Take Home Message
Salvage radical prostatectomy is a therapeutic option still associated with a high postoperative morbidity rate. Salvage focal treatments could be a viable therapeutic alternative, but there are few studies on the topography of prostate cancer recurrence and a lack of theoretical confirmation of the viability of such an approach.
Keywords: Salvage radical prostatectomy, Recurrent prostate cancer, Focal therapy
Abstract
Background
Most prostate cancer (PCa) recurrences after nonsurgical first-line treatment are managed with androgen deprivation therapy (ADT). When local treatment is indicated, salvage focal treatment (FT) may achieve outcomes similar to those after salvage radical prostatectomy (sRP), with lower morbidity. However, descriptions of the topography of PCa recurrence are scarce.
Objective
To describe the characteristics and topography of recurrent PCa at sRP.
Design, setting, and participants
We performed a review of the final pathology for consecutive men undergoing sRP at a single centre between 2007 and 2021.
Outcome measurements and statistical analysis
Clinical and pathological outcomes and recurrence localisation (standardised map) were recorded. Suitability for salvage FT was evaluated using criteria defined a priori.
Results and limitations
We included 41 men who underwent sRP after whole-gland treatment (82.9% primary radiotherapy). Of these, 68.3% had grade group ≥3 and 46.3% had pT3 disease, including nine men (22%) with seminal vesicle involvement >1 cm. The pN+ rate was 29.3%. Surgical margins were positive in 39% (mostly at the apex, 21.9%). PCa was located at <3 mm from the apex in 68% of cases. The segment most frequently involved was the mid-gland (93%). The median prostate and index lesion (IL) volume was 31.4 cm3 (interquartile range [IQR] 23–37) and 2 cm3 (IQR 0.5–6), respectively. A solitary IL was present in 63.4% of cases, while 7.3% had whole-gland PCa involvement. Overall, 56% of the men (n = 23) were deemed suitable for salvage FT (although seven had pN+ disease). The sample size, single-centre retrospective design, and unavailability of magnetic resonance imaging data are the main limitations.
Conclusions
According to sRP pathology, radiorecurrent PCa is an aggressive disease, frequently showing extraprostatic extension, positive margins, and apical involvement. The majority of cases still harbour a solitary index lesion and a consistent proportion may be suitable for a gland-preserving strategy.
Patient summary
In this report we looked at the location of prostate cancer recurrence within the prostate gland after radiotherapy or ablation, in which energy (such as heat, cold, or laser energy) is used to kill cells. We found that although these recurrences are often high-grade locally advanced disease, around half of cases might be suitable for a gland-preserving salvage treatment.
1. Introduction
The rate of prostate cancer (PCa) recurrence after radiotherapy (RT) and/or brachytherapy has been reported as 10–30% at 5 yr up to 50–60% at 10 yr [1], [2], [3]. Considering the average rate of recurrence, radiorecurrent PCa would represent the fourth most common male genitourinary cancer [4], [5].
In the past few decades, epidemiological studies reported that more than 90% of patients with radiorecurrent PCa indiscriminately received palliative androgen deprivation therapy (ADT), losing the chance of a definitive cure [6] and experiencing the side effects associated with ADT and a decrease in quality of life [7].
At first glance, these figures do not seem justified considering that half of men with radioresistant PCa have not experienced metastases by 5 yr, which thus represents a significant window for cure. More recently, prostate-specific membrane antigen (PSMA) positron emission tomography (PET) imaging studies revealed that more than half of recurrences are confined to the prostate [8], [9].
However, this disturbing compromise is not surprising given the results from salvage radical prostatectomy (sRP) series. Despite renewed interest in sRP in recent years and better results in comparison to historical series, outcomes remain suboptimal and surgery constitutes a challenging option, even in expert hands [10], [11], [12]. High-grade complications are experienced by one in ten men and overall complications by one in three men, one in four men have severe incontinence (>3 pads/d), and erectile function is poorly preserved [12].
To date, results for more than 500 men who received salvage focal treatment (FT) have been detailed. Although the follow-up is relatively short, oncological control seems acceptable and similar to that with sRP, with half of men not having evidence of recurrence at 3–5 yr [13]. Functional outcomes are promising, as continence can be maintained in up to 90% of cases, erectile function (among those who were potent preoperatively) generally only slightly decreases, and complications are relatively rare [13].
The rationale for FT is treatment of the index lesion, which is the largest and more aggressive cancer focus that is likely to drive PCa progression and metastatic spread, eventually leading to death. By contrast, satellite nonsignificant lesions are unlikely to evolve [14]. Interestingly, PCa recurrence has been found at the same site as for the original index lesion in up to 90–100% of cases. First-line whole-gland treatment may definitively silence nonsignificant foci, while failure may be related to radioresistant clones emerging within the index lesion. However, this evidence remains questionable, as analysis is sometimes based on biopsy specimens and/or imaging only, and performed in cohorts with a limited number of patients or including men who underwent sRP more than 20 yr previously [15], [16], [17], [18], [19].
Detailed information on the location and characteristics of radiorecurrent PCa is key to confirming the feasibility of salvage FT and defining an ad hoc approach. Hence, we performed a pathological analysis of sRP specimens to describe the characteristics and topography of recurrent PCa after nonsurgical whole-gland primary treatment.
2. Patients and methods
2.1. Collection of clinical data
We retrospectively reviewed data for consecutive men undergoing sRP for recurrent PCa at Molinette Hospital, Turin, between 2007 and 2021. The quality of the clinical data was independently reviewed by two physicians (G.C. and E.M.). Patients were contacted by phone in cases with relevant information missing and/or discrepancies. All men had negative conventional staging on imaging, including axial imaging (computed tomography scan or prostate multiparametric magnetic resonance imaging [mpMRI]) and a bone scan.
Functional outcomes were recorded at baseline and 12 mo. Postoperative erectile function was categorised as: (1) spontaneous erections with/without use of a phosphodiesterase type 5 inhibitor; (2) use of prostaglandin E or a vacuum device; (3) a penile prosthesis; or (4) no erectile function, as previously described [10]. Continence was recorded as the number of pads used per day and categorised as full continence (no pads), terminal dribbling, or mild (1 pad/d), moderate (2 pads/d), or severe incontinence (≥3 pads/d). Complications were graded using the Clavien-Dindo classification and in accordance with the European Association of Urology guidelines (major complication if Clavien ≥3); complication onset was categorised as <30 d, 30–90 d, or >90 d after sRP.
2.2. Pathological review
2.2.1. Processing and classification systems
All available specimens were processed using a standard whole-mount method [20]. All prostate specimens were fixed in 10% formalin solution for 24–48 h. The inferior‐most 0.5-cm and the superior‐most 5–10-mm portions of the gland were amputated, serially sectioned parallel to the urethra, and divided into the apex and base, respectively. The seminal vesicles were dissected perpendicular to the junction with the prostate. The mid‐gland was cut into 5‐mm‐thick slices. All specimens were embedded in paraffin, cut into 3-μm-thick sections, and stained with haematoxylin and eosin [21].
Pathological review was carried out by two expert uropathologists (E.V. and L.M.) according to the 2014 International Society of Urological Pathology (ISUP) consensus conference [22] and the 2017 Union for International Cancer Control pTNM classification [23]. An example of the specimen analysis is shown in Figure 1.
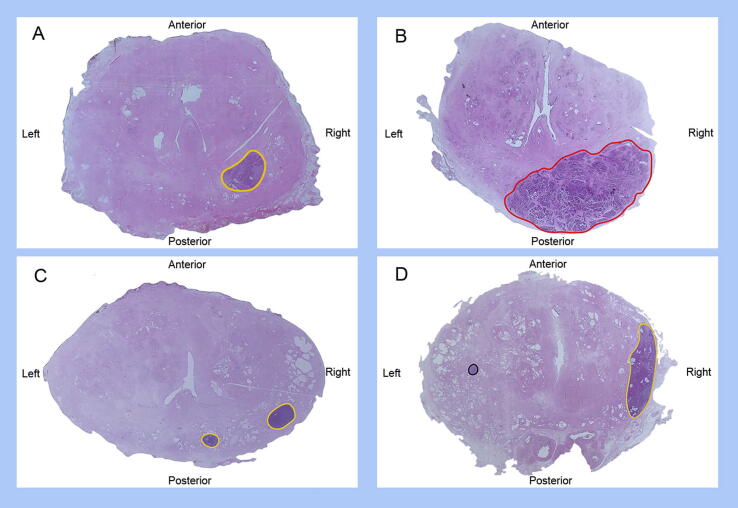
Fig. 1.
Examples of radical prostatectomy sections suitable for focal therapy (in particular, focal ablation, hemiablation, or hockey-stick ablation): (A) unifocal lesion; (B) unifocal lesion with <50% invasion on the contralateral side and suitable for hockey-stick ablation; (C) unilateral multifocal lesion (two tumour foci with the same ISUP grade group); and (D) bilateral unifocal lesions with a clinically insignificant secondary focus. Lesions are outlined in different colours according to the ISUP grade group: black = grade group 1; yellow = grade group 2–3; red = grade group 4–5. ISUP = International Society of Urological Pathology.
2.2.2. Topography definition
Specimens for which pathological review was not feasible because of poor slide quality were excluded (n = 3). In each slide, areas of adenocarcinoma were identified and the contours were marked with ink.
Zonal PCa sites were categorised using both the Prostate Imaging-Recording and Data System v2.1 map and by dividing the prostate in 12 zones according to distinction of: (1) anterior versus posterior zones, with the urethra considered as the delineation limit; (2) the base, mid-gland, and apex; and (3) left versus right zones, with the midline crossing the urethra. Seminal vesicle involvement (SVI) was considered as a separate zone. Further methodological details regarding the pathology review are provided in the Supplementary material.
2.3. Suitability for salvage FT
Suitability for salvage FT was considered according to criteria established a priori. In contrast to FT for treatment-naïve PCa, Gleason score and extracapsular extension were not considered as exclusion criteria.
Patients were deemed suitable for salvage focal ablation (ablation of PCa with a 0.5-cm margin of normal tissue) in the following cases:
-
(1)
Unifocal cases with the possibility of performing unilateral (focal or hemiablation; Fig. 1A) up to hockey-stick ablation (for lesions involving one prostate side and <50% on the contralateral side; Fig. 1B) [24].
-
(2)
Multifocal unilateral cases (more than one lesion confined to the hemi-gland; Fig. 1C).
-
(3)
Multifocal cases with clinically significant PCa as for point (1) and clinically insignificant secondary foci, defined as tumour foci <0.5 cm3 and ISUP grade group 1 (Fig. 1D).
The criteria for unsuitability for salvage focal ablation were as follows:
-
(1)
Two or more index lesions on opposite prostate quadrants (Fig. 2A,B) or more extensive disease (Fig. 2C).
-
(2)
Cases with a bilateral solitary lesion invading >50% of the contralateral quadrant (Fig. 2D).
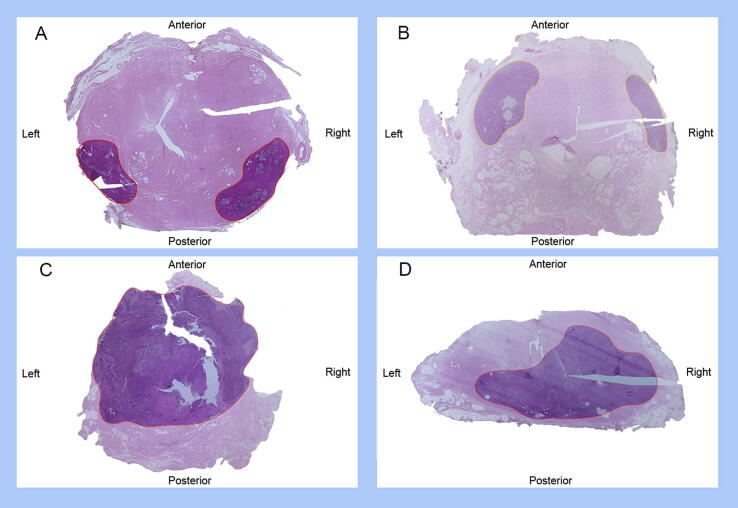
Fig. 2.
Examples of radical prostatectomy sections from patients unsuitable for focal therapy: (A,B) bilateral lesions (two tumor foci in opposite quadrants); (C) lesion extending to the whole gland; and (D) bilateral unifocal lesion invading >50% of the contralateral quadrant. Lesions are outlined in different colours according to the International Society of Urological Pathology grade group: black = grade group 1; yellow = grade group 2–3; red = grade group 4–5.
2.4. Statistical analysis
Results for continuous and categorical variables are expressed as the median and interquartile range (IQR), and the frequency and proportion, respectively. Kaplan-Meier curves were used to assess time to biochemical recurrence (BCR) and castrate-resistant prostate cancer (CRPC), as well as PCa survival (PCa-specific or overall survival).
Statistical analysis was conducted using SAS v9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Baseline characteristics, morbidity, and outcomes
The baseline characteristics for the 41 patients in the study cohort are listed in Table 1. All patients were Caucasian and the median age at sRP was 70 yr (IQR 66.5–73). All the men had localised disease (cN0M0) at first PCa diagnosis, with only one patient having cT3 disease. The majority (82.9%) underwent RT, while five men (12.2%) underwent whole-gland high-intensity focal ultrasound (HIFU). The median time to BCR was 57 mo (35.7–118.8).
Table 1.
Baseline characteristics of the study cohort at first-line and secondary (salvage radical prostatectomy) treatment; all men had negative preoperative staging according to conventional imaging (axial imaging using computed tomography or mpMRI and a bone scan)
| Parameter | Result |
|---|---|
| First-line prostate cancer treatment | |
| Median prostate-specific antigen, ng/ml (interquartile range) | 7.5 (6.4–45) |
| Median number of cores taken on prostate biopsy, n (interquartile range) | 11.5 (8–12) |
| Median number of positive cores on prostate biopsy, n (interquartile range) | 3.5 (2–6) |
| ISUP grade group, n (%) | |
| Grade group 1 | 20 (48.8) |
| Grade group 2 | 6 (14.6) |
| Grade group 3 | 4 (9.7) |
| Grade group 4 | 3 (7.3) |
| Grade group 5 | 6 (14.6) |
| cT stage, n (%) | |
| cT1 | 20 (48.8) |
| cT2 | 11 (29.3) |
| cT3 | 1 (2.4) |
| cNM0 | 41 (100.0) |
| First-line treatment type, n (%) | |
| Radiotherapy | 34 (82.9) |
| Median dose, Gy (interquartile range) | 76 (74–78) |
| Brachytherapy | 2 (4.8) |
| Whole-gland high-intensity focused ultrasound | 5 (12.2) |
| Androgen deprivation therapy, n (%) | |
| Neoadjuvant | 6 (14.6) |
| Adjuvant | 14 (34.1) |
| Both | 3 (7.3) |
| Median time to BCR after first-line treatment, mo (interquartile range) | 57 (35.7–118.85) |
| Characteristics at salvage radical prostatectomy | |
| Median age, yr (interquartile range) | 70 (66.5–73) |
| Caucasian race, n (%) | 41 (100.0) |
| Family history of prostate cancer, n (%) | 4 (10.0) |
| Previous transurethral resection of the prostate, n (%) | 3 (7.3) |
| American Society of Anesthesiologists score, n (%) | |
| 1 | 2 (4.9) |
| 2 | 18 (43.9) |
| 3 | 17 (41.5) |
| 4 | 1 (2.4) |
| Charlson comorbidity score, n (%) | |
| 1 | 4 (9.8) |
| 2 | 6 (14.6) |
| 3 | 12 (29.3) |
| 4 | 7 (17.0) |
| 5 | 6 (14.6) |
| 6 | 2 (4.9) |
| Positive prostate findings on MRI, n (%) a | 11 (84.6) |
| Positive findings on choline positron emission tomography, n (%) | 32 (78.0) |
| Prostate | 21 (65.6) |
| Lymph nodes | 1 (3.1) |
| Prostate + lymph nodes | 3 (9.4) |
| Negative | 7 (17.0) |
| Positive findings on PSMA positron emission tomography, n (%) b | 5 (12.2) |
| Prostate | 3 (7.3) |
| Lymph nodes | 0 (0.0) |
| Prostate + lymph nodes | 1 (2.4) |
| Negative | 1 (2.4) |
| Median number of cores taken on prostate biopsy, n (interquartile range) | 11.5 (8–12) |
| Median number of positive cores on prostate biopsy, n (interquartile range) | 3.5 (2–6) |
| ISUP grade group c | |
| Grade group 1 | 5 (12.2) |
| Grade group 2 | 5 (12.2) |
| Grade group 3 | 2 (4.9) |
| Grade group 4 | 4 (9.8) |
| Grade group 5 | 1 (2.4) |
| Not attributable | 1 (2.4) |
BCR = biochemical recurrence; ISUP = International Society of Urological Pathology; mpMRI = multiparametric resonance imaging; PSMA = prostate-specific membrane antigen.
Percentage calculated among the patients who underwent prostate mpMRI (n = 13).
Two men underwent both choline and PSMA positron emission tomography that showed suspicious nodes, which were then confirmed at final pathology.
Data missing for 23 men.
Before sRP, the median prostate-specific antigen level was 7.5 ng/ml (IQR 5.25–9.75). Thirty-five men underwent a preoperative PET scan, of whom five had suspected nodal involvement. The median number of positive biopsy cores was 3.5 (IQR 2–6).
Intraoperative sRP characteristics, morbidity data, and oncological and functional outcomes are presented in Supplementary Table 1. Approximately one in four men was severely incontinent at 1 yr (28%) and high-grade complications were experienced by 10%. Approximately one-third of the men (34.1%) had BCR at a median of 14 mo; systemic progression was experienced by 24.4% and 17% developed CRPC. At 5 yr, PCa-specific was 90% and overall survival was 74%.
3.2. Pathological features
Table 2 summarises the sRP pathology. ISUP grade group was ≥3 in 68.3% (n = 28). Almost half (46.3%) had pT3 disease, including 12 (29.3%) with SVI, which was >1 cm in nine cases (22%). Surgical margins were positive in 39% (n = 16) of the men, mainly unilaterally (n = 10) and at the apex (n = 9). Positive surgical margins were multifocal in eight cases and were most frequently positive at the apex (21.9%), while extracapsular extension was more common at the base (31.7%) and was not infrequently multifocal (21.9%). In the majority of cases, PCa was located <3 mm from the apex (68%). The median prostate volume and index lesion volume were 31.4 cm3 (IQR 23–37) and 2 cm3 (IQR 0.5–6), respectively.
Table 2.
Pathological analysis and topography of salvage radical prostatectomy
| Parameter | Result |
|---|---|
| Year of salvage radical prostatectomy, n (%) | |
| <2010 | 7 (17.0) |
| 2011–2015 | 17 (41.5) |
| 2016–2021 | 17 (41.5) |
| International Society of Urological Pathology grade group, n (%) | |
| Grade group 1 | 4 (9.8) |
| Grade group 2 | 7 (17.0) |
| Grade group 3 | 12 (29.3) |
| Grade group 4 | 3 (7.3) |
| Grade group 5 | 13 (31.7) |
| Not evaluable | 2 (4.9) |
| pT stage, n (%) | |
| pT2 | 22 (53.6) |
| pT3a | 7 (17.0) |
| pT3b | 12 (29.3) |
| Positive surgical margin status (R+), n (%) | 16 (39.0) |
| Unilateral | 10 (24.4) |
| Bilateral | 6 (14.6) |
| R+ focality | |
| Unifocal | 8 (19.5) |
| Multifocal | 8 (19.5) |
| R+ site a | |
| Apex | 9 (21.9) |
| Mid-gland | 7 (17.0) |
| Base | 7 (17.0) |
| R+ extent | |
| 1/3 segments | 10 (24.4) |
| 2/3 segments | 4 (9.8) |
| 3/3 segments | 2 (4.9) |
| Extracapsular extension, n (%) | 19 (46.3) |
| Anterior only | 0 (0.0) |
| Posterior only | 15 (36.6) |
| Anterior + posterior | 4 (9.7) |
| Laterality | |
| Unilateral | 10 (24.4) |
| Bilateral | 9 (21.9) |
| Site a | |
| Apex | 9 (21.9) |
| Mid-gland | 9 (21.9) |
| Base | 13 (31.7) |
| Extent | |
| 1/3 segments | 13 (31.7) |
| 2/3 segments | 1 (2.4) |
| 3/3 segments | 5 (12.2) |
| Focality | |
| Unifocal | 10 (24.4) |
| Multifocal | 9 (21.9) |
| Index lesion status, n (%) b | |
| Whole gland | 3 (7.3) |
| Solitary index lesion | 26 (63.4) |
| Unilateral/minor contralateral involvement | 13 (50.0) |
| Unilateral/minor contralateral involvement + nsPCa focus | 7 (27.0) |
| Unilateral/contralateral involvement >50% | 6 (23.0) |
| More than one index lesion | 12 (29.3) |
| Same quadrant | 3 (25.0) |
| Opposite quadrants | 9 (75.0) |
| Seminal vesicle invasion >1 cm | 9 (22.0) |
| Median maximum diameter of the index lesion, mm (IQR) | 17 (10–25) |
| Median maximum volume of the index lesion, cm3 (IQR) | 1.07 (0.3–3.0) |
| Median prostate volume, cm3 (IQR) | 31.4 (23.5–36.7) |
| Lesion distance from the urethra, n (%) | |
| Invasion | 11 (26.8) |
| <2 mm | 7 (17.0) |
| >2 mm | 23 (56.0) |
| Lesion distance from the apex, n (%) | |
| <3 mm | 28 (68.0) |
| >3 mm | 13 (32.0) |
| Lymph nodes | |
| Lymphadenectomy template, n (%) | |
| Limited | 29 (70.7) |
| Extended | 3 (7.3) |
| Superextended | 7 (17.0) |
| Median number of nodes taken, n (IQR) | 17 (11–28) |
| pN+ status, n (%) | 12 (29.3) |
| Median number of positive nodes, n (IQR) c | 2 (1.75–3) |
| Unilateral, n (%) | 10 (25.0) |
| Bilateral, n (%) | 2 (5.0) |
IQR = interquartile range; nsPCa = nonsignificant prostate cancer.
Percentage calculated according to the total number of salvage radical prostatectomies, and considering the positivity for individual areas. 1/3 segment = apex or mid-gland or base; 2/3 segments = apex and mid-gland, or apex and base, or mid-gland and base; 3/3 segments = apex, mid-gland, and base.
Percentages for lesion subgroups were calculated according to the number of patients belonging to the respective group; limited = external iliac and obturator nodes; extended = external iliac, internal iliac, obturator, and presacral nodes; superextended = extended template plus the retroperitoneum.
Calculated among the men with positive nodes.
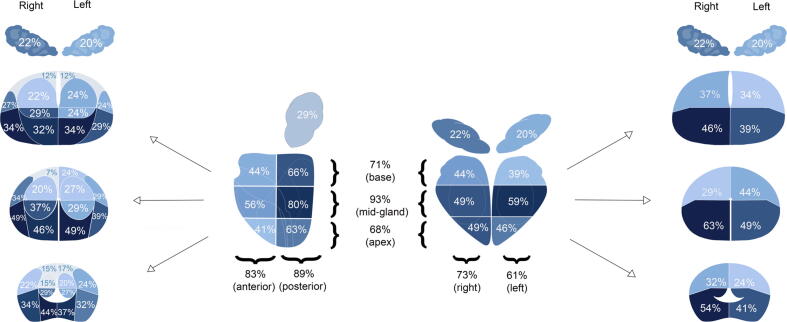
Overall, the area most frequently involved was the mid-gland (93%), whilst disease involved the apex in 68% of cases. Anterior quadrant involvement was not infrequent (83%; Fig. 3).
Fig. 3.
Prostate zones involved by recurrent prostate cancer.
A solitary index lesion was present in 26 of the men (63%), which was unilateral in 13 patients, unilateral with another nonsignificant PCa focus in seven patients, and unilateral with extended contralateral involvement in six patients. Twelve men (29%) had two or more index lesions, which were ipsilateral in three and contralateral in nine patients. PCa involved the whole gland in 7% (n = 3) of cases. The number of neoplastic foci per patient is shown in Supplementary Figure 1.
The majority of cases had PCa at a distance of >2 mm from the urethra. However, 11 men (26.8%) had urethral invasion.
Lymph nodes were positive in 29.3% of cases (n = 13). The topography of nodal positivity is shown in Supplementary Figure 2.
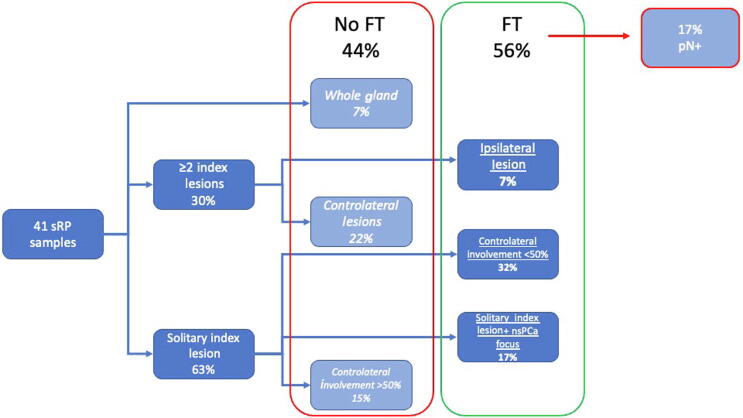
3.3. Suitability for salvage FT
Overall, 56% (n = 23) of the men were deemed suitable for salvage focal ablation on the basis of final pathology alone (Fig. 4). In particular, six men were suitable for hockey-stick ablation (minimal, <50% contralateral lobe involvement in less than 1 quadrant), seven men for hemi-ablation (PCa involving more than one quadrant unilaterally), and ten men for focal ablation (PCa involving less than half of a hemi-gland).
Fig. 4.
Flowchart showing patients who would or would not have been candidates for salvage FT (percentages were calculated using the total number of sRP samples as the denominator). FT = focal therapy; nsPCa = nonsignificant prostate cancer; sRP = salvage radical prostatectomy.
Seven patients suitable for salvage focal ablation had pathologically positive nodes. If node positivity were considered unsuitable for focal ablation, this would have led to a total of 16 candidates (39%) suitable for focal salvage treatment.
4. Discussion
We performed a pathological review of sRP specimens to detail the topography and characteristics of recurrent PCa and to verify whether FT would be feasible in this context. Several findings are of interest.
First, more than half of the men would have been suitable for a gland-preserving approach on the basis of sRP pathology. Fewer than one-third of our cases had multiple significant foci. Importantly, a relatively high proportion of patients had nodal involvement despite negative clinical staging, and some cases with extensive PCa involvement of the whole gland were found. In our cohort, although the majority underwent choline PET imaging, almost nine in ten did not undergo PSMA PET, which can now be regarded as the standard of care for guiding management of significant PCa recurrences [8]. The benefits of potential local treatment in men with nodal disease are unclear [25]. However, it is likely that earlier diagnosis of recurrence, before nodal and/or systemic dissemination, would further increase the number of men suitable for such approaches. In our series, the proportion of patients potentially suitable for salvage FT remained high even after exclusion of those with nodal positivity.
Second, radiorecurrent PCa generally has aggressive features, as previously highlighted [10], [12]. Almost half of our cohort had extracapsular extension and a high ISUP grade group, suggesting the need for appropriate safety margins when envisaging whole-gland or focal salvage treatments. SVI was also a common feature. When present, SVI was often extensive, with involvement of >1 cm. Not surprisingly, positive margins at the base were also not rare. While previous studies did not highlight details regarding the seminal vesicles [18], [19], [26], we believe that this point also deserves attention. During surgery, the possible need for a wide excision should always be kept in mind. Similarly, inclusion of the seminal vesicles should also be considered when performing salvage FTs so that complete PCa ablation can be achieved.
Third, the apical region is frequently involved. In our series, recurrent PCa was mainly located in the mid-gland. This slightly differs from previous studies reporting that the apex was involved in up to 95% of cases [19], [26]. However, our result confirms that this region plays a major role in the context of previous treatment and relatively small glands, as the majority of PCa foci were still located at <3 mm from the apex. In fact, the apical region was not the most frequently involved, but still harboured disease in two-thirds of our patients and was the most frequent site of positive margins. Hence, apical dissection should be performed carefully during surgery. Moreover, in the case of ablation, choice of the best energy modality should be guided by the ability to selectively target apical disease while sparing the surrounding apical anatomy, possibly reducing morbidity [27], [28]. Irreversible electroporation proved appropriate in treating apical disease in a first-line setting [29]. According to expert recommendations, laser ablation, photodynamic therapy, and brachytherapy are among the most promising energies for treating the apex [27], [28], [30]. All these energies should be strongly considered as preferred modalities in the radiorecurrent setting.
From a clinical perspective, we provide an evidence-based and pathological rationale supporting salvage FT in the context of radiorecurrent PCa. Given the high morbidity of sRP and promising results for salvage focal ablation, this may represent a significant advance in the management of radiorecurrent PCa. Other authors have suggested that sRP pathology shows that focal ablation is not applicable to this setting [19]. However, this was postulated on the basis of apical and periurethral vicinity because of the limitations of the energy modalities available at the time, namely cryotherapy and HIFU, and dates to >15 yr ago. In our view, greater imaging, diagnostic, and targeting precision, together with improvements in energy delivery and new ablative modalities, means that this may no longer be true in the contemporary era [19]. In addition, pathological information on radiorecurrent PCa, in particular apical involvement and SVI, has to be kept in mind to achieve radicality when performing surgical or ablative treatment of recurrence with a curative intent.
From a research perspective we provide additional insights into sRP pathology that supplements the existing literature [18], [19], [26]; to the best of our knowledge, this is the third sRP pathological analysis available to date. Nonetheless, the previous two series [18], [19], [26] included patients mainly from the late 1990s and early 2000s. The vast majority of men in our study underwent surgery after 2010 and thus our cohort may better account for RT and diagnostic advances that are now standards of care.
Unfortunately, first-line and second-line biopsy specimens, as well as preoperative imaging results, were scarce. This constitutes a major interest for future research to better understand the links between recurrent and primary PCa. A multicentre collaboration is ongoing to provide timely answers for these unmet needs.
However, our work has some limitations. Although the oldest case was from 2007, some men had to be excluded because of poor slide quality. Because sRP is much less common than first-line robot-assisted RP, the number of patients in our cohort is relatively low and the study is a single-centre retrospective analysis.
mpMRI was performed preoperatively in a minority of patients. Appropriate correlation of mpMRI and targeted biopsy results with final sRP pathology needs to be confirmed before concluding that focal salvage treatments are feasible. The strengths of the present study include the centralised and detailed pathological review of specimens and an a priori systematic definition for assessing FT suitability.
5. Conclusions
According to sRP specimens, radiorecurrent PCa treated with surgery is an aggressive disease, frequently showing extracapsular extension, extensive seminal vesicle invasion, positive margins, and apical involvement. However, the majority of men have a solitary index lesion and may be suitable for gland-preserving strategies with adequate safety margins, although a significant proportion may already harbour pN+ disease. Future studies involving larger series assessing the impact of recent diagnostic advances on patient selection, including mpMRI, are needed.
Author contributions: Giancarlo Marra had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Marra, Gontero.
Acquisition of data: Massari.
Analysis and interpretation of data: Marra, Calleris.
Drafting of the manuscript: Marra, Calleris.
Critical revision of the manuscript for important intellectual content: Marra, Calleris, Massari, Vissio, Molinaro, Cassoni, D’Agate, Oderda, Valerio, Raskin, Joniau, Papotti, Gontero.
Statistical analysis: Marra, Massari, Calleris.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Gontero.
Other (pathological review): Molinaro, Vissio.
Financial disclosures: Giancarlo Marra certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Ethics statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.11.017.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bolla M., van Tienhoven G., Warde P., et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/s1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 2.Dearnaley D., Syndikus I., Mossop H., et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalski J.M., Moughan J., Purdy J., et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4:e180039. doi: 10.1001/jamaoncol.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Jones J.S. Radiorecurrent prostate cancer: an emerging and largely mismanaged epidemic. Eur Urol. 2011;60:411–412. doi: 10.1016/j.eururo.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Lee W.R., Hanks G.E., Hanlon A. Increasing prostate-specific antigen profile following definitive radiation therapy for localized prostate cancer: clinical observations. J Clin Oncol. 1997;15:230–238. doi: 10.1200/jco.1997.15.1.230. [DOI] [PubMed] [Google Scholar]
- 7.Alibhai S.M.H., Breunis H., Timilshina N., et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/jco.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 8.Perera M., Papa N., Roberts M., et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77:403–417. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Lawal I.O., Lengana T., Popoola G.O., et al. Pattern of prostate cancer recurrence assessed by 68Ga-PSMA-11 PET/CT in men treated with primary local therapy. J Clin Med. 2021;10:3883. doi: 10.3390/jcm10173883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra G., Karnes R.J., Calleris G., et al. Oncological outcomes of salvage radical prostatectomy for recurrent prostate cancer in the contemporary era: a multicenter retrospective study. Urol Oncol. 2021;39:296.e21–296.e29. doi: 10.1016/j.urolonc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Chade D.C., Eastham J., Graefen M., et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol. 2012;61:961–971. doi: 10.1016/j.eururo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Calleris G., Marra G., Dalmasso E., et al. Is it worth to perform salvage radical prostatectomy for radio-recurrent prostate cancer? A literature review. World J Urol. 2019;37:1469–1483. doi: 10.1007/s00345-019-02749-z. [DOI] [PubMed] [Google Scholar]
- 13.Khoo C.C., Miah S., Connor M.J., et al. A systematic review of salvage focal therapies for localised non-metastatic radiorecurrent prostate cancer. Transl Androl Urol. 2020;9:1535–1545. doi: 10.21037/tau.2019.08.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marra G., Gontero P., Valerio M. Changing the prostate cancer management pathway: why focal therapy is a step forward. Arch Esp Urol. 2016;69:271–280. [PubMed] [Google Scholar]
- 15.Arrayeh E., Westphalen A.C., Kurhanewicz J., et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. 2012;82:e787–e793. doi: 10.1016/j.ijrobp.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Son M., Peters M., Moerland M., Kerkmeijer L., Lagendijk J., van der Voort van Zyp J. Focal salvage treatment of radiorecurrent prostate cancer: a narrative review of current strategies and future perspectives. Cancers. 2018;10:480. doi: 10.3390/cancers10120480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalloh M., Leapman M.S., Cowan J.E., et al. Patterns of local failure following radiation therapy for prostate cancer. J Urol. 2015;194:977–982. doi: 10.1016/j.juro.2015.04.111. [DOI] [PubMed] [Google Scholar]
- 18.Leibovici D., Chiong E., Pisters L.L., et al. Pathological characteristics of prostate cancer recurrence after radiation therapy: implications for focal salvage therapy. J Urol. 2012;188:98–102. doi: 10.1016/j.juro.2012.02.2571. [DOI] [PubMed] [Google Scholar]
- 19.Huang W.C., Kuroiwa K., Serio A.M., et al. The anatomical and pathological characteristics of irradiated prostate cancers may influence the oncological efficacy of salvage ablative therapies. J Urol. 2007;177:1324–1329. doi: 10.1016/j.juro.2006.11.069. [DOI] [PubMed] [Google Scholar]
- 20.Compérat E., Camparo P., Srigley J., et al. Prise en charge de la pièce de prostatectomie radicale. Résultats de la conférence de consensus de la Société Internationale d’Uropathologie (ISUP) Ann Pathol. 2013;33:155–161. doi: 10.1016/j.annpat.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Mai Z., Yan W., et al. The characteristics and spatial distributions of prostate cancer in autopsy specimens. Prostate. 2020;81:135–141. doi: 10.1002/pros.24091. [DOI] [PubMed] [Google Scholar]
- 22.Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J.R., Humphrey P.A. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/pas.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 23.Brierley J.D., Gospodarowicz M.K., Wittekind C., editors. UICC TNM classification of malignant tumours. ed 8. John Wiley & Sons; Hoboken, NJ: 2017. [Google Scholar]
- 24.Lebastchi A.H., George A.K., Polascik T.J., et al. Standardized nomenclature and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer: an international multidisciplinary consensus. Eur Urol. 2020;78:371–378. doi: 10.1016/j.eururo.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ventimiglia E., Seisen T., Abdollah F., et al. A systematic review of the role of definitive local treatment in patients with clinically lymph node–positive prostate cancer. Eur Urol Oncol. 2019;2:294–301. doi: 10.1016/j.euo.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Takeda T., Tin A.L., Corradi R.B., et al. Topography of prostate cancer recurrence after radiation therapy: a detailed mapping study of salvage radical prostatectomy specimens. Eur Urol. 2018;73:488–490. doi: 10.1016/j.eururo.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivaraman A., Barret E. Focal therapy for prostate cancer: an “à la carte” approach. Eur Urol. 2016;69:973–975. doi: 10.1016/j.eururo.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Marra G., Shah T.T., D’Agate D., et al. The SAFE pilot trial—Salvage Focal Irreversible Electroporation—for recurrent localized prostate cancer: rationale and study protocol. Front Surg. 2022;9:719. doi: 10.3389/FSURG.2022.900528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blazevski A., Amin A., Scheltema M.J., et al. Focal ablation of apical prostate cancer lesions with irreversible electroporation (IRE) World J Urol. 2021;39:1107–1114. doi: 10.1007/S00345-020-03275-Z/TABLES/2. [DOI] [PubMed] [Google Scholar]
- 30.Ganzer R., Arthanareeswaran V.K.A., Ahmed H.U., et al. Which technology to select for primary focal treatment of prostate cancer? European Section of Urotechnology (ESUT) position statement. Prostate Cancer Prostat Dis. 2018;21:175–186. doi: 10.1038/S41391-018-0042-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.