Abstract
Both neonatal and C57BL/6 gamma interferon (IFN-γ) knockout (C57BL/6-GKO) mice are susceptible to Cryptosporidium parvum, but the course of infection is different. Neonatal mice are able to clear the parasite within 3 weeks, whereas C57BL/6-GKO mice, depending on age, die rapidly or remain chronically infected. The mechanism by which IFN-γ leads to a protective immunity is yet poorly understood. In order to investigate the effect of IFN-γ on other cytokines expressed in the intestinal mucosa during C. parvum infection, we studied cytokine mRNA expression in the neonates and GKO (neonatal and adult) mice by quantitative reverse transcription-PCR (RT-PCR) at 4 and 9 days after infection. IFN-γ mRNA levels were quickly and strongly up-regulated in the mucosa of neonatal mice. In GKO mice, the Th1-type response was dramatically altered during the infection, whereas the mRNA expression levels of the Th2-type cytokines interleukin 4 (IL-4) and IL-10 were increased in both mouse models. In the absence of IFN-γ, the adult knockout mice up-regulated the mRNA levels of inflammatory cytokines, such as IL-1β, IL-6, and granulocyte-macrophage colony-stimulating factor, in the mucosa, but not tumor necrosis factor alpha (TNF-α), whereas all these cytokines were up-regulated in the infected neonatal mice. Further experiments indicated that injections of TNF-α into GKO adult mice significantly reduced oocyst shedding. The results of the present study indicate that the resolution of infection is dependent on the expression of Th1-type cytokines in the mucosa of C57BL/6 mice and that TNF-α may participate in the control of parasite development.
Cryptosporidium parvum is an obligate intracellular protozoan parasite that infects intestinal epithelial cells of humans and various other mammals. C. parvum causes protracted diarrhea in young and immunodeficient individuals and can lead to death for AIDS patients. Cryptosporidiosis is frequent in young farm animals and has economic and environmental consequences. In immunocompetent hosts, the disease is self-limited, suggesting a major role for host defense factors in controlling the infection.
Most of the studies of experimental cryptosporidiosis have been performed with rodents whose immune systems were impaired, e.g., neonatal mice (14, 25, 35), rats immunosuppressed with dexamethasone (27), or congenitally mutated nude (21, 23) and SCID mice (17, 34). More recent studies have used mice with targeted mutations for the genes of major histocompatibility complex class II (1), CD40, CD40L (7), or gamma interferon (IFN-γ) (33, 38). The key role of IFN-γ in resistance to C. parvum infection initially demonstrated with antibody depletion was confirmed more recently with IFN-γ knockout mice (GKO) (6, 33, 34). However, the mechanisms whereby IFN-γ intervenes in the clearance of C. parvum are still not well understood. Some possibilities, not mutually exclusive, include a direct toxic effect of IFN-γ on the parasite or the infected cells or the induction of other cytokines that can be toxic for the parasite or of chemoattractants for immune cells. Mead and You reported that susceptible BALB/c-GKO mice recover from infection whereas C57BL/6-GKO mice remain chronically infected (24), suggesting that other immune components related to the genetic background of the mice play a role in the susceptibility of mice to C. parvum infection.
To determine what IFN-γ-dependent components of the immune response could be involved in the clearance of infection, we compared differential cytokine expression in a healing neonatal mouse model and in a nonhealing mouse model (neonates and adults) of the same genetic background that was deficient in IFN-γ. The cytokine mRNA levels were measured by quantitative reverse transcription-PCR (RT-PCR) in the intestinal mucosa, at the site where parasites are actively multiplying. In this study, we report that the mRNA for both Th1- and Th2-type cytokines was up-regulated during C. parvum infection and that Th1 cytokines play a major role in the resolution of infection in the C57BL/6 genetic background. The overexpression of tumor necrosis factor alpha (TNF-α) observed during infection in neonatal mice was absent in GKO adult mice. This prompted us to explore the role of TNF-α in protection against C. parvum in a model devoid of IFN-γ. Intraperitoneal injections of TNF-α during the first days of the infection resulted in a significant reduction in oocyst shedding, suggesting that TNF-α can take part in protective immunity against C. parvum.
MATERIALS AND METHODS
Mice.
C57BL/6J-Ifgtml and wild-type mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). The dams with 1- to 2-day-old litters and the adults were housed individually in cages under pathogen-free conditions. In another experiment, 1-day-old GKO neonates were cross-fostered onto normal C57BL/6 mothers. Food and water were available ad libitum. For RT-PCR analysis, 3-day-old GKO and wild-type neonatal mice and 6- or 7-week-old GKO mice were inoculated with 106 oocysts of C. parvum by the oral route. For TNF-α experiments, 10-week-old GKO mice were inoculated with 105 oocysts and received repeated intraperitoneal injections of murine TNF-α (6.5 μg/kg of body weight) at days 0, 1, 2, and 4 after infection.
Parasite.
C. parvum oocysts were initially isolated from the feces of an infected child (3) and were maintained by regular passages in newborn calves. Fecal samples were stored in 2.5% potassium dichromate at 4°C until use. C. parvum oocysts, isolated from feces by filtration and diethyl ether sedimentation, were treated with 1.25% sodium hypochlorite, washed, and stored until use at 4°C in phosphate-buffered saline (pH 7.4) containing 50 U of penicillin G/ml and 0.25 mg of amphotericin B/ml. Oocysts were less than 2 months old when used as an inoculum.
Intestinal and fecal C. parvum oocyst determination.
The level of infection in individual neonatal mice was assessed by the number of oocysts in the intestinal content. Since infection is not always spread homogeneously along the intestine, the whole intestines were removed from neonatal mice. They were individually homogenized in 1 ml of water with an Ultra-turax, and oocyst quantification was performed in Sheather's solution on a Thoma cell.
Adult GKO mice were housed individually on a grating, and the numerations of daily shed oocysts were performed. Feces were filtered successively through two sieves of porosities of 425 and 100 μm. After centrifugation, the pellets were weighed. A part of each pellet was resuspended, and the oocysts were counted in the Sheather's solution on a Thoma cell.
RNA extraction.
Mice were killed at 4 and 9 days of infection, and ilea were removed. Peyer's patches were removed from ilea of adult mice before mRNA extraction processing. For the neonatal mice, controls were killed at identical ages. In order that the immune response in the upper layer of the infected mucosa might be studied, the ilea were not crushed but were split lengthwise and shaken in TRIzol (Gibco-BRL Life Technologies, Cergy Pontoise, France). After 5 min of incubation, ilea were removed and TRIzol solutions were centrifuged for 5 min at 8,000 × g to eliminate debris. The supernatants were stored at −70°C until further processing. RNAs were extracted according to the manufacturer's instructions and were quantified by measuring the optical density at 260 nm. RNA quality was estimated with agarose gel electrophoresis using ethidium bromide for staining.
Analysis of cytokine mRNA levels by reverse transcription-PCR.
One microgram of total RNA was reverse transcribed using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase, according to the manufacturer's instructions (Eurogentec, Angers, France). Ten percent of the synthesized cDNA was then subjected to PCR amplification (total volume of reaction mixture, 25 μl). Ten microliters of each RT-PCR mixture was electrophoresed on a 2% agarose gel. mRNA expression levels were quantified by competitive RT-PCR as described previously (16). Briefly, the standard RNA for β-actin, IFN-γ, interleukin 4 (IL-4), IL-6, IL-10, IL-12p40, inducible nitric oxide synthase (iNOS), TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) obtained after in vitro transcription of the different plasmids pMCQ1, -2, -3, and -4 (9) carries primer binding sites identical with those used to amplify target RNA. A similar plasmid was constructed for quantification of iNOS and IL-1β (F. Laurent and S. Lacroix, unpublished data). The distances between specific 5′ and 3′ primer sequences and, therefore, the sizes of PCR amplification products differ for standard and target RNAs. Serial threefold dilutions of standard RNA molecules were mixed with 1 μg of total sample RNA and reverse transcribed. PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining, and band intensities were quantitated by densitometry (Molecular Analyst; Bio-Rad S.A., Ivry-sur-Seine, France). The ratios of the band intensities of the PCR products from the standard RNA and target RNA were plotted against the starting number of standard RNA molecules using a double logarithmic scale. When the ratio equals 1, the number of target RNA molecules is equivalent to the number of standard RNA molecules.
RESULTS
Course of infection and weight changes.
To investigate the role of IFN-γ in the pathogenesis of C. parvum, we studied the susceptibility to infection of C57BL/6 mice which recover from infection and C57BL/6-GKO mice which do not recover (33).
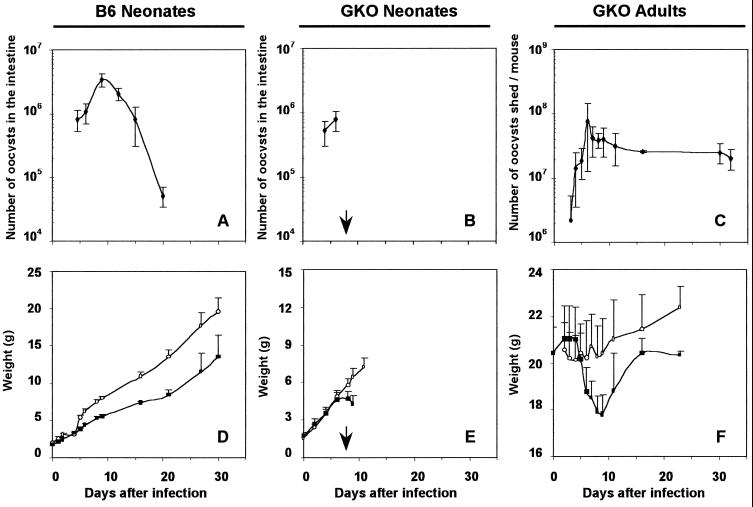
In C57BL/6 adult mice, under our experimental conditions, no oocysts could be detected in the feces of mice inoculated with 106 oocysts of C. parvum. In neonatal mice, the oocyst production in intestines were first detected 4 days postinfection (p.i.), increased until 9 days p.i., and then declined until no oocysts were detected under our experimental conditions by day 20 (Fig. 1A). The infected neonatal mice had a lower level of growth than the controls. The difference in weight was maintained until 30 days p.i. despite the clearance of infection (Fig. 1D). GKO neonates did not survive after 5 days p.i. when they were grown with their mother, because the dams became infected and did not feed their young. For GKO neonates cross-fostered onto C57BL/6 mothers, the course of infection and the weight time course were similar to those of wild-type C57BL/6 neonates in the first 6 days after inoculation (Fig. 1B). However, despite the fact that neonates were suckled normally, 80% of GKO neonatal mice died between days 7 and 9 p.i. During this period, the surviving mice were moribund and continued to lose weight, and none survived more than 10 days p.i. (Fig. 1E).
FIG. 1.
Course of infection and weight changes in C57BL/6 and GKO neonatal mice and GKO adult mice infected with 106 oocysts of C. parvum. Each point represents the mean ± the standard deviation of the number of oocysts (upper panels) and of the weight (lower panels). Parasite loads in the intestine of infected C57BL/6 neonatal mice (n = 5) (A) and GKO neonatal mice (n = 5) (B) are shown. (C) Oocyst excretion in GKO mice (n = 21 to 33 through day 9; n = 6 through day 32). (D) Weight changes in infected (■) (n = 6) and control (○) (n = 7 to 11) C57BL/6 neonatal mice. (E) Weight changes in infected (■) (n = 23 to 46 through day 8; n = 6 through day 9) and control (○) (n = 20) GKO neonatal mice. (F) Weight changes in control (○) (n = 6) and infected (■) (n = 10 to 15 through day 9; n = 3 through day 23) GKO mice. Arrows indicate the time point at which GKO neonates began to die.
For the GKO adult mice, excretion of oocysts in feces was first detected 3 days after inoculation and reached maximum excretion by day 6 p.i. The magnitude of oocyst shedding was maintained until death or euthanasia of the mice (Fig. 1C). Body weights of the infected mice decreased when oocyst shedding was at maximum and until 9 days p.i. Afterwards, the GKO mice gained back some weight despite the chronic infection; however, the weight difference persisted at least until the end of the experiment (30 days p.i.) (Fig. 1F).
Th1 and Th2 cytokines and iNOS mRNA expression is up-regulated during infection.
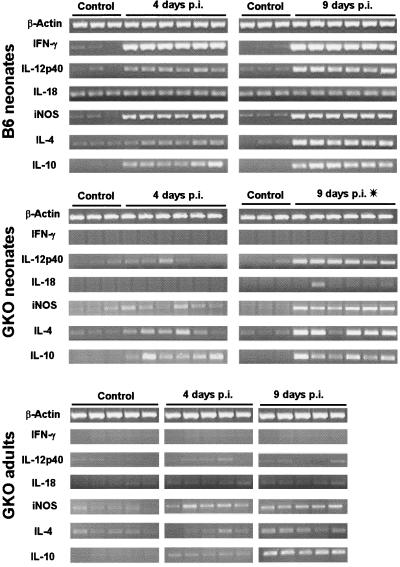
Control of parasitic infections is generally dependent on the production of cytokines; we therefore studied the expression of IFN-γ, IL-12p40, IL-18, and iNOS mRNA for the Th1 pattern and IL-4 and IL-10 mRNA for the Th2 pattern. To analyze cytokine expression during C. parvum infection, two time points were selected: 4 and 9 days p.i. for the beginning and the maximum of oocyst production, respectively, because of the course of infection in wild-type neonates and GKO adult mice. We first used qualitative RT-PCR to screen the cytokines that were regulated during infection and may therefore play a role in the resolution of cryptosporidiosis. Thereafter, in order to measure the levels of cytokine expression, we performed quantitative RT-PCR on total RNA pooled from the different mice.
In C57BL/6 adult mice, there were no modifications of the cytokine levels, probably due to the absence of significant infection. Figure 2 shows the individual cytokine response in wild-type neonates and in GKO (neonate and adult) mice. In neonatal mice, both Th1 and Th2 mRNA expression increased by days 4 and 9 p.i. in the ileum, except for IL-18, for which the expression levels did not change. This up-regulation of mRNA expression was markedly elevated at day 9 p.i., with a strong Th1-type cytokine expression (900× for IFN-γ and 666× for iNOS mRNA), although IFN-γ mRNA was already increased by 250-fold at 4 days p.i. (Table 1), suggesting that IFN-γ may be one of the first cytokines involved in the response to C. parvum.
FIG. 2.
Qualitative RT-PCR amplification of Th1 and Th2 cytokines and iNOS mRNA of C. parvum-infected C57BL/6 and GKO neonatal mice and GKO adult mice. mRNA was extracted from the ilea of mice, as reported in Materials and Methods. The data shown are the results from individual mice. Thirty-five amplification cycles were performed, except for β-actin (28 cycles) and iNOS (GKO adult mice) (33 cycles). Asterisk, ileum extracted from moribund GKO neonates.
TABLE 1.
Cytokine mRNA levels in the ilea of C. parvum-infected mice
| Mouse group | Cytokine | Result
|
|||||
|---|---|---|---|---|---|---|---|
| 4 Days after infection
|
9 Days after infection
|
||||||
| No. of transcripts/μga
|
Infected/control ratio | No. of transcripts/μga
|
Infected/control ratio | ||||
| Control | Infected | Control | Infected | ||||
| B6 neonates | β-Actin | 2.7 × 108 | 2.5 × 108 | 0.9 | 2.6 × 108 | 3.0 × 108 | 1.1 |
| IFN-γ | 2.0 × 103 | 5.0 × 105 | 250.0 | 1.0 × 103 | 9.0 × 105 | 900.0 | |
| IL-12p40 | 5.0 × 103 | 8.0 × 103 | 1.6 | 1.0 × 103 | 1.1 × 104 | 11.0 | |
| iNOS | 2.0 × 103 | 2.5 × 105 | 125.0 | 3.0 × 103 | 2.0 × 106 | 666.0 | |
| IL-4 | <103 | 3.5 × 103 | NCb | 1.0 × 103 | 6.5 × 104 | 65.0 | |
| IL-10 | 9.0 × 103 | 9.0 × 104 | 10.0 | 6.0 × 103 | 8.0 × 105 | 133.0 | |
| GKO neonates | β-Actin | 2.0 × 108 | 3.0 × 108 | 1.5 | 3.0 × 108 | 6.0 × 108 | 2.0∗c |
| IL-12p40 | 5.0 × 103 | 6.5 × 103 | 1.3 | 4.0 × 103 | 1.0 × 105 | 25.0∗ | |
| iNOS | 7.0 × 103 | 3.0 × 104 | 4.3 | 7.0 × 103 | 1.0 × 106 | 142.8∗ | |
| IL-4 | 1.8 × 103 | 5.6 × 103 | 3.1 | 1.8 × 103 | 7.0 × 104 | 38.9∗ | |
| IL-10 | 7.0 × 103 | 8.0 × 104 | 11.4 | 5.6 × 103 | 8.5 × 105 | 151.8∗ | |
| GKO adults | β-Actin | 5.0 × 108 | 6.0 × 108 | 1.2 | 5.0 × 108 | 5.0 × 108 | 1.0 |
| IL-12p40 | <103 | <103 | NC | <103 | <103 | NC | |
| iNOS | 1.3 × 106 | 3.0 × 106 | 2.3 | 1.3 × 106 | 7.0 × 106 | 5.4 | |
| IL-4 | 1.2 × 103 | 5.5 × 103 | 4.6 | 1.2 × 103 | 3.0 × 104 | 25.0 | |
| IL-10 | <103 | 1.0 × 105 | NC | <103 | 7.0 × 105 | NC | |
Values are numbers of cytokine transcripts/μg of total RNA. A total of 103 transcripts/μg of RNA was taken as a lower limit of quantitation.
NC, value cannot be calculated.
Asterisk indicates moribund mice.
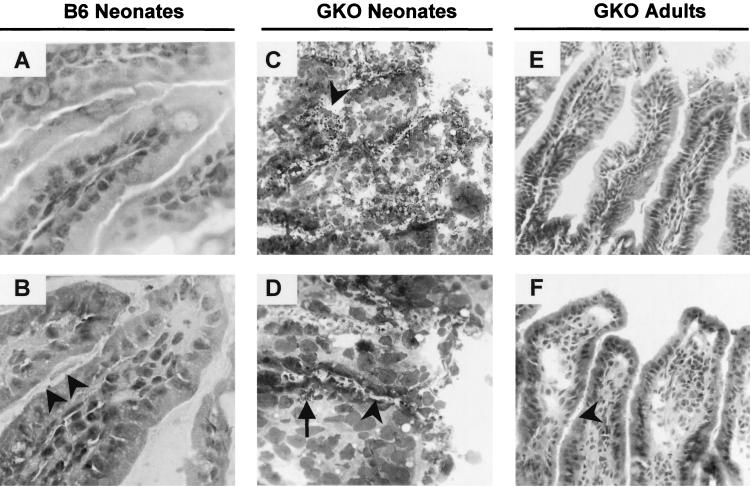
Although basal levels of mRNA were in some cases different between GKO neonates and GKO adult mice, the extent of the cytokine mRNA response was similar at day 4 p.i. In the absence of IFN-γ, the expression of the Th2-type cytokines, IL-4 and IL-10, increased as in wild-type neonates, whereas iNOS mRNA up-regulation was weak. By day 9 p.i., when the levels of iNOS (5.4×) and IL-12p40 mRNA remained weak or undetectable in GKO adult mice despite the extent of the infection, mRNA levels in GKO neonates were increased (143× for iNOS and 25× for IL-12p40). It should be noted that the cytokine response in GKO neonates at day 9 p.i. was studied with moribund mice, which manifested acute injury of the villi (Fig. 3C and D).
FIG. 3.
Histological analysis of hematoxylin- and eosin-stained section of ileum from wild-type neonates (A and B) (magnification, ×800), GKO neonates (C and D) (magnification, ×300 and ×800, respectively), and GKO adult mice (E and F) (magnification, ×300). Panels A and E show ileum from control mice. Panels B, C, D, and E show ileum from mice infected for 9 days with C. parvum (C. parvum organisms are indicated by arrowheads). Note in panels C and D the detachment of enterocytes at the tip of the villi and the infiltration of the lamina propria with bacteria (arrow).
mRNA expression of proinflammatory cytokines is upregulated during infection.
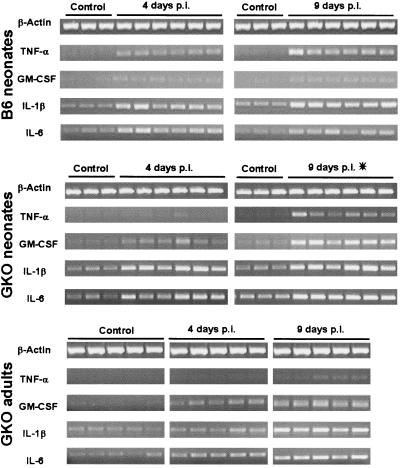
Among a spectrum of pathological changes observed in the intestine after C. parvum infection, mucosal inflammation with neutrophils and mononuclear infiltrates in the lamina propria is frequently observed. In wild-type neonates the inflammation was moderate (Fig. 3B), whereas in GKO adult mice, which were more infected, the villi were heavily infiltrated by inflammatory cells (Fig. 3F). In GKO neonates still alive at day 9 p.i., C. parvum infection induced ileal mucosa injury, with a detachment of enterocytes at the tip of the villi (Fig. 3C and D). TNF-α and IL-1β are the main cytokines produced at the inflammatory sites and subsequently induce mRNA expression of GM-CSF and IL-6. To investigate the inflammatory response, we studied mRNA expression of these proinflammatory cytokines in the ilea of C. parvum-infected mice. The mRNA expression of TNF-α, IL-1β, and IL-6 increased moderately in the wild-type neonatal mice 4 and 9 days after infection (Fig. 4; Table 2). In the GKO mice (adults and neonates), IL-1β and IL-6 mRNA levels increased by day 4 p.i., at which time the TNF-α mRNA was not up-regulated. At day 9 p.i., in surviving GKO neonates, IL-6, GM-CSF, TNF-α, and mainly IL-1β mRNA levels were higher than for the wild-type neonates. The strong overexpression of proinflammatory cytokines was probably due to the destruction of the ileal epithelium. Therefore, the immune response at this time point may not reflect only the immune response to C. parvum infection. By day 9 p.i., in the GKO adult mice which presented nondestructive inflammation of the intestine, GM-CSF, IL-6, and IL-1β mRNA were overexpressed, whereas TNF-α mRNA was still not up-regulated, despite significant infection.
FIG. 4.
Qualitative RT-PCR amplification of the mRNA of inflammatory cytokines of C. parvum-infected C57BL/6 and GKO neonatal mice and GKO adult mice. mRNA was extracted from the ilea of mice, as reported in Materials and Methods. The data shown are the results from individual mice. Thirty-three amplification cycles were performed, except for β-actin (28 cycles) and TNF-α (35 cycles). Asterisk, ileum extracted from moribund GKO neonates.
TABLE 2.
Proinflammatory cytokine mRNA levels in the ilea of C. parvum-infected mice
| Mouse group | Cytokine | Result
|
|||||
|---|---|---|---|---|---|---|---|
| 4 Days after infection
|
9 Days after infection
|
||||||
| No. of transcripts/μga
|
Infected/control ratio | No. of transcripts/μga
|
Infected/control ratio | ||||
| Control | Infected | Control | Infected | ||||
| B6 neonates | β-Actin | 2.7 × 108 | 2.5 × 108 | 0.9 | 2.6 × 108 | 3.0 × 108 | 1.1 |
| TNF-α | 4.0 × 104 | 2.0 × 105 | 5.0 | 7.0 × 104 | 1.0 × 106 | 14.0 | |
| IL-1β | 5.6 × 103 | 2.0 × 104 | 3.5 | 9.0 × 103 | 5.0 × 104 | 5.5 | |
| IL-6 | 6.5 × 103 | 2.5 × 104 | 3.8 | 5.0 × 103 | 2.5 × 104 | 5.0 | |
| GM-CSF | <103 | <103 | NCb | <103 | <103 | NC | |
| GKO neonates | β-Actin | 2.0 × 108 | 3.0 × 108 | 1.5 | 3.0 × 108 | 6.0 × 108 | 2.0∗c |
| TNF-α | 8.0 × 104 | 9.0 × 104 | 1.1 | 8.0 × 104 | 4.1 × 106 | 51.2∗ | |
| IL-1β | 4.5 × 103 | 5.0 × 104 | 11.1 | 7.0 × 103 | 1.3 × 106 | 185.7∗ | |
| IL-6 | 4.0 × 104 | 8.5 × 105 | 21.2 | 1.7 × 104 | 6.0 × 105 | 35.3∗ | |
| GM-CSF | <103 | <103 | NC | <103 | 8.0 × 105 | NC∗ | |
| GKO adults | β-Actin | 5.0 × 108 | 6.0 × 108 | 1.2 | 5.0 × 108 | 5.0 × 108 | 1.0 |
| TNF-α | <103 | <103 | NC | <103 | <103 | NC | |
| IL-1β | 2.0 × 104 | 4.0 × 104 | 2.0 | 2.0 × 104 | 3.0 × 105 | 15.0 | |
| IL-6 | 4.0 × 103 | 3.0 × 104 | 7.5 | 4.0 × 103 | 6.0 × 104 | 15.0 | |
| GM-CSF | <103 | 1.1 × 103 | NC | <103 | 1.6 × 104 | NC | |
Values are numbers of cytokine transcripts/μg of total RNA. 103 transcripts/μg of RNA was taken as a lower limit of quantitation.
NC, value cannot be calculated.
Asterisk indicates moribund mice.
Exogenous TNF-α injections in GKO mice decrease oocyst shedding.
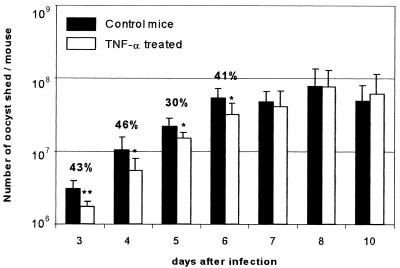
To investigate the role of TNF-α in the development of cryptosporidiosis, murine recombinant TNF-α was administrated repetitively by the intraperitoneal route in infected GKO mice. Daily C. parvum oocyst excretion was measured individually to determine the severity of infection. From day 3 p.i., TNF-α-treated mice shed significantly fewer oocysts than controls (Fig. 5). After 7 days, at which point the last injection of TNF-α had been performed 3 days earlier, there was no longer any difference in oocyst excretion.
FIG. 5.
TNF-α decreases the shedding of oocysts in C. parvum-infected GKO mice. Ten-week-old mice were infected with 105 oocysts of C. parvum and received repeated intraperitoneal injections of murine rTNF-α (6.5 μg/kg) at days 0, 1, 2, and 4. This graph represents the results of two experiments. Each experiment was performed with four mice per group, and the results were individually significant. The percentages represent the reduction of oocyst shedding after TNF-α treatment. Significant differences were observed (double asterisk, P < 0.01; single asterisk, P < 0.05 [Mann-Whitney U test]).
DISCUSSION
The role of IFN-γ in enhancing resistance to C. parvum was clearly demonstrated by several groups using administration of neutralizing IFN-γ antibody or GKO mice (6, 22, 35). Recent findings showed that BALB/c-GKO mice were able to clear the parasite within 2 weeks after infection, suggesting that in the absence of IFN-γ, other mechanisms can lead to protection in BALB/c mice (38). In chronically infected adult C57BL/6-GKO mice, these mechanisms, if they exist, are not sufficient to eliminate the infection. C57BL/6 mouse healing is therefore dependent on the presence of IFN-γ, but the way in which IFN-γ has its protective effect still needs to be clarified.
In this study, we investigated the effect of IFN-γ on other cytokines that could play a role in the resolution of infection using C57BL/6 neonates and neonatal and adult GKO mice. The balance between a Th1 and Th2 cytokine response often regulates the outcome of infection with many organisms (15). C. parvum infection of wild-type neonates induces strong up-regulation of IFN-γ, iNOS, and IL-12p40 mRNA expression in the mucosa, whereas in the adult GKO mice mRNA expression of IL-12p40 and iNOS was not increased or was poorly increased. Culshaw et al. previously reported that intraepithelial lymphocytes (IEL) could be a source of IFN-γ that conferred protection against cryptosporidiosis in mice (8). Both IL-12 (35) and NO (19) have been shown to also participate in the clearance of infection. Urban et al. showed that the treatment of mice with IL-12 before experimental inoculation prevented or greatly reduced the severity of the infection via an IFN-γ-dependent mechanism. Our data show that the increase of IFN-γ mRNA expression preceded the maximum level of IL-12p40 mRNA expression, suggesting that IL-12 may not be the only cytokine participating in the initial increase in IFN-γ mRNA expression in the mucosa. IL-18, first designated IGIF (interferon gamma inducing factor), is another strong inductor of IFN-γ and is expressed in intestinal epithelial cells (31). However, although a low level of mRNA expression was measured by RT-PCR, we did not observe any increase of the expression level during the infection. To release the IL-18 mature form, pro-IL-18 needs to be cleaved by a protease, ICE or caspase 1 (10). IL-18 is unlikely to play a role in the initial IFN-γ response after C. parvum infection, since injection of caspase 1 inhibitor in neonates did not modify the IFN-γ mRNA response (data not shown). A recent study by Leitch and He demonstrated a modest but significant role for reactive nitrogen produced by epithelial cells in limiting the severity and course of infection in neonatal mice (19). Our results confirm the increased expression of iNOS in the ileum following infection of neonatal mice. Our results also extend those findings by demonstrating that IFN-γ is most probably responsible for the majority of iNOS mRNA up-regulation after infection. In fact, in the absence of IFN-γ, GKO neonates did not up-regulate iNOS mRNA levels compared to wild-type neonates by day 4 p.i. In addition, in adult GKO mice, iNOS mRNA expression was not strongly increased despite significant infection. The strong up-regulation of iNOS mRNA observed in surviving GKO neonates at day 9 p.i. was probably due to the presence of bacteria in the injured mucosa. Several in vitro studies reported that bacterial infections induce strong and rapid up-regulation of iNOS mRNA levels in intestinal epithelial cells (28, 36). Moreover, in vitro stimulation of epithelial cells with IFN-γ increased iNOS mRNA levels and NO production, whereas C. parvum infection alone did not (unpublished data).
In both wild-type neonates and GKO (neonatal and adult) mice, mRNA expression levels for IL-4 and IL-10 increased during infection. In IFN-γ-deficient mice, C. parvum infection did not result in a shift to a higher level of Th2 cytokine mRNA expression, as observed for other infectious agents like herpesvirus or influenza virus (5, 12). It was assumed that the increased level of IL-4 mRNA observed in our study with infected neonatal and knockout (KO) mice was produced by intraepithelial lymphocytes, as shown by Aguirre et al. (2). The role of IL-4 in the termination of infection was demonstrated using antibody depletion and C57BL/6 IL-4 KO mice. However, increased expression of IL-10 has never been observed in murine or bovine mucosa infected by C. parvum, and its role in protection has not been demonstrated. Thus, the role of IL-10 in the resolution of C. parvum infection deserves to be investigated. However, the presence of IL-4 and IL-10 in the mucosa during the infection of adult C57BL/6-GKO mice did not prevent the chronic infection, suggesting that in the C57BL/6 mice, the Th1 cytokine response is indispensable for the clearance of the parasite.
C. parvum infection results in mucosal inflammation of the intestine with infiltration of inflammatory cells, such as monocytes and neutrophils (11). The extent of the inflammation in the mucosa is generally related to the severity of infection. Members of our laboratory and others have previously shown that C. parvum-infected epithelial cells can participate in mucosal inflammation by producing C-X-C chemokines (IL-8 and Gro-α) (18, 29). Proinflammatory cytokines like IL-1β and TNF-α, produced by many different cell types, can induce and amplify the secretion of various chemokines and therefore promote the recruitment of inflammatory cells in the mucosa. Our finding that IL-1β and TNF-α transcripts were produced in response to C. parvum infection in wild-type neonates confirms the findings of Seydel et al. using the human intestinal xenograft model (29). Our results extend those findings by demonstrating that IL-6 and GM-CSF transcripts are also produced in response to C. parvum infection. In the complete absence of IFN-γ, C. parvum infection resulted in a more extensive inflammation of the ileum than in the wild-type neonates. In adult GKO mice, expression of the proinflammatory cytokine was elevated except for TNF-α mRNA, which was undetectable despite severe infection. Moreover, TNF-α mRNA up-regulation at day 4 p.i. was lower in GKO neonates (1.1×) than in wild-type neonates (5×). The recent study of Smith et al. (30), who demonstrated the absence of TNF-α mRNA in the splenocytes of C57BL/6-GKO mice after infection with C. parvum, is consistent with our results with GKO adult mice. It seems likely, therefore, that IFN-γ could contribute to the overexpression of TNF-α mRNA observed in the ileum during C. parvum infection. In this study, we demonstrated that exogenous TNF-α significantly decreased excretion of oocysts in infected adult C57BL/6-GKO mice which did not overexpress this proinflammatory cytokine, suggesting that TNF-α could participate in protection against C. parvum. Despite increased expression of TNF-α mRNA levels by moribund GKO neonates at day 9 p.i., none survived more than 10 days p.i. We hypothesize that the increased TNF-α mRNA levels in moribund mice were probably not related only to C. parvum infection and, in any case, the TNF-α would have been produced too late to have an effect on the extent of infection, since at this time point there was already extensive damage to the intestine. The role of TNF-α in cryptosporidiosis had already been studied with mice, but the injection of neutralizing TNF-α-specific antibody did not affect the course of infection (6, 21). The discrepancy between these results and our new findings that TNF-α can participate in protection may be due to the presence of IFN-γ in mice, which may mask any ameliorating effects of TNF-α. Furthermore, it should be emphasized that the mouse strain can have a large effect on the outcome of infection. Smith et al. showed that an increase of TNF-α mRNA occurs in the splenocytes of infected BALB/c-GKO mice, contrary to what is observed in C57BL/6-GKO mice (30). The fact that the BALB/c-GKO strain can resolve infection is in favor of a role of TNF-α in the resolution of cryptosporidiosis. Moreover, the role of TNF-α in the control of infection has already been demonstrated for leishmania (32, 37) and Trichuris muris (4) disease. Many different cell types in the mucosa can release this cytokine, including IEL, which are in close contact with epithelial cells. TNF-α can be chemotactic and activate inflammatory cells and IEL. Moreover, TNF-α can induce the death of infected or senescent epithelial cells by apoptosis (13). We recently showed that the infection of intestinal epithelial cells by C. parvum induced apoptosis (20, 26); however, the involvement of TNF-α in this mechanism remains to be demonstrated in vivo.
In conclusion, the mucosal immune response to C. parvum in C57BL/6 neonatal and GKO mice demonstrates a concomitant Th1 and Th2 cytokine mRNA expression, with a crucial role for IFN-γ in the resolution of the infection. IFN-γ acts most probably via more than one single mechanism. IL-12 and NO have been reported to participate in the protection of mice against C. parvum. In this study, we showed that IFN-γ facilitates the up-regulation of IL-12, iNOS, and TNF-α mRNA expression in the intestinal mucosa after C. parvum infection. The injection of exogenous TNF-α in C57BL/6-GKO mice, which significantly decreased oocyst shedding, suggests that this cytokine may take part in the IFN-γ-mediated protective immune response.
ACKNOWLEDGMENTS
We thank Geneviève Fort for technical help in oocyst preparation, Sébastien Lavilatte for mice breeding, and David Ojcius (Institut Pasteur, Paris, France) for critical reviewing of the manuscript and for helpful discussion and encouragements. We are also very grateful to Martin Kagnoff (UCSD, San Diego, Calif.) for kindly providing plasmids pMCQ1, pMCQ2, pMCQ3, pMCQ4, and pCpNOSQ1.
REFERENCES
- 1.Aguirre S A, Mason P H, Perryman L E. Susceptibility of major histocompatibility complex (MHC) class I- and MHC class II-deficient mice to Cryptosporidium parvum infection. Infect Immun. 1994;62:697–699. doi: 10.1128/iai.62.2.697-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre S A, Perryman L E, Davis W C, McGuire T C. IL-4 protects adult C57BL/6 mice from prolonged Cryptosporidium parvum infection: analysis of CD4+αβ+IFN-γ+ and CD4+αβ+IL-4+ lymphocytes in gut-associated lymphoid tissue during resolution of infection. J Immunol. 1998;161:1891–1900. [PubMed] [Google Scholar]
- 3.Arnaud-Battandier F, Naciri M, Fisher A, Ricour C, Griscelli C, Yvore P. Intestinal cryptosporidiosis: a new cause of human diarrhoea. Gastroenterol Clin Biol. 1982;6:1045–1046. . (In French.) [PubMed] [Google Scholar]
- 4.Artis D, Humphreys N E, Bancroft A J, Rothwell N J, Potten C S, Grencis R K. Tumor necrosis factor α is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J Exp Med. 1999;190:953–962. doi: 10.1084/jem.190.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouley D M, Kanangat S, Wire W, Rouse B T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-γ knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 6.Chen W X, Harp J A, Harmsen A G, Havell E A. Gamma interferon functions in resistance to Cryptosporidium parvum infection in severe combined immunodeficient mice. Infect Immun. 1993;61:3548–3551. doi: 10.1128/iai.61.8.3548-3551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward A R. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culshaw R J, Bancroft G J, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect Immun. 1997;65:3074–3079. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckmann L, Fierer J, Kagnoff M F. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J Immunol. 1996;156:2894–2900. [PubMed] [Google Scholar]
- 10.Fantuzzi G, Dinarello C A. Interleukin-18 and interleukin-1 β: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 11.Genta R M, Chappell C L, White A C, Jr, Kimball K T, Goodgame R W. Duodenal morphology and intensity of infection in AIDS-related intestinal cryptosporidiosis. Gastroenterology. 1993;105:1769–1775. doi: 10.1016/0016-5085(93)91075-s. [DOI] [PubMed] [Google Scholar]
- 12.Graham M B, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon γ gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy-Grand D, DiSanto J P, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Harp J A, Sacco R E. Development of cellular immune functions in neonatal to weanling mice: relationship to Cryptosporidium parvum infection. J Parasitol. 1996;82:245–249. [PubMed] [Google Scholar]
- 15.Infante-Duarte C, Kamradt T. Th1/Th2 balance in infection. Springer Semin Immunopathol. 1999;21:317–338. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- 16.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhls T L, Mosier D A, Abrams V L, Crawford D L, Greenfield R A. Inability of interferon-gamma and aminoguanidine to alter Cryptosporidium parvum infection in mice with severe combined immunodeficiency. J Parasitol. 1994;80:480–485. [PubMed] [Google Scholar]
- 18.Laurent F, Eckmann L, Savidge T C, Morgan G, Theodos C, Naciri M, Kagnoff M F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitch G J, He Q. Reactive nitrogen and oxygen species ameliorate experimental cryptosporidiosis in the neonatal BALB/c mouse model. Infect Immun. 1999;67:5885–5891. doi: 10.1128/iai.67.11.5885-5891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCole D F, Eckmann L, Laurent F, Kagnoff M F. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect Immun. 2000;68:1710–1713. doi: 10.1128/iai.68.3.1710-1713.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald V, Deer R, Uni S, Iseki M, Bancroft G J. Immune responses to Cryptosporidium muris and Cryptosporidium parvum in adult immunocompetent or immunocompromised (nude and SCID) mice. Infect Immun. 1992;60:3325–3331. doi: 10.1128/iai.60.8.3325-3331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald V, Robinson H A, Kelly J P, Bancroft G J. Cryptosporidium muris in adult mice: adoptive transfer of immunity and protective roles of CD4 versus CD8 cells. Infect Immun. 1994;62:2289–2294. doi: 10.1128/iai.62.6.2289-2294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mead J R, Arrowood M J, Sidwell R W, Healey M C. Chronic Cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J Infect Dis. 1991;163:1297–1304. doi: 10.1093/infdis/163.6.1297. [DOI] [PubMed] [Google Scholar]
- 24.Mead J R, You X. Susceptibility differences to Cryptosporidium parvum infection in two strains of gamma interferon knockout mice. J Parasitol. 1998;84:1045–1048. [PubMed] [Google Scholar]
- 25.Novak S M, Sterling C R. Susceptibility dynamics in neonatal BALB/c mice infected with Cryptosporidium parvum. J Protozool. 1991;38:102S–104S. [PubMed] [Google Scholar]
- 26.Ojcius D M, Perfettini J L, Bonnin A, Laurent F. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 1999;1:1163–1168. doi: 10.1016/s1286-4579(99)00246-4. [DOI] [PubMed] [Google Scholar]
- 27.Rehg J E. Effect of interferon-γ in experimental Cryptosporidium parvum infection. J Infect Dis. 1996;174:229–232. doi: 10.1093/infdis/174.1.229. [DOI] [PubMed] [Google Scholar]
- 28.Salzman A L, Eaves-Pyles T, Linn S C, Denenberg A G, Szabo C. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology. 1998;114:93–102. doi: 10.1016/s0016-5085(98)70637-7. [DOI] [PubMed] [Google Scholar]
- 29.Seydel K B, Zhang T, Champion G A, Fichtenbaum C, Swanson P E, Tzipori S, Griffiths J K, Stanley S L., Jr Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect Immun. 1998;66:2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith L M, Bonafonte M T, Mead J R. Cytokine expression and specific lymphocyte proliferation in two strains of Cryptosporidium parvum-infected γ-interferon knockout mice. J Parasitol. 2000;86:300–307. doi: 10.1645/0022-3395(2000)086[0300:CEASLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi M, Nishizaki Y, Sano O, Ohta T, Ikeda M, Kurimoto M. Immunohistochemical and immuno-electron-microscopic detection of interferon-γ-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- 32.Taylor A P, Murray H W. Intracellular antimicrobial activity in the absence of interferon-γ: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-γ gene-disrupted mice. J Exp Med. 1997;185:1231–1239. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theodos C M, Sullivan K L, Griffiths J K, Tzipori S. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect Immun. 1997;65:4761–4769. doi: 10.1128/iai.65.11.4761-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungar B L P, Kao T C, Burris J A, Finkelman F D. Cryptosporidium infection in an adult mouse model: independent roles for IFN-γ and CD4+ T lymphocytes in protective immunity. J Immunol. 1991;147:1014–1022. [PubMed] [Google Scholar]
- 35.Urban J F, Jr, Fayer R, Chen S, Gause W C, Gately M K, Finkelman F D. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J Immunol. 1996;156:263–268. [PubMed] [Google Scholar]
- 36.Witthoft T, Eckmann L, Kim J M, Kagnoff M F. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am J Physiol. 1998;275:G564–G571. doi: 10.1152/ajpgi.1998.275.3.G564. [DOI] [PubMed] [Google Scholar]
- 37.Xu D, McSorley S J, Tetley L, Chatfield S, Dougan G, Chan W L, Satoskar A, David J R, Liew F Y. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-α, and IFN-γ administered orally via attenuated Salmonella typhimurium. J Immunol. 1998;160:1285–1289. [PubMed] [Google Scholar]
- 38.You X, Mead J R. Characterization of experimental Cryptosporidium parvum infection in IFN-γ knockout mice. Parasitology. 1998;117:525–531. doi: 10.1017/s0031182098003424. [DOI] [PubMed] [Google Scholar]