Abstract
Education is integral to the American Academy of Sleep Medicine (AASM) mission. The AASM Emerging Technology Committee identified an important and evolving piece of technology that is present in many of the consumer and clinical technologies that we review on the AASM #SleepTechnology (https://aasm.org/consumer-clinical-sleep-technology/) resource—photoplethysmography. As more patients with sleep tracking devices ask clinicians to view their data, it is important for sleep providers to have a general understanding of the technology, its sensors, how it works, targeted users, evidence for the claimed uses, and its strengths and weaknesses. The focus in this review is photoplethysmography—a sensor type used in the familiar pulse oximeter that is being developed for additional utilities and data outputs in both consumer and clinical sleep technologies.
Citation:
Ryals S, Chang A, Schutte-Rodin S, et al. Photoplethysmography–new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189–195.
Keywords: photoplethysmography, PPG, sleep technology, wearables
BACKGROUND
Photoplethysmography (PPG) is a noninvasive optical technique from which many physiologic parameters may be derived, as it produces a waveform which correlates with the circulatory volume in skin tissue (Table 1). The technique was first discovered in the 1930s. The first commercially available continuous monitor of arterial oxyhemoglobin saturation was the Hewlett-Packard 47201A ear oximeter in the early 1970s.1 It was cumbersome to use and was quickly replaced when pulse oximetry was discovered by Dr. Takuo Aoyagi at Nihon Kohden in 1972 and later became available in the mid-1970s.2 The most common clinical application of PPG is the modern pulse oximeter, developed in 1991. With advances in PPG signal processing, added inputs, and artificial intelligence/machine learning/deep learning (AI/ML/DL) algorithms, additional data outputs such as sleep tracking have been incorporated into wearables since 2013. These advances have given these PPG-utilizing devices evolving consumer as well as clinical capabilities detailed below.3,4
Table 1.
Physiological parameters that may potentially be derived from photoplethysmography in sleep.
| Oxyhemoglobin saturation* |
| Sleep time and sleep stage |
| Blood pressure |
| Pulse rate* |
| Pulse rate variability* |
| Parasympathetic and sympathetic activity |
| Rhythm regularity |
| Respiratory rate |
| Respiratory events |
| Arterial stiffness and endothelial function |
*Parameters that have a substantial body of evidence and are more directly measured by PPG.
BASIC CONCEPTS OF PPG AND MODES OF SIGNAL COLLECTION
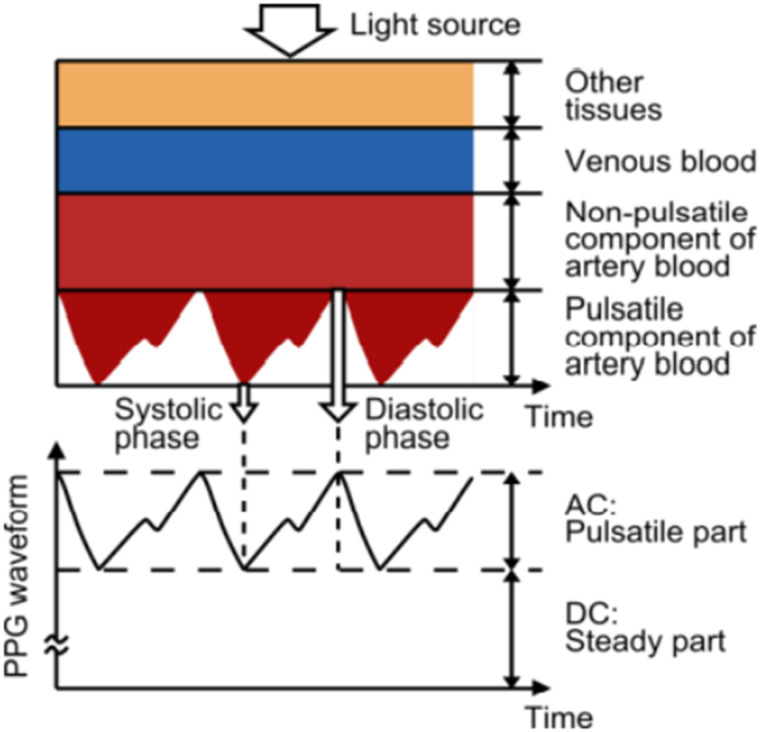
The fundamental basis of PPG technology can be explained by the Beer-Lambert law. When tissue is interrogated with the light signal of specific wavelengths, the light absorbance within tissue fluctuates with pulsation. As light passes through tissue it is attenuated and the resulting signal is then amplified, filtered, and processed to form the characteristic PPG waveform of a dicrotic notch occurring with each heartbeat.5 During the cardiac cycle the nonpulsatile tissue provides the DC (direct current) component, and light absorbance in the pulsatile tissue provides the systolic and diastolic AC (alternating current) component (Figure 1).6 Notably, the DC signal baseline is not steady but displays low-frequency AC oscillations (not depicted in Figure 1) due to changes in capillary density (eg, due to episodic sympathetic outflow and local autoregulation) and venous volume fluctuations (eg, due to respiration-induced fluctuations in central venous pressure).7 This low frequency AC baseline variation contains data that can be used to measure parameters such as respiratory rate.
Figure 1. Variation in light attenuation by tissue.
Note the photoplethysmography (PPG) waveform with systolic phase, diastolic phase, and characteristic dicrotic notch gleaned from the AC component of data. Note the pulsatile component of arterial blood is inverted due to the increase in absorption (or decrease in reflectance) from the changes in blood volume. Also, the “AC: Pulsatile part” of PPG is inverted. AC = alternating current, DC = direct current. Image reproduced under Creative Commons Attribution license (https://creativecommons.org/licenses/by/3.0/). Source: Tamura T, Maeda Y, Sekine M, and Yoshida M. Wearable photoplethysmographic sensors–past and present. Electronics 2014, 3 282–302; DOI:10.3390/electronics3020282.
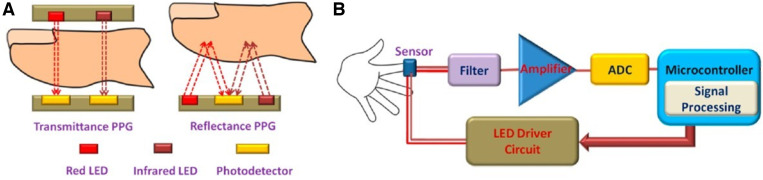
There are 2 PPG modes: transmission/absorption and reflection/reflectance (Figure 2). For the transmission/absorption mode, a light source is positioned opposite a light-detecting photosensor on the other side of an appendage (eg, fingertip or earlobe) to calculate absorption. For the reflection/reflectance mode, the light source and photosensor are on the same surface (eg, placement on the forehead or wrist), and the amount of reflected light is calculated.
Figure 2. Illustration of transmittance and reflectance photoplethysmography (PPG) and signal processing.
(A) Transmittance (transmission) and reflectance PPG. (B) Light signal is collected, amplified, filtered, and processed by an ADC (analog-to-digital converter). Image adapted and reproduced under Creative Commons Attribution license (https://creativecommons.org/licenses/by/4.0/). LED = light-emitting diode. Source: Majumder S, Mondal T, and Deen MJ. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130; DOI:10.3390/s17010130.
Pulse oximetry, the most common application of PPG, has been extensively investigated in the literature. A detailed discussion of its operating principle is beyond the scope of this review. But in short, under red illumination (650–750 nm), the absorption of deoxygenated hemoglobin is greater than that of oxygenated hemoglobin, whereas the relative absorption is reversed under infrared (IR) light (850–1,000 nm). By measuring the absorption by the blood of both red and IR light, it is possible to estimate the value of SpO2. However, in the presence of carboxyhemoglobin, methemoglobin, or sulfhemoglobin, the SpO2 reading can be unreliable.8
USE OF RED AND INFRARED LIGHT vs GREEN LIGHT
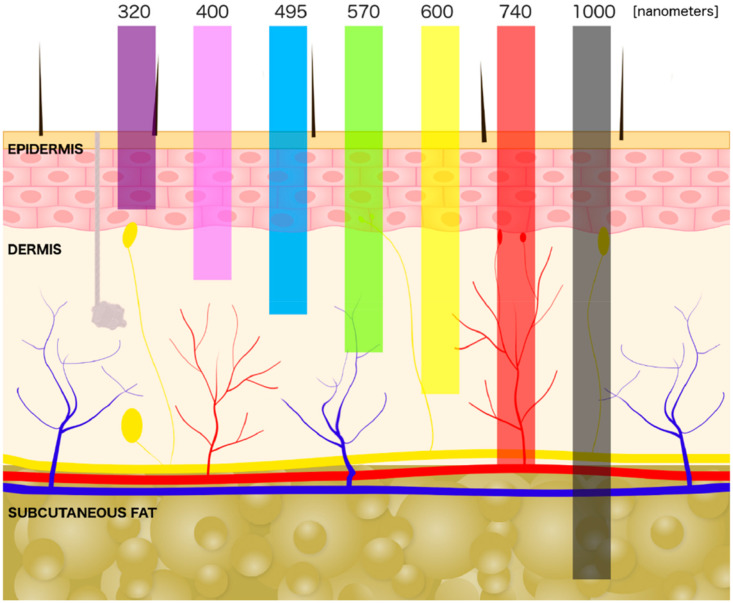
With either PPG mode, different light wavelengths may be employed.3,9,10 Red and infrared light reach deeper vascular tissues (Figure 3), measure SpO2 more accurately, are less affected by skin tone, and are more often used in medical-grade equipment. It is worth noting that most consumer-grade PPG devices use green light which is less affected by motion artifact. When only 1 wavelength of green light is used, it is meant to measure pulse rate only. However, utilization of 2 different wavelengths of green light (eg, 560 nm and 577 nm) can also be used to measure oxyhemoglobin saturation and may be more suited to the reflectance mode of PPG. Green light does not reach as far into vascular tissue and is thus less suitable for oxygen assessment. This is mainly because melanin is a strong absorber of green light, limiting depth penetration in dark-skinned individuals. Green light is also well absorbed by hemoglobin itself, which limits deeper penetration needed to obtain more accurate measurements in high cutaneous blood flow areas.5 Due to the varying levels of absorption, particularly for green light used in consumer devices, some manufacturers recommend using their device only in light skin tones or at rest.11 In response to differences in sensor accuracy during activity or in individuals with different skin tones or characteristics such as obesity, other manufacturers may boost or enhance the green light intensity and/or adjust the proprietary algorithms to attempt to improve accuracy.10,12
Figure 3. Schematic of light penetration through the skin by wavelength.
Note the depth of dermal vasculature in relation to light penetration. Image reproduced under Creative Commons Attribution license (https://creativecommons.org/licenses/by/4.0/). Source: Cios A, Ciepielak M, Szymanski L, et al. Effect of different wavelengths of laser irradiation on the skin cells. Int J Mol Sci. 2021, 22, 2437. DOI:10.3390/ijms22052437.
To highlight the importance of large and diverse datasets to address combinations of factors affecting PPG, Ajmal et al13 showed that obesity, especially obesity combined with darker skin tone, could cause a relative signal loss up to 61.2% Factors that can affect the PPG signal are myriad and are further detailed below. While red and IR light have the advantage of deeper tissue penetration, red photosensors have a lower signal-to-noise ratio compared to green photosensors when it comes to motion artifact.14 This means that devices using green light are in general more resistant to motion artifacts. A summary of the differences between red and IR compared to green light can be found in Table 2.
Table 2.
Differences of red and infrared (IR) light vs green light in photoplethysmography.
| Red and IR Light | Green Light |
|---|---|
| Reaches deeper tissue | Reaches more superficial tissue |
| Less affected by epidermal melanin | More affected by epidermal melanin |
| More suitable for SpO2 measurement | More suitable for pulse rate measurement |
| Mainly medical-grade devices | Mainly consumer-grade devices |
| More susceptible to motion artifact | Less susceptible to motion artifact |
LOCATION OF PPG MEASUREMENT
Location of measurement is important, as the efficacy of this technology depends on the quality and strength of the signal obtained. Examples of locations for measurement are found in Table 3.14 In general, locations with high vascular density and good sensor contact yield a higher quality signal. Less vascular density or poor sensor contact due to motion or hair can yield a poorer signal. As a result of these factors, in addition to device-dependent differences in algorithms used for PPG analysis, wearables like wristbands and watches vary in reliability (more below).
Table 3.
Example locations and mode of photoplethysmography measurement.
| Transmission Mode |
| Fingertip |
| Earlobe |
| Toe in infants |
| Reflectance Mode |
| Forehead |
| Wrist |
| Torso, arms, and legs |
| Ear canal (“earables”) |
| Bridge of nose |
| Mastoid |
| Scalp of fetus during labor |
FACTORS AFFECTING THE PPG SIGNAL
PPG can be influenced by numerous individual, physiologic, and external factors. In addition to skin tone,10,15,16 skin thickness, tattoos, sweat, and hair,10 PPG shape and amplitude also can be affected by individual and physiologic characteristics such as age,12,17 sex,12,16 obesity, local body temperature, activity,12 and cardiovascular status, including arterial stiffness, arrhythmias, left ventricular function, arterial and venous tissue perfusion, and autonomic function.7,18 Further, external factors such as poor sensor positioning,8 motion, ambient light,12 fingernail polish8 or artificial nails, and applied pressure at the sensor site may affect PPG and pulse oximetry data quality.12,19 While these factors may affect PPG, not all factors affect the PPG signal equally and combinations of these factors may have more impact on the PPG signal and data outputs (eg, a combination of obesity and skin tone as noted earlier). Large and diverse datasets and learning AI/ML/DL/neural networks are needed to analyze these factors in diverse population datasets.
FURTHER ADVANCES IN PPG SIGNAL ANALYSIS
Advances in PPG signal analysis have fueled its implementation into clinical and consumer sleep technologies and given them many new capabilities (Table 1). Presently, PPG data yields information on heart rate, heart rhythm, respiratory rate,20,21 and pulse rate variability. PPG analysis can also be used to estimate arterial stiffness22,23 and blood pressure (with limited accuracy).24 PPG has been used in nonclinical applications to fitness by helping to estimate baseline energy expenditure and maximum O2 consumption (VO2 max). However, recent studies have found PPG on wrist-worn activity trackers not accurate enough to be used in sports or health care applications.25,26 Analysis of pulse rate variability is performed similarly to and is a surrogate marker of heart rate variability (HRV), which is known to estimate autonomic system activity.27 Pulse rate variability derived from wearables has been shown to have a very good to excellent correlation with classic electrocardiogram-derived HRV when at rest.28 An overall increase in HRV is associated with parasympathetic activity while decreased HRV is associated with sympathetic activity. Time-domain or frequency-domain analysis can also be calculated, yielding commonly used metrics, including high frequency (HF, 0.15–0.4 Hz, commonly accepted as a marker of parasympathetic activity), low frequency (LF, 0.04–0.15 Hz, likely a reflection of both sympathetic and vagal activity or a marker of sympathetic modulation), and the LF/HF ratio (often considered as an index for sympatho-vagal balance).18 HRV changes have been studied in obstructive sleep apnea,29–31 insomnia,31,32 depression,33 and anxiety. PPG is also utilized in peripheral arterial tonometry devices, which are now used in some clinical sleep technologies such as home sleep apnea testing.
IMPACT OF PPG ON THE SLEEP CLINICIAN AND US FOOD AND DRUG ADMINISTRATION CLEARANCE OF DEVICES
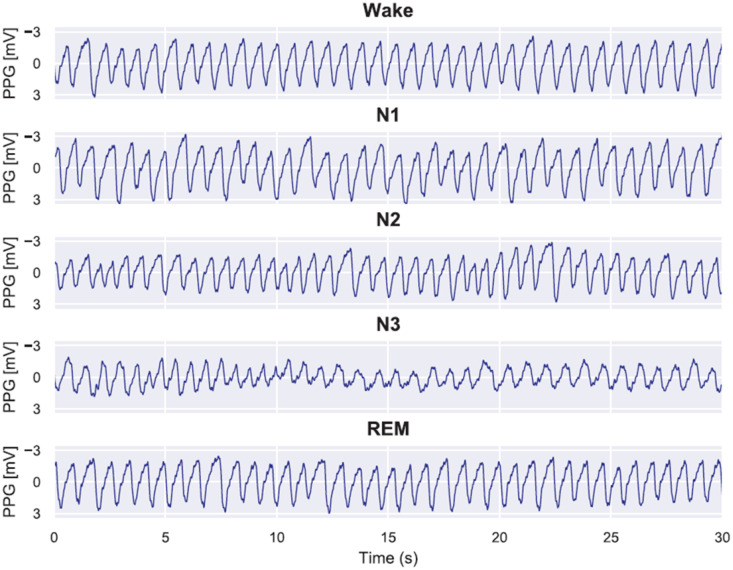
The most significant clinical impact of PPG on both consumer and clinical sleep technologies is due to its combination with actigraphy in many devices. This combination has allowed the approximation of many familiar parameters, including sleep stages, sleep times, and respiratory events. With sleep trackers now purporting to track and report total sleep time, time in wake vs light vs deep sleep, and potential respiratory events, the obvious question is how they compare to each other as well as to traditional sleep testing. All of the individual sleep technology devices that utilize PPG are too numerous to recount here, but AASM members are urged to visit the #SleepTechnology resource (https://aasm.org/consumer-clinical-sleep-technology/). It is important to note that while various devices utilize PPG, it does not mean they are similar in efficacy. The efficacy of each device depends on the light wavelength and collection variations that are used, the analysis of the PPG signal (and the proprietary algorithm used), the other technologies (eg, actigraphy) with which PPG is combined in a specific device, and additional (proprietary) AI/ML/DL using other inputs on specified (or unspecified) dataset populations. Figure 4 gives examples of PPG outputs in different stages of sleep. Korkalainen et al34 trained a deep learning model to perform sleep staging on the PPG signals from diagnostic polysomnograms from 894 suspected OSA patients. Differences in signal characteristics, including amplitude, frequency, and variability, can be appreciated between signals obtained during different stages of sleep. While differences between the signals can be appreciated visually, it is essential to note that these are outputs obtained from a single, undisclosed, deep learning algorithm and specific datasets. These proprietary algorithms and datasets are company-and device-specific and cannot be generalized across different devices/data platforms. It follows then that when a PPG-based device is Food and Drug Administration (FDA)–cleared that the clearance is for that specific device for a specific indication.
Figure 4. Examples of photoplethysmography (PPG) signals during correctly identified sleep stages.
In these examples, it can be seen that during wake the PPG signal remains stable, and the frequency and amplitude are fairly constant. During N1 sleep, irregular variation in the signal amplitude occurs and the frequency decreases. When progressing to N2 and further to N3 sleep, the amplitude decreases and low frequency oscillations in the PPG signal begin to occur. In contrast, rapid eye movement sleep is highly similar to wake but with slightly higher variation in the signal amplitude. It may be difficult to discern PPG profile changes of various sleep stages with naked eyes, but the sleep stages may be sufficiently discriminated by rule-based or AI-enabled algorithms. AI = artificial intelligence. Caption and figure adapted and reproduced under Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/). Source: Korkalainen, H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11). DOI:10.1093/sleep/zsaa098.
It is crucial that readers are cognizant that consumer-grade devices are only lightly regulated, and even medical-grade devices are often not subject to an extensive approval process due to the 510(k) process. To better understand the complexity of FDA clearance for PPG and its uses in unique devices, an inspection of the FDA standard for medical-grade pulse oximetry device clearance deserves attention. Consumer pulse oximeters that are FDA registered are sports/wellness devices that are not FDA cleared. Medical-grade pulse oximeters are FDA cleared, but the meaning of this clearance requires clinical examination, particularly when used for clinical decision-making. Per the FDA website: “if an FDA-cleared pulse oximeter reads 90%, then the true oxygen saturation in the blood is generally between 86% and 94%. Pulse oximeter accuracy is highest at saturations of 90%–100%, intermediate at 80%–90%, and lowest below 80%.”35 On February 19, 2021 and June 21, 2022, the FDA posted 2 pulse oximetry updates and specific warnings of SpO2 inaccuracies for darker-skinned individuals. In addition, FDA clearance may not address all claimed uses for a particular device. For example, the pulse oximeter output, but not other outputs such as sleep assessment, may be cleared for a device. Further, depending on the levels of FDA-categorized risk, changes in proprietary AI/ML/DL algorithms and datasets may or may not require oversight by the FDA.
Many devices have different levels of sensitivity not just between ages in adult and pediatric populations, but also when used outside of a healthy population. Often, devices are tested in healthy datasets and therefore may not be accurate when comorbid medical or sleep disorders are present. As described above, large and diverse testing datasets are required to improve PPG sensor, device algorithm, and device data output accuracy. Therefore, when evaluating the devices themselves, sleep outputs should be approached with a great degree of caution particularly if the inclusion/exclusion criteria and descriptive population datasets used in the proprietary AI/ML/DL algorithms are not publicly available, as is often the case.
SLEEP DATA OUTPUTS
When it comes to devices that purport to track sleep, PPG is often combined with actigraphy to provide the sleep data outputs (such as sleep times, restlessness, movement) that are familiar to clinicians. Consider that many device validation studies are comparing “apples and oranges” by comparing overnight polysomnogram (electroencephalogram [EEG], electromyogram [EMG], and electrooculography [EOG]) “apples” sensors and PPG/actigraphy “oranges” sensors. It should be noted that PPG-based wearables have an output of PPG-estimated sleep or sleep stages, in contrast to the gold standard of polysomnography (PSG) that uses different sensors and yields the conventional sleep stages defined by EEG, EMG, and EOG. PPG-estimated sleep stages are currently not standardized and vary from device to device, commonly grouping sleep stages into 2-stage categorization (simply wake and sleep), 3-stage categorization (wake/NREM/REM), 4-stage categorization (light sleep, deep sleep, REM, wake), or 5-stage categorization (stages N1, N2, N3, REM, and wake). While sleep-stage reporting may vary across devices, wearables have the benefit of gathering longitudinal data, with the ability to evaluate sleep daily over weeks/months in contrast to 1 night in the sleep laboratory or 1 to 2 nights at home. While many studies compare these estimates with PSG for sleep stage validation, these again require a measure of caution in data interpretation. Many of these studies use limited datasets (which may lack diversity) and post-hoc algorithmic adjustments to “validate” the devices. At this time, the estimated parameters of consumer-grade devices should not take the place of the extensively validated use of PSG testing in the diagnosis and evaluation of treatment efficacies of sleep disorders. For clinical use, clear standards of practice for specified populations and indications for diagnostic or therapeutic efficacy monitoring are warranted. Again, proprietary device technology is generally device-specific, and its uses should not be generalized across different device technologies. Another consideration for clinical uses is cost and reimbursement of device integration into the clinical workflow.
MOVING FORWARD
Current and future uses of PPG technology in sleep medicine for diagnosis, treatment, remote data monitoring, and population health are exciting. As both consumer and clinical PPG-based technologies continue to evolve, it is certain that the sleep provider will need continued awareness of this technology and its capabilities especially when navigating clinical uses as well as consumer sleep technologies presented in the clinic by patients. AASM members will continue to receive updates through AASM communications, #SleepTechnology reviews, and other resources. Going forward, validating consumer sleep technology against in-lab PSG or outcomes-based performance, clear clinical practice guidelines for specific populations, and more research on the performance of diagnostic/screening/monitoring/therapeutic tools are warranted. Ultimately, as the capabilities of PPG-based and multisensor (PPG plus actigraphy or other) technologies evolve, so do the potential uses to further advance consumer and clinical sleep technology.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AI
artificial intelligence
- DL
deep learning
- FDA
US Food and Drug Administration
- HRV
heart rate variability
- IR
infrared
- ML
machine learning
- PPG
photoplethysmography
- PSG
polysomnography
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors of this paper compose the 2021–2022 AASM Emerging Technology Committee. This technology review was developed by the AASM Emerging Technology Committee and presented to the Executive Committee. It is published as an advisory that is to be used for educational and informational purposes only. Dr. Chandrakantan was supported by the AASM Bridge to Independence and Baylor College of Medicine OOR awards. Currently, he is supported by NHLBI Grant # 1K08HL161263-01. The other authors report no conflicts of interest.
REFERENCES
- 1. Merrick EB , Hayes TJ . Continuous, non-invasive measurements of arterial blood oxygen levels . Hewlett-Packard J. 1976. ; 28 ( 2 ): 2 – 9 . http://www.hparchive.com/Journals/HPJ-1976-10.pdf; Accessed September 26, 2022. [Google Scholar]
- 2. Severinghaus JW . Takuo Aoyagi: discovery of pulse oximetry . Anesth Analg. 2007. 105 ( 6 , Suppl ): S1 – S4 . [DOI] [PubMed] [Google Scholar]
- 3. Budhida K , Kyriacou P . Photoplethysmography Technology . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 43 – 67 . [Google Scholar]
- 4. Ghamari M , Castaneda D , Esparza A , Soltanpur C , Nazeran H . A review on wearable photoplethysmography sensors and their potential future applications in health care . Int J Biosens Bioelectron. 2018. ; 4 ( 4 ): 195 – 202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kyriacou P , Chatterjee S . The Origin of Photoplethysmography . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 17 – 42 . [Google Scholar]
- 6. Tamura T , Maeda Y , Sekine M , Yoshida M . Wearable photoplethysmographic sensors – past and present . Electronics (Basel). 2014. ; 3 ( 2 ): 282 – 302 . [Google Scholar]
- 7. Reisner A , Shaltis PA , McCombie D , Asada HH . Utility of the photoplethysmogram in circulatory monitoring . Anesthesiology. 2008. ; 108 ( 5 ): 950 – 958 . [DOI] [PubMed] [Google Scholar]
- 8. Chan ED , Chan MM , Chan MM . Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations . Respir Med. 2013. ; 107 ( 6 ): 789 – 799 . [DOI] [PubMed] [Google Scholar]
- 9. Ash C , Dubec M , Donne K , Bashford T . Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods . Lasers Med Sci. 2017. ; 32 ( 8 ): 1909 – 1918 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colvonen PJ , DeYoung PN , Bosompra NA , Owens RL . Limiting racial disparities and bias for wearable devices in health science research . Sleep. 2020. ; 43 ( 10 ): zsaa159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bent B , Goldstein BA , Kibbe WA , Dunn JP . Investigating sources of inaccuracy in wearable optical heart rate sensors . NPJ Digit Med. 2020. ; 3 ( 1 ): 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fine J , Branan KL , Rodriguez AJ , et al . Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring . Biosensors (Basel). 2021. ; 11 ( 4 ): 126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ajmal B-AT , Boonya-Ananta T , Rodriguez AJ , Du Le VN , Ramella-Roman JC . Monte Carlo analysis of optical heart rate sensors in commercial wearables: the effect of skin tone and obesity on the photoplethysmography (PPG) signal . Biomed Opt Express. 2021. ; 12 ( 12 ): 7445 – 7457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charlton PH , Marozas V . Wearable Photoplethysmography Devices . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 401 – 438 . [Google Scholar]
- 15. Andrist E , Nuppnau M , Barbaro RP , Valley TS , Sjoding MW . Association of race with pulse oximetry accuracy in hospitalized children . JAMA Netw Open. 2022. ; 5 ( 3 ): e224584 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nowara EM , McDuff D , Veeraraghavan A . A meta-analysis of the impact of skin type and gender on non-contact photoplethysmography measurements. In: 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW). 2020. :1148–1155.
- 17. Allen J , Murray A . Age-related changes in the characteristics of the photoplethysmographic pulse shape at various body sites . Physiol Meas. 2003. ; 24 ( 2 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 18. Mejía-Mejía E , Allen J , Budidha K , et al . Photoplethysmography Signal Processing and Synthesis . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 69 – 145 . [Google Scholar]
- 19. Lee JH , Yang S , Park J , et al . Time to consider the contact force during photoplethysmography measurement during pediatric anesthesia: a prospective, nonrandomized interventional study . Paediatr Anaesth. 2018. ; 28 ( 7 ): 660 – 667 . [DOI] [PubMed] [Google Scholar]
- 20. Charlton PH , Bonnici T , Tarassenko L , Clifton DA , Beale R , Watkinson PJ . An assessment of algorithms to estimate respiratory rate from the electrocardiogram and photoplethysmogram . Physiol Meas. 2016. ; 37 ( 4 ): 610 – 626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlton PH , Marozas V . Wearable Photoplethysmography Devices . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 415 – 416 . [Google Scholar]
- 22. Millasseau SC , Kelly RP , Ritter JM , Chowienczyk PJ . Determination of age-related increases in large artery stiffness by digital pulse contour analysis . Clin Sci (Lond). 2002. ; 103 ( 4 ): 371 – 377 . [DOI] [PubMed] [Google Scholar]
- 23. Yousef Q , Reaz M , Ali MAM . The analysis of PPG morphology: investigating the effects of aging onarterial compliance . Meas Sci Rev. 2012. ; 12 ( 6 ): 266 – 271 . [Google Scholar]
- 24. Mukkamala R , Hahn J , Chandrekhar A . Photoplethysmography in Noninvasive Blood Pressure Monitoring . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 359 – 399 . [Google Scholar]
- 25. Passler S , Bohrer J , Blöchinger L , Senner V . Validity of wrist-worn activity trackers for estimating VO2max and energy expenditure . Int J Environ Res Public Health. 2019. ; 16 ( 17 ): 3037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charlton PH , Marozas V . Wearable Photoplethysmography Devices . In: Kyriacou P , Allen J , eds. Photoplethysmography: Technology, Signal Analysis, and Applications. Amsterdam: : Elsevier; ; 2022. : 417 – 418 . [Google Scholar]
- 27. Herhaus B , Kalin A , Gouveris H , Petrowski K . Mobile heart rate variability biofeedback improves autonomic activation and subjective sleep quality of healthy adults – a pilot study . Front Physiol. 2022. ; 13 : 821741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Georgiou K , Larentzakis AV , Khamis NN , Alsuhaibani GI , Alaska YA , Giallafos EJ . Can wearable devices accurately measure heart rate variability? A systematic review . Folia Med (Plovdiv). 2018. ; 60 ( 1 ): 7 – 20 . [DOI] [PubMed] [Google Scholar]
- 29. Ucak S , Dissanayake HU , Sutherland K , de Chazal P , Cistulli PA . Heart rate variability and obstructive sleep apnea: current perspectives and novel technologies . J Sleep Res. 2021. ; 30 ( 4 ): e13274 . [DOI] [PubMed] [Google Scholar]
- 30. Qin H , Steenbergen N , Glos M , et al . The different facets of heart rate variability in obstructive sleep apnea . Front Psychiatry. 2021. ; 12 : 642333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tobaldini E , Nobili L , Strada S , Casali KR , Braghiroli A , Montano N . Heart rate variability in normal and pathological sleep . Front Physiol. 2013. ; 4 : 294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonnet MH , Arand DL . Heart rate variability in insomniacs and matched normal sleepers . Psychosom Med. 1998. ; 60 ( 5 ): 610 – 615 . [DOI] [PubMed] [Google Scholar]
- 33. Koch C , Wilhelm M , Salzmann S , Rief W , Euteneuer F . A meta-analysis of heart rate variability in major depression . Psychol Med. 2019. ; 49 ( 12 ): 1948 – 1957 . [DOI] [PubMed] [Google Scholar]
- 34. Korkalainen H , Aakko J , Duce B , et al . Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea . Sleep. 2020. ; 43 ( 11 ): zsaa098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. US Food and Drug Administration . Pulse Oximeter Accuracy and Limitations. FDA Safety Communication. https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication ; Accessed August 8, 2022. .