, Hirakawa M, Honda H, Mimori K. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 2016;76(11):3265–3276. doi: 10.1158/0008-5472.CAN-15-2601. [DOI] [PubMed] [Google Scholar]
- 33.Kuramitsu S, Masuda T, Hu Q, Tobo T, Yashiro M, Fujii A, Kitagawa A, Abe T, Otsu H, Ito S, Oki E, Mori M, Mimori K. Cancer-associated fibroblast-derived spondin-2 promotes motility of gastric cancer cells. Cancer Genomics Proteomics. 2021;18(4):521–529. doi: 10.21873/cgp.20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Q, Masuda T, Koike K, Sato K, Tobo T, Kuramitsu S, Kitagawa A, Fujii A, Noda M, Tsuruda Y, Otsu H, Kuroda Y, Ito S, Oki E, Mimori K. Oxysterol binding protein-like 3 (OSBPL3) is a novel driver gene that promotes tumor growth in part through R-Ras/Akt signaling in gastric cancer. Sci Rep. 2021;11(1):19178. doi: 10.1038/s41598-021-98485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garmire LX, Subramaniam S. Evaluation of normalization methods in mammalian microRNA-Seq data. RNA. 2012;18(6):1279–1288. doi: 10.1261/rna.030916.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuno H, Kitada K, Nakai K, Sarai A. PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genomics. 2009;2:18. doi: 10.1186/1755-8794-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobin LH, Gospodarowicz M, Wittekind C. New York, Wiley-Liss. 2010. International union against cancer (uicc) tnm classification of malignant tumours, 7th edition. [Google Scholar]
- 38.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng G, Qu J, Zhang Y, Che X, Cheng Y, Fan Y, Zhang S, Na D, Liu Y, Qu X. Gastric cancer-derived exosomes promote peritoneal metastasis by destroying the mesothelial barrier. FEBS Lett. 2017;591(14):2167–2179. doi: 10.1002/1873-3468.12722. [DOI] [PubMed] [Google Scholar]
- 40.Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Ogino S, Fujita Y, Hiramoto H, Hamada J, Shoda K, Kosuga T, Fujiwara H, Okamoto K, Otsuji E. Tumor exosome-mediated promotion of adhesion to mesothelial cells in gastric cancer cells. Oncotarget. 2016;7(35):56855–56863. doi: 10.18632/oncotarget.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Che Y, Geng B, Xu Y, Miao X, Chen L, Mu X, Pan J, Zhang C, Zhao T, Wang C, Li X, Wen H, Liu Z, You Q. Helicobacter pylori-induced exosomal MET educates tumour-associated macrophages to promote gastric cancer progression. J Cell Mol Med. 2018;22(11):5708–5719. doi: 10.1111/jcmm.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao J, Ni Y, Qin H, Xu L, Ge Z, Zhan F, Zhu H, Zhao J, Zhou X, Tang X, Tang L. Rab27b is a potential predictor for metastasis and prognosis in colorectal cancer. Gastroenterol Res Pract. 2014;2014:913106. doi: 10.1155/2014/913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H, Wang Q, Wang X, Zhu H, Zhang S, Wang W, Wang Z, Huang J. Correlation between RAB27B and p53 expression and overall survival in pancreatic cancer. Pancreas. 2016;45(2):204–210. doi: 10.1097/MPA.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu F, Wu W, Liang M, Huang Y, Chen C. Prognostic significance of Rab27A and Rab27B expression in esophageal squamous cell cancer. Cancer Manag Res. 2020;12:6353–6361. doi: 10.2147/CMAR.S258940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An HJ, Lee JS, Yang JW, Kim MH, Na JM, Song DH. RAB27A and RAB27B expression may predict lymph node metastasis and survival in patients with gastric cancer. Cancer Genomics Proteomics. 2022;19(5):606–613. doi: 10.21873/cgp.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer SR. Two Rabs for exosome release. Nat Cell Biol. 2010;12(1):3–4. doi: 10.1038/ncb0110-3. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14(9):949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Li B, Li Q, Wei S, He Z, Huang X, Wang L, Xia Y, Xu Z, Li Z, Wang W, Yang L, Zhang D, Xu Z. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9(9):854. doi: 10.1038/s41419-018-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]