Abstract
In young Asian patients with Wilson disease and no cardiac abnormalities or late gadolinium enhancement, cardiac MRI depicted elevated native T1, T2, and extracellular volume, suggesting early cardiac involvement.
Keywords: Wilson Disease, Cardiac MRI, Extracellular Volume Fraction, T1 Mapping, Myocardial Strain, Late Gadolinium Enhancement
Summary
In young Asian patients with Wilson disease and no cardiac abnormalities or late gadolinium enhancement, cardiac MRI depicted elevated native T1, T2, and extracellular volume, suggesting early cardiac involvement.
Introduction
Wilson disease (WD) is a rare autosomal recessive genetic disorder characterized by the accumulation of copper in the liver, brain, and musculoskeletal system (1). Recent cardiac MRI studies have also indicated that copper deposits and fibrosis formation may develop in the heart, contributing to varying degrees of abnormalities in cardiac function, strain, native T1, extracellular volume fraction (ECV), and late gadolinium enhancement (LGE) (2,3). To our knowledge, such studies investigating myocardial involvement in WD have not been reported in Asian patients. This study aims to evaluate the phenotypic characteristics of Chinese patients with WD using cardiac MRI.
Materials and Methods
The local ethics committee agency approved this study. Written informed consent was obtained from controls and was waived for patients. We retrospectively reviewed cardiac MRI examinations of Chinese patients diagnosed with WD between January 2021 and April 2022. We retrieved their clinical history and electrocardiographic and echocardiographic images. WD severity was determined by using the Unified Wilson Disease Rating Scale, and disease was categorized into with or without neurologic symptoms. We also recruited individuals without cardiovascular disease matched to our WD study sample for age, sex, and body surface area as controls. Cardiac MRI was performed with a 1.5-T whole-body MRI system (Ingenia; Philips Healthcare) using a 32-element body array coil. Cine cardiac MRI was performed using a balanced steady-state free precession sequence with breath holding, comprising a stack of contiguous parallel short-axis sections covering the entire left ventricle (LV) and right ventricle (RV) from base to apex and three LV long-axis sections (two-, three-, and four-chamber views). Cardiac MRI native T1 mapping and postcontrast T1 mapping were performed at the basal, middle, and apical levels of the LV short-axis view using a modified Look-Locker inversion recovery sequence, with a 5(3)3 protocol for T1 mapping and a 4(1)3(1)2 protocol for postcontrast T1 mapping. Cardiac MRI T2 mapping was performed at the basal, middle, and apical levels of the LV short-axis view using a multiecho gradient spin-echo sequence. LGE imaging was performed using a segmented phase-sensitive inversion recovery sequence within 10 to 15 minutes after injection of a gadolinium-based contrast agent, comprising a stack of contiguous parallel short-axis sections covering the entire LV and RV from base to apex and three LV long-axis sections. We used cvi42 (version 5.1, Circle Cardiovascular Imaging) software to generate cardiac function, myocardial strain, and T1 and T2 relaxation time values by delineating the endocardium and epicardium in the short-axis and long-axis views of the cine images and T1 and T2 maps.
Statistical analysis was performed using SPSS (version 26.0, IBM SPSS Statistics). Differences between two unrelated groups for continuous variables were tested using the independent t test. The statistical significance level was set to P < .05.
Results
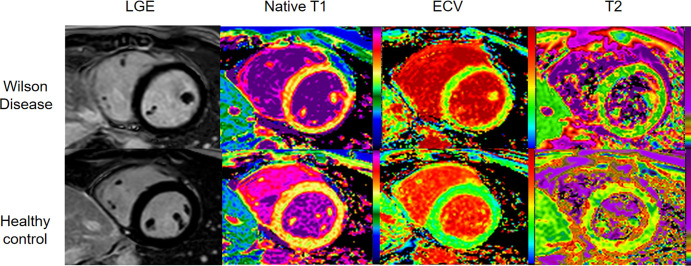
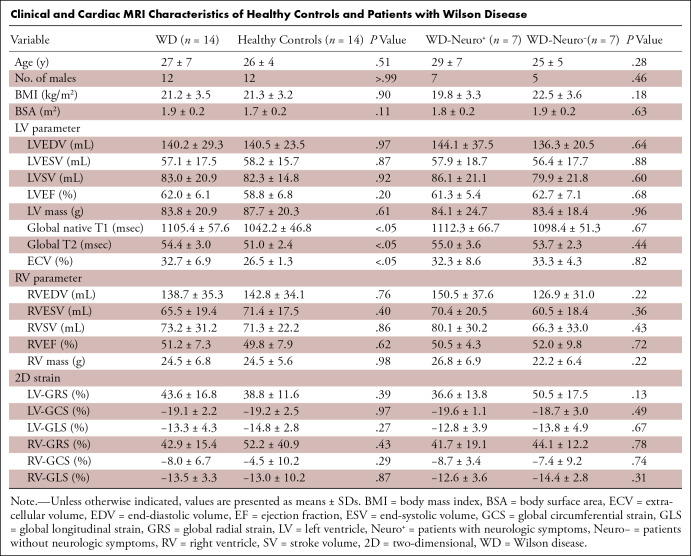
Fourteen patients with WD (mean age, 27 years ± 7 [SD]; 12 males) and 14 healthy controls (mean age, 26 years ± 4; 12 males) were included. None of the patients had obvious cardiac symptoms or echocardiographic abnormalities, but all had abnormal electrocardiograms with nonspecific findings. T1 (1105.4 msec ± 57.6 vs 1042.2 msec ± 46.8; P = .005), T2 (54.4 msec ± 3.0 vs 51.0 msec ± 2.4; P = .003), and ECV (32.7% ± 6.9 vs 26.5% ± 1.3; P = .006) values were higher in patients than healthy controls (Figure). We found no evidence of differences in native T1, T2, and ECV between WD subgroups or in age, body mass index, body surface area, cardiac function, and two-dimensional myocardial strain between patients and healthy controls (P > .05) (Table).
Cardiac short-axis late gadolinium enhancement (LGE) image and native T1, extracellular volume (ECV), and T2 maps in a 25-year-old woman with Wilson disease with neurologic symptoms (top row) and a healthy control (26-year-old woman) (bottom row). Both the patient and control had negative LGE. Global native T1 times (1101 msec vs 1019 msec), ECV values (30% vs 26%), and global native T2 times (56 msec vs 49 msec) were higher in the patient.
Clinical and Cardiac MRI Characteristics of Healthy Controls and Patients with Wilson Disease
Discussion
Our study showed that young Chinese patients with WD had no obvious clinical cardiac symptoms, cardiac function or myocardial strain abnormalities, or LGE; however, patients had higher native T1, T2, and ECV compared with healthy controls, suggesting early myocardial tissue abnormality. To our knowledge, this is the first cardiac MRI study assessing patients with WD in the Asian population. Our findings are consistent with those of previous cardiac MRI studies performed in older patients with WD (2–4). However, in contrast to these studies (2–5), we did not find reduced RV ejection fraction and RV and LV myocardial strain values or LGE in our study sample. We suspect this might be related to the younger age of patients in our study and earlier stage of cardiac involvement, as well as patient race. This study is limited by its retrospective, single-center design and small sample size, and further investigation is required.
W.D. and J.Z. contributed equally to this work.
Supported in part by the National Natural Science Foundation of China (grants 82071897 and 81970446).
Disclosures of conflicts of interest: W.D. No relevant relationships. J.Z. No relevant relationships. R.Z. No relevant relationships. Y.Q. No relevant relationships. Y.H. NIH R01 grant (not relevant to this work); consulting fees from Vertex (not relevant to this work); payment or honoraria from GE Healthcare (not relevant to this work); patent for Real-time CMR (not relevant to this work); SCMR translation committee, SCMR program committee. Y.Y. No relevant relationships. X.L. No relevant relationships.
Abbreviations:
- ECV
- extracellular volume fraction
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- RV
- right ventricle
- WD
- Wilson disease
Keywords: Wilson Disease, Cardiac MRI, Extracellular Volume Fraction, T1 Mapping, Myocardial Strain, Late Gadolinium Enhancement
References
- 1. Dusek P , Lescinskij A , Ruzicka F , et al . Associations of Brain Atrophy and Cerebral Iron Accumulation at MRI with Clinical Severity in Wilson Disease . Radiology 2021. ; 299 ( 3 ): 662 – 672 . [DOI] [PubMed] [Google Scholar]
- 2. Salatzki J , Mohr I , Heins J , et al . The impact of Wilson disease on myocardial tissue and function: a cardiovascular magnetic resonance study . J Cardiovasc Magn Reson 2021. ; 23 ( 1 ): 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quick S , Weidauer M , Heidrich FM , et al . Cardiac Manifestation of Wilson’s Disease . J Am Coll Cardiol 2018. ; 72 ( 22 ): 2808 – 2809 . [DOI] [PubMed] [Google Scholar]
- 4. Zhang K , Reuner U , Hempel C , et al . Evaluation of Myocardial Strain Using Cardiac Magnetic Resonance in Patients with Wilson’s Disease . J Clin Med 2021. ; 10 ( 2 ): 335 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang K , Reuner U , Weidauer M , et al . Left ventricular clefts - incidental finding or pathologic sign of Wilson’s disease? Orphanet J Rare Dis 2019. ; 14 ( 1 ): 244 . [DOI] [PMC free article] [PubMed] [Google Scholar]