Abstract
Study Objectives:
Recent findings indicate that noradrenergic and muscarinic processes are crucial for pharyngeal muscle control during sleep. However, to date, reductions in obstructive sleep apnea (OSA) severity have only been detected when noradrenergic agents are combined with an antimuscarinic. Accordingly, this study aimed to determine if reboxetine alone and combined with oxybutynin reduces OSA severity. The pathophysiological mechanisms underpinning the effects of these agents were also investigated via endotyping analysis.
Methods:
Sixteen people (6 women) with OSA completed 3 polysomnograms (∼1-week washout) according to a double-blind, placebo-controlled, three-way crossover design across 2 sites. Single doses of 4 mg reboxetine, placebo, or 4 mg reboxetine + 5 mg oxybutynin were administered before sleep (order randomized).
Results:
Reboxetine reduced the apnea-hypopnea index (primary outcome) by 5.4 (95% confidence interval −10.4 to −0.3) events/h, P = .03 (−24 ± 27% in men; −0.7 ± 32% in women). Oxybutynin did not cause additional reductions in apnea-hypopnea index. Reboxetine alone reduced the 4% oxygen desaturation index by (mean ± standard deviation) 5.2 ± 7.2 events/h and reboxetine+oxybutynin by 5.1 ± 10.6 events/h vs placebo, P = .02. Nadir oxygen saturation also increased by 7 ± 11% with reboxetine and 5 ± 9% with reboxetine+oxybutynin vs placebo, P = .01. Mechanistically, reboxetine and reboxetine+oxybutynin improved pharyngeal collapsibility and respiratory control (loop gain). Larger reductions in apnea-hypopnea index with reboxetine in men were associated with higher baseline loop gain.
Conclusions:
These findings show the first evidence that reboxetine alone reduces OSA severity. The data provide novel insight into the role of norepinephrine reuptake inhibitors on upper airway stability during sleep and are important to inform future pharmacotherapy development for OSA.
Clinical Trial Registration:
Registry: Australian New Zealand Clinical Trials Registry; Name: Reboxetine and Combination Therapy with AD128 in Sleep Apnoea Trial: A Double-Blind, 3-Way Cross-Over Study; URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374614&isReview=true; Identifier: ACTRN12620000662965.
Citation:
Altree TJ, Aishah A, Loffler KA, Grunstein RR, Eckert DJ. The norepinephrine reuptake inhibitor reboxetine alone reduces obstructive sleep apnea severity: a double-blind, placebo-controlled, randomized crossover trial. J Clin Sleep Med. 2023;19(1):85–96.
Keywords: sleep-disordered breathing, pharmacotherapy, precision medicine, respiratory, upper airway physiology
BRIEF SUMMARY
Current Knowledge/Study Rationale: The noradrenergic reboxetine combined with antimuscarinic drugs reduces obstructive sleep apnea severity. Reboxetine alone may also reduce obstructive sleep apnea severity but has not been assessed as a single agent.
Study Impact: This study shows that reboxetine alone reduces obstructive sleep apnea severity and provides insight into the underlying pathophysiological mechanisms by which reboxetine stabilizes breathing during sleep. These findings are important to inform future development of drugs to treat obstructive sleep apnea.
INTRODUCTION
Global estimates indicate that nearly 1 billion people have obstructive sleep apnea (OSA).1,2 OSA is characterized by repetitive narrowing and partial or complete collapse of the pharyngeal airway, hypoxia, hypercapnia, and frequent arousals during sleep. Untreated OSA is associated with a range of adverse health outcomes including cardiovascular,3,4 neurocognitive,5,6 and metabolic disease.7 Continuous positive airway pressure is efficacious and is currently the first-line treatment for moderate-severe OSA. However, 46 to 83% of those prescribed continuous positive airway pressure are not adherent to therapy.8 Other therapies such as mandibular advancement splints have better adherence but variable and unpredictable efficacy.9 Thus, there is an urgent need to develop new therapies to treat this highly prevalent chronic health condition.
Sleep-dependent reductions in pharyngeal dilator muscle control combined with vulnerable upper airway anatomy are key contributors to OSA pathophysiology.10 Recent animal studies highlight the critical role of noradrenergic and antimuscarinic processes in pharyngeal muscle control during sleep.11,12 These studies indicate that loss of noradrenergic activity is the major mechanism responsible for sleep-related pharyngeal muscle hypotonia during nonrapid eye movement (NREM) sleep.11 Muscarinic activity further contributes to atonia during rapid eye movement (REM) sleep.12 These findings suggest that medications targeting noradrenergic processes during NREM sleep and antimuscarinic processes during REM sleep may reduce OSA severity by augmenting pharyngeal dilator muscle activity.
The importance of these mechanisms in humans was supported by the recent findings of Taranto-Montemurro et al where the selective noradrenaline reuptake inhibitor atomoxetine (80 mg) combined with the antimuscarinic agent oxybutynin (5 mg) reduced the apnea-hypopnea index (AHI) by ∼60% and improved nadir overnight oxygen saturation from ∼85% to the high 90s compared with placebo.13 These beneficial effects were driven by a 3-fold improvement in pharyngeal muscle responsiveness and a reduction in loop gain (improved respiratory control).14 The wake-promoting effects of atomoxetine also modestly increased the propensity for awakening during respiratory events (lowered the respiratory arousal threshold).14 However, unlike the animal data, reductions in OSA severity did not occur when either atomoxetine or oxybutynin was administered alone.13 An alternative noradrenergic agent, reboxetine (4 mg), combined with oxybutynin (5 mg) administered orally daily for 7 days was recently shown to cause a median reduction in AHI of ∼60% in 16 people with severe OSA.15 A single dose of reboxetine (4 mg) combined with an alternative antimuscarinic, hyoscine butylbromide (20 mg), improved upper airway stability during sleep in healthy adults16 and reduced the AHI via increased tonic genioglossus muscle activity and reductions in loop gain in 12 people with OSA.17 However, hyoscine butylbromide minimally crosses the blood-brain barrier, so the reduction in OSA severity with reboxetine and hyoscine butylbromide may have been predominantly driven by reboxetine alone.18 However, no studies have investigated the effects of reboxetine alone. Accordingly, this study aimed to determine the acute effects of a single presleep dose of reboxetine alone (primary outcome) and in combination with oxybutynin on OSA severity and on next day sleepiness and alertness (secondary outcomes). In addition, we also explored the effects of these agents on OSA pathophysiological mechanisms.
METHODS
Participants
People with OSA (AHI ≥ 10 events/h confirmed via in-laboratory polysomnography within the past 12 months) aged between 18 and 65 years and not currently on OSA treatment were eligible to participate. Individuals were excluded if they used antidepressants, strong cytochrome P450 3A4 and 2D6 inhibitors, any medication known to influence breathing during sleep or daytime alertness (ie hypnotics, respiratory stimulants, antipsychotics, anxiolytics, psychostimulants), were pregnant, smoked > 10 cigarettes per day (due to potential sleep disruption effects), had narcolepsy, a clinically significant mood disorder, cardiac disease including uncontrolled blood pressure, significant craniofacial malformation, epilepsy, schizophrenia, previous diagnosis of insomnia, history of benign prostatic hyperplasia or urinary retention, narrow angle glaucoma, or known allergy to reboxetine or oxybutynin. Participants were asked to abstain from alcohol on the days of the study and limit caffeine intake to a maximum of 400 mg per day, and none in the 3 hours prior to bedtime. Participants were enrolled from sleep medicine clinics, a database of previous research participants and a clinical trial matching agency (HealthMatch). No participant had taken reboxetine previously. The study was approved by Bellberry Human Research Ethics Committee (2019-12-1081-A-1) and participants provided informed written consent prior to enrollment. The research was performed in accordance with relevant guidelines and regulations including the Declaration of Helsinki and all local Human Research Ethics Committee requirements.
Protocol
Three overnight sleep studies were performed with an approximately 1-week washout between each visit according to a double-blind, randomized, placebo-controlled, three-way, crossover design (Figure 1). This was a multicenter study with two recruitment and data collection sites: (1) Adelaide Institute for Sleep Health, Flinders University, Adelaide, Australia and (2) the Woolcock Institute for Medical Research, Sydney, Australia. At each of the three visits, participants received oral reboxetine alone (4 mg) or reboxetine (4 mg) with oxybutynin (5 mg) or placebo in randomized order immediately before bedtime. Study medications were prepared by Optima Ovest and were placed in identical capsules that could not be identified by study personnel or participants. The study pharmacist prepared the randomization code in blocks of 4. All participants, investigators, and outcome assessors were blinded to the treatment allocation. Bedtime was kept constant between study visits and participants were given an 8-hour sleep opportunity on each occasion. The predefined primary endpoint was the AHI (events/h sleep) using 3% desaturation criteria (AHI3). Secondary outcomes included other polysomnography outcomes such as sleep efficiency, the arousal index, measures of hypoxemia, snoring using a calibrated sound meter, AHI using the 4% desaturation criteria (AHI4) and markers of next day sleepiness and alertness. All data analyses were performed before unblinding of the intervention allocation. The protocol was prospectively registered on the Australian New Zealand Clinical Trials Registry (ACTRN12620000662965).
Figure 1. Consort flow diagram.
Enrollment and participant flow through the protocol and analysis for this double-blind, randomized, placebo-controlled, 3-way crossover study.
Measurements and equipment
Blood pressure and heart rate were measured 3 times each in the evening and the following morning during each visit. A standard clinical montage was used during overnight polysomnography including nasal flow, thermistor, respiratory bands, oximetry, chin and leg electromyogram, electroencephalogram, and electrooculogram (Grael 4K PSG:EEG, Compumedics, Abbotsford, Australia).19 Participants completed a 30-min simulated driving task (AusEd Driving Simulator)20 approximately 30 minutes after waking at each visit to assess next-day alertness. Subjective sleepiness was measured approximately 1 hour after waking using the Karolinska Sleepiness Scale21 and the Leeds Sleep Evaluation Questionnaire was administered.22
Data analysis
Sleep staging, arousals, and respiratory events were scored at each site using standard American Academy of Sleep Medicine guidelines23 by an experienced sleep technologist blinded to the study intervention. Hypopneas were defined as a reduction in flow of 30% or more from baseline lasting at least 10 seconds, associated with either an arousal from sleep or an oxyhemoglobin desaturation ≥ 3% (AHI3) or ≥ 4% (AHI4).
OSA endotypic traits to explore pathophysiological mechanisms were quantified using a validated custom-designed algorithm from the polysomnography recordings (MATLAB, MathWorks, Natick, Massachusetts).24,25 Ventilation was estimated using the square root transform of the nasal pressure signal (tidal volume × respiratory frequency). This was integrated breath-by-breath to provide a time series of ventilation data that was normalized (mean ventilation = 1.0, apnea = 0) for analysis as per the methodology described by Terrill et al and Sands et al.24,25 The following traits were measured on each night during NREM sleep in supine and lateral positions as a percentage of eupneic ventilation (V̇eupnea):
mean pharyngeal collapsibility (V̇passive): the estimated average ventilation during sleep at eupneic drive when the pharyngeal muscles are relatively passive.26 A higher value represents a less-collapsible upper airway;
nadir pharyngeal collapsibility (V̇passivemin): the estimated ventilation when the pharyngeal muscles are at their most hypotonic level/the airway is most collapsible, quantified at the lowest estimated decile of ventilatory drive from the V̇passive measures (analogous to the passive critical closing pressure of the upper airway).27 A higher value represents a less collapsible airway at the point of highest likelihood of collapse;
pharyngeal muscle recruitment (V̇active): the estimated ventilation at maximum ventilatory drive. A higher value indicates increased muscle recruitment;
pharyngeal muscle compensation (V̇comp): the estimated change in ventilation that accompanies an increase in ventilatory drive, ie, the ventilatory equivalent of the active minus passive critical closing pressures measured as the difference between V̇active and V̇passive. A higher value represents greater muscle compensation;
the ventilatory response to arousal (VRA): the estimated ventilatory overshoot during a transient cortical arousal from sleep. A higher value represents greater ventilatory overshoot and increased propensity for subsequent respiratory instability;
ventilatory control stability (loop gain): LG1, breathing response to a 1-cycle-per-minute reduction in ventilation and LGn, including circulatory delay effects. Higher values represent greater ventilatory control instability;
respiratory arousal threshold: the estimated respiratory drive that causes an arousal from sleep. A higher value represents a larger fall in ventilation that can be sustained before an arousal from sleep occurs.
The hypoxic burden was also quantified using previously described methodology.28
Statistical analysis
We performed a power analysis based on detection of a change in AHI of 9 events/h using an alpha of 0.05 and a power of 0.8. We determined the minimum number of participants required was 15. Note that based on our previous reboxetine and hyoscine butylbromide study17 we anticipated a larger effect size. However, we elected to use a more conservative effect size estimate in the current study. One-way repeated-measures analysis of variance (ANOVA) was used to test for differences in polysomnography parameters, OSA endotypes, and next-day measures of alertness and subjective sleep quality between reboxetine, placebo, and reboxetine+oxybutynin or one-way ANOVA on ranks for nonnormally distributed data (according to a Shapiro-Wilk normality test). Where significant main effects were detected, pairwise comparisons were performed using Student-Newman-Keuls post hoc test or chi-square tests as appropriate. Post hoc exploratory analyses to investigate potential sex differences in AHI responses, oxygen parameters, and OSA endotypes were performed using unpaired Students t tests or Mann-Whitney rank sum tests for nonnormally distributed data. Polysomnography and endotype data were analyzed with SigmaPlot V14.5 (Systat Software, San Jose, California). All other analyses were performed using SPSS V25 (IBM SPSS Statistics, IBM Corporation, Armonk, New York). Statistical significance was inferred when P < .05.
RESULTS
Participants
Data collection for the study was undertaken from June to December 2020. Of 45 potential participants screened, 17 met the inclusion criteria. One was excluded after providing consent due to high blood pressure prior to drug administration on night 1 (Figure 1). Data were acquired in all the remaining 16 participants who commenced the study. Data collection was ceased when the prespecified sample size completed the study. On average, the 16 participants who completed all 3 nights were middle-aged, overweight to obese, had subclinical insomnia (according to Insomnia Severity Index scores collected on night 1 of the study), did not have significant daytime sleepiness, and had moderate–severe OSA (Table 1). Comorbidities and medication use were as expected for a cohort of people with OSA.
Table 1.
Participant characteristics.
| Sex | 6 female, 10 male |
| Age, y | 49 ± 12 |
| BMI, kg/m2 | 30.5 ± 4.7 |
| Neck circumference, cm | 41 ± 4 |
| Waist circumference, cm | 103 ± 12 |
| Comorbidities, n (%) | |
| Hypertension | 5 (31.25) |
| Hyperlipidemia | 3 (18.75) |
| Type 2 diabetes mellitus | 3 (18.75) |
| Hypothyroidism | 1 (6.25) |
| Medications, n (%) | |
| Proton pump inhibitors | 1 (6.25) |
| Statins | 3 (18.75) |
| Antihypertensives | 2 (12.5) |
| Oral hypoglycemics | 1 (6.25) |
| Thyroid hormones | 1 (6.25) |
| Epworth Sleepiness Scale (0–24-point scale) | 5.5 (3.5–7.5) |
| Insomnia severity index | 14.0 (8.0–16.5) |
| Key baseline polysomnography parameters | |
| AHI (events/h) | 32 ± 14 |
| Sleep efficiency (%) | 81 (72–90) |
| NREM AHI (events/h) | 31 ± 16 |
| REM AHI (events/h) | 35 ± 15 |
| Nadir overnight oxygen saturation (%) | 84 (79–89) |
Key baseline polysomnographic data were acquired from sleep studies performed prior to study enrolment. Data are presented as mean ± SD or median (interquartile range) as appropriate, unless otherwise indicated. AHI = apnea-hypopnea index, BMI = body mass index, NREM = nonrapid eye movement, REM = rapid eye movement.
No serious adverse events were observed during the study. Seven participants reported mild–moderate adverse events related to reboxetine, 5 reported mild adverse events related to reboxetine+oxybutynin, and 1 reported a mild adverse event on placebo (Table 2). The adverse events recorded were known side effects of either reboxetine or oxybutynin and had no major impact on sleep efficiency (Table 3). No adverse event was serious enough to warrant unblinding of the allocation in any participant.
Table 2.
Adverse events.
| Reboxetine | Placebo | Reb-Oxy | |
|---|---|---|---|
| Total number of adverse events | 10 | 1 | 9 |
| Participants with ≥ 1 adverse event, n (%) | 3 (18.75) | 0 | 3 (18.75) |
| Total number of serious adverse events | 0 | 0 | 0 |
| Total number of moderate adverse events | 2 | 0 | 0 |
| Total number of mild adverse events | 8 | 1 | 9 |
| Total number of adverse events leading to participant withdrawal | 0 | 0 | 0 |
| Adverse events by system organ class | |||
| Gastrointestinal | |||
| Abdominal pain | 0 | 0 | 1 |
| Constipation | 1 | 0 | 0 |
| Dyspepsia | 1 | 0 | 0 |
| Nausea | 1 | 0 | 3 |
| General | |||
| Chills | 2 | 0 | 1 |
| Nervous system | |||
| Dizziness | 1 | 0 | 0 |
| Dysgeusia | 0 | 0 | 1 |
| Headache | 0 | 1 | 0 |
| Paresthesia | 1 | 0 | 1 |
| Psychiatric | |||
| Anxiety | 1 | 0 | 0 |
| Renal | |||
| Urinary hesitancy | 2 | 0 | 2 |
Mild adverse event defined as “easily tolerated, causing minimal discomfort, not interfering with activities.” Moderate adverse event defined as “sufficient discomfort to interfere with everyday activities.” Reb-Oxy = reboxetine+oxybutynin.
Table 3.
Polysomnography parameters.
| Reboxetine | Placebo | Reb-Oxy | P value | |
|---|---|---|---|---|
| Supine AHI (events/h) | 43 ± 20 | 46 ± 15 | 42 ± 25 | .578 |
| Supine sleep (%TST) | 49 (27–80) | 52 (31–94) | 54 (35–71) | .121 |
| NREM AHI (events/h) | 31 ± 15 | 35 ± 17 | 32 ± 17 | .253 |
| Obstructive apnea index (events/h) | 0 (0–3) | 3 (0–10) | 0 (0–3) | .072 |
| 3% ODI (events/h) | 8.9 (2.1–21.1) | 13.1 (10.1–35.5) | 13.1 (2.0–20.7) | .029*† |
| Snoring index (snores/h)‡ | 341 ± 179 | 469 ± 176 | 252 ± 177 | .001*† |
| Arousal index (events/h) | 33 ± 13 | 32 ± 12 | 30 ± 12 | .609 |
| Total sleep time (min) | 376 ± 44 | 391 ± 51 | 400 ± 38 | .218 |
| Sleep efficiency (%TIB) | 79 ± 10 | 80 ± 10 | 85 ± 6 | .113 |
| Wake after sleep onset (min) | 90 ± 35 | 83 ± 42 | 64 ± 28 | .105 |
| Sleep stage (% of TST) | ||||
| N1 | 25 ± 17 | 21 ± 11 | 27 ± 14 | .185 |
| N2 | 56 ± 16 | 45 ± 11 | 56 ± 14 | .003*† |
| N3 | 18 ± 8 | 21 ± 11 | 16 ± 7 | .100 |
| REM | 2 ± 2 | 13 ± 7 | 2 ± 4 | <.001*† |
| Morning measurements | ||||
| Heart rate (beats/min) | 83 ± 14 | 69 ± 11 | 83 ± 13 | <.001*† |
| Systolic blood pressure (mm Hg) | 134 ± 21 | 134 ± 15 | 134 ± 17 | .984 |
| Diastolic blood pressure (mm Hg) | 89 (83–96) | 88 (79–91) | 90 (83–97) | .103 |
AHI values refer to AHI scored using 3% desaturation criteria. Data are presented as mean ± SD or median (interquartile range) as appropriate. *Reboxetine vs placebo pairwise comparison P < .05. †Reboxetine+oxybutynin vs placebo pairwise comparison P < .05. ‡n = 13. Three participants’ snoring data were incomplete and therefore were not included in the analysis. AHI = apnea-hypopnea index, N1 = stage 1 sleep, N2 = stage 2 sleep, N3 = stage 3 sleep, NREM = nonrapid eye movement sleep, ODI = oxygen desaturation index, Reb-Oxy = reboxetine+oxybutynin, REM = rapid eye movement sleep, TIB = time in bed, TST = total sleep time.
Effects of reboxetine and reboxetine+oxybutynin on OSA severity and oxygenation
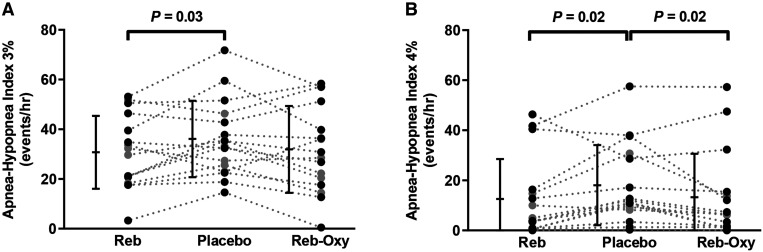
There was an overall treatment effect on AHI3 (ANOVA P = .049; Figure 2A). Reboxetine alone reduced the AHI3 by 5.4 events/h (95% confidence interval, −10.4 to −0.3], P = .04 (−8 ± 9 events/h in men from a baseline of 39 ± 18 events/h; −1 ± 9 events/h in women from a baseline of 32 ± 9 events/h) compared to placebo. AHI3 with reboxetine+oxybutynin compared to placebo was not significantly different (4.2 events/h [95% confidence interval, −9.6 to 1.1]; P = .11, −6 ± 9 events/h in men; −2 ± 12 events/h in women). There was also an overall treatment effect for AHI4 (ANOVA P = .002; Figure 2B). Both reboxetine alone and reboxetine+oxybutynin reduced the AHI4 vs placebo (Figure 2B).
Figure 2. Effect of reboxetine (Reb) and reboxetine–oxybutynin combination (Reb-Oxy) on apnea-hypopnea index (AHI).
AHI using the 3% (A) and 4% desaturation criteria (B) are shown. Plots show mean and standard deviation (A) and median and interquartile range (B) plus individual values (gray circles indicate women, black circles indicate men). Significant pairwise comparisons P < .05 are indicated above the individual values.
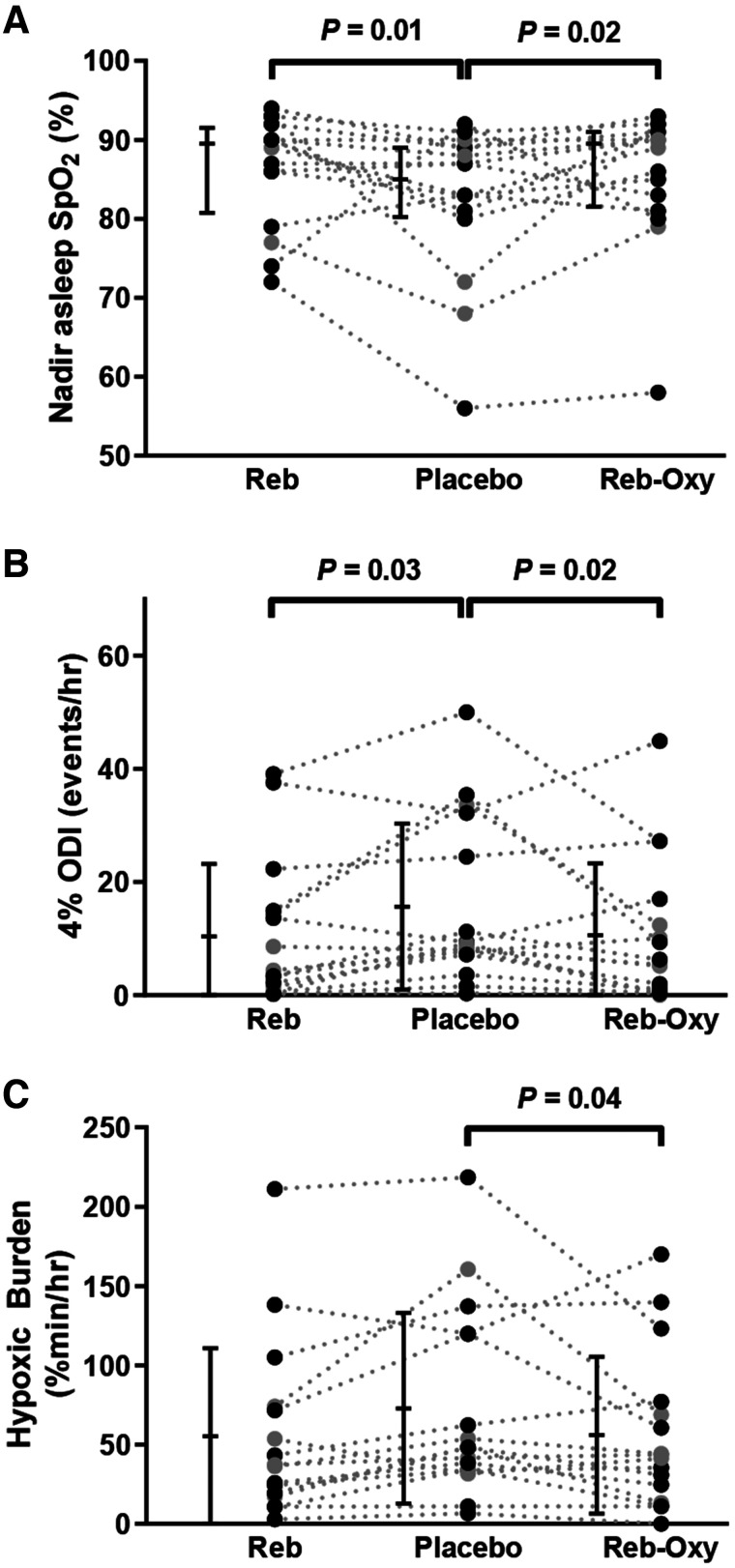
Nadir oxygen saturation increased by 7 ± 11% (mean ± SD) with reboxetine and 5 ± 9% with reboxetine+oxybutynin vs placebo (Figure 3A, ANOVA P = .013). Reboxetine and reboxetine+oxybutynin both reduced 4% oxygen desaturation index compared to placebo (Figure 3B, ANOVA P = .018). Similarly, the hypoxic burden was reduced with treatment vs placebo (Figure 3C, ANOVA P = 0.049). Reboxetine and reboxetine+oxybutynin improved the 3% oxygen desaturation index and snoring index vs placebo (Table 3).
Figure 3. Effect of reboxetine (Reb) and reboxetine-oxybutynin combination (Reb-Oxy) on measures of overnight hypoxemia compared to placebo.
(A) Nadir O2 saturation, (B) 4% oxygen desaturation index, and (C) hypoxic burden. Plots show mean and standard deviation and individual values (gray circles indicate women, black circles indicate men). Significant pairwise comparisons P < .05 are indicated above the individual values.
Effects of reboxetine and reboxetine+oxybutynin on sleep parameters
Percent sleep time spent supine, sleep efficiency, wake after sleep onset, arousal index, NREM AHI, supine AHI, and obstructive apnea index were not different between conditions. Reboxetine and reboxetine+oxybutynin reduced the proportion of REM sleep and increased stage N2 sleep, with no changes in stages N1 or N3 sleep vs placebo. Reboxetine and reboxetine+oxybutynin increased morning heart rate by 14 ± 11 and 14 ± 8 beats per minute compared to placebo, respectively. Despite the increased morning heart rate, there were no changes in morning systolic or diastolic blood pressure, and no participants experienced palpitations during the study.
OSA endotypes
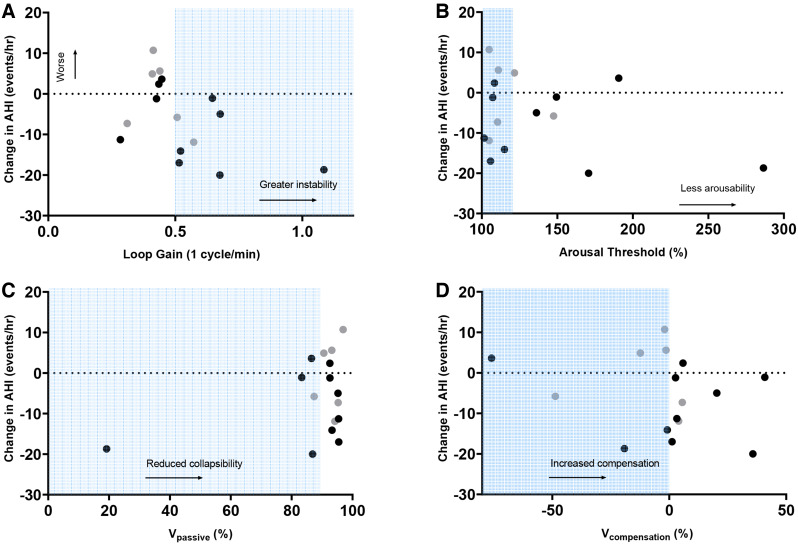
Reboxetine alone and in combination with oxybutynin improved pharyngeal collapsibility at the lowest decile of respiratory drive (V̇passivemin) compared to placebo (median 7.7% [interquartile range 4.4 to 10.7] and 6.4% [interquartile range 2.7 to 6.4] respectively, both P < .001). Reboxetine and reboxetine+oxybutynin both reduced LGn and the ventilatory response to arousal vs placebo. Reboxetine+oxybutynin increased upper airway muscle compensation, although reboxetine alone did not. Overall estimated pharyngeal collapsibility was not significantly different between conditions. Placebo night loop gain was higher in men vs women (0.44 ± 0.09 vs 0.35 ± 0.06, P = .042). The other OSA endotypes were not systematically different between men and women (eg, V̇passive 93 [86 to 95] vs 94 [90 to 96]). AHI tended to improve with reboxetine in participants with high loop gain and high muscle compensation (Table 4 and Figure 4).
Table 4.
OSA endotypes.
| Reboxetine | Placebo | Reb-Oxy | P value | |
|---|---|---|---|---|
| V̇passive (%eupnea) | 93 (89 to 95) | 93 (87 to 95) | 92 (89 to 95) | .472 |
| V̇passivemin (%eupnea) | 66 (57 to 74) | 54 (43 to 66) | 66 (54 to 76) | <.001*† |
| V̇active (%eupnea) | 96 (84 to 100) | 96 (82 to 100) | 98 (95 to 105) | .075 |
| V̇comp (%eupnea) | 2 (−6 to 4) | 2 (−10 to 6) | 4 (2 to 9) | .009† |
| VRA (%eupnea) | 37 ± 20 | 49 ± 25 | 29 ± 13 | <.001*† |
| Loop gainn (dimensionless) | 0.37 (0.31 to 0.41) | 0.40 (0.35 to 0.46) | 0.34 (0.32 to 0.43) | .039*† |
| Loop gain1 (dimensionless) | 0.46 ± 0.16 | 0.52 ± 0.19 | 0.45 ± 0.10 | .097 |
| Arousal threshold (%eupnea) | 114 (107 to 134) | 113 (106 to 149) | 114 (109 to 127) | .368 |
Data are presented as mean ± SD or median (interquartile range) as appropriate. *Reboxetine vs placebo pairwise comparison P < .05. †Reboxetine+oxybutynin vs placebo pairwise comparison P < .05. Loop gain = estimated change in ventilatory drive in response to a ventilatory disturbance (LG1, breathing response to a 1-cycle-per-minute reduction in ventilation and LGn, including circulatory delay effects), OSA = obstructive sleep apnea, Reb-Oxy = reboxetine+oxybutynin, respiratory arousal threshold = estimated respiratory drive that causes an arousal from sleep, V̇active = estimated ventilation at maximum ventilatory drive, V̇comp = the change in estimated ventilation that accompanies an increase in ventilatory drive, measured as the difference between V̇active and V̇passive, V̇passive = estimated ventilation (pharyngeal collapsibility) at normal/eupneic ventilatory drive, V̇passivemin = estimated ventilation when pharyngeal muscles are at their most hypotonic level, quantified at the lowest estimated decile of ventilatory drive, ventilatory response to arousal = estimated ventilatory overshoot to an arousal from sleep, VRA = ventilatory response to arousal.
Figure 4. Change in AHI (events/h, 3% criteria) on reboxetine compared to baseline obstructive sleep apnea endotypes (as measured on placebo).
(A) Loop gain (LG1) representing ventilatory control hypersensitivity, (B) arousal threshold, (C) collapsibility (V̇passive), and (D) muscle compensation (V̇compensation) are presented as a percentage of eupneic levels. Refer to text for further details. Shading indicates unfavorable trait characteristics (ie, high loop gain, low arousal threshold, collapsible pharyngeal airway, and poor muscle compensation) as defined previously.25,46–48 Gray circles indicate women, black circles indicate men. AHI = apnea-hypopnea index.
Effects of reboxetine and reboxetine+oxybutynin on next-day alertness and subjective sleep quality
There were no differences in driving simulator performance measures between reboxetine, placebo, and reboxetine+oxybutynin conditions. There were also no differences in morning subjective sleepiness scores as measured by the Karolinska Sleepiness Scale. However, participants reported worse perceived sleep quality on reboxetine (mean difference in Leeds Sleep Evaluation Questionnaire “Quality of Sleep” domain score, −3.46 ± 5.97; P = .04) and reboxetine+oxybutynin (−3.98 ± 5.38; P = .01) vs placebo (Table 5).
Table 5.
Measures of morning alertness.
| Reboxetine | Placebo | Reb-Oxy | P value | |
|---|---|---|---|---|
| AusEd driving simulator | ||||
| Steering deviation from median lane position, cm | 34.9 ± 13.0 | 36.6 ± 16.8 | 37.3 ± 13.2 | .436 |
| Braking reaction time, s | 0.93 ± 0.17 | 0.93 ± 0.20 | 0.96 ± 0.14 | .523 |
| Karolinska Sleepiness Scale, total score | 5 ± 2 | 5 ± 2 | 5 ± 2 | .994 |
| Leeds Sleep Evaluation Questionnaire | ||||
| GTS | 11.90 ± 3.74 | 13.57 ± 7.21 | 10.92 ± 3.80 | .291 |
| QOS | 5.62 ± 2.78 | 9.08 ± 6.13 | 5.10 ± 3.46 | .014*† |
| AFS | 10.04 ± 3.75 | 8.61 ± 3.84 | 10.93 ± 4.09 | .161 |
| BFW | 13.03 ± 4.33 | 10.98 ± 6.40 | 14.26 ± 4.90 | .252 |
Data are presented as mean ± SD or median (interquartile range) as appropriate. *Reboxetine vs placebo pairwise comparison P < .05. †Reboxetine+oxybutynin vs placebo pairwise comparison P < .05. AFS = awake following sleep, BFW = behavior following wakening, GTS = getting to sleep, Reb-Oxy = reboxetine+oxybutynin, QOS = quality of sleep.
DISCUSSION
The main finding from our study is that a single 4-mg dose of reboxetine alone prior to sleep modestly reduces the AHI by an average of ∼5 events/h of sleep. Reboxetine as a single agent or when combined with oxybutynin also improves overnight oxygenation and snoring indices. These effects appear to be mediated largely through improvements in ventilatory control stability. In addition, reboxetine with and without oxybutynin markedly reduces REM sleep, which is replaced with stage 2 sleep without altering sleep efficiency, does not change perceived next-day sleepiness, alertness, or blood pressure vs placebo but does increase morning heart rate and reduces perceived sleep quality. These findings provide novel insight into the pathophysiological mechanisms by which reboxetine reduces OSA severity and its potential safety and tolerability profile to inform longer-term trials.
Our study supports and extends recent upper airway physiology16 and clinical findings from Lim et al17 with reboxetine plus hyoscine butylbromide and 1-week clinical findings from Perger et al15 with reboxetine plus oxybutynin and indicates that reboxetine alone can reduce OSA severity. However, the magnitude of the effect was less than the > 15 event/h reductions in AHI seen in the recent Lim et al17 and Perger et al15 studies. The reasons for these differences between studies are unclear but may relate to differences in participant characteristics and methodology. For example, while body mass index, age, and perceived daytime sleepiness as measured by Epworth Sleepiness Scale were comparable between all 3 studies, the current participants had less-severe OSA. Consistent with less-severe OSA, participants in the current study had higher overall sleep efficiency and proportionally more slow-wave sleep and spent less time supine. In addition to the ∼20 events/h lower baseline AHI in the current study compared to the 2 other recent reboxetine in OSA studies,15,17 respiratory events were predominantly hypopnea-driven and associated with cortical arousals rather than marked hypoxemia. Given the potential wake-promoting effects of noradrenergic agents, these drugs may be less effective at resolving respiratory events purely associated with arousals vs more severe events associated with hypoxemia. Indeed, noradrenergic agents appear particularly effective at improving hypoxic burden,13,15 which was comparatively small in the current study. Furthermore, the current study included both men and women rather than just men as per the Lim et al study17 and ∼90% men in the Perger et al study.15 Indeed, in the current study, reductions in AHI with reboxetine occurred in men but not women. While this may indicate sex differences in response to reboxetine, as highlighted below, a more likely explanation is that the larger reductions in men are explained by higher loop gain values and sex differences in the ventilatory response to arousal.
Conversely, Taranto-Monetemurro et al’s recent findings with a different noradrenergic agent, atomoxetine, as a single agent did not reduce the AHI but when combined with oxybutynin caused marked reductions in OSA severity.13 The addition of oxybutynin to reboxetine in the current study did not yield additive improvements in AHI. This may also be due to differences in participant characteristics (ie, mostly men, more overweight, with greater upper airway collapsibility at baseline in the Taranto-Monetemurro et al study14), differences in noradrenergic potency between reboxetine vs atomoxetine, or unique and currently incompletely understood interactions between atomoxetine and oxybutynin. As highlighted, recently published findings with 1 week of reboxetine plus oxybutynin also yielded larger reductions in OSA severity compared to the current study.15 Possible differences in participant characteristics aside, this finding may suggest that a longer duration of administration could be required to achieve greater therapeutic efficacy.
Analyses of the effects of atomoxetine+oxybutynin on OSA endotypic traits found that the drug combination was most effective in patients with mild to moderate upper airway collapsibility and a predominance of hypopneas over apneas.14 The median placebo night Vpassive (%eupnea) value in our study was 93%. This indicates that the current cohort generally did not have highly collapsible pharyngeal airways. Our findings therefore suggest that nonanatomical mechanisms such as improvements in respiratory control stability, which also occurred with atomoxetine+oxybutynin,14 atomoxetine with other antimuscarinics,29 and reboxetine with hyoscine butylbromide,17 contributed to the reduction in AHI with reboxetine in our study. Indeed, while the reported reductions in loop gain with noradrenergic and antimuscarinic agents of ∼10–20% is less pronounced than with oxygen therapy and acetazolamide (∼50%),30,31 consistent with OSA endotyping concepts, the greatest reductions in OSA severity tended to occur in those with ventilatory control instability on placebo (high loop gain). These participants were mostly male. Given that male sex is associated with higher baseline loop gain32 and an increased ventilatory response to arousal33 as discussed below, these findings indicate that reboxetine reduces OSA, at least in part, via improvements in ventilatory control stability.
Sleep efficiency and wake after sleep onset tended to improve with the reboxetine+oxybutynin combination compared to reboxetine alone. These findings are consistent with a mild sedative effect with oxybutynin that attenuated the alerting effects of increased central nervous system norepinephrine levels from reboxetine. Anticholinergics are known to have mild sedative effects at low doses.34 Indeed, atomoxetine has been shown to reduce the arousal threshold (ie, easier to wake up), but the effect is offset by the addition of oxybutynin14 and can be further offset with the addition of the hypnotic zolpidem.35 Our analysis showed no major differences in arousal threshold between reboxetine, placebo, and reboxetine+oxybutynin. Reboxetine and reboxetine+oxybutynin both improved nadir oxygen saturation and oxygen desaturation indices, indicating that the residual respiratory events were predominantly due to cortical arousals without major oxygen desaturations.
The reasons for reduced perceived sleep quality with the drug conditions vs placebo in the current single-night study are likely driven by the excitatory noradrenergic properties of reboxetine as reflected by a shift toward lighter stages of sleep and potentially its mild side effects. While any reductions in perceived sleep quality are not favorable, the magnitude was mild. Indeed, overall objective sleep efficiency, next-day perceived sleepiness, and driving simulator performance were not different between conditions. Furthermore, subjective sleep quality was not different following 1 week of nightly reboxetine plus oxybutynin vs placebo in the recent Perger et al15 study and psychomotor vigilance improved, presumably because of reduced OSA severity. This suggests that any perceived worsening in sleep quality with reboxetine may be transient. Indeed, most acute sleep architecture changes associated with reboxetine alone in people with persistent mild depression resolve over time36 apart from reduced REM sleep which only partially returns.
Thus, marked REM suppression as observed with reboxetine in the current study may only be partially restored over time. However, while the proportion of REM sleep was low at baseline, 1 week of nightly reboxetine plus oxybutynin in people with OSA did not significantly reduce REM sleep vs placebo in the recent Perger et al study.15 Nonetheless, reduced REM sleep is common with most antidepressants.16,37,38 However, it does not appear to cause major adverse outcomes in this context.
While REM was suppressed by reboxetine and reboxetine+oxybutynin, which may have, at least in part, contributed to the overall reduction in total AHI, this is unlikely to be the predominant mechanism of AHI reduction for several reasons. First, for REM suppression to be the major mechanism the REM AHI would be expected to be much higher than the NREM AHI at baseline. However, this was not the case. Thus, in the context of similar baseline REM and NREM AHI values, removal of REM sleep alone, which was ∼13% of total sleep time, and replacement with NREM would be expected to yield similar AHI values rather than an overall reduction in total AHI as detected in the current study. Second, although there was no statistically significant reduction in NREM AHI with reboxetine vs placebo, the mean point estimate reduction in NREM AHI was of similar magnitude to the overall mean reduction in total AHI with reboxetine and reboxetine+oxybutynin. Furthermore, consistent with the NREM endotype changes detected in the current study, other recent noradrenergic and antimuscarinic combination therapy studies13,15,17 have detected significant reductions in NREM AHI vs placebo, indicating that total AHI reductions were not driven solely by REM suppression.
Reboxetine and reboxetine+oxybutynin both caused similar improvements in nadir pharyngeal collapsibility (V̇passivemin). Based on these and previous findings,14 it is likely that the changes were predominantly due to the noradrenergic effects of reboxetine. Although reboxetine was anticipated to reduce AHI primarily through improvements in upper airway dilator muscle activity,16 estimates of dilator muscle compensation were not significantly different with reboxetine alone in the current study. However, the addition of oxybutynin with reboxetine increased pharyngeal muscle compensation during sleep in the current study, albeit to a much lesser extent than other recent combination therapy studies with noradrenergic and antimuscarinic agents.13,14,16 Thus, as highlighted earlier, the beneficial effect on upper airway stability in the current study during the reboxetine conditions was likely driven primarily via improvements in ventilatory control stability.
In addition to overall respiratory control stability as quantified by loop gain, the ventilatory response to arousal is an important contributor to OSA pathogenesis.33,39 Respiratory drive increases during partial airway obstruction, stimulating upper airway dilator muscle activity that eventually restores airway patency, at which point ventilation briefly exceeds baseline ventilation. If the restoration in airway patency is associated with a cortical arousal, the excessive ventilatory response may be sufficiently high to reduce respiratory drive and upper airway dilator muscle activity that feeds into a repetitive cycle of airway obstruction and arousals. On average, the ventilatory response to arousal is higher in men than women.33 The carbonic anhydrase inhibitor acetazolamide and the serotonin–norepinephrine reuptake inhibitor venlafaxine reduce the ventilatory response to arousal40,41 and in the case of acetazolamide reduces OSA severity.30 Thus, reductions in the ventilatory response to arousal with reboxetine may also contribute to breathing stability and the observed reductions in OSA severity.
Methodological considerations
While this study has several strengths including rigorous clinical trial design and provides both clinical and mechanistic insight, there are several limitations. First, the cohort was not selected based on individual endotypes. Thus, preselection based on endotype characterization may have yielded larger changes in OSA severity with reboxetine. However, despite predominately severe OSA as measured by the AHI, most participants had minimally collapsible upper airways at baseline, which is typically associated with favorable therapeutic responses with similar drug combinations.14 This may have been, at least in part, due to participants spending on average approximately 50% of the night lateral on each of the study nights, which reduces upper airway collapsibility compared to the supine position.42,43 Thus, it will be important to carefully control body position in future endotype studies. Second, detailed physiology quantification of OSA endotypes was not performed in the current study. However, the signal processing methodology that we used to estimate OSA endotypes is far less intrusive than the detailed physiology methodology and has recently been shown to have acceptable repeatability of measurement over time.44 In addition, intervention studies aimed to modify one or more of the OSA endotypes, including previous OSA pharmacotherapy studies,13,14,17,29,45 have consistently yielded quantifiable differences in endotypes vs placebo. Third, our study only assessed the effects of the medications over a single night. Thus, a longer-duration study would be useful to determine if OSA severity is further decreased by reboxetine alone once the drug concentration reaches steady state, as recently published findings with combined reboxetine and oxybutynin suggest may be the case,15 and if the adverse effects of reboxetine (with and without oxybutynin), including increased heart rate and reduced perceived sleep quality, are clinically significant and persist or reduce over time. Based on previous findings from longer-term studies in people who have not been screened for OSA, it would be expected that most of the acute changes in sleep architecture and elevated heart rate with reboxetine resolve within months.36,37 Fourth, as highlighted, some of the characteristics of the current cohort including predominance of respiratory events associated with arousals rather than desaturations, subclinical insomnia, and minimal daytime sleepiness may not be ideally suited for noradrenergic pharmacotherapy. Thus, the current findings may not be generalizable to all patients with OSA. Finally, we only studied a standard dose of reboxetine. Higher doses may have produced larger reductions in OSA severity. Thus, these unresolved clinically relevant questions require further investigation.
CONCLUSIONS
In this cohort with predominantly severe OSA with mostly arousal-associated hypopneas, subclinical insomnia, and minimal daytime sleepiness, a single dose of reboxetine alone modestly reduces the frequency of respiratory events and improves overnight oxygenation and snoring. These beneficial effects are likely driven largely by improvements in ventilatory control stability (reductions in loop gain and the ventilatory response to arousal). The addition of oxybutynin has mild sedative effects but does not produce additive benefit in reducing OSA severity on a single night despite modest improvements in pharyngeal muscle compensation. People with unstable ventilatory control (high loop gain endotype, mostly men in the current study) tend to respond most favorably to reboxetine. However, acutely, morning heart rate increases and perceived sleep quality decreases, although neither objective sleep quality, next-day alertness, nor blood pressure change with a single dose of reboxetine. Thus, longer-term mechanistic and clinical studies to carefully study the effects of different doses of reboxetine and its efficacy, safety, and tolerability profile in different patient populations that include both men and women are warranted. In summary, this study shows for the first time that reboxetine alone reduces OSA severity, provides new insight into the importance of noradrenergic mechanisms in OSA, and will inform future pharmacotherapy investigations for OSA.
DISCLOSURE STATEMENT
All authors approve of the final version to be published and agree to be accountable for all aspects of the work. Work for this study was performed at Adelaide Institute for Sleep Health and Woolcock Institute of Medical Research. This study was supported by an investigator-initiated research grant from Apnimed. The funder did not play a role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. D.J.E. is supported by a National Health and Medical Research Council (NHMRC) of Australia Leadership Fellowship (1196261). D.J.E. reports a Collaborative Research Centre (CRC) Consortium Grant between the Australian Government, Academia and Industry (Industry partner: Oventus Medical) and has research grants and serves as a consult/advisory board member for Bayer, Invicta Medical, Takeda, and Apnimed. R.R.G. is supported by an NHMRC Senior Research Fellowship (1106974) and an NHMRC Investigator Grant (1197439). The other authors report no financial or competing interests.
ACKNOWLEDGMENTS
The authors thank the study participants for volunteering their time toward the conduct of this research. We also thank the staff at the Woolcock Institute of Medical Research and the Adelaide Institute for Sleep Health who assisted with the conduct of the trial, especially Carla Evans, Alison Wheaton, Jack Anderson, Richard Lim, Sophie Carter, Alan Chiang, Henry Ainge-Allen, Roy Sweeney, Laura Harris, Fiona Fletcher, Ibrahim Basic, Alison Teare, Ashwin Whitelaw, Carolin Tran, Gemma Robertson, Georgina Rawson, Lady Calonzo, Hannah Long, Jack Manners, Tessa Liebich, and Duc Phuc Nguyen. T.J.A., A.A., and K.A.L. contributed substantially to the acquisition, analysis, interpretation of the data, and writing of the manuscript. R.R.G. and D.J.E. contributed substantially to the study design, data analysis, interpretation, and writing of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- NREM
nonrapid eye movement sleep
- OSA
obstructive sleep apnea
- REM
rapid eye movement
REFERENCES
- 1. Benjafield AV , Ayas NT , Eastwood PR , et al . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis . Lancet Respir Med. 2019. ; 7 ( 8 ): 687 – 698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lechat B , Naik G , Reynolds A , et al . Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea . Am J Respir Crit Care Med. 2022. ; 205 ( 5 ): 563 – 569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marin JM , Carrizo SJ , Vicente E , Agusti AG . Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study . Lancet. 2005. ; 365 ( 9464 ): 1046 – 1053 . [DOI] [PubMed] [Google Scholar]
- 4. Yaggi HK , Concato J , Kernan WN , Lichtman JH , Brass LM , Mohsenin V . Obstructive sleep apnea as a risk factor for stroke and death . N Engl J Med. 2005. ; 353 ( 19 ): 2034 – 2041 . [DOI] [PubMed] [Google Scholar]
- 5. Yaffe K , Laffan AM , Harrison SL , et al . Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women . JAMA. 2011. ; 306 ( 6 ): 613 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackwell T , Yaffe K , Laffan A , et al. Osteoporotic Fractures in Men Study Group . Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study . J Am Geriatr Soc. 2015. ; 63 ( 3 ): 453 – 461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Punjabi NM , Shahar E , Redline S , Gottlieb DJ , Givelber R , Resnick HE ; Sleep Heart Health Study Investigators . Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study . Am J Epidemiol. 2004. ; 160 ( 6 ): 521 – 530 . [DOI] [PubMed] [Google Scholar]
- 8. Weaver TE , Grunstein RR . Adherence to continuous positive airway pressure therapy: the challenge to effective treatment . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 173 – 178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osman AM , Carter SG , Carberry JC , Eckert DJ . Obstructive sleep apnea: current perspectives . Nat Sci Sleep. 2018. ; 10 : 21 – 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eckert DJ , White DP , Jordan AS , Malhotra A , Wellman A . Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets . Am J Respir Crit Care Med. 2013. ; 188 ( 8 ): 996 – 1004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan E , Steenland HW , Liu H , Horner RL . Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states . Am J Respir Crit Care Med. 2006. ; 174 ( 11 ): 1264 – 1273 . [DOI] [PubMed] [Google Scholar]
- 12. Grace KP , Hughes SW , Horner RL . Identification of the mechanism mediating genioglossus muscle suppression in REM sleep . Am J Respir Crit Care Med. 2013. ; 187 ( 3 ): 311 – 319 . [DOI] [PubMed] [Google Scholar]
- 13. Taranto-Montemurro L , Messineo L , Sands SA , et al . The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial . Am J Respir Crit Care Med. 2019. ; 199 ( 10 ): 1267 – 1276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taranto-Montemurro L , Messineo L , Azarbarzin A , et al . Effects of the combination of atomoxetine and oxybutynin on OSA endotypic traits . Chest. 2020. ; 157 ( 6 ): 1626 – 1636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perger E , Taranto Montemurro L , Rosa D , et al . Reboxetine plus oxybutynin for OSA treatment: a 1-week, randomized, placebo-controlled, double-blind crossover trial . Chest. 2022. ; 161 ( 1 ): 237 – 247 . [DOI] [PubMed] [Google Scholar]
- 16. Lim R , Carberry JC , Wellman A , Grunstein R , Eckert DJ . Reboxetine and hyoscine butylbromide improve upper airway function during nonrapid eye movement and suppress rapid eye movement sleep in healthy individuals . Sleep. 2019. ; 42 ( 4 ): 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim R , Messineo L , Grunstein RR , Carberry JC , Eckert DJ . The noradrenergic agent reboxetine plus the antimuscarinic hyoscine butylbromide reduces sleep apnoea severity: a double-blind, placebo-controlled, randomised crossover trial . J Physiol. 2021. ; 599 ( 17 ): 4183 – 4195 . [DOI] [PubMed] [Google Scholar]
- 18. Tytgat GN . Hyoscine butylbromide - a review on its parenteral use in acute abdominal spasm and as an aid in abdominal diagnostic and therapeutic procedures . Curr Med Res Opin. 2008. ; 24 ( 11 ): 3159 – 3173 . [DOI] [PubMed] [Google Scholar]
- 19. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research . The report of an American Academy of Sleep Medicine task force . Sleep. 1999. ; 22 ( 5 ): 667 – 689 . [PubMed] [Google Scholar]
- 20. Desai AV , Wilsmore B , Bartlett DJ , Unger G , Constable B , Joffe D , Grunstein RR . The utility of the AusEd driving simulator in the clinical assessment of driver fatigue . Behav Res Methods. 2007. ; 39 ( 3 ): 673 – 681 . [DOI] [PubMed] [Google Scholar]
- 21. Akerstedt T , Gillberg M . Subjective and objective sleepiness in the active individual . Int J Neurosci. 1990. ; 52 ( 1–2 ): 29 – 37 . [DOI] [PubMed] [Google Scholar]
- 22. Hindmarch I . A 1,4-benzodiazepine, temazepam (K 3917), its effect on some psychological parameters of sleep and behaviour . Arzneimittelforschung. 1975. ; 25 ( 11 ): 1836 – 1839 . [PubMed] [Google Scholar]
- 23. Berry RB , Budhiraja R , Gottlieb DJ , et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terrill PI , Edwards BA , Nemati S , et al . Quantifying the ventilatory control contribution to sleep apnoea using polysomnography . Eur Respir J. 2015. ; 45 ( 2 ): 408 – 418 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sands SA , Edwards BA , Terrill PI , et al . Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea . Am J Respir Crit Care Med. 2018. ; 197 ( 9 ): 1187 – 1197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messineo L , Eckert DJ , Taranto-Montemurro L , et al . Ventilatory drive withdrawal rather than reduced genioglossus compensation as a mechanism of obstructive sleep apnea in REM sleep . Am J Respir Crit Care Med. 2022. ; 205 ( 2 ): 219 – 232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vena D , Taranto-Montemurro L , Azarbarzin A , et al . Clinical polysomnographic methods for estimating pharyngeal collapsibility in obstructive sleep apnea . Sleep. 2022. ; 45 ( 6 ): 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azarbarzin A , Sands SA , Stone KL , et al . The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study . Eur Heart J. 2019. ; 40 ( 14 ): 1149 – 1157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aishah A , Lim R , Sands SA , et al . Different antimuscarinics when combined with atomoxetine have differential effects on obstructive sleep apnea severity . J Appl Physiol 1985. 2021. ; 130 ( 5 ): 1373 – 1382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edwards BA , Sands SA , Eckert DJ , et al . Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea . J Physiol. 2012. ; 590 ( 5 ): 1199 – 1211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wellman A , Malhotra A , Jordan AS , Stevenson KE , Gautam S , White DP . Effect of oxygen in obstructive sleep apnea: role of loop gain . Respir Physiol Neurobiol. 2008. ; 162 ( 2 ): 144 – 151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Won CHJ , Reid M , Sofer T , et al . Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis . Sleep. 2020. ; 43 ( 5 ): 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jordan AS , Eckert DJ , Catcheside PG , McEvoy RD . Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women . Am J Respir Crit Care Med. 2003. ; 168 ( 12 ): 1512 – 1519 . [DOI] [PubMed] [Google Scholar]
- 34. Thornton WE . Sleep aids and sedatives . JACEP. 1977. ; 6 ( 9 ): 408 – 412 . [PubMed] [Google Scholar]
- 35. Messineo L , Carter SG , Taranto-Montemurro L , et al . Addition of zolpidem to combination therapy with atomoxetine-oxybutynin increases sleep efficiency and the respiratory arousal threshold in obstructive sleep apnoea: a randomized trial . Respirology. 2021. ; 26 ( 9 ): 878 – 886 . [DOI] [PubMed] [Google Scholar]
- 36. Ferini-Strambi L , Manconi M , Castronovo V , Riva L , Bianchi A . Effects of reboxetine on sleep and nocturnal cardiac autonomic activity in patients with dysthymia . J Psychopharmacol. 2004. ; 18 ( 3 ): 417 – 422 . [DOI] [PubMed] [Google Scholar]
- 37. Göder R , Seeck-Hirschner M , Stingele K , et al . Sleep and cognition at baseline and the effects of REM sleep diminution after 1 week of antidepressive treatment in patients with depression . J Sleep Res. 2011. ; 20 ( 4 ): 544 – 551 . [DOI] [PubMed] [Google Scholar]
- 38. Riemann D , Krone LB , Wulff K , Nissen C . Sleep, insomnia, and depression . Neuropsychopharmacology. 2020. ; 45 ( 1 ): 74 – 89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jordan AS , McEvoy RD , Edwards JK , et al . The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans . J Physiol. 2004. ; 558 ( Pt 3 ): 993 – 1004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edwards BA , Connolly JG , Campana LM , et al . Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea . Sleep. 2013. ; 36 ( 2 ): 281 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmickl CN , Li Y , Orr JE , et al . Effect of venlafaxine on apnea-hypopnea index in patients with sleep apnea: a randomized, double-blind crossover study . Chest. 2020. ; 158 ( 2 ): 765 – 775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ong JS , Touyz G , Tanner S , Hillman DR , Eastwood PR , Walsh JH . Variability of human upper airway collapsibility during sleep and the influence of body posture and sleep stage . J Sleep Res. 2011. ; 20 ( 4 ): 533 – 537 . [DOI] [PubMed] [Google Scholar]
- 43. Penzel T , Möller M , Becker HF , Knaack L , Peter JH . Effect of sleep position and sleep stage on the collapsibility of the upper airways in patients with sleep apnea . Sleep. 2001. ; 24 ( 1 ): 90 – 95 . [DOI] [PubMed] [Google Scholar]
- 44. Alex RM , Sofer T , Azarbarzin A , et al . Within-night repeatability and long-term consistency of sleep apnea endotypes: the Multi-Ethnic Study of Atherosclerosis and Osteoporotic Fractures in Men Study . Sleep. 2022. : zsac129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Messineo L , Loffler K , Chiang A , Osman A , Taranto-Montemurro L , Eckert DJ . The combination of betahistine and oxybutynin increases respiratory control sensitivity (loop gain) in people with obstructive sleep apnea: a randomized, placebo-controlled trial . Nat Sci Sleep. 2022. ; 14 : 1063 – 1074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sands SA , Edwards BA , Terrill PI , et al . Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy . Eur Respir J. 2018. ; 52 ( 3 ): 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taranto-Montemurro L , Sands SA , Edwards BA , et al . Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation . Eur Respir J. 2016. ; 48 ( 5 ): 1340 – 1350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bamagoos AA , Cistulli PA , Sutherland K , et al . Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances . Ann Am Thorac Soc. 2019. ; 16 ( 11 ): 1422 – 1431 . [DOI] [PMC free article] [PubMed] [Google Scholar]