Abstract
BACKGROUND
New approaches for the prevention and elimination of malaria, a leading cause of illness and death among infants and young children globally, are needed.
METHODS
We conducted a phase 1 clinical trial to assess the safety and pharmacokinetics of L9LS, a next-generation antimalarial monoclonal antibody, and its protective efficacy against controlled human malaria infection in healthy adults who had never had malaria or received a vaccine for malaria. The participants received L9LS either intravenously or subcutaneously at a dose of 1 mg, 5 mg, or 20 mg per kilogram of body weight. Within 2 to 6 weeks after the administration of L9LS, both the participants who received L9LS and the control participants underwent controlled human malaria infection in which they were exposed to mosquitoes carrying Plasmodium falciparum (3D7 strain).
RESULTS
No safety concerns were identified. L9LS had an estimated half-life of 56 days, and it had dose linearity, with the highest mean (±SD) maximum serum concentration (Cmax) of 914.2±146.5 μg per milliliter observed in participants who had received 20 mg per kilogram intravenously and the lowest mean Cmax of 41.5±4.7 μg per milliliter observed in those who had received 1 mg per kilogram intravenously; the mean Cmax was 164.8±31.1 in the participants who had received 5 mg per kilogram intravenously and 68.9±22.3 in those who had received 5 mg per kilogram subcutaneously. A total of 17 L9LS recipients and 6 control participants underwent controlled human malaria infection. Of the 17 participants who received a single dose of L9LS, 15 (88%) were protected after controlled human malaria infection. Parasitemia did not develop in any of the participants who received 5 or 20 mg per kilogram of intravenous L9LS. Parasitemia developed in 1 of 5 participants who received 1 mg per kilogram intravenously, 1 of 5 participants who received 5 mg per kilogram subcutaneously, and all 6 control participants through 21 days after the controlled human malaria infection. Protection conferred by L9LS was seen at serum concentrations as low as 9.2 μg per milliliter.
CONCLUSIONS
In this small trial, L9LS administered intravenously or subcutaneously protected recipients against malaria after controlled infection, without evident safety concerns. (Funded by the National Institute of Allergy and Infectious Diseases; VRC 614 ClinicalTrials.gov number, NCT05019729.)
Malaria is a life-threatening, mosquito-borne disease caused by plasmodium parasites. In 2020, the disease affected 241 million persons and led to 627,000 deaths worldwide — a 12% increase in mortality since 2019.1 Sub-Saharan Africa continues to bear a disproportionate burden of malarial disease, and deaths among children younger than 5 years of age account for approximately 80% of all deaths from malaria in that region.1 Development of a highly efficacious malaria vaccine remains a long-sought public health goal. The RTS,S/AS01 vaccine (Mosquirix), recommended by the World Health Organization (WHO) in October 2021 for widespread use in infants, provides only partial protection against clinical malaria, with a reported vaccine efficacy of 36.3% after 4 years of follow-up.2 Despite progress in vaccine development, there remains a need for additional strategies to reduce the increasing global incidence of malaria and related mortality, with an eventual goal of elimination of this disease.
Monoclonal antibodies offer a new approach to passive protection against malaria over a prolonged period.3 They have been shown to prevent Plasmodium falciparum malaria at the pre-erythrocytic stage that precedes clinical blood-stage infection by neutralizing the infecting sporozoites through binding to the major P. falciparum circumsporozoite protein, an essential mediator of infection.4 Passive administration of monoclonal antibodies can consistently provide a defined concentration at a protective titer. This approach differs from vaccines that may have variable immune priming and can be influenced by previous exposure to malaria, age, and immunocompetence, which can vary across persons.5-7 Finally, monoclonal antibodies directed at conserved sites of P. falciparum circumsporozoite protein are expected to be broadly efficacious against circulating parasite strains.8,9
As a proof of principle for the use of monoclonal antibodies in the prevention of malaria, we reported the safety and efficacy of a highly potent human antimalarial monoclonal antibody, CIS43LS, which targets the junctional region of the P. falciparum circumsporozoite protein and contains an LS mutation in the Fc region that extends serum half-life.10,11 CIS43LS provided protection against malaria in all nine participants who received intravenous doses of 20 or 40 mg per kilogram of body weight, followed by controlled human malaria infection, without identified safety concerns.3
The use of this strategy in an environment where malaria is endemic will depend on the durability and potency of the monoclonal antibody. Improving potency is critical for increasing protection and reducing the volume of monoclonal antibody required, thus limiting costs and allowing for subcutaneous dosing across all age groups. Mediating protection by means of subcutaneous administration, which is more feasible than intravenous administration in the pediatric population, is key to limiting morbidity and mortality among infants and young children.
In the current trial, we assessed the safety, pharmacokinetics, and protective efficacy of L9LS, a next-generation antimalarial monoclonal antibody, which was approximately three times more potent than CIS43, the parent antibody of CIS43LS, in preclinical models.12 L9 was modified within the Fc region with an LS mutation that increases neonatal Fc receptor binding and antibody half-life through increased antibody recirculation.13 L9LS targets highly conserved, minor NVDP repeats on the P. falciparum circumsporozoite protein and then achieves both cytolytic destruction of sporozoites and prevention of hepatocyte infection by limiting parasite (sporozoite) egress from liver sinusoids.12 In this phase 1 clinical trial involving healthy adults in the United States who had not previously had malaria or received a vaccine for malaria, we assessed whether protection could be achieved with various doses of L9LS administered intravenously or subcutaneously.
METHODS
TRIAL DESIGN AND PARTICIPANTS
VRC 614 was a phase 1, open-label, dose-escalation clinical trial. The primary objectives of the trial were to evaluate the safety and side-effect profile of L9LS administered at intravenous doses of 1, 5, and 20 mg per kilogram of body weight and at a subcutaneous dose of 5 mg per kilogram. The secondary objectives were to evaluate the pharmacokinetic properties and protective efficacy of L9LS after controlled human malaria infection approximately 2 to 6 weeks after the participants had received L9LS.
Eligible participants were healthy adults who were 18 to 50 years of age and had not previously had malaria or received a vaccine for malaria. Full details of the inclusion and exclusion criteria are provided in the protocol, available with the full text of this article at NEJM.org.
TRIAL OVERSIGHT
The trial was designed, funded, and conducted by the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), at the NIH Clinical Center in Bethesda, Maryland. Controlled human malaria infection was conducted at the U.S. Army facility at the Walter Reed Army Institute of Research in Silver Spring, Maryland. The NIH institutional review board approved the clinical trial protocol. All the participants provided written informed consent, and the trial followed the Department of Health and Human Services guidelines for the protection of human research participants. Data were collected and analyzed by the Vaccine Research Center and the Walter Reed Army Institute of Research. All the authors vouch for the accuracy and completeness of the data and analyses and for the adherence of the trial to the protocol.
TRIAL PRODUCT
L9LS, a human IgG1 monoclonal antibody that was produced in accordance with current good manufacturing practices by cell-culture expression in a recombinant Chinese hamster ovary cell line, consists of purified, formulated L9LS glycoprotein. Processes and analytic methods were developed at the Vaccine Research Center Vaccine Production Program and transferred to the Vaccine Clinical Materials Program, operated under contract with Leidos Biomedical Research in Frederick, Maryland, for current good manufacturing practices production and vialing in a buffered formulation at a concentration of 150 mg per milliliter.
TRIAL PROCEDURES
L9LS was administered intravenously over a period of 30 minutes at a dose of 1 mg per kilogram of body weight, 5 mg per kilogram, or 20 mg per kilogram. Participants who received subcutaneous injections received 5 mg per kilogram, with the total dose divided into one or two injections, not exceeding 2.0 ml each, according to the weight of the participant. Most injections were abdominal, but the upper arm could be used if preferred by the participant and clinician. Participants were observed in the clinic for 1 to 2 hours after administration of L9LS.
Interim safety data reviews were conducted to assess for any dose-related safety concerns before escalation to doses of 5 mg per kilogram and 20 mg per kilogram. Unsolicited adverse events were recorded for 28 days after L9LS administration and controlled human malaria infection and were graded according to a modified Division of Acquired Immunodeficiency Syndrome Table for Grading the Severity of Adult and Pediatric Adverse Events.14 Serious adverse events and new chronic medical conditions were recorded for the entire duration of the trial.
Participants were followed for 24 weeks after L9LS administration. Control participants were followed for 7 weeks after controlled human malaria infection.
CONTROLLED HUMAN MALARIA INFECTION
Participants were exposed to bites on the forearm from Anopheles stephensi mosquitoes infected with P. falciparum (3D7 strain). The participants met standard infectivity criteria consisting of five qualifying bites from mosquitoes with a salivary gland score of 2 or greater (scores range from 0 to 4, with higher scores indicating more microscopically observed sporozoites).15 The participants were evaluated by means of two telephone calls in the first 7 days after controlled human malaria infection, followed by in-clinic visits on days 7 through 17 and on day 21 to assess for parasitemia with a highly sensitive and specific polymerase-chain-reaction (PCR) test to detect early blood-stage malaria infection.15-17 Day 21 was chosen as the upper end of the range of assessment days in order to minimize the risk of exposure to coronavirus disease 2019 while ensuring sufficient time to assess for parasitemia.
Parasitemia was defined as a single positive PCR result. Participants were considered protected if parasitemia did not develop through day 21 after controlled human malaria infection. Directly observed therapy with a standard treatment of 1 g of atovaquone and 400 mg of proguanil hydrochloride for 3 consecutive days was initiated in all the participants either on confirmation of parasitemia or on day 21 if the participant had not already been treated.
PHARMACOKINETICS
Serum concentrations of L9LS were quantified with the use of an L9LS anti-idiotype antibody on the Meso Scale Discovery platform, as previously described, at prespecified time points up to 8 weeks after administration of the monoclonal antibody.3 Pharmacokinetic analysis of L9LS concentrations was performed with both compartmental and noncompartmental approaches. Descriptive statistics for the maximum serum concentration (Cmax) and for the time of maximum concentration (Tmax), along with the concentrations at trial days 28 and 56, were calculated on the basis of observed data. The area under the curve was calculated with the use of the linear trapezoid method. Additional details of the quantification method and pharmacokinetic analysis are described in the Supplemental Methods section in the Supplementary Appendix, available at NEJM.org.
STATISTICAL ANALYSIS
The target sample size was determined on the basis of the probability of observing serious adverse events. The efficacy analysis included all enrolled participants who underwent controlled human malaria infection. The primary efficacy analysis was performed with the use of a two-sided Barnard test in which the percentage of participants who had malaria infection among those who had received L9LS was compared with the percentage among control participants. The secondary efficacy analysis was based on the time to parasitemia; Kaplan–Meier curves were provided for each group and compared with the use of a log-rank test. To assess the comparability of the challenge between the treatment and control groups, the median and interquartile ranges of the salivary gland scores were reported for each group. Owing to the exploratory nature of the trial, no adjustment was made for multiplicity.
RESULTS
PARTICIPANTS
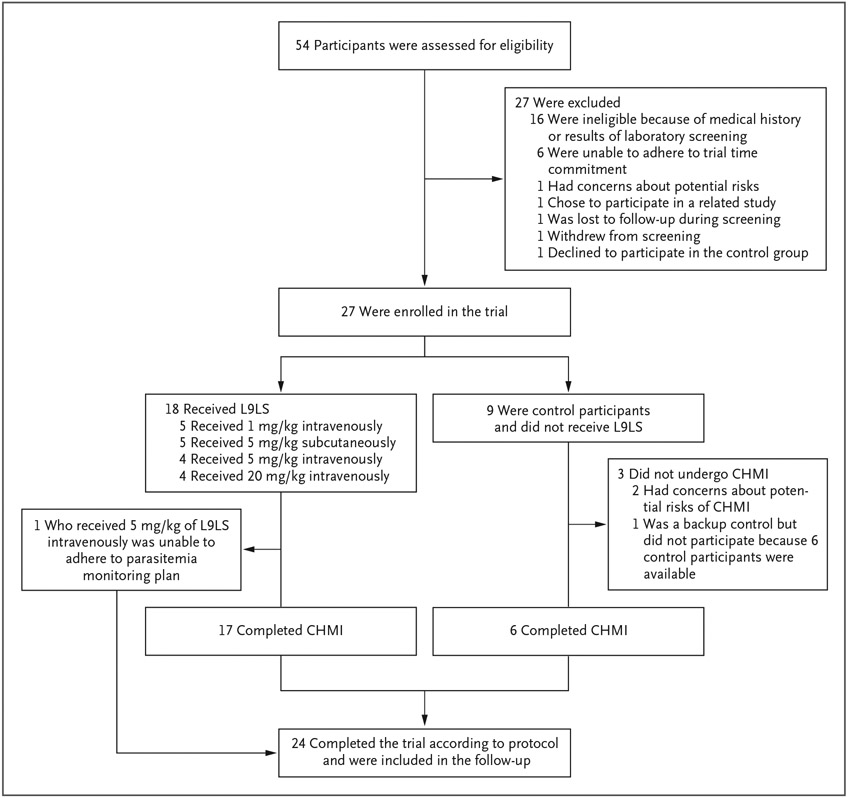
From September 13 to October 25, 2021, a total of 27 participants were enrolled in the trial (Fig. 1, and Table S1 in the Supplementary Appendix). Of the 27 participants, 18 received L9LS. Five participants received L9LS intravenously at a dose of 1 mg per kilogram, 4 received 5 mg per kilogram intravenously, 5 received 5 mg per kilogram subcutaneously, and 4 received 20 mg per kilogram intravenously. The remaining 9 participants served as controls and did not receive L9LS.
Figure 1. Enrollment, Administration of L9LS, and Controlled Human Malaria Infection.
Trial enrollment occurred from September 13 to October 25, 2021. Controlled human malaria infection occurred on October 26, 2021. All participants who received L9LS were followed for 24 weeks after administration of L9LS. Control participants were followed for 7 weeks after controlled human malaria infection (CHMI).
The trial design called for the 6 control participants to undergo controlled human malaria infection, and an additional 3 participants were enrolled to ensure that at least 6 control participants would be available to undergo the controlled human malaria infection in the event of unplanned dropout of participants. The controlled human malaria infection was administered to 23 participants (17 L9LS recipients and 6 control participants) on October 26, 2021, at the U.S. Army facility at the Walter Reed Army Institute of Research. Ten L9LS recipients were challenged within 4 weeks after administration and 7 were challenged 5 to 6 weeks after administration. The 21-day parasitemia monitoring period was completed on November 16, 2021. One participant who received L9LS intravenously at a dose of 5 mg per kilogram did not undergo controlled human malaria infection because of an inability to adhere to the parasitemia monitoring plan specified in the protocol, but that participant remained in the trial for safety and pharmacokinetic evaluations.
SAFETY
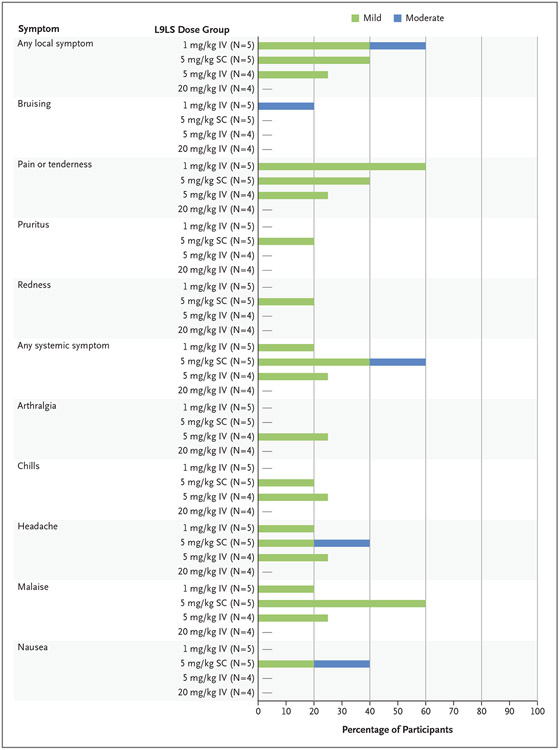
All 18 L9LS recipients completed the solicited and unsolicited local and systemic safety assessments. When present, solicited symptoms were mild to moderate in severity and self-limited (Fig. 2). No infusion-related reactions or serious adverse events were reported. A single unsolicited adverse event — mild cervical lymphadenopathy that occurred 9 days after administration of L9LS in a participant who had received a dose of 1 mg per kilogram — was attributed by the investigators to administration of L9LS. The temporal relationship between the administration of L9LS and the clinical finding was the basis for the attribution. The lymphadenopathy resolved after 29 days without intervention or residual effects.
Figure 2. Maximum Local and Systemic Solicited Reactogenicity.
The percentage of participants who reported a mild or moderate local or systemic symptom in the 7 days after administration of L9LS intravenously (IV) or subcutaneously (SC) is shown. After administration of L9LS, none of the participants reported systemic symptoms of myalgia or fever or the local symptom of swelling.
PHARMACOKINETIC ASSESSMENTS
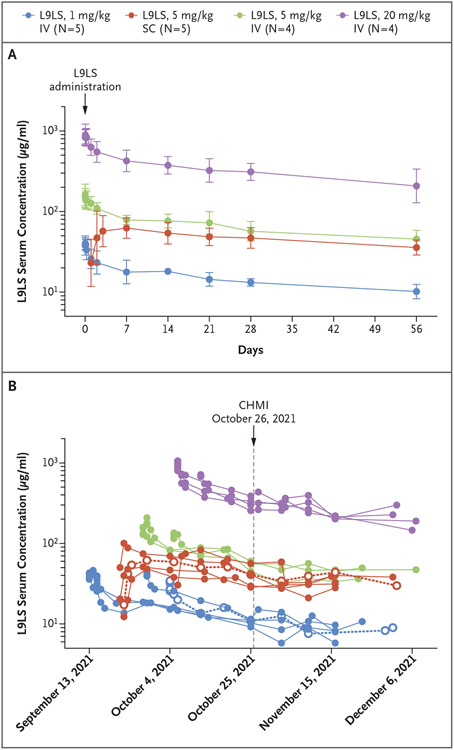
The serum L9LS pharmacokinetic antibody profile was analyzed in all L9LS recipients for whom at least 8 weeks of pharmacokinetic data were available (Fig. 3A and 3B). L9LS had dose linearity, with the highest mean (±SD) Cmax of 914.2±146.5 μg per milliliter observed in the group of participants who had received 20 mg per kilogram intravenously and the lowest mean Cmax of 41.5±4.7 μg per milliliter observed in those who had received 1 mg per kilogram intravenously; the mean Cmax was 164.8±31.1 in the group of participants who had received 5 mg per kilogram intravenously and 68.9±22.3 in those who had received 5 mg per kilogram subcutaneously. Concentrations of L9LS exceeded 10 μg per milliliter within 1 day after subcutaneous administration in all participants, and Tmax occurred approximately 6 days after subcutaneous administration.
Figure 3. Serum Concentrations of L9LS.
Panel A shows the geometric mean serum concentrations of L9LS with 95% confidence intervals (indicated by I bars) for each dose group after administration of a single dose of L9LS. Panel B shows the serum concentrations of L9LS over time in individual trial participants who underwent CHMI. Individual horizontal dotted lines with open circles indicate malaria infection (in one participant who received 1 mg per kilogram intravenously and in one participant who received 5 mg per kilogram subcutaneously).
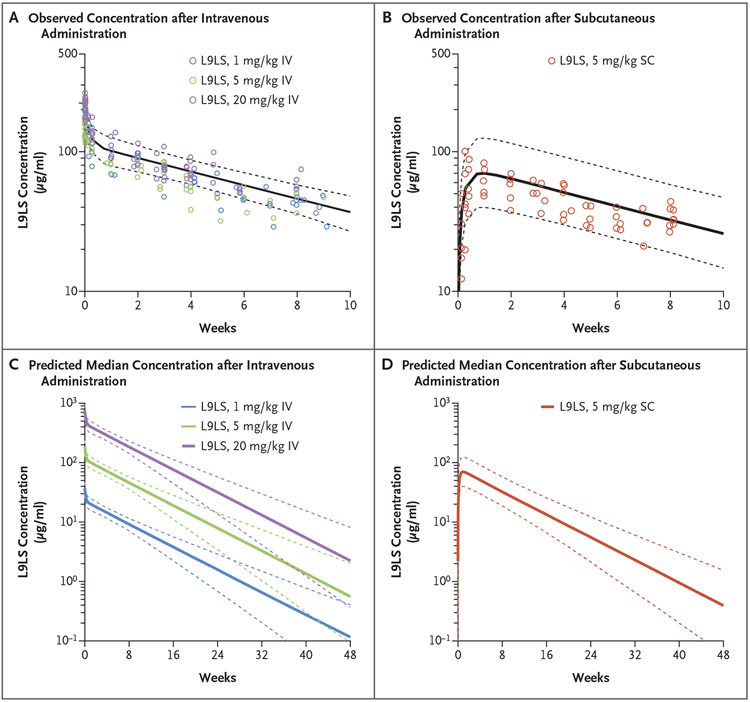
According to the population pharmacokinetic model, the estimate of overall L9LS clearance was 46.1 milliliter per day (95% bootstrap confidence interval [CI], 43.1 to 53.0), the half-life (T1/2β) was 56 days (95% bootstrap CI, 47 to 66), and the volume of distribution (Vdss) was 3.67 liters (95% bootstrap CI, 3.31 to 4.09) (Table S4). The population estimate for subcutaneous bioavailability was 69% (95% bootstrap CI, 51 to 88). According to population modeling and simulation, the predicted L9LS concentration 6 months after administration ranged from 11 to 16 μg per milliliter in the participants who received 5 mg per kilogram intravenously, 28 to 48 μg per milliliter in those who received 20 mg per kilogram intravenously, 5 to 11 μg per milliliter in those who received 5 mg per kilogram subcutaneously, and 1.7 to 2.7 μg per milliliter in those who received 1 mg per kilogram intravenously (Fig. 4C and 4D).
Figure 4. Predictions for Serum Concentrations after Intravenous or Subcutaneous Administration of L9LS.
Panels A and B show pharmacokinetic modeling for intravenous and subcutaneous administration of L9LS. Observed L9LS concentrations (normalized for each dose administered intravenously or subcutaneously) are overlayed for the comparison. The predicted median serum concentrations of L9LS (solid lines) and 90% prediction intervals (5th and 95th percentiles) (dashed lines) are shown. Panels C and D show the predicted median L9LS serum concentrations (solid lines) 48 weeks after administration of a single dose according to intravenous dose groups (1 mg per kilogram, 5 mg per kilogram, and 20 mg per kilogram) and the subcutaneous dose group (5 mg per kilogram) with 90% prediction intervals (5th and 95th percentiles) (dashed lines). Values were calculated on the basis of Monte Carlo simulations with the use of a population pharmacokinetic model.
EFFICACY
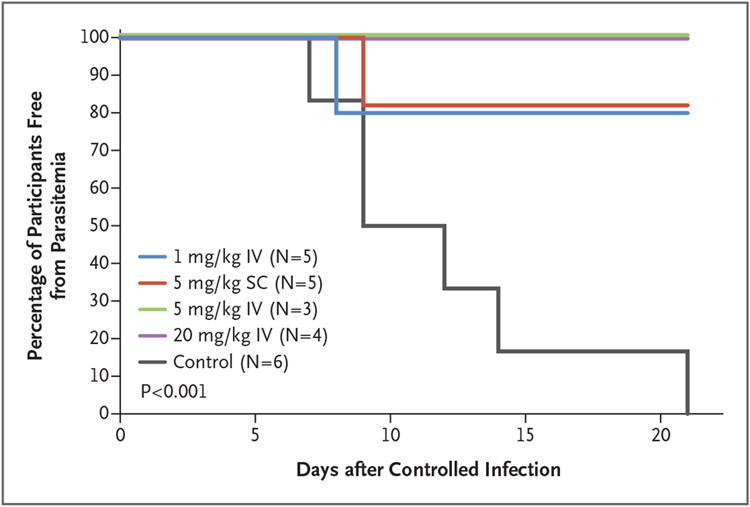
Parasitemia, as determined by PCR through day 21 after controlled human malaria infection, developed in 2 of the 17 L9LS recipients and in all 6 control participants (P<0.001 by two-sided Barnard test) (Fig. 5). Parasitemia did not develop in any of the participants who had received intravenous L9LS at doses of 5 mg per kilogram intravenously or 20 mg per kilogram intravenously, whereas parasitemia developed on day 8 after controlled human malaria infection in 1 participant who had received 1 mg per kilogram intravenously and on day 9 in 1 participant who had received 5 mg per kilogram subcutaneously. Parasitemia developed on days 7, 9, 12, 14 or 21 after controlled human malaria infection in control participants who had not received L9LS. At the time of controlled human malaria infection, serum concentrations of L9LS ranged from 9.2 to 11.5 μg per milliliter in participants who had received 1 mg per kilogram intravenously, 28.6 to 56.4 μg per milliliter in those who had received 5 mg per kilogram subcutaneously, 45.2 to 60.4 μg per milliliter in those who had received 5 mg per kilogram intravenously, and 256.0 to 387.9 μg per milliliter in those who had received 20 mg per kilogram intravenously (Table S5).
Figure 5. Parasitemia after Controlled Human Malaria Infection.
A Kaplan–Meier analysis shows the time to blood-stage parasitemia as measured by polymerase-chain-reaction analysis. Participants were monitored daily starting on day 7 after controlled human malaria infection through day 17, with a final test performed on day 21. A primary efficacy analysis performed with the use of a two-sided Barnard test comparing parasitemia among the participants who received L9LS with that among the control participants yielded a P value of less than 0.001.
Controlled human malaria infection was based on prespecified malaria exposure criteria, which consisted of five qualifying bites from mosquitoes with a salivary gland rating of 2 or greater. These criteria were consistent with those used in previous studies involving controlled human malaria infection.3,15,18,19 The median salivary score was 3.4 (interquartile range, 2.8 to 3.4) in mosquitoes that bit participants who had received L9LS and 2.8 (interquartile range, 2.6 to 2.9) in those that bit control participants (Fig. S3 and Table S6).
DISCUSSION
Monoclonal antibodies are a promising solution to the problem of overcoming host and parasite factors that can present challenges to the generation of highly protective and durable vaccine-induced immunity to malaria. A durable and potent monoclonal antibody delivered subcutaneously to infants and children could be an effective intervention to limit malaria-related morbidity and mortality worldwide over prolonged periods. Here, we report the findings of a phase 1 clinical trial assessing a next-generation human antimalarial monoclonal antibody, L9LS, administered intravenously or subcutaneously.
In this trial involving a small number of participants, L9LS was protective against controlled human malaria infection, and no safety concerns were identified. The safety profile was favorable, with no reported infusion-related reactions after intravenous administration, and subcutaneous injections had a similar side-effect profile, with some mild-to-moderate reactogenicity that resolved shortly after administration of L9LS.
After administration of a single intravenous or subcutaneous dose of L9LS, the serum half-life was estimated at 56 days, a finding that indicated the potential for sustained protection against malaria. Protection after controlled human malaria infection was observed with serum concentrations of L9LS as low as 9.2 μg per milliliter. Pharmacokinetic modeling indicated that a concentration of L9LS in the range of 5 to 11 μg per milliliter would remain in circulation at 6 months in participants who received 5 mg per kilogram subcutaneously. Because of its solubility and stability, L9LS was vialed at a concentration of 150 mg per milliliter. Therefore, a single 1-ml subcutaneous injection could readily translate to a dose of 5 mg per kilogram or greater in infants and in children younger than years of age, possibly providing protection for up to 6 to 12 months.
These findings may have important public health and clinical implications because they establish the potential to advance protection against malaria in regions with seasonal and perennial transmission. For instance, the current standard of care in the Sahel region of West Africa is seasonal malaria chemoprevention administered three or four times per season.20 Although seasonal malaria chemoprevention can be effective, lack of compliance may result in fatal malaria infection. In addition, the use of antimalarial drugs over time has been associated with the emergence of drug-resistant strains.21
Administration of a single subcutaneous dose of a monoclonal antibody at the beginning of the transmission season could provide protection, overcome adherence issues, and potentially limit the emergence of drug-resistant strains associated with long-term use of seasonal malaria chemoprevention. On the basis of the half-life and potency of L9LS, it could also be considered for use in East Africa, where perennial transmission of malaria occurs. To assess these two approaches, two phase 2 clinical trials of subcutaneous administration of L9LS in Africa are planned — one in Mali involving children 6 to 10 years of age to assess protection against seasonal transmission for 6 months (ClinicalTrials.gov number, NCT05304611) and one in Kenya involving children 6 months to 5 years of age to assess protection against perennial transmission (NCT05400655).
Monoclonal antibodies also have the potential to prevent malaria in special populations. Given that monoclonal antibodies do not contain adjuvants and are not intended to stimulate an immune response, the ability to provide several months of protection with administration of a single dose would be useful for prevention of malaria in pregnancy. Moreover, in theory, the immediate effect of immunoprophylaxis with L9LS could make it an effective tool for clinical use in areas where malaria is not endemic, such as prophylaxis for military personnel or other travelers to regions where malaria is endemic. Such use could eliminate daily chemoprophylaxis and concerns regarding longer-term adherence.
The limitations of this trial include its small size and the safety and efficacy data that were restricted to healthy adults in the United States who had never had malaria or received a vaccine for malaria and who underwent controlled human malaria infection.18,19 Although overall protection against controlled human malaria infection was observed, two L9LS recipients lacked protection despite the presence of serum concentrations that were similar to those in participants who were protected. Thus, larger field studies are required to better define and potentially establish protective serum concentrations. The phase 2 clinical trials in Mali and Kenya are planned to address some of these limitations and to help establish the effective dose and inform product-development costs. On the basis of preliminary cost modeling, a dose of L9LS of up to 10 mg per kilogram would probably be feasible for a critical purpose — protection of infants and children younger than 5 years of age.
The current trial provides a proof of principle that prevention of malaria can be achieved with a next-generation monoclonal antibody, L9LS. This subcutaneous regimen of a single low dose warrants further study to define its potential to limit malaria-associated morbidity and mortality among infants and young children in regions where malaria is endemic.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH).
We thank the clinical trial participants for their contributions and commitment to malaria research and Manjula Basappa, Colin M. Smith, staff members of the Emmes Corporation (Phyllis Zaia, Meghan Kunchai, Eugeania Burch, Jeanine May, Amy Bray, Kaitlyn Paster, Taylor Rowley, and Jeetika Sainani), staff members of Technical Resources International (Neta Nelson, Melynda Holmes, Rebecca Hochman, and Nurat Quadri), and staff members of the NIH Department of Laboratory Medicine, the Vaccine Clinical Materials Program at the Frederick National Laboratory for Cancer Research, and the U.S. Army Medical Research and Development Command, Office of Research Protections, Human Research Protections Office, for their contributions to this trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Richard L. Wu, M.D., Azza H. Idris, M.D., Ph.D., Nina M. Berkowitz, M.P.H., Myra Happe, Ph.D., Martin R. Gaudinski, M.D., Christian Buettner, Ph.D., Larisa Strom, M.P.H., Seemal F. Awan, M.D., La-Sonji A. Holman, F.N.P., Floreliz Mendoza, R.N., Ingelise J. Gordon, R.N., Zonghui Hu, Ph.D., Andrezza Campos Chagas, Ph.D., Lawrence T. Wang, B.A., Lais Da Silva Pereira, Ph.D., Joseph R. Francica, Ph.D., Neville K. Kisalu, Ph.D., Barbara J. Flynn, M.S., Wei Shi, Ph.D., Wing-Pui Kong, Ph.D., Sarah O’Connell, M.S., Sarah H. Plummer, C.R.N.P, Allison Beck, P.A., Adrian McDermott, Ph.D., Sandeep R. Narpala, M.S., Leonid Serebryannyy, Ph.D., Mike Castro, M.S., Rosa Silva, B.S., Marjaan Imam, B.S., Iris Pittman, B.S., C.C.R.P., Somia P. Hickman, Ph.D., Andrew J. McDougal, Ph.D., Ashly E. Lukoskie, M.H.S., Jittawadee R. Murphy, Ph.D., Jason G. Gall, Ph.D., Kevin Carlton, M.S., Patricia Morgan, P.A., Ellie Seo, Ph.D., Judy A. Stein, M.B.A., Sandra Vazquez, M.S., Shinyi Telscher, Pharm.D., Edmund V. Capparelli, Pharm.D., Emily E. Coates, Ph.D., John R. Mascola, M.D., Julie E. Ledgerwood, D.O., Lesia K. Dropulic, M.D., and Robert A. Seder, M.D.
The authors’ affiliations are as follows: the Vaccine Research Center (R.L.W., A.H.I., N.M.B., M.H., M.R.G., C.B., L. Strom, S.F.A., L.A.H., F.M., I.J.G., L.T.W., L.D.S.P., J.R.F., N.K.K., B.J.F., W.S., W.-P.K., S.O., S.H.P., A.B., A.M., S.R.N., L. Serebryannyy, M.C., R.S., M.I., I.P., S.P.H., A.J.M., A.E.L., J.G.G., K.C., P.M., E.S., J.A.S., S.V., S.T., E.E.C., J.R. Mascola, J.E.L., L.K.D., R.A.S.) and the Biostatistics Research Branch, Division of Clinical Research (Z.H.), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, the U.S. Public Health Service Commissioned Corps, Rockville (R.L.W., M.R.G.), and the Entomology Branch, Walter Reed Army Institute of Research, Silver Spring (A.C.C., J.R. Murphy) — all in Maryland; the Ragon Institute, Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard University, Cambridge, MA (A.H.I.); and the School of Medicine and Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California San Diego, San Diego (E.V.C.).
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
R.L. Wu, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; U.S. Public Health Service Commissioned Corps, Rockville, Maryland
A.H. Idris, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; Ragon Institute, Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard University, Cambridge, MA
N.M. Berkowitz, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
M. Happe, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
M.R. Gaudinski, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; U.S. Public Health Service Commissioned Corps, Rockville, Maryland
C. Buettner, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
L. Strom, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S.F. Awan, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
L.S.A. Holman, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
F. Mendoza, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
I.J. Gordon, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
Z. Hu, Biostatistics Research Branch, Division of Clinical Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
A. Campos Chagas, Entomology Branch, Walter Reed Army Institute of Research, Silver Spring, Maryland
L.T. Wang, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
L. Da Silva Pereira, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
J.R. Francica, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
N.K. Kisalu, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
B.J. Flynn, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
W. Shi, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
W.-P. Kong, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S. O’Connell, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S.H. Plummer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
A. Beck, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
A. McDermott, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S.R. Narpala, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
L. Serebryannyy, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
M. Castro, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
R. Silva, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
M. Imam, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
I. Pittman, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S.P. Hickman, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
A.J. McDougal, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
A.E. Lukoskie, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
J.R. Murphy, Entomology Branch, Walter Reed Army Institute of Research, Silver Spring, Maryland
J.G. Gall, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
K. Carlton, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
P. Morgan, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
E. Seo, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
J.A. Stein, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S. Vazquez, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
S. Telscher, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
E.V. Capparelli, School of Medicine and Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California San Diego, San Diego
E.E. Coates, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
J.R. Mascola, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
J.E. Ledgerwood, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
L.K. Dropulic, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
R.A. Seder, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Global Malaria Programme. World malaria report 2021. Geneva: World Health Organization, 2021. [Google Scholar]
- 2.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015;386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudinski MR, Berkowitz NM, Idris AH, et al. A monoclonal antibody for malaria prevention. N Engl J Med 2021;385:803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julien J-P, Wardemann H Antibodies against plasmodium falciparum malaria at the molecular level. Nat Rev Immunol 2019;19:761–75. [DOI] [PubMed] [Google Scholar]
- 5.Portugal S, Tipton CM, Sohn H, et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015;4:e07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jongo SA, Church LWP, Mtoro AT, et al. Safety and differential antibody and T-cell responses to the plasmodium falciparum sporozoite malaria vaccine, PfSPZ vaccine, by age in Tanzanian adults, adolescents, children, and infants. Am J Trop Med Hyg 2019;100:1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oneko M, Steinhardt LC, Yego R, et al. Safety, immunogenicity and efficacy of PfSPZ vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med 2021;27:1636–45. [DOI] [PubMed] [Google Scholar]
- 8.Thera MA, Doumbo OK, Coulibaly D, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 2011;365:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med 2015;373:2025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisalu NK, Idris AH, Weidle C, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 2018;24:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010;28:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LT, Pereira LS, Flores-Garcia Y, et al. A potent anti-malarial human monoclonal antibody targets circumsporozoite protein minor repeats and neutralizes sporozoites in the liver. Immunity 2020;53(4):733–744.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisalu NK, Pereira LD, Ernste K, et al. Enhancing durability of CIS43 monoclonal antibody by Fc mutation or AAV delivery for malaria prevention. JCI Insight 2021;6(3):e143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1 July 2017. (https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf). [Google Scholar]
- 15.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013;341:1359–65. [DOI] [PubMed] [Google Scholar]
- 16.Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016;22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mejia R, Booth GS, Fedorko DP, et al. Peripheral blood stem cell transplant-related plasmodium falciparum infection in a patient with sickle cell disease. Transfusion 2012;52:2677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J Infect Dis 2014;209:Suppl 2:S40–S45. [DOI] [PubMed] [Google Scholar]
- 19.Garver LS, Dowler M, Davidson SA. Controlled human malaria infection at the Walter Reed Army Institute of Research: the past, present, and future from an entomological perspective. US Army Med Dep J 2015:16–24. [PubMed] [Google Scholar]
- 20.World Health Organization. WHO policy recommendation: seasonal malaria chemoprevention (SMC) for plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel subregion in Africa. March 2012. (https://apps.who.int/iris/handle/10665/337978). [Google Scholar]
- 21.Balikagala B, Fukuda N, Ikeda M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 2021;385:1163–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.