Abstract
This study examined the maximum joint angles and moments, and electromyography (EMG) activity of the lower limbs in an experienced Tai Chi (TC) practitioner in performing four dynamic (Repulse Monkey, Wave-hand in Clouds, Brush Knee Twist Step, and Lateral Forward Step) and three static TC movements (Starting Form, Hero Touch Sky, and Push Hand Back) and compared them with the measures from walking. Integrated EMG (iEMG) and peak EMG of the rectus femoris, adductor longus, tibialis anterior, semitendinosus, erector spinae, gluteus medius, tensor fasciae latae, medial and lateral gastrocnemius muscles were analyzed. One-way analysis of variance showed that compared with walking, TC presented 1) significantly larger hip flexion (71.4° vs. 42.2°) and abduction angles (11.9° vs. 5.3°), smaller knee flexion (45.2° vs. 71.1°) and abduction angles (13.0° vs. 27.7°), larger ankle dorsiflexion (41.4° vs. 11.3°) and abduction angles (8.8° vs. 7.2°); 2) hip flexion moment and knee flexion and abduction moment were significantly larger. Ankle dorsiflexion moment were significantly smaller, whereas ankle abduction moment was significantly larger in two TC movements; and 3) the EMG activity of the muscles in TC varied from 10% to 610% of walking. The knee extensors, hip adductors and abductors had significantly higher peak EMG (430% ± 40%, 240% ± 30%, and 320% ± 90%) and iEMG values (610% ± 30%, 311% ± 30%, and 1.4% ± 20%), respectively. The findings suggested that these TC movements could be a good option for the improvement of muscle strength and range of motion of the lower limbs.
Keywords: Joint angles, Joint moments, EMG, Tai Chi, Walking
Abbreviations
- 3D
Three-dimensional
- AL
Adductor Longus
- ANOVA
Analysis of Variance
- AP
Anterior-Posterior
- BKTS
Brush Knee Twist Step
- BOS
Base of Support
- COM
Center of Mass
- EMG
Electromyography
- ES
Erector Spinae
- GM
Gluteus Medius
- iEMG
Integrated Electromyography
- LFS
Lateral Forward Step
- LG
Lateral Gastrocnemius
- MG
Medial Gastrocnemius
- ML
Medial-Lateral
- RF
Rectus Femoris
- RM
Repulse Monkey
- ROM
Range of Motion
- SENIAM
Surface Electromyography for the Non-Invasive Assessment of Muscles
- ST
Semitendinosus
- TA
Tibialis Anterior
- TC
Tai Chi
- TFL
Tensor Fasciae Latae
- WHIC
Wave-hand in Cloud
Introduction
Tai Chi (TC) is a traditional Chinese exercise practiced in China and around the world owing to its beneficial effects on health. Regular TC practice is well documented to enhance physical and mental health.1,2 Particularly, TC training leads to improved muscle strength, balance, and posture that contribute to reducing the risk of falls in the elderly.3,4 The American and British Geriatric Societies recommends practicing TC to prevent falls in older adults.5 Given that TC can be practiced at home or outdoors, require no facility, and has gentle motion features, TC training has big potential for health promotion and the management of some chronic conditions associated with poor posture control and falls, such as Parkinson's disease.
One of the most remarkable findings in TC training is its beneficial effects on postural stability in the medial-lateral (ML) direction.6 The terms “balance” and “postural stability” are often used interchangeably. Balance refers to a state in which the body is in equilibrium,7 whereas postural stability is defined as the ability to control of the body's position in space for the purposes of balance and orientation.8 Research has shown that the decline in postural stability in the ML direction is closely associated with falls and the re-occurrence of falls in elderly people.9 Moreover, TC training can improve awareness of the body's movements and joints' proprioception and can provide appropriate joint loading of the lower limb.10 Thus, TC training could strengthen and enhance the neuromuscular reaction of muscles in the lower extremities related to postural control and balance.11,12 To improve postural stability in the ML direction could help to reduce falls in the elderly. However, why regular TC training can improve postural stability in the ML direction is unknown. To enhance our understanding of this question, a biomechanical analysis of TC movement is needed.
Several kinematic and kinetic studies of TC movements have been published. These studies have examined the biomechanical characteristics of a few TC movements such as Push Hand, Brush Knee and Twist Step (BKTS), as well as TC stepping.13, 14, 15 Kinematic analysis of TC movements demonstrates that compared with walking, TC movements present a significantly larger three-dimensional (3D) movement in the forward, backward, and sideway directions, a significantly larger range of motion (ROM) of the joint of the lower limbs,15, 16, 17 and double-limb support times.14,15 A kinetic study of TC stepping has shown that compared with walking, TC stepping has significantly smaller peak compressive forces, larger peak shear forces in the ankle, knee and hip joints, and larger peak moments in the knee and hip joints.18 Larger hip abduction and extension moments have been reported during the performance of two TC movements, the Repulse Monkey (RM) and Wave-hand in Clouds (WHIC) compared with the hip joint abduction and extension moments of walking.19 Results reveal that TC differs from the joint-moment patterns of walking, the peak moment generation time is much longer during TC performance, and the joint moments are active across an entire gait cycle. Thus, TC could be a safe and effective training for the elderly.
Muscle activity during TC performance has also been explored. The earliest study, published in 2003, examined the muscle activity of the trunk and lower limb during push-hand movement and BKTS using electromyography (EMG).13,20 Push-hand movement generates high muscle activity in the lumbar erector spinae and the rectus femoris. Concentric and eccentric contractions occur in muscles of the lower limbs.13 During the performance of BKTS and TC stepping, the activation of the lower limb muscles is slow and coordinated, and the activity level is higher than that during walking.16,20 In 2007, an EMG study revealed that the TC activation level of knee muscles is higher than that during walking.21 Nevertheless, muscle activity during TC is poorly understood despite the growing interest and popularity of TC.22 Moreover, an integrative and synchronized study on kinematics, kinetics, and muscle activity during TC is lacking, which limits the understanding of the biomechanics of TC movements.
To practice TC for health promotion and chronic diseases management for the elderly, particularly to improve postural stability and fall prevention, attention must be paid to two aspects. First is its feasibility. Many traditional TC styles exist, and they vary in the number of TC movements, ranging from 24, 48, and up to 108 forms. Learning and practicing these TC movements would be very difficult for the elderly. Second is effectiveness. To structure a short, feasible, and practical TC program for the elderly, the selection of TC forms is critical because the movement characteristics are some of the factors influencing the training effects. Thus, selecting the TC movements should be based on the biomechanical study of TC movement that would help in developing a feasible, practical, and effective TC program.
The present study aimed to examine the kinematics, kinetics, and muscle activity of the lower limb in performing seven selected TC movements. Biomechanical measures from TC were compared with the measures from walking, a popular physical activity for the elderly. The selected TC movements are common forms of the traditional TC styles such as Wu, Yang, and Taoist styles of TC and are believed to represent the classical movements of TC.23,24 These TC movements are Starting Form, Hero Touch Sky, and Push Hand Back, BKTS, RM, WHIC, and Lateral Forward Step (LFS). We hypothesized that significant differences existed in joint angles, joint moments, and muscle activity of the lower limbs between TC and walking. The information obtained from this study could enable further understanding of the mechanism of the beneficial effects of TC on improving postural stability and could provide scientific recommendation in developing a TC training program for the elderly.
Method
Participant and TC movement selection
One TC master (n = 1; age, 38 years old; body weight, 84.2 kg; height, 176.5 cm) with more than four years of Wu style TC experience was recruited for this study. This study was approved by the University of Ottawa Health Science and Science Research Ethics Board. Informed consent from the participant was obtained. Selection of TC movements were based on our previous TC biomechanical research15,19 and TC literature.23,24 These TC movements are typical TC movements included in the Wu, Yang, and Chen TC styles.23,25 The selection considerations were as follows: 1) easy to learn, 2) feasible to practice, 3) involves large motion in different direction, such as anterior-posterior (AP) and ML directions, and 4) includes static and dynamic motion. A static TC movement was defined as the movement in which the center of mass (COM) was maintained within the limits of the body's base of support (BOS), whereas a dynamic TC movement was defined as the movement in which the body's COM was outside the limits of the body's BOS.
Data collection
Thirty-nine reflective markers were attached to the skin or over the participant's clothing using adhesive tape in accordance with the Plug-in-Gait marker set (Oxford Matrices), as modified from the Helen Hayes marker set.26 A Vicon motion-analysis system comprising 10 infrared tracking cameras (two Vicon Vantage cameras (model v5) and eight Vicon Vero cameras (model v1.3); Oxford Metrics, Oxford, UK)) was used to capture the TC movements and walking trials at 200 Hz. Ground reaction forces were recorded using four force plates at 2 000 Hz (models 9286AA, Kistler Instruments Corporation, Winterthur, Switzerland; FP 4060–08, Bertec Corporation, Columbus, OH, USA) embedded in the middle of an 8 m-long walkway. The muscle activity of the right lower extremity and right side of the trunk was recorded using a 16-channel Trigno™ Wireless EMG System (SP–W01D, Delsys Inc., Boston, MA, USA) at 2 000 Hz and bandpass filtered at 20–450 Hz with a 6 db/octave-filter slope. Wireless sensors were placed on the following 10 muscles: rectus femoris (RF), adductor longus (AL), tibialis anterior (TA), semitendinosus (ST), erector spinae (ES), gluteus medius (GM), tensor fasciae latae (TFL), medial gastrocnemius (MG), and lateral gastrocnemius (LG). EMG placement was performed in accordance with the Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) Project's guidelines.27 The participant was asked to perform seven TC movements, including three static (Starting Form, Hero Touch Sky, and Push Hand Back) and four dynamic (BKTS, RM, WHIC, and LFS) movements, as well as walking for 10 trials at a comfortable pace.
Data processing and analysis
The 3D joint angles and moments for the lower limbs were obtained using Vicon Nexus (Oxford Metrics) and the Plug-in-Gait model (Oxford Metrics). A total of five collected trials for each TC movement were selected for analysis based on the consistency of the visually inspected TC stepping pattern along the sagittal and frontal planes. One gait cycle was identified from each trial for data analysis. A gait cycle was defined from the instance of foot contact to the subsequent foot contact of the same foot for walking and the four dynamic TC movements. Foot-strike and foot-off were determined by a combination of visual inspection of the position of the virtual heel and toe markers and the detection of the contact and take-off points by the force plates. For the kinematic and kinetics parameters, the computed joint angles and joint moments of the hip, knee, and ankle along the frontal and sagittal planes were exported to MATLAB (MathWorks, Natick, USA) to average and calculate the maximum and minimal values of each trial of the movement. Joint angle and moment data were normalized to one gait cycle.
EMG data were processed using a custom MATLAB (MathWorks, Natick, USA) script. The EMG signals of the lower limb muscles was filtered with a third-order Butterworth bandpass filter at 20–500 Hz, full-wave rectified, down sampled to 200 Hz, and filtered with a second-order low-pass filter at 6 Hz to remove any noise in the signal. For the TC movements and walking trials, a 5-s sample of the EMG signal was collected from the start of the first right-heel-strike event. The peak EMG amplitude (peak EMG) value and integrated EMG (iEMG) value were calculated within a 5-s window for each trial of TC movements and walking trials. The maximum EMG and iEMG values of the TC movements were normalized by the averaged value of the walking trials and reported as a ratio of the value obtained from walking.
Descriptive statistics were reported for the measures using SPSS version 20 for Windows (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) (TC movements: seven levels vs. walking) was used to compare the joint angles, joint moments, iEMG, and peak EMG values. Bonferroni correction was used for post-hoc analysis when needed, and the significance level was set at 0.05.
Results
Table 1 shows the mean and standard deviation of the maximum joint angles and moments during the performance of the four dynamic TC movements and walking. The hip flexion and abduction angles were significantly greater for the TC movements except for WHIC and LFS, respectively, (p ≤ 0.001 vs. walking). Knee flexion and abduction angles were smaller for all TC movements, notably for BKTS and WHIC (p ≤ 0.05 vs. walking). Dorsiflexion angles for the TC movements were significantly larger than walking. Compared with walking, hip flexion moments were significantly larger for LFS and RM (p ≤ 0.001 vs. walking). Knee flexion and abduction moments were significantly larger for the TC movements, except the abduction moment during WHIC. Dorsiflexion moments were significantly smaller during LFS and RM movements, whereas abduction moments were significantly larger during LFS and WHIC.
Table 1.
Mean and standard deviation (brackets) of the maximum hip, knee, and ankle angle and moment for the four dynamic Tai Chi movements and walking during a gait cycle (n = 1).
| Measures | Walking | BKTS | LFS | RM | WHIC |
|---|---|---|---|---|---|
| Angles (°) | |||||
| Hip Flexion | 42.0 (2.4) | 71.4 (1.6)a | 61.2 (2.1)a | 67.2 (1.1)a | 40.7 (1.6) |
| Hip Abduction | 5.3 (1.2) | 11.9 (0.9)a | 5.6 (1.1) | 9.4 (0.8)a | 9.2 (0.5)a |
| Knee Flexion | 71.1 (1.9) | 69.0 (1.2)b | 65.1 (3.1) | 45.2 (2.3) | 68.3 (2.7)a |
| Knee Abduction | 27.7 (2.4) | 13.0 (2.4)a | 25.1 (1.5) | 26.9 (0.8) | 22.4 (2.7)b |
| Ankle Dorsiflexion | 11.3 (0.9) | 37.7 (1.7)a | 40.9 (1.0)a | 41.4 (1.1)a | 29.5 (1.8)a |
| Ankle Abduction | 7.2 (1.0) | 3.4 (0.2)a | 8.6 (0.5)b | 8.8 (0.4)b | 6.1 (0.4)b |
| Moments (N·m/kg) | |||||
| Hip Flexion | 1.0 (0.2) | 1.1 (0.0) | 1.5 (0.1)a | 1.2 (0.1)a | 0.9 (0.1) |
| Hip Abduction | 0.7 (0.4) | 0.8 (0.1) | 0.5 (0.1) | 0.7 (0.1) | 0.4 (0.1) |
| Knee Flexion | 0.5 (0.2) | 1.3 (0.1)a | 0.9 (0.1)a | 1.0 (0.0)a | 0.8 (0.1)a |
| Knee Abduction | 0.6 (0.2) | 0.9 (0.1)b | 0.9 (0.0)b | 1.0 (0.1)a | 0.6 (0.1) |
| Ankle Dorsiflexion | 1.2 (0.1) | 1.1 (0.1) | 1.2 (0.1) | 0.9 (0.0)b | 0.8 (0.1)a |
| Ankle Abduction | 0.1 (0.1) | 0.1 (0.0) | 0.3 (0.0)b | 0.2 (0.0) | 0.4 (0.1)a |
BKTS: Brush Knee & Twist Step, LFS: Lateral Forward Step, RM: Repulse Monkey, WHIC: Wave-hand in Cloud.
p ≤ 0.001, vs. walking.
p ≤ 0.05, vs. walking.
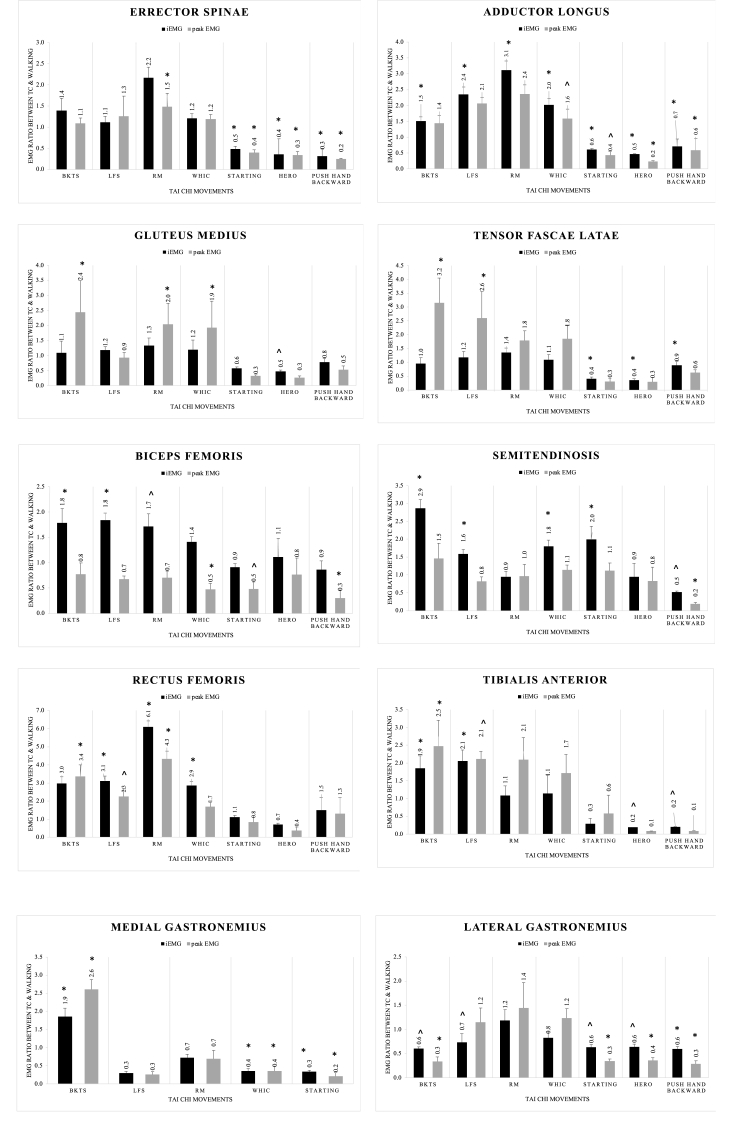
Fig. 1 illustrates the ratio of iEMG and maximum EMG of the 10 selected muscles of each TC movement compared with walking. The EMG activity of the lower limb muscles in TC varied from 10% to 610% of walking. The adductor longus iEMG and maximum EMG values were significantly larger for all TC movements, except for the maximum EMG value of BKTS. The maximum values of the gluteus medius were significantly larger for three of the four dynamic TC movements, whereas the maximum values of the tensor fasciae latae were significantly larger for BKTS and LFS. The iEMG values from biceps femoris were significantly larger during the dynamic TC movements, except for WHIC. The iEMG and maximum EMG values of the semitendinosus were significantly larger during BKTS (p ≤ 0.001). The semitendinosus iEMG values were significantly larger for all TC movements, except RM and Hero. Compared with walking, significantly larger iEMG and maximum EMG values of rectus femoris were observed in the four dynamic TC movements, except the maximum EMG values for WHIC. Significantly larger iEMG and maximum values of the medial gastrocnemius were found for all TC movements, except for the maximum EMG value of WHIC.
Fig. 1.
Illustration of the ratio of integrated electromyography and maximum electromyography (EMG) values of each Tai Chi movement to walking values of each muscle. BKTS: Brush Knee & Twist Step, EMG: electromyography, iEMG: integrated EMG, LFS: Lateral Forward Step, RM: Repulse Monkey, TC: Tai Chi, WHIC: Wave-hand in Cloud. The left bottom chart of medial gastrocnemius doesn’t include the EMG data values from two static Tai Chi movements because the EMG signal were missing due to problems with the EMG sensors’ batteries. ∗ p ≤ 0.001, vs. walking; ˆ p ≤ 0.05, vs. walking.
Discussion
This study examined the joint angles, joint moments, and muscle activity of seven TC movements and compared them with the measures from walking. Our main findings were as follows: compared with walking, 1) most dynamic TC movements presented significantly larger hip flexion and abduction angles and ankle dorsiflexion and abduction angles; 2) significant joint moment between TC and walking were found at the hip, knee, and ankle joints, particularly in hip and knee flexion moments and knee abduction moments; and 3) TC resulted in stronger muscle activity of the lower limb, particularly in hip abductor and adductor muscles.
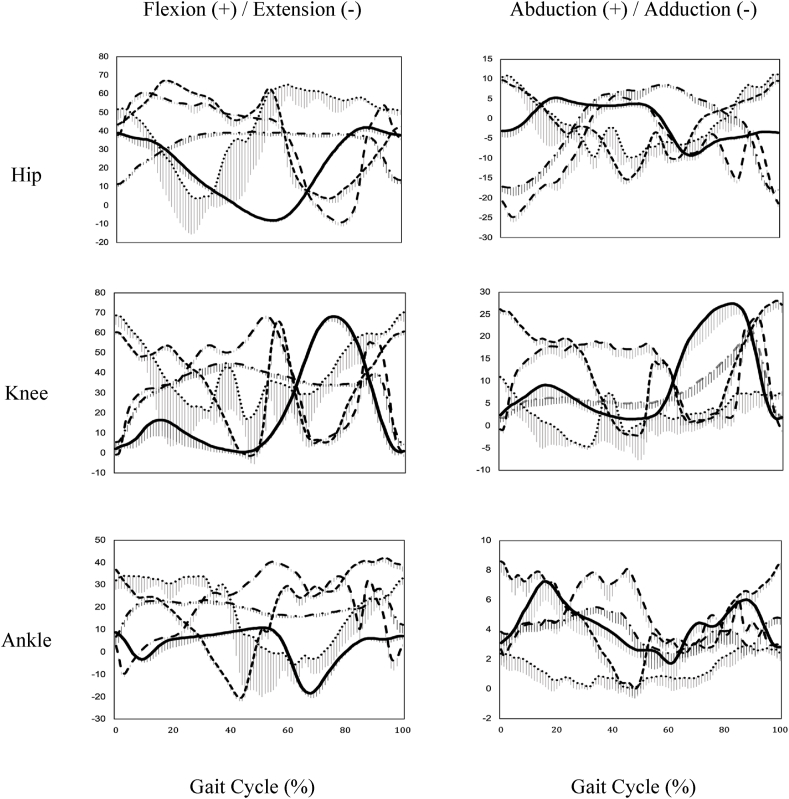
Significant differences were found between TC and walking in the joint angles of the hip, knee, and ankle. TC had significantly larger maximum hip flexion and abduction angles and ankle dorsiflexion and abduction angles than walking. Changes in joint angle (Fig. 2) were found in the early part a gait cycle, and the hip joint remained flexed in stance phase (0%–60% gait cycle) when performing TC, whereas the hip started into extension in the early-stance phase (0%–20% gait cycle) when walking. At the knee joint, more joint angle variations and changes occurred in the early-stance phase during TC than during walking. The evidence was consistent with the findings reported from a few published TC kinematic studies.15,28 The joint angles of the lower limb have been studied in several TC movements, including BKTS, RM, and WHIC. The earliest study examined joint kinematics during BKTS performance in six experienced TC masters and reported a wide ROM of the knee and ankle joints.20 Later, kinematics analysis of gait cycle time, single leg and double leg stance time, and joint angles, as well as ROM of lower limb, were examined during RM and WHIC performing by older TC practitioners with more than four years of experiences.15 Their results show that WHIC has significantly larger movement angles at hip and ankle joint than walking, whereas RM shows significantly larger knee joint angles than walking.
Fig. 2.
Angular displacement of the hip, knee, & ankle in sagittal (left column) and frontal plane (right column) during one gait cycle of walking (solid line) and four Tai Chi movements, lateral forward step (wide dash), repulse monkey (narrow dash line), wave-hand in cloud (dash-dot line) and brush knee & twist step (dotted line).
The larger joint angles and motion of lower limb in three of four TC movements reported in the current study further supported a previous study on the ROM of the ankle and knee joints during five movements of 42-form TC (BKTS, RM, WHIC, Kick Heel to Right, and Grasping the Bird's Tail) in a young TC master.17 A larger ROM of the ankle and knee joints was reported in TC than normal walking data.15 The current study showed larger angles of the hip and ankle during TC but not of the knee. The difference in knee joint angles may be related to the variation in TC style between the Wu style TC studied in the current study and the 42-form TC in the aforementioned previous study. 42-form TC is defined as a TC form for professional competition with a high requirement of knee-joint motion. Studies have demonstrated that larger angles of hip rotation and ankle dorsiflexion ROM are positively correlated with dynamic balance in young and older adults.29,30 Larger hip ROM and ankle dorsiflexion ROM correspond with better performance on balance tests. Thus, larger ROM or maximum angles of the lower extremity during TC could help improve muscle strength and balance control. Regular TC practice could be a good exercise option for improving the ROM of the lower limb and subsequently contributing to improved postural stability.
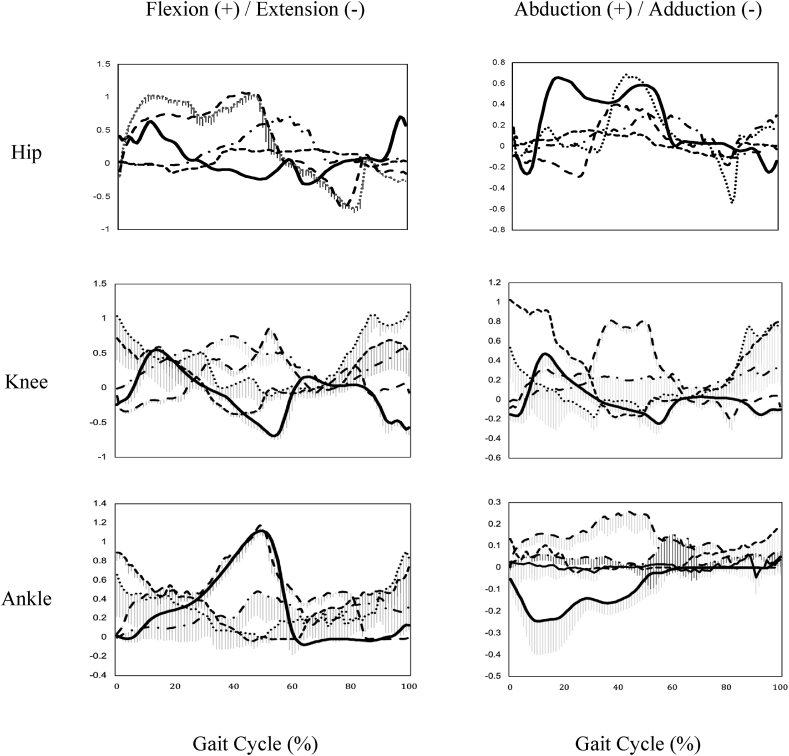
This study found significant differences in the flexion and abduction moments of the hip, knee, and ankle joints between TC movements and walking, respectively. Our previous study19 has examined the joint moment of hip, knee, and ankle during RM and WHIC performance and found larger flexion/extension moment at the hip. Significantly larger flexion moments at the hip were found in two TC movements, namely, LFS and RM movements, consistent with previous findings. The current and the previous studies showed smaller dorsiflexion moments in for RM and WHIC. Wu and colleagues studied joint kinetics in young (n = 6) and old (n = 6) TC practitioners.31 They found that abduction/adduction moments at the hip, knee, and ankle joints during TC stepping increased compared with walking.18 Together with our study, research evidence in joint moments of the hip, knee, and ankle during TC were basically consistent. The EMG activity of the lower limb muscles from this study also indicated that stronger muscle activity that could contribute to higher joint moment.
The joint-moment patterns (Fig. 3) suggested that the loading pattern at the joint of the lower limb during TC performance differed from that in walking. During a gait cycle, most TC movements were maintained at a higher level and longer lasting period than those in walking. During TC, semi-squatting posture and gentle foot touchdown keeps the hip, knee, and ankle in a flexed position,19 generating higher flexion moments at the joints. Our previous study has shown that the time-to-maximum moment generation takes a significantly longer time to reach for TC than for walking.19 This finding suggested that muscles of the hip, knee, and ankle are in relatively higher active in TC than walking.
Fig. 3.
Joint moment patterns of in sagittal plane (left column) and frontal plane (right column) during one gait cycle of walking (solid line) and four Tai Chi movements, Lateral Forward Step (wide dash), Repulse Monkey (narrow dash line), Wave-hand in Cloud (dash-dot line) and Brush Knee & Twist Step (dotted line).
The higher flexion joint moment at hip and knee during TC performance are similar to those observed during the performance of squat exercises. One study has examined joint moment during a squat to self-selected depth (normal squat) and a squat onto a chair with a standardized height of 43.8 cm in 20 older adults (aged 70–85).32 Research has shown that the squats can increase either hip or knee joint moments, which can be recommended to health workers in prescribing exercise for older adults to improve muscle strength. In the current study, four dynamic TC movements showed increased flexion moment of the hip and knee than walking, suggesting that performing the four TC movements could require more muscle activity of the hip and knee. Thus, the four TC movements could be prescribed for older adults to improve muscle strength.
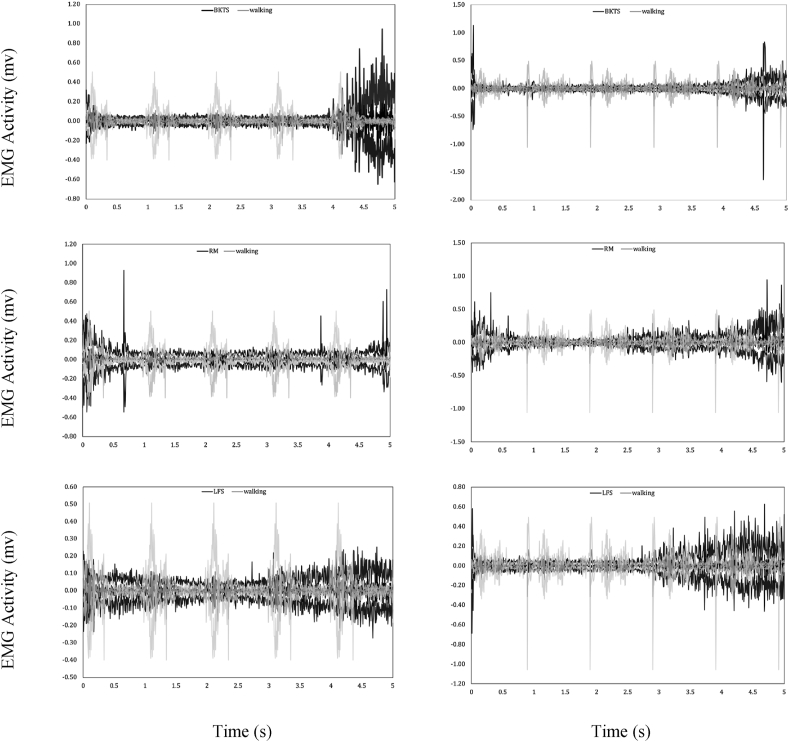
We conducted synchronized analysis on the EMG activity of the lower limb with joint angles and joint moments during TC performance for the first time. Moreover, the muscle activities of hip abductor/adductor muscles closely attributed to postural stability in the ML direction were examined for the first time. Results showed that the hip abductors, gluteus medius and tensor fasciae latae, hip adductor, and adductor longus presented either significantly stronger iEMG or maximum EMG activities during dynamic TC movements than walking. The EMG signal patterns of gluteus medius and tensor fasciae latae (Fig. 4) showed the higher magnitude of EMG signals and longer duration of EMG activity in TC movements than walking.
Fig. 4.
Electromyography activity of the gluteus medius (left column) and tensor fasciae latae (right column) during performing Brush Knee & Twist Step (top), Repulse Monkey (middle), and Lateral Forward Step (bottom). BKTS: Brush Knee & Twist Step, EMG: electromyography, LFS: Lateral Forward Step, RM: Repulse Monkey.
Our findings suggested that performing these TC movements enabled the activation of more muscle fibers and had stronger training effects on these muscles than walking. Gluteus medius, tensor fasciae latae, and adductor longus muscles were attributed to ML postural stability and countermovement stabilization during the event of a sideward fall. Neuromuscular control from the muscles of the hip joint helps regulate the movement of the pelvis and trunk while moving.33,34 Research has demonstrated that older people seem to prioritize the hip strategy to maintain postural stability.35 However, aging-related deficit in hip strategy, especially in the ML direction, has been documented.36 The decline in postural stability in the ML direction is closely associated with falls and their re-occurrence in elderly people.9 Muscle weakness is one of factors leading to postural-stability deficiency in elderly people.37 Weak hip muscles affect the stability of the joint and reduce the effectiveness of hip-stabilization strategies, thereby increasing the risk of falls and injuries.38,39 Thus, maintaining and enhancing the muscle strength of hip abductors and adductors have been addressed for fall prevention. Based on the EMG findings of dynamic TC movements, regular TC practice can help train the hip muscles, particularly those responsible for ML stability.
EMG analysis of erector spinae, rectus femoris, tibialis anterior, semitendinosus, medial gastrocnemius, and lateral gastrocnemius during the performance of dynamic TC movements showed higher muscle activity than in walking. Chan and colleagues examined the iEMG of lower limb muscles when performing push hand performance by one TC master with more than 22 years of TC practice.13 They found that iEMG in the medial hamstring and gastrocnemius muscles during push hand were about 20%–68.3% of the iEMG of maximum voluntary muscle contraction. Push-hand performance requires a double leg-standing posture. In the present study, the static TC movements were in double leg-standing posture. The EMG activity of the lower limb during static TC movements ranged from 0.2 to 2.0 times of EMG in walking (∼20%–200% of walking's EMG). The difference in EMG data normalization between the abovementioned study and the current one makes comparison of the findings difficult. Wu and colleagues studied the EMG activity of lower limb during TC gait/stepping in young TC practitioners with four months of experience compared with the EMG activity during walking.16,31 They found a higher magnitude (200%–400%) of the tibialis anterior, rectus femoris, and tensor fascia latae muscle activities and a longer duration of coactivation of most leg muscle pairs (130%–380%). A significantly higher EMG activity, ranging from 0.3 to 6.1 times of EMG in walking (∼30%–610%), have been found in the pairs of flexors/extensors of hip, knee, and ankle during TC movements in this study, consistent with that of Wu and colleagues.31 Tseng and colleagues reported their EMG findings from the vastus lateralis, vastus medialis, bicep femoris, and gastrocnemius muscles during TC and walking in 11 experienced TC practitioners.21 They found that compared with walking, the EMG activity for the quadriceps and vastus lateralis muscle was significantly greater in TC stepping. Obviously, the findings from the aforementioned two studies and this study supported the conclusion that TC movements generated more active muscle activity in the lower limb than walking.
One limitation of this study was the EMG signal analysis. The average single TC gait cycle in this study was approximately 10 s. Owing to technical limitation, EMG signals were unable to be collected throughout the entire TC movement, and only 5 s of EMG signals (half of one gait cycle time of TC movement) for each TC movement was selected for analysis. EMG data from a full TC gait cycle would be very interesting to analyze.
Conclusion
This study examined the joint angles, joint moment, and EMG activity of the lower limb during the performance of seven TC movements and compared them with the measures from walking. Compared with walking, practicing dynamic TC movements required greater joint movement angles of the lower limb; most dynamic TC movements generated larger flexion and abduction joint moment at the joints of the lower limb; and performing TC resulted in higher muscle activity of the lower limb, particularly in the hip abductors and adductors. The hip adductors and adductors have important role in postural stability for all population, particularly for older adults. These TC movements could be a good option for people, especially to improve the muscle strength and ROM of their lower limbs.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Authors’ contributions
Dr. Li contributed to the conception and principal guidance to the study. Mr. Law contributed to the data collection and data processing. Both authors contributed to drafting of the manuscript. Both authors have read and approved the final manuscript.
Ethics approval statement
The study was approved by the human ethics committee of the University of Ottawa. The participant was a volunteer, without a monetary incentive and were informed about the use of their information. Informed consent from the participant was obtained.
Conflict of interest
The authors have no conflicts of interest to report.
Acknowledgements
The authors would like to thank Dr. Qingguang Zhu for his help with the study and guidance in the selection of the seven TC movements, Ms. Sara Naghdlou for her help with the data processing.
References
- 1.Li JX, Xu DQ, Hong Y. Changes in muscle strength, endurance, and reaction of the lower extremities with Tai Chi intervention. J Biomech. 2009;42(8):967–971. doi: 10.1016/j.jbiomech.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hong Y, Xian J, Robinson PD. Balance control, flexibility, and cardiorespiratory fitness among older Tai Chi practitioners. Br J Sports Med. 2000;34(1):29–34. doi: 10.1136/bjsm.34.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu G. Evaluation of the effectiveness of Tai Chi for improving balance and preventing falls in the older population--a review. J Am Geriatr Soc. 2002;50(4):746–754. doi: 10.1046/j.1532-5415.2002.50173.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolfson L, Whipple R, Derby C, Judege J, King M, Amerman P. Balance and strength training in older adults: intervention gains and tai chi maintenance. J Am Geriatr Soc. 1996;44(5):498–506. doi: 10.1111/j.1532-5415.1996.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society, British Geriatrics Society . 2010. Prevention of Falls in Older Persons: AGS/BGS Clinical Practice Guideline.https://www.archcare.org/sites/default/files/pdf/2010-prevention-of-falls-in-older-persons-ags-and-bgs-clinical-practice-guideline.pdf Accessed August 5 2020. [Google Scholar]
- 6.Wang SJ, Xu DQ, Su LN, Li JX. Effect of long-term exercise training on static postural control in older adults: a cross-sectional study. Res Sports Med. 2020;28(4):553–562. doi: 10.1080/15438627.2020.1795661. [DOI] [PubMed] [Google Scholar]
- 7.Ragnarsdóttir M. The concept of balance. Physiotherapy. 1996;82(6):368–375. doi: 10.1016/S0031-9406(05)66484-X. [DOI] [Google Scholar]
- 8.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/S0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 9.Lord SR, Rogers MW, Howland A, Fitzpatrick R. Lateral stability, sensorimotor function and falls in older people. J Am Geriatr Soc. 1999;47(9):1077–1081. doi: 10.1111/j.1532-5415.1999.tb05230.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu DQ, Li JX, Hong Y. Effects of long term Tai Chi practice and jogging exercise on muscle strength and endurance in older people. Br J Sports Med. 2006;40(1):50–54. doi: 10.1136/bjsm.2005.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SJ, Xu DQ, Li JX. Effects of regular Tai Chi practice and jogging on neuromuscular reaction during lateral postural control in older people. Res Sports Med. 2017;25(1):111–117. doi: 10.1080/15438627.2016.1258649. [DOI] [PubMed] [Google Scholar]
- 12.Fong SM, Ng GY. The effects on sensorimotor performance and balance with Tai Chi training. Arch Phys Med Rehabil. 2006;87(1):82–87. doi: 10.1016/j.apmr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Chan SP, Luk TC, Hong Y. Kinematic and electromyographic analysis of the push movement in tai chi. Br J Sports Med. 2003;37(4):339–344. doi: 10.1136/bjsm.37.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao DW, Hong Y, Li JX. Characteristics of foot movement in Tai Chi exercise. Phys Ther. 2006;86(2):215–222. doi: 10.1093/ptj/86.2.215. [DOI] [PubMed] [Google Scholar]
- 15.Law NY, Li JX. The temporospatial and kinematic characteristics of typical tai chi movements: repulse monkey and wave-hand in cloud. Res Sports Med. 2014;22(2):111–123. doi: 10.1080/15438627.2014.881819. [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Liu W, Hitt J, Millon D. Spatial, temporal and muscle action patterns of Tai Chi gait. J Electromyogr Kinesiol. 2004;14(3):343–354. doi: 10.1016/j.jelekin.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Mao D., Hong Y., Li J. ISBS Conference Proceedings. 2004. The Kinematical Characteristics of the Lower Extremities During Tai Chuan Exercise; pp. 1–4.https://ojs.ub.uni-konstanz.de/cpa/article/view/1394 Accessed February 22, 2022. [Google Scholar]
- 18.Wu G, Millon D. Joint kinetics during Tai Chi gait and normal walking gait in young and elderly Tai Chi Chuan practitioners. Clin Biomech. 2008;23(6):787–795. doi: 10.1016/j.clinbiomech.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Li JX, Law NY. Kinetics of the lower limb during two typical Tai Chi movements in the elderly. Res Sports Med. 2018;26(1):112–123. doi: 10.1080/15438627.2017.1393753. [DOI] [PubMed] [Google Scholar]
- 20.Xu D, Li J, Hong Y. Tai chi movement and proprioceptive training: a kinematics and EMG analysis. Res Sports Med. 2003;11(2):129–144. doi: 10.1080/0308352. [DOI] [Google Scholar]
- 21.Tseng SC, Liu W, Finley M, McQuade K. Muscle activation profiles about the knee during Tai-Chi stepping movement compared to the normal gait step. J Electromyogr Kinesiol. 2007;17(3):372–380. doi: 10.1016/j.jelekin.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Hackney ME, Wolf SL. Impact of tai chi chu’an practice on balance and mobility in older adults: an integrative review of 20 years of research. J Geriatr Phys Ther. 2014;37(3):127–135. doi: 10.1519/JPT.0b013e3182abe784. [DOI] [PubMed] [Google Scholar]
- 23.Man-Ch’ing C, Smith RW. Tuttle Publishing; 2011. T’ai Chi: the“ Supreme Ultimate” Exercise for Health, Sport, and Self-Defense. [Google Scholar]
- 24.Yang J. YMAA Publication Center; 2010. Tai Chi Chuan: Classical Yang Style the Complete Long Form and Qigong. [Google Scholar]
- 25.Mark BS. Chinese Wushu Research Institute; 1979. Combined Tai-Chi Chuan. [Google Scholar]
- 26.Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10(5):575–587. doi: 10.1016/0167-9457(91)90046-Z. [DOI] [Google Scholar]
- 27.Sensor Locations: Recommendations for sensor locations on individual muscles. SENIAM. Accessed January 10, 2018. http://www.seniam.org.
- 28.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8(3):383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 29.Mecagni C, Smith JP, Roberts KE, O’Sullivan SB. Balance and ankle range of motion in community-dwelling women aged 64 to 87 years: a correlational study. Phys Ther. 2000;80(10):1004–1011. doi: 10.1093/ptj/80.10.1004. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa TH, Petersen RS. Relationship of hip and ankle range of motion, trunk muscle endurance with knee valgus and dynamic balance in males. Phys Ther Sport. 2018;34:174–179. doi: 10.1016/j.ptsp.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Wu G. Age-related differences in tai chi gait kinematics and leg muscle electromyography: a pilot study. Arch Phys Med Rehabil. 2008;89(2):351–357. doi: 10.1016/j.apmr.2007.08.147. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan S, Salem GJ, Wang MY, Sanker SE, Greendale GA. Squatting exercises in older adults: kinematic and kinetic comparisons. Med Sci Sports Exerc. 2003;35(4):635–643. doi: 10.1249/01.MSS.0000058364.47973.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorstensson A, Nilsson J, Carlson H, et al. Trunk movements in human locomotion. Acta Physiol Scand. 1984;121(1):9–22. doi: 10.1111/j.1748-1716.1984.tb10452.x. [DOI] [PubMed] [Google Scholar]
- 34.Allum JHJ, Bloem BR, Carpenter MG, Hulliger M, Hadders-Algra M. Proprioceptive control of posture: a review of new concepts. Gait Posture. 1998;8(3):214–242. doi: 10.1016/S0966-6362(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 35.Amiridis IG, Hatzitaki V, Arabatzi F. Age-induced modifications of static postural control in humans. Neurosci Lett. 2003;350(3):137–140. doi: 10.1016/S0304-3940(03)00878-4. [DOI] [PubMed] [Google Scholar]
- 36.Maki BE, McIlroy WE. Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age Ageing. 2006;35(Suppl 2):ii12–ii18. doi: 10.1093/ageing/afl078. [DOI] [PubMed] [Google Scholar]
- 37.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, et al. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89(9):1708–1713. doi: 10.1016/j.apmr.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers MW, Mille MLL. Lateral stability and falls in older people. Exerc Sport Sci Rev. 2003;31(4):182–187. doi: 10.1097/00003677-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Johnson ME, Mille ML, Martinez KM, Crombie G, Rogers MW. Age-related changes in hip abductor and adductor joint torques. Arch Phys Med Rehabil. 2004;85(4):593–597. doi: 10.1016/j.apmr.2003.07.022. [DOI] [PubMed] [Google Scholar]