Abstract
Study Objectives:
To evaluate the effects of bupropion on periodic limb movements during sleep (PLMS) and chin electromyography tone in children taking it for their mood disorder, compared to the effects of selective serotonin reuptake inhibitors (SSRIs) and of bupropion combined with SSRIs.
Methods:
Six adolescents (aged 16.0 ± 0.63 years) taking bupropion alone and 6 adolescents (aged 15.9 ± 1.36 years) taking bupropion in combination with an SSRI antidepressant were recruited, along with 10 adolescents (aged 16.2 ± 0.2 years) taking different SSRIs, and they were also enrolled together with 17 age- and sex-matched control patients (aged 15.5 ± 1.26 years). Polysomnographic studies were obtained, and participants’ leg movement activity during sleep and muscle tone were assessed quantitatively (atonia index) during all sleep stages.
Results:
Participants taking SSRIs showed PLMS indices significantly higher than those of control patients, whereas adolescents taking bupropion showed only slightly increased indexes of nonperiodic leg movements during sleep. No differences in PLMS were observed between adolescents taking bupropion alone or in association with SSRIs. The atonia index showed, within each sleep stage, the lowest values in the 2 groups taking SSRIs and the highest in the control patients; adolescents taking bupropion alone tended to show values slightly smaller than those of the control patients.
Conclusions:
We found that similar to adults, in adolescents SSRIs but not bupropion are associated with increased PLMS. Bupropion also seems to counteract the SSRI-induced increase of PLMS, when administered in combination; thus, the dopaminergic effect of bupropion seems to outmatch the antidopaminergic action of SSRIs. Conversely, bupropion does not counteract the effects of SSRIs on chin electromyography tone.
Citation:
DelRosso LM, Mogavero MP, Fickensher A, Bruni O, Schenck CH, Ferri R. Effects of bupropion and SSRI antidepressants on leg movement activity and chin muscle tone during sleep in adolescents. J Clin Sleep Med. 2023;19(1):151–161.
Keywords: bupropion, SSRI antidepressants, periodic leg movements during sleep, leg movement activity during sleep, periodicity index, atonia index, chin EMG tone, adolescents
BRIEF SUMMARY
Current Knowledge/Study Rationale: In adults, selective serotonin reuptake inhibitors but not bupropion have been reported to affect periodic limb movements during sleep and rapid eye movement sleep atonia. We analyzed the different effects of these 2 classes of antidepressants on periodic limb movements during sleep and chin electromyography tone during sleep in adolescents and evaluated the same measures when they were administered in association.
Study Impact: In adolescents, selective serotonin reuptake inhibitors but not bupropion are associated with increased periodic limb movements during sleep. Bupropion also seems to counteract the selective serotonin reuptake inhibitor–induced increase of periodic limb movements during sleep but not the changes in chin electromyography tone, when administered in combination; thus, the dopaminergic effect of bupropion seems to outmatch the antidopaminergic action of selective serotonin reuptake inhibitors.
INTRODUCTION
Bupropion is a dopamine and norepinephrine reuptake inhibitor commonly indicated for the treatment of major depressive disorder and for smoking cessation and is a promising alternative for the treatment of attention-deficit/hyperactivity disorder (ADHD) in both adolescents and adults.1 Furthermore, regarding the treatment of major depressive disorder, the combination of bupropion and selective serotonin reuptake inhibitors (SSRIs) or serotonin and noradrenalin reuptake inhibitors is generally well tolerated, capable of increasing the antidepressant response and reducing the adverse effects associated with SSRIs or serotonin and noradrenalin reuptake inhibitors. For example, the latter can induce long-term adverse effects, such as sexual dysfunction or weight gain2; moreover, SSRIs can induce emotional detachment, apathy, or fatigue,3 whereas bupropion has been reported to be effective on the cluster of depressive symptoms of fatigue, low energy, and hypersomnia and seems to induce these adverse effects less often than SSRIs.4 In addition, bupropion is not associated with major adverse cardiovascular events,5 unlike many SSRIs.6
A significant association between a large range of movement disorders, such as akathisia, bruxism, dystonia, myoclonus, parkinsonism, tardive dyskinesia, tic, and tremor and the intake of various types of antidepressants has been reported, whereas restless legs syndrome (RLS) seems to be associated only with SSRIs.7 RLS is a sleep movement disorder whose etiopathogenesis is still under study; an involvement of the dopaminergic system has been proposed.8 RLS and periodic leg movements during sleep (PLMS) worsening with some antidepressants, especially SSRIs and mirtazapine, are known phenomena, with the exception of bupropion9; however, data regarding the use of bupropion in the treatment of mood disorders in patients with RLS are still scarce.10
RLS is very often associated with PLMS, which can also be present in the absence of RLS as a separate sleep movement disorder (periodic limb movement disorder), if associated with sleep or daytime disturbance.11 In the general population, PLMS have a prevalence of approximately 30%,12 with 2 peaks, in childhood and in older adults13; PLMS can negatively impact the quality of sleep and health status in both children and adults.14–16 There are no accepted guidelines for the treatment of PLMS; moreover, they are often associated with psychiatric conditions,16 with consequent worsening of PLMS after treatment with SSRIs, in both adults and children, further complicating the clinical symptomatology of patients with mental disorders and sleep disruption.17,18
Despite the high prevalence of both PLMS12 and psychiatric disorders,19 studies investigating the effects of antidepressants on PLMS are few (especially in children).9 Furthermore, PLMS and RLS are very often associated with ADHD, a very frequent pathology in childhood and adolescence,20,21 in which bupropion has shown good efficacy and good tolerance.1
Among all antidepressants, as mentioned above, bupropion has different characteristics in regard to the mechanism of action and the tolerability profile, with only few reports in the literature evaluating its impact on PLMS.
In addition, antidepressants, especially SSRIs and serotonin and noradrenalin reuptake inhibitors, have long been known to be often associated with rapid eye movement (REM) sleep without atonia22 and less often with REM sleep behavior disorder23 in adults, in whom antidepressants may act as factors “unmasking” REM sleep behavior disorder without causing it.22,24 One study recently reported that in children SSRI antidepressants increase the chin electromyography (EMG) tone during REM sleep, similar to adults, and in all nonrapid eye movement (NREM) sleep stages.25 However, the clinical meaning of this effect in children has yet to be determined.
For all these reasons, the aim of this new study was to evaluate the effects of bupropion on PLMS and chin EMG tone in a small convenience sample of children taking bupropion chronically for their mood disorder, compared to the effects of SSRIs and of bupropion associated with SSRIs.
METHODS
Participants
This study was part of a retrospective chart review from every sleep study performed at Seattle Children’s Hospital from June 14, 2020, until December 8, 2021. Participants using antidepressants at the time of polysomnography (PSG) were included. For each patient data collected included age, sex, current use of antidepressant medication, name of medication, and dosage. Among 3,371 children and adolescents, 361 were taking 1 or more antidepressant medications, and only 14 were taking bupropion. Of these 14 participants, 2 had other sleep/medical disorders and were not included. Consequently, for this study, a convenience group of 6 adolescents (4 girls and 2 boys; mean age, 16.0 years; standard deviation, 0.63) taking bupropion alone (150–300 mg/d) and another convenience sample of 6 adolescents (5 girls and 1 boy; mean age, 15.9 years; standard deviation, 1.36) taking bupropion (150–300 mg/d) in combination with an SSRI antidepressant (3 taking sertraline, 2 taking fluoxetine, and 1 taking escitalopram) was also recruited. In addition, 10 adolescents (6 girls and 4 boys; mean age, 16.2 years; standard deviation, 0.2) taking different SSRIs alone were also enrolled; 4 were taking sertraline (150–200 mg/d), 4 were taking escitalopram (10–30 mg/d), and 2 were taking fluoxetine (10–30 mg/d). Antidepressants were prescribed for depression, anxiety, or both. None of the adolescents had another medical or psychiatric disorder. PSG recording had been ordered, in all patients, because of snoring, fatigue, or sleepiness. Treatment duration ranged between 2 and 12 months. All adolescents had an apnea-hypopnea index < 1 event/h and none were diagnosed with RLS.
Finally, an age- and sex-matched group of 17 control adolescents (13 girls and 4 boys; mean age, 15.5 years; standard deviation, 1.26) was selected from our database (including adolescents recruited by the authors at their respective centers who had participated in previous studies26). None of these adolescents were taking drugs or were affected by another medical, psychiatric, or sleep disorder other than primary snoring. In all groups, physical and neurological examinations were conducted. In addition, sleep apnea was ruled out in each patient, independently, by 2 different sleep experts.
The sex composition and age of the groups were not significantly different. No sample size/power analysis was possible because this was a convenience sample. The study was approved by the local ethics committee, and all participants or their parents/guardians provided informed written consent.
PSG recording and scoring of leg movements and of the submentalis muscle EMG amplitude during sleep
Routine full-night PSG recordings in the sleep laboratory were carried out in each participant, including EMG of the submentalis and both tibialis anterior muscles and electrocardiogram. Sleep stages and arousals were visually scored following standard criteria.27
Leg movements during sleep (LMS) were detected and scored according to the most recent criteria by the World Association of Sleep Medicine,28 followed by the calculation of a series of measures including total LMS index; PLMS index; short-interval LMS (SILMS) index; isolated LMS (ISOLMS) index; percentage of bilateral PLMS; periodicity index (PLMS/total LMS ratio); PLMS, SILMS, and ISOLMS durations; and PLMS index in REM sleep and in NREM sleep. In particular, the periodicity index can vary from 0 (complete absence of PLMS within the total LMS activity) to 1 (the entire LMS activity formed by PLMS). Moreover, all sleep leg movement onset-to-onset inter movement intervals (IMIs) IMIs from each recording were counted in each patient for 2-second classes, and group grand averages were obtained and used for statistical analysis. Finally, hourly night-distribution histograms of the number of PLMS during the first 8 recording hours were obtained for each group of participants.
In addition, we computed the atonia index for the quantification of the submentalis muscle EMG amplitude, as previously reported29–31; this amplitude has been shown to be a reliable measure also in children and adolescents.25,31–33 Briefly, the atonia index can vary from 0 (which means the complete absence of EMG atonia) to 1 or continuous stable EMG atonia during each sleep stage.
Statistical analysis
The nonparametric Kruskal–Wallis analysis of variance was used for between-group comparisons, followed by posthoc comparisons using the Mann–Whitney U test. Frequencies were compared using the chi-square test. The commercially available software STATISTICA version 6 (StatSoft Inc., Palo Alto, CA, USA) was used for the statistical analysis, and the significance level was set at P < .05.
RESULTS
Table 1 reports the sleep architecture measures found in control patients and patients taking SSRIs or bupropion. Overall, adolescents taking SSRIs showed more variables different from those of control patients than patients taking bupropion, with increased sleep latency, number of stage shifts, and number of awakenings, along with an increased percentage of wakefulness after sleep onset and percentage of sleep stage N1. These differences, together with the decreased sleep efficiency and percentage of sleep stage N2, point at a significant sleep architecture impairment in these patients. Conversely, patients taking bupropion only differed from control patients because of their increased number of awakenings and percentage of stage W sleep, and for a decrease in the percentage of sleep stage N3. With respect to patients taking SSRIs, those taking bupropion had only a significantly shorter sleep latency. When patients taking bupropion were compared to those taking bupropion associated with SSRIs, no significant differences were found regarding sleep architecture (Table 2).
Table 1.
Sleep variables found in control patients and participants taking SSRIs or bupropion.
| Control Patients (n = 17) | SSRIs (n = 10) | Bupropion (n = 6) | Kruskal–Wallis ANOVA | Mann–Whitney U Test, P < | ||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | H2, n = 33 | P < | 1 vs 2 | 2 vs 3 | 1 vs 3 | |
| Time in bed, min | 476.0 (461.0–505.0) | 510.3 (476.5–549.5) | 486.8 (480.0–512.0) | 2.951 | NS | — | — | — |
| Sleep period time, min | 453.0 (439.0–492.0) | 470.3 (443.5–504.0) | 475.8 (432.5–493.5) | 1.091 | NS | — | — | — |
| Total sleep time, min | 428.5 (406.0–467.0) | 394.3 (326.0–434.0) | 401.0 (321.0–432.0) | 3.117 | NS | — | — | — |
| Sleep latency, min | 10.0 (6.0–13.0) | 37.0 (32.0–46.0) | 14.8 (5.5–19.0) | 11.248 | .0036 | 0.001 | 0.045 | NS |
| Stage R latency, min | 91.5 (67.5–120.5) | 123.8 (93.5–157.5) | 76.3 (57.5–94.0) | 3.762 | NS | — | — | — |
| Stage shifts/h | 5.5 (4.4–11.7) | 16.4 (12.2–22.9) | 12.0 (8.8–15.3) | 13.796 | .001 | 0.0007 | NS | 0.042 |
| Awakenings/h | 1.2 (0.8–3.9) | 4.2 (3.3–9.5) | 5.1 (4.6–5.5) | 7.230 | .027 | 0.02 | NS | NS |
| Sleep efficiency, % | 91.8 (87.4–93.4) | 73.6 (67.8–85.0) | 85.1 (78.4–88.1) | 8.717 | .013 | 0.0084 | NS | NS |
| Stage W, % | 3.9 (2.1–6.0) | 18.4 (8.0–28.0) | 11.2 (5.9–21.5) | 7.703 | .021 | 0.033 | NS | 0.019 |
| Stage N1, % | 5.0 (3.7–5.9) | 12.4 (7.9–17.0) | 8.6 (4.0–9.7) | 10.876 | .0043 | 0.0017 | NS | NS |
| Stage N2, % | 51.5 (44.0–55.3) | 35.9 (28.5–39.7) | 39.1 (36.9–58.0) | 7.646 | .022 | 0.0046 | NS | NS |
| Stage N3, % | 19.9 (17.8–24.9) | 14.8 (12.9–20.8) | 12.6 (10.2–16.9) | 7.361 | .025 | NS | NS | 0.013 |
| Stage R, % | 17.4 (12.3–21.6) | 16.6 (8.0–18.7) | 19.8 (16.3–21.9) | 1.296 | NS | — | — | — |
ANOVA = analysis of variance, IQR = interquartile range, SSRs = selective serotonin reuptake inhibitors.
Table 2.
Sleep variables found in participants taking bupropion alone or bupropion in combination with SSRIs.
| Bupropion (n = 6) | Bupropion + SSRIs (n = 6) | Mann–Whitney U Test | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | P < | |
| Time in bed, min | 486.8 (480.0–512.0) | 520.7 (489.0–525.5) | NS |
| Sleep period time, min | 475.8 (432.5–493.5) | 493.0 (478.0–510.0) | NS |
| Total sleep time, min | 401.0 (321.0–432.0) | 419.5 (288.0–469.0) | NS |
| Sleep latency, min | 14.8 (5.5–19.0) | 11.5 (10.5–14.5) | NS |
| Stage R latency, min | 76.3 (57.5–94.0) | 93.7 (89.5–96.0) | NS |
| Stage shifts/h | 12.0 (8.8–15.3) | 14.3 (8.3–20.1) | NS |
| Awakenings/h | 5.1 (4.6–5.5) | 4.6 (3.4–5.7) | NS |
| Sleep efficiency, % | 85.1 (78.4–88.1) | 83.2 (55.7–95.2) | NS |
| Stage W, % | 11.2 (5.9–21.5) | 14.8 (2.9–43.1) | NS |
| Stage N1, % | 8.6 (4.0–9.7) | 9.3 (3.9–16.9) | NS |
| Stage N2, % | 39.1 (36.9–58.0) | 36.1 (31.9–42.1) | NS |
| Stage N3, % | 12.6 (10.2–16.9) | 24.3 (13.1–27.6) | NS |
| Stage R, % | 19.8 (16.3–21.9) | 7.7 (6.7–16.0) | NS |
IQR = interquartile range, SSRIs, selective serotonin reuptake inhibitors.
Table 3 shows the measures of leg movement activity during sleep found in control patients and patients taking SSRIs or bupropion. Again, participants taking SSRIs showed more variables different from those of control patients than adolescents taking bupropion, with an increase in all indexes and categories of LMS, including the periodicity index. The duration of the different LMS categories (PLMS, SILMS, and ISOLMS) was not different. A similar pattern of difference was evident for the comparison between SSRIs and bupropion, but in this case, only PLMS were clearly more numerous in adolescents taking SSRIs (not SILMS or ISOLMS) because these were also significantly increased in these patients as compared to in the control patients. No significant differences regarding LMS parameters were found between adolescents taking bupropion alone or in association with SSRIs (Table 4). In these analyses, data from only 5 participants for each bupropion group were used because of the presence of artifacts in the leg EMG signals in 1 adolescent in each group, which did not allow a reliable use of their data for statistical analysis.
Table 3.
Leg movement activity during sleep measures found in control patients and participants taking SSRIs or bupropion.
| Control Patients (n = 17) | SSRIs (n = 10) | Bupropion (n = 5) | Kruskal–Wallis ANOVA | Mann–Whitney U Test, P < | ||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | H2, n = 32 | P < | 1 vs 2 | 2 vs 3 | 1 vs 3 | |
| PLMS index, n/h | 0.6 (0.0–2.1) | 13.2 (5.6–15.6) | 0.5 (0.0–1.6) | 19.391 | .0001 | 0.00004 | 0.0027 | NS |
| SILMS index, n/h | 1.9 (1.7–3.8) | 6.4 (4.8–20.6) | 5.1 (3.0–6.1) | 12.435 | .002 | 0.0012 | NS | 0.042 |
| ISOLMS index, n/h | 7.2 (5.6–9.0) | 15.9 (10.3–22.7) | 11.8 (9.5–13.6) | 14.745 | .0006 | 0.0004 | NS | 0.034 |
| Total LMS index, n/h | 12.3 (7.3–13.8) | 34.1 (23.7–47.0) | 16.9 (14.6–19.7) | 19.887 | .0001 | 0.00003 | 0.012 | NS |
| Bilateral PLMS, % | 0.4 (0.0–0.8) | 3.1 (2.0–7.6) | 0.2 (0.0–0.3) | 18.607 | .0001 | 0.0001 | 0.0033 | NS |
| Periodicity index | 0.092 (0.000–0.118) | 0.303 (0.205–0.554) | 0.047 (0.000–0.048) | 14.573 | .0007 | 0.0008 | 0.004 | NS |
| PLMS duration, s | 2.9 (2.0–3.5) | 2.5 (2.3–3.2) | 2.3 (1.2–3.1) | 0.839 | NS | — | — | — |
| SILMS duration, s | 2.3 (2.0–2.8) | 2.9 (2.5–3.4) | 2.3 (1.9–2.9) | 5.621 | NS | — | — | — |
| ISOLMS duration, s | 3.1 (2.7–3.3) | 2.9 (2.7–3.3) | 2.5 (2.1–3.3) | 1.056 | NS | — | — | — |
| REM sleep PLMS index, n/h | 0.0 (0.0–0.0) | 4.5 (3.5–5.7) | 0.0 (0.0–0.0) | 11.747 | .0028 | 0.013 | 0.023 | NS |
| NREM sleep PLMS index, n/h | 0.7 (0.0–1.7) | 15.1 (5.5–17.8) | 0.6 (0.0–1.7) | 17.777 | .0001 | 0.000081 | 0.004 | NS |
ANOVA = analysis of variance, IQR = interquartile range, ISOLMS = isolated leg movements during sleep, LMS = leg movements during sleep, NREM = nonrapid eye movement, PLMS = periodic leg movements during sleep, REM = rapid eye movement, SILMS = short-interval leg movements during sleep, SSRIs = selective serotonin reuptake inhibitors.
Table 4.
Leg movement activity during sleep measures found in participants taking bupropion alone or bupropion associated with SSRIs.
| Bupropion (n = 5) | Bupropion + SSRIs (n = 5) | Mann–Whitney U Test | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | P < | |
| PLMS index, n/h | 0.5 (0.0–1.6) | 3.4 (1.0–4.3) | NS |
| SILMS index, n/h | 5.1 (3.0–6.1) | 2.0 (1.4–2.1) | NS |
| ISOLMS index, n/h | 11.8 (9.5–13.6) | 8.9 (5.7–10.2) | NS |
| Total LMS index, n/h | 16.9 (14.6–19.7) | 10.9 (9.6–19.1) | NS |
| Bilateral PLMS, % | 0.2 (0.0–0.3) | 0.8 (0.6–2.5) | NS |
| Periodicity index | 0.047 (0.000–0.048) | 0.129 (0.092–0.356) | NS |
| PLMS duration, s | 2.3 (1.2–3.1) | 2.2 (1.7–3.0) | NS |
| SILMS duration, s | 2.3 (1.9–2.9) | 2.8 (2.3–2.9) | NS |
| ISOLMS duration, s | 2.5 (2.1–3.3) | 3.0 (2.3–3.4) | NS |
| REM sleep PLMS index, n/h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | NS |
| NREM sleep PLMS index, n/h | 0.6 (0.0–1.7) | 3.7 (1.4–4.4) | NS |
IQR = interquartile range, ISOLMS = isolated leg movements during sleep, LMS = leg movements during sleep, NREM = nonrapid eye movement, PLMS = periodic leg movements during sleep, REM = rapid eye movement, SILMS = short-interval leg movements during sleep, SSRIs = selective serotonin reuptake inhibitors.
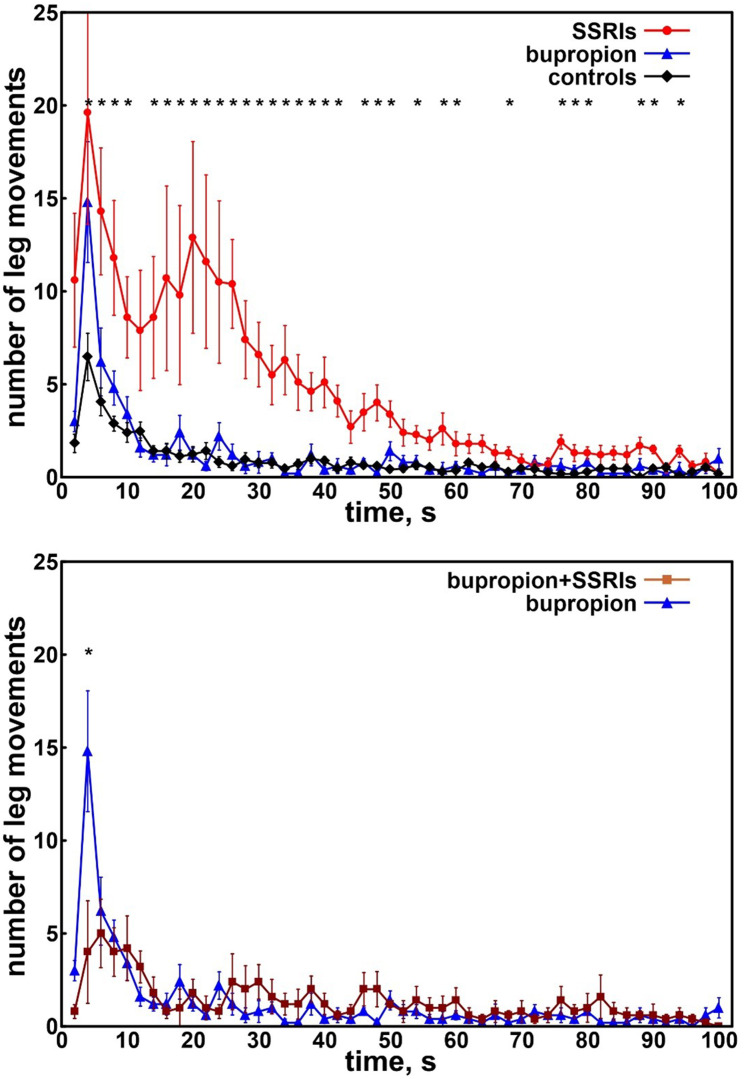
The top panel of Figure 1 shows the distribution of intervals between consecutive LMS in adolescents taking SSRIs or bupropion and in control patients. Patients taking SSRIs showed 2 clearly increased peaks, one extending from approximately 2–10 seconds and another from 10–60 seconds, respectively, with the second peak essentially representing PLMS and the first peak representing SILMS. Adolescents taking bupropion and control patients (not taking any medication) showed only the first SILMS peak, which was less evident in the control patients. When adolescents taking bupropion were compared to those taking bupropion associated with SSRIs, no significant difference was found, with the exception of the peak at 4 seconds, which was less evident in the latter group (Figure 1, bottom panel).
Figure 1. Distribution of intervals between consecutive LMS in adolescents.
Top panel: Distribution of intervals between consecutive LMS in adolescents taking SSRIs (red circles) or bupropion (blue triangles) and in control patients (black diamonds). Bottom panel: Distribution of intervals between consecutive LMS in adolescents taking bupropion alone (blue triangles) or bupropion plus SSRIs (brown squares). Data are shown as mean and standard errors (whiskers). Asterisks along the top indicate a significant difference (P < .015) obtained with the Kruskal–Wallis ANOVA comparing the 3 groups and computed for all points of the graphs. ANOVA = analysis of variance, LMS = leg movements during sleep, SSRIs = selective serotonin reuptake inhibitors.
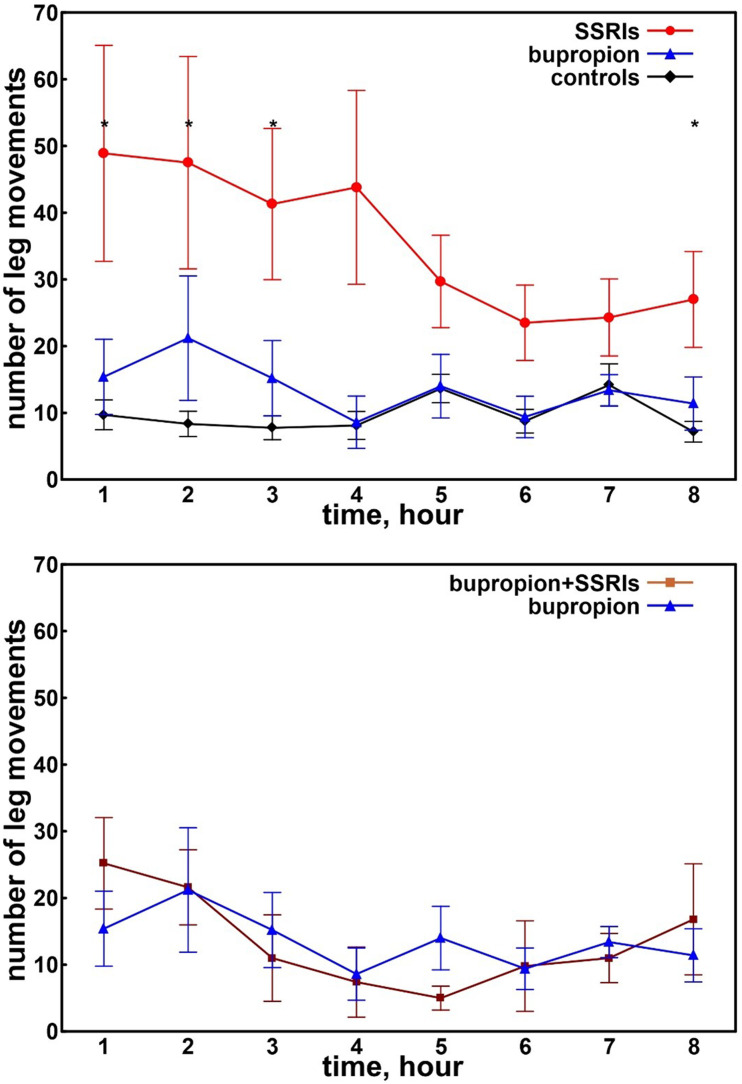
Figure 2 (top panel) displays the number of leg movements recorded during the first 8 hours of sleep in adolescents taking SSRIs or bupropion and in the control patients. A clearly decreasing trend was evident only in adolescents taking SSRIs who also showed values higher than those of the other 2 groups for the entire night, reaching statistical significance during the first 3 hours and during the last hour of sleep. The bottom panel of Figure 2 reports the distribution of intervals between consecutive leg movements during the first 8 hours of sleep in adolescents taking bupropion alone or those taking bupropion and SSRIs. No significant difference was found in this comparison.
Figure 2. Number of leg movements recorded during the first 8 hours of sleep in adolescents.
Top panel: Number of leg movements recorded during the first 8 hours of sleep in adolescents taking SSRIs (red circles) or bupropion (blue triangles) and in control patients (black diamonds). Bottom panel: Number of leg movements recorded during the first 8 hours of sleep in adolescents taking bupropion alone (blue triangles) or bupropion plus SSRIs (brown squares). Data are shown as mean and standard errors (whiskers). Asterisks on the top indicate a significant difference (P < .015) obtained with the Kruskal–Wallis ANOVA comparing the 3 groups and computed for all points of the graphs. ANOVA = analysis of variance, SSRIs = selective serotonin reuptake inhibitors.
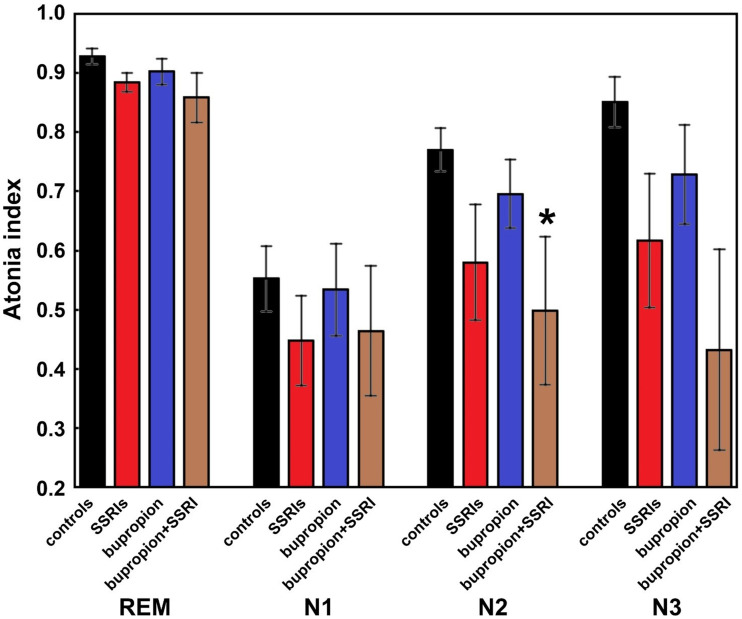
Finally, Figure 3 shows the atonia index computed during REM sleep and NREM sleep stages in control patients and adolescents taking SSRIs, bupropion alone, or bupropion and SSRIs; the corresponding data are reported in Table 5. As expected, the atonia index was highest during REM sleep and lowest during NREM sleep stage N1 in all groups. However, the differences between groups showed a similar pattern within each sleep stage, with the lowest stage values in the 2 groups taking SSRIs and the highest in the control patients. Adolescents taking bupropion alone tended to show values slightly smaller than the control patients; however, most of the between-group comparisons were statistically nonsignificant, most probably because of the insufficient power of this analysis with respect to the effect size found. Only a marginally significant difference was found between the atonia index in sleep stage N2 in patients taking bupropion and SSRIs versus control patients not taking any medication.
Figure 3. Atonia index computed during REM sleep and NREM sleep stages.
Atonia index computed during REM and NREM sleep stages in control patients (black-filled columns), adolescents taking SSRIs (red-filled columns), adolescents taking bupropion alone (blue-filled columns), or adolescents taking bupropion plus SSRIs (brown-filled columns). Data are shown as mean (column) and standard errors (whiskers). The asterisk indicates a statistically significant difference versus control patients at P < .05 (Mann–Whitney U test). NREM = nonrapid eye movement, REM = rapid eye movement, SSRIs = selective serotonin reuptake inhibitors.
Table 5.
Atonia index computed during REM sleep and NREM sleep stages in control patients and adolescents taking SSRIs, bupropion alone, or bupropion plus SSRIs.
| Control Patients (n = 17) | SSRIs (n = 10) | Bupropion (n = 6) | Bupropion + SSRIs (n = 6) | |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| REM sleep atonia index | 0.920 (0.890–0.977) | 0.882 (0.849–0.901) | 0.918 (0.858–0.947) | 0.908 (0.780–0.931) |
| Stage N1 atonia index | 0.554 (0.403–0.699) | 0.484 (0.244–0.571) | 0.588 (0.389–0.692) | 0.438 (0.239–0.704) |
| Stage N2 atonia index | 0.792 (0.668–0.883) | 0.654 (0.246–0.876) | 0.745 (0.593–0.790) | 0.463 (0.218–0.732)* |
| Stage N3 atonia index | 0.916 (0.813–0.961) | 0.708 (0.494–0.935) | 0.746 (0.580–0.935) | 0.302 (0.085–0.866) |
*Statistically significantly different versus control patients at P < .05 (Mann–Whitney U test). IQR = interquartile range, NREM = nonrapid eye movement, REM = rapid eye movement, SSRIs = selective serotonin reuptake inhibitors.
DISCUSSION
The main results of this study on a small series of patients taking bupropion, SSRIs, or a combination of both show that SSRIs but not bupropion are associated with increased overall LMS, including not only PLMS but all categories of LMS, such as SILMS and ISOLMS. These results represent a novel finding in the adolescent population, but could be expected, based on the data already available in the literature, especially regarding adults.9 Definitely novel is our finding of the effectiveness of bupropion to counteract the SSRI-induced increase of PLMS, indicating an inhibitory effect stronger than the facilitatory effect of SSRIs on PLMS and sleep-related leg movements in general. Essentially, the dopaminergic effect of bupropion seems to outmatch the antidopaminergic action of SSRIs, suggesting the possibility to use a combination of these 2 categories of agents when treating mood disorders in adolescents, with the possible added value of a lower risk of other adverse effects along with increased efficacy.2,4 A study on adults investigated the trend of PLMS in patients treated using SSRIs and using bupropion and a control group, showing an increase in PLMS in the group taking SSRIs and a reduction in PLMS in those taking bupropion and in the control group17; however, there seem to be no data in the literature on the effect of bupropion on PLMS in children. The beneficial effect of bupropion on both PLMS, as shown here and previously in adults,17 and RLS10 could be hypothesized on the basis of the target areas of the drug (reward system and limbic system, subserved by the mesolimbic dopamine pathway34) and their potential involvement in PLMS and RLS; a recent neuroimaging study on patients with RLS and PLMS showed involvement of the subcortical gray structures embedded within the mesolimbic dopaminergic pathway.35
Very few studies have evaluated the PSG effects of bupropion in adults or children. A study on 19 adult patients taking bupropion showed increased REM sleep latency but no other change in sleep variables.36 Our study showed a very small prolongation in REM sleep latency in the group of children taking bupropion that did not achieve statistical significance, likely because of the small cohort. Interestingly, this prolongation in REM sleep latency has been correlated with response to treatment in patients with depression.37
Bupropion has not been reported to induce or aggravate REM sleep without atonia/REM sleep behavior disorder38; however, studies are scarce, and our report indicates the need to deepen our understanding of this aspect and, if confirmed, to clarify the mechanism by which bupropion might affect sleep EMG tone not only during REM sleep. Studies in rodent models have identified the core circuits of REM sleep within the sublaterodorsal tegmental nucleus or subcoeruleus nucleus in humans at the level of the brainstem. Neurons in the sublaterodorsal tegmental nucleus are glutamatergic and control muscle atonia through gamma aminobutyric acid-ergic and glycinergic motor neurons.39 In agreement with the findings of the current study and those of a previous report,25 SSRIs can increase serotonin and consequently decrease dopaminergic activity in the descending pathways from the brainstem to the spinal cord motor neurons, not only during REM sleep but also during NREM sleep.40 Given the apparently different magnitude of the effect of SSRIs and bupropion on muscle tone found in this study and the possible summation of their effects when taken together, we hypothesize that the mechanism by which bupropion might affect muscle tone is different from that of SSRIs. In addition, the lack of bupropion-induced REM sleep behavior disorder in the few reports available may indicate that unlike for LMS, bupropion could not counteract the effects of SSRIs that reduced REM sleep atonia, as expected22,25; on the contrary, in this case bupropion seemed to show an effect on the atonia index, slightly reducing it with respect to control patients, and the association of bupropion plus SSRIs produced the lowest values, with a marginally significant difference between the atonia index in sleep stage N2 in patients taking bupropion plus SSRIs and control patients. Some studies suggest a cholinergic modulation of REM sleep atonia circuits41; in fact, acetylcholine has pre- and postsynaptic excitatory effects on glutamatergic sublaterodorsal tegmental nucleus neurons that project to the spinal cord.42 It has been proposed that these cells promote REM sleep atonia, thus suggesting a cholinergic mechanism in the regulation of REM sleep atonia.43 Bupropion acts on the reward system,44 with excellent long-term effects, unlike SSRIs45; several studies have shown that this action is made possible by its ability to modulate different types of cholinergic receptors46 and its influence on the nicotinic acetylcholine receptors of neurons from the dorsal raphe nucleus and the hippocampus.47 The reduction in muscle atonia observed in our study following the intake of bupropion alone could therefore be attributable to the blockade of cholinergic receptors induced by bupropion.
Bupropion is a dopamine and norepinephrine reuptake inhibitor1; therefore, our results can be viewed as a further proof of the involvement of the dopaminergic system in PLMS, whose etiopathogenesis, as previously mentioned, is still not completely known.7,46 In particular, recent neuroimaging studies have evaluated the brain structures on which bupropion acts, giving indications on the mechanisms of action of this drug and therefore indirect indications on the conditions that could benefit from its effects (in this case PLMS). A functional magnetic resonance imaging study revealed increased functional connectivity between the dorsal medial prefrontal cortex and posterior cingulate cortex and precuneus in patients receiving bupropion48; another study evaluated the degree of neural activation within the reward system, showing reduced activation of the ventral striatum and ventral tegmental area after the administration of paroxetine (an SSRI), but not with bupropion, which instead caused an increase in activation within the sublenticular amygdala.49 And finally, a recent review evaluated several neuroimaging reports on the effect of bupropion on brain areas, all of which agreed on the involvement of the anterior cingulate cortex and the limbic system.50
Our study has also shown that bupropion is not commonly used in the pediatric population, with 14 children taking the medication out of 361 (3.7%) of children on antidepressants undergoing a sleep study. Other therapeutic uses in children and adolescents include ADHD,1,51 smoking prevention in high-risk children with ADHD,1,51 smoking cessation therapy in adolescents,52 conduct disorder,53 bipolar disorder,54 and severe morning sleep inertia.55 It is important to note that ADHD is often associated with RLS and PLMS.19,20 As evidenced by our study, bupropion did not cause a worsening of PLMS, and it also may counteract the negative effects of SSRIs on them, strengthening the validity of this therapeutic option for the treatment of RLS and PLMS in the presence of ADHD or another comorbidity. Controlled clinical trials would be welcome in this field. It seems that bupropion is an underutilized medication in the pediatric and adolescent population; it has benign56 and even beneficial effects on sleep, as shown herein, and it does not carry the withdrawal problems that often occur and can be long-lasting with SSRIs/serotonin and noradrenalin reuptake inhibitors that are frequently prescribed.
The main limitation of this study is the small sample size of the groups of adolescents recruited, which was imposed by the retrospective observational nature of the recruitment of participants and by the need to exclude as many confounders as possible, such as the use of additional drugs acting at the level of the central nervous system, in the first place. This important limitation significantly decreased the power of our statistical analysis, which found clearly significant differences only for the parameters involving LMS (with large effect sizes) but not for those related to the chin EMG and the atonia index, in particular, characterized by smaller effect sizes. For this reason, the interpretation of the atonia index results can only be highly speculative and needs to be replicated in larger samples. In any case, a general trend can be noticed that should be considered with great caution; indeed, the lowest stage values were found in the 2 groups taking SSRIs (alone or taken with bupropion) and the highest in the control patients, with adolescents taking bupropion alone who tended to show values slightly smaller than control patients.
In addition to the small sample size, other limitations of this study include the single-center recruitment of patients, the variable dosage and duration of the therapy, and the absence of PSG recordings in the same patients without therapy, before treatment or after a drug wash out.
CONCLUSIONS
Similar to what has been reported in adults,9,17,57 this study indicates that in adolescents, SSRIs but not bupropion are also associated with increased PLMS. Bupropion also seems to counteract the SSRI-induced increase of PLMS, but not the changes in chin EMG tone,25,58 when administered in combination with SSRIs; thus, regarding PLMS, the dopaminergic effect of bupropion seems to outmatch the antidopaminergic action of SSRIs whereas it may reinforce that of SSRIs on muscle tone. The results of this study strongly encourage future studies on larger series of patients, from whom important clues can be derived for understanding the underlying mechanisms and treatment. In conclusion, the information provided by our findings represents an important starting point for more powered analyses and controlled studies.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Seattle Children’s Hospital, Seattle, Washington. This study was partially supported by a fund from the Italian Ministry of Health Ricerca Corrente (RC number 2773798) to RF. RF has been a consultant for Jazz Healthcare Italy, unrelated to this study. The remaining authors report no conflicts of interest.
ABBREVIATIONS
- ADHD
attention-deficit/hyperactivity disorder
- EMG
electromyography
- ISOLMS
isolated leg movements during sleep
- LMS
leg movements during sleep
- NREM
nonrapid eye movement
- PLMS
periodic limb movements during sleep
- PSG
polysomnography
- REM
rapid eye movement
- RLS
restless legs syndrome
- SILMS
short-interval leg movements during sleep
- SSRI
selective serotonin reuptake inhibitor
REFERENCES
- 1. Ng QX . A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents . J Child Adolesc Psychopharmacol. 2017. ; 27 ( 2 ): 112 – 116 . [DOI] [PubMed] [Google Scholar]
- 2. Zisook S , Rush AJ , Haight BR , Clines DC , Rockett CB . Use of bupropion in combination with serotonin reuptake inhibitors . Biol Psychiatry. 2006. ; 59 ( 3 ): 203 – 210 . [DOI] [PubMed] [Google Scholar]
- 3. Barnhart WJ , Makela EH , Latocha MJ . SSRI-induced apathy syndrome: a clinical review . J Psychiatr Pract. 2004. ; 10 ( 3 ): 196 – 199 . [DOI] [PubMed] [Google Scholar]
- 4. Demyttenaere K , Jaspers L . Review: bupropion and SSRI-induced side effects . J Psychopharmacol. 2008. ; 22 ( 7 ): 792 – 804 . [DOI] [PubMed] [Google Scholar]
- 5. Sposito AC , Bonilha I , Luchiari B , et al . Cardiovascular safety of naltrexone and bupropion therapy: systematic review and meta-analyses . Obes Rev. 2021. ; 22 ( 6 ): e13224 . [DOI] [PubMed] [Google Scholar]
- 6. Carvalho AF , Sharma MS , Brunoni AR , Vieta E , Fava GA . The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature . Psychother Psychosom. 2016. ; 85 ( 5 ): 270 – 288 . [DOI] [PubMed] [Google Scholar]
- 7. Revet A , Montastruc F , Roussin A , Raynaud JP , Lapeyre-Mestre M , Nguyen TTH . Antidepressants and movement disorders: a postmarketing study in the world pharmacovigilance database . BMC Psychiatry. 2020. ; 20 ( 1 ): 308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manconi M , Garcia-Borreguero D , Schormair B , et al . Restless legs syndrome . Nat Rev Dis Primers. 2021. ; 7 ( 1 ): 80 . [DOI] [PubMed] [Google Scholar]
- 9. Kolla BP , Mansukhani MP , Bostwick JM . The influence of antidepressants on restless legs syndrome and periodic limb movements: a systematic review . Sleep Med Rev. 2018. ; 38 : 131 – 140 . [DOI] [PubMed] [Google Scholar]
- 10. Winkelmann J , Allen RP , Högl B , et al . Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (revised 2017) . Mov Disord. 2018. ; 33 ( 7 ): 1077 – 1091 . [DOI] [PubMed] [Google Scholar]
- 11. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 12. Haba-Rubio J , Marti-Soler H , Marques-Vidal P , et al . Prevalence and determinants of periodic limb movements in the general population . Ann Neurol. 2016. ; 79 ( 3 ): 464 – 474 . [DOI] [PubMed] [Google Scholar]
- 13. Ferri R , DelRosso LM , Silvani A , et al . Peculiar lifespan changes of periodic leg movements during sleep in restless legs syndrome . J Sleep Res. 2020. ; 29 ( 3 ): e12896 . [DOI] [PubMed] [Google Scholar]
- 14. Kendzerska T , Kamra M , Murray BJ , Boulos MI . Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review . Sleep. 2017. ; 40 ( 3 ): zsx013 . [DOI] [PubMed] [Google Scholar]
- 15. DelRosso LM , Mogavero MP , Ferri R . Restless sleep disorder, restless legs syndrome, and periodic limb movement disorder—sleep in motion! Pediatr Pulmonol. 2022. ; 57 ( 8 ): 1879 – 1886 . [DOI] [PubMed] [Google Scholar]
- 16. Wipper B , Winkelman JW . The long-term psychiatric and cardiovascular morbidity and mortality of restless legs syndrome and periodic limb movements of sleep . Sleep Med Clin. 2021. ; 16 ( 2 ): 279 – 288 . [DOI] [PubMed] [Google Scholar]
- 17. Yang C , White DP , Winkelman JW . Antidepressants and periodic leg movements of sleep . Biol Psychiatry. 2005. ; 58 ( 6 ): 510 – 514 . [DOI] [PubMed] [Google Scholar]
- 18. Ferri R , Mogavero MP , Bruni O , Picchietti DL , Kapoor V , DelRosso LM . Leg movements during sleep in children treated with serotonergic antidepressants . Sleep. 2022. ; 45 ( 3 ): zsab236 . [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . The Global Burden of Disease: 2004 Update. Geneva, Switzerland: : World Health Organization; ; 2008. . [Google Scholar]
- 20. Angriman M , Cortese S , Bruni O . Somatic and neuropsychiatric comorbidities in pediatric restless legs syndrome: a systematic review of the literature . Sleep Med Rev. 2017. ; 34 : 34 – 45 . [DOI] [PubMed] [Google Scholar]
- 21. Picchietti DL , Stevens HE . Early manifestations of restless legs syndrome in childhood and adolescence . Sleep Med. 2008. ; 9 ( 7 ): 770 – 781 . [DOI] [PubMed] [Google Scholar]
- 22. Lee K , Baron K , Soca R , Attarian H . The prevalence and characteristics of REM sleep without atonia (RSWA) in patients taking antidepressants . J Clin Sleep Med. 2016. ; 12 ( 3 ): 351 – 355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan L , Zhou J , Yang L , et al . Duloxetine-induced rapid eye movement sleep behavior disorder: a case report . BMC Psychiatry. 2017. ; 17 ( 1 ): 372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Postuma RB , Gagnon JF , Tuineaig M , et al . Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 2013. ; 36 ( 11 ): 1579 – 1585 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferri R , Mogavero MP , Bruni O , Plazzi G , Schenck CH , DelRosso LM . Increased chin muscle tone during all sleep stages in children taking SSRI antidepressants and in children with narcolepsy type 1 . Sleep. 2021. ; 44 ( 11 ): zsab147 . [DOI] [PubMed] [Google Scholar]
- 26. Ferri R , DelRosso LM , Aricò D , et al . Leg movement activity during sleep in school-age children and adolescents: a detailed study in normal controls and participants with restless legs syndrome and narcolepsy type 1 . Sleep. 2018. ; 41 ( 4 ): zsy010 . [DOI] [PubMed] [Google Scholar]
- 27. Berry RB , Quan SF , Abreu AR , et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: : American Academy of Sleep Medicine; ; 2020. . [Google Scholar]
- 28. Ferri R , Fulda S , Allen RP , et al. International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) . World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) . Sleep Med. 2016. ; 26 : 86 – 95 . [DOI] [PubMed] [Google Scholar]
- 29. Ferri R , Manconi M , Plazzi G , et al . A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder . J Sleep Res. 2008. ; 17 ( 1 ): 89 – 100 . [DOI] [PubMed] [Google Scholar]
- 30. Ferri R , Rundo F , Manconi M , et al . Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder . Sleep Med. 2010. ; 11 ( 9 ): 947 – 949 . [DOI] [PubMed] [Google Scholar]
- 31. Ferri R , Bruni O , Fulda S , Zucconi M , Plazzi G . A quantitative analysis of the submentalis muscle electromyographic amplitude during rapid eye movement sleep across the lifespan . J Sleep Res. 2012. ; 21 ( 3 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 32. Silvani A , Vandi S , Pizza F , Antelmi E , Ferri R , Plazzi G . Combining information on nocturnal rapid eye movement sleep latency and atonia to facilitate diagnosis of pediatric narcolepsy type 1 . Sleep. 2021. ; 44 ( 3 ): zsaa203 . [DOI] [PubMed] [Google Scholar]
- 33. Antelmi E , Filardi M , Pizza F , et al . REM sleep behavior disorder in children with type 1 narcolepsy treated with sodium oxybate . Neurology. 2021. ; 96 ( 2 ): e250 – e254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wise RA . Drug-activation of brain reward pathways . Drug Alcohol Depend. 1998. ; 51 ( 1–2 ): 13 – 22 . [DOI] [PubMed] [Google Scholar]
- 35. Mogavero MP , Mezzapesa DM , Savarese M , DelRosso LM , Lanza G , Ferri R . Morphological analysis of the brain subcortical gray structures in restless legs syndrome . Sleep Med. 2021. ; 88 : 74 – 80 . [DOI] [PubMed] [Google Scholar]
- 36. Schramm PJ , Poland RE , Rao U . Bupropion response on sleep quality in patients with depression: implications for increased cardiovascular disease risk . Eur Neuropsychopharmacol. 2014. ; 24 ( 2 ): 207 – 214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ott GE , Rao U , Nuccio I , Lin KM , Poland RE . Effect of bupropion-SR on REM sleep: relationship to antidepressant response . Psychopharmacology (Berl). 2002. ; 165 ( 1 ): 29 – 36 . [DOI] [PubMed] [Google Scholar]
- 38. Hoque R , Chesson AL Jr . Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis . J Clin Sleep Med. 2010. ; 6 ( 1 ): 79 – 83 . [PMC free article] [PubMed] [Google Scholar]
- 39. Fraigne JJ , Torontali ZA , Snow MB , Peever JH . REM sleep at its core—circuits, neurotransmitters, and pathophysiology . Front Neurol. 2015. ; 6 : 123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang B , Hao Y , Jia F , et al . Sertraline and rapid eye movement sleep without atonia: an 8-week, open-label study of depressed patients . Prog Neuropsychopharmacol Biol Psychiatry. 2013. ; 47 : 85 – 92 . [DOI] [PubMed] [Google Scholar]
- 41. Torontali ZA , Grace KP , Horner RL , Peever JH . Cholinergic involvement in control of REM sleep paralysis . J Physiol. 2014. ; 592 ( 7 ): 1425 – 1426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weng FJ , Williams RH , Hawryluk JM , et al . Carbachol excites sublaterodorsal nucleus neurons projecting to the spinal cord . J Physiol. 2014. ; 592 ( 7 ): 1601 – 1617 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Figorilli M , Lanza G , Congiu P , et al . Neurophysiological aspects of REM sleep behavior disorder (RBD): a narrative review . Brain Sci. 2021. ; 11 ( 12 ): 1588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menossi HS , Goudriaan AE , de Azevedo-Marques Périco C , et al . Neural bases of pharmacological treatment of nicotine dependence—insights from functional brain imaging: a systematic review . CNS Drugs. 2013. ; 27 ( 11 ): 921 – 941 . [DOI] [PubMed] [Google Scholar]
- 45. Hughes JR , Stead LF , Hartmann-Boyce J , Cahill K , Lancaster T . Antidepressants for smoking cessation . Cochrane Database Syst Rev. 2014. ; 2014 ( 1 ): CD000031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tomaz PRX , Santos JR , Scholz J , et al . Cholinergic receptor nicotinic alpha 5 subunit polymorphisms are associated with smoking cessation success in women . BMC Med Genet. 2018. ; 19 ( 1 ): 55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vázquez-Gómez E , Arias HR , Feuerbach D , et al . Bupropion-induced inhibition of α7 nicotinic acetylcholine receptors expressed in heterologous cells and neurons from dorsal raphe nucleus and hippocampus . Eur J Pharmacol. 2014. ; 740 : 103 – 111 . [DOI] [PubMed] [Google Scholar]
- 48. Rzepa E , Dean Z , McCabe C . Bupropion administration increases resting-state functional connectivity in dorso-medial prefrontal cortex . Int J Neuropsychopharmacol. 2017. ; 20 ( 6 ): 455 – 462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abler B , Seeringer A , Hartmann A , et al . Neural correlates of antidepressant-related sexual dysfunction: a placebo-controlled fMRI study on healthy males under subchronic paroxetine and bupropion . Neuropsychopharmacology. 2011. ; 36 ( 9 ): 1837 – 1847 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cabrera EA , Wiers CE , Lindgren E , Miller G , Volkow ND , Wang GJ . Neuroimaging the effectiveness of substance use disorder treatments . J Neuroimmune Pharmacol. 2016. ; 11 ( 3 ): 408 – 433 . [DOI] [PubMed] [Google Scholar]
- 51. Conners CK , Casat CD , Gualtieri CT , et al . Bupropion hydrochloride in attention deficit disorder with hyperactivity . J Am Acad Child Adolesc Psychiatry. 1996. ; 35 ( 10 ): 1314 – 1321 . [DOI] [PubMed] [Google Scholar]
- 52. Gray KM , Carpenter MJ , Lewis AL , Klintworth EM , Upadhyaya HP . Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial . Nicotine Tob Res. 2012. ; 14 ( 2 ): 234 – 239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Riggs PD , Leon SL , Mikulich SK , Pottle LC . An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder . J Am Acad Child Adolesc Psychiatry. 1998. ; 37 ( 12 ): 1271 – 1278 . [DOI] [PubMed] [Google Scholar]
- 54. Kowatch RA , Sethuraman G , Hume JH , Kromelis M , Weinberg WA . Combination pharmacotherapy in children and adolescents with bipolar disorder . Biol Psychiatry. 2003. ; 53 ( 11 ): 978 – 984 . [DOI] [PubMed] [Google Scholar]
- 55. Schenck CH , Golden EC , Millman RP . Treatment of severe morning sleep inertia with bedtime long-acting bupropion and/or long-acting methylphenidate in a series of 4 patients . J Clin Sleep Med. 2021. ; 17 ( 4 ): 653 – 657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krystal DA , Thase EM , Tucker LV , Goodale PE . Bupropion HCL and sleep in patients with depression . Curr Psychiatry Rev. 2007. ; 3 ( 2 ): 123 – 128 . [Google Scholar]
- 57. McCall CA , Winkelman JW . Respiratory-related leg movements of sleep are associated with serotonergic antidepressants but not bupropion . J Clin Sleep Med. 2018. ; 14 ( 9 ): 1569 – 1576 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Winkelman JW , James L . Serotonergic antidepressants are associated with REM sleep without atonia . Sleep. 2004. ; 27 ( 2 ): 317 – 321 . [DOI] [PubMed] [Google Scholar]