Abstract
Aims
Diabetes mellitus is one of the largest global health concerns of recent times. Women with diabetes mellitus have a higher excess risk of all-cause mortality and more vascular events than men. Focusing on type 1 diabetes, this could be caused by gender inequalities in delivered diabetes care. This study aims to assess gender differences in type 1 diabetes outpatient care, particularly diagnostics and outcomes.
Methods
This cross-sectional cohort study included all adult type 1 diabetes patients in the Dutch Pediatric and Adult Registry of Diabetes (DPARD) visiting diabetes outpatient clinics between 2016–2021. The frequency of process measurements, including physical examination and laboratory testing, was assessed among both sexes after adjustment for age and body mass index. Gender differences in eGFR ≥ 60, BMI-, and control in blood pressure and LDL-cholesterol were evaluated. Hospital variation in achieving HbA1c targets of 53 mmol/mol and median HbA1c were assessed. Cardiovascular risk scores were calculated in men and women using the Systematic Coronary Risk Evaluation (SCORE) European low-risk chart.
Results
Our study showed a 17% higher odds of reaching weight control and a 23% lower odds of achieving blood pressure targets in men than women. Gender-skewed cardiovascular mortality risk scores were found. Gender disparities in outcomes appear not to be caused by gender-biased attitudes in healthcare professionals since no gender differences were found in the performance of process measurements in type 1 diabetes care. In addition, hospitals appear to vary by extent of gender differences in achieving a target HbA1c of 53 mmol/mol.
Conclusion
Gender equality exists in the diagnostic process of diabetes care. However, differences in weight control, blood pressure control, and cardiovascular mortality risk scores remain between both sexes, most likely due to multifactorial causes. Indications for interhospital variation in gender disparities in HbA1c control exist. Further focus on performance of process measurements between hospitals may identify areas for improvement of gender-skewed outcomes to further enhance Dutch diabetes care for both sexes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00592-022-02023-6.
Keywords: Gender equality, Registries, Outpatients, Type 1 diabetes mellitus
Introduction
Diabetes mellitus is one of the largest global health concerns of recent times, with a staggering financial burden of USD 760 billion in 2019 and an expected rise in the decades to come [1]. The grave cardiovascular consequences of diabetes pose a need for more effective options to treat and prevent cardiovascular complications. There is accumulating evidence for clinically relevant differences in outcomes in diabetes care between men and women. Women with type 1 diabetes have a roughly 40% greater excess risk of all-cause mortality and twice the excess risk of fatal and nonfatal vascular events than men [2]. Furthermore, in women, the development of childhood type 1 diabetes before ten years of age resulted in an additional loss of 3,5 years on top of the 14.2 lost life years in men [3].
Gender differences in diabetes care have been found in target achievement of diabetes, cardiovascular risk factor assessment and management, and quality of diabetes care parameters. Multiple studies have found worse glycemic control in women with type 1 diabetes than men, yet women are more likely to use an insulin pump and have higher rates of intensive insulin therapy [4]. Women show a higher rate of hypoglycemia [5]. Moreover, overweight and obesity are more prevalent in patients with female sex. In 1991, the Yentl syndrome was postulated to describe women with coronary artery disease being underdiagnosed and undertreated [6]. Since then, multiple studies have shown a gender gap along the entire spectrum of cardiovascular medicine, leading to poorer treatment outcomes and a more adverse prognosis in women than men [7]. Despite the wide attention paid to this phenomenon, the Yentl syndrome is still very much alive three decades later. In women, risk factors for cardiovascular disease are assessed less frequently compared to their male counterparts [8]. In addition, women are less likely than men to receive lipid-lowering therapy and antithrombotic therapy for primary and secondary prevention of cardiovascular disease [9]. Furthermore, dyslipidemia control is achieved less in female patients [4]. Not surprisingly, gender differences are also ubiquitously present in patients with diabetes mellitus. In type 2 diabetic outpatients, women have a lower quality of care [10]. Furthermore, women have higher out-of-pocket and healthcare expenditures compared to men [11]. While valuable in their own right, these gender-skewed associations found in diabetes care are scattered across the scientific landscape.
Up until now, studies examining the diabetes care process concerning gender were focused mainly on type 2 diabetes, yet a gender gap in outcomes and prognosis unequivocally exists in type 1 diabetes [2, 4]. This makes gender differences in the care process of type 1 diabetes patients quite likely, yet current data on process parameters in type 1 diabetes is scarce [4].
Using a nationwide clinical diabetes registry, this study aims to assess whether gender differences exist in the process of type 1 diabetes outpatient care, and to examine if treatment patterns and clinical outcomes differ between sexes.
Methods
Study population
The Dutch Pediatric and Adult Registry of Diabetes (DPARD) is a national quality registry of adult and pediatric patients with diabetes mellitus treated in secondary and tertiary outpatient care across the Netherlands [12]. The registry was launched in 2017 by the BIDON foundation, a Dutch nationwide consortium of diabetologists, pediatric endocrinologists, and diabetes patients. DPARD is governed by the Dutch Institute for Clinical Auditing (DICA), known for facilitating nationwide audits in a uniform format. Exclusion criteria for DPARD are gestational diabetes and treatment of diabetes mellitus in primary care. Data are collected directly from electronic health records of participating hospitals and entered into batch files, which are a series of data. Batches are uploaded to Medical Research Data Management (MRDM) [13], a trusted third party responsible for securely processing and storing data compliant with all Dutch and European privacy laws. Each year, hospitals deliver data about the preceding year, thus including data from 2016 onwards. All data are encrypted directly after entry to prevent data from being traced back to individual patients. Unique non-traceable identification numbers are assigned to every patient to allow for follow-up over time. According to Dutch and European Privacy Protection laws, no ethical approval or informed consent was required, as DPARD is primarily designed to assess and improve the quality of care. Hospitals must inform diabetes patients on DPARD participation and the possibility of withdrawing participation. Up until now, patients have not withdrawn participation. In this cross-sectional cohort study of observational nature, we included all adult patients (≥ 18 years) with type 1 diabetes who visited Dutch diabetes outpatient clinics between January 1, 2016, and January 1, 2021, allowing for a maximum of five years of follow-up in individual patients. Patients with no outpatient visits during these years and patients without known type 1 diabetes were excluded. Classification data was missing at random as a relation between entering diabetes classification into electronic health records, and diabetes type itself is highly unlikely.
Data collection
The DPARD dataset includes 141 parameters concerning patient and disease characteristics, process parameters used in the diagnostic process and follow-up, complications, comorbidity, and treatment. Age at the last outpatient visit was used. Diabetes mellitus is diagnosed according to the American Diabetes Association (ADA) and International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines [14, 15]. Diabetes type is derived from the clinical classification entered in electronic health records by medical professionals. We assessed process measures expressed as percentages of patients who underwent the reported diagnostic procedure systematically aiming for at least once during the previous 12 months. Process parameters analyzed were lifestyle advice, physical examination, BMI measurement, blood pressure, foot examination, eye examination, laboratory testing, HbA1c-, lipid-, kidney function-, antibody- and MODY testing. In addition, we assessed intermediate outcome measures, defined as short-term endpoints associated with long-term outcomes, including BMI < 30 (kg/m2), blood pressure ≤ 130/80 mmHg, HbA1c ≤ 53, ≤ 64, and ≤ 86 mmol/mol (respectively, up and until 7, 8 and 10%), LDL-cholesterol < 2.6 mmol/l and, eGFR ≥ 60 ml/min. BMI was calculated as weight in kilograms divided by height square in meters, using a cutoff value of 25 kg/m2 for overweight and 30 kg/m2 for obesity. In patients with multiple outpatient visits during one calendar year, the last outpatient visit in this year was considered. Furthermore, we evaluated the outcome 10-year risk of a cardiovascular event in all adult patients with type 1 in our database using the Systematic COronary Risk Evaluation (SCORE) European low-risk chart as the closest reflection of the Dutch setting.[16] The SCORE risk score is considered an outcome.
The rationale for choosing the SCORE chart is its validity and close proximity to the Dutch population, its generalizability since the population on which the chart was validated included diabetes patients, and its feasibility on our data. Median SCORE-risk scores of all imputed datasets were calculated for each patient. Finally, we evaluated the proportion of patients achieving HbA1c targets of up and including 53 mmol/mol by hospital and in median HbA1c per hospital in mmol/mol. Missing values were not included in the calculation of the median HbA1c. Medical centers were numbered in ascending order of patient volume to prevent disclosing hospital identity; the centers were subdivided into academic hospitals (tertiary care) and non-academic treatment centers (secondary care and private care clinics).
Statistical analysis
Descriptive statistics including median and range were used to assess patient, disease, and treatment characteristics. Due to the non-parametrical distribution of our data, medians and ranges were used for descriptives. Rates of missing data were shown in tables or described in the results. Missing data were included in all analyses unless mentioned otherwise. Both present data and missing data were analyzed on gender differences as this could also provide insight into the variation in the diabetes care process among men and women. Multiple imputation was used for missing body mass index, smoking status, systolic blood pressure, and total cholesterol. After adjustment for age and body mass index, odds ratios at receiving process parameter measurements were calculated among men and women using logistic regression analysis. Age and BMI were chosen as adjusting factors, since there were significant differences between men and women in age and patients with a BMI up and above 30 kg/m2. Odds ratios for physical examination, BMI measurement, MODY testing, and BMI < 30 kg/m2 were only adjusted for age due to multicollinearity with BMI. Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, version 26.0) and R (RStudio, version 1.4.1106).
Results
Between November 2017 and January 2021, 22,692 patients were included in DPARD. Patients were treated across eight medical centers (two tertiary care centers, five secondary hospitals, and one independent diabetes treatment center), comprising approximately 11% of all Dutch general hospitals with a level of care distribution representative of all diabetic outpatients in the Netherlands. Diabetes classification was not provided in 37.0% of adult men and 44.1% of adult women. Among adult men with known diabetes type, 40.7% was diagnosed with type 1 diabetes, 58.0% with type 2, and 1.3% with secondary or other causes of diabetes mellitus. In adult women, 41.3% had type 1 diabetes mellitus, 57.7% type 2, and 1.0% was diagnosed with secondary diabetes mellitus or other causes. From January 2016 to January 2021, 4,655 patients with confirmed type 1 diabetes visited the outpatient clinic at least once. Of these patients, 559 were treated in tertiary care and 4096 in secondary-care centers.
Table 1 shows the characteristics of type 1 patients included in DPARD by gender, comprising 2,489 male and 2,166 female patients. Women were younger than male subjects (37.0 vs. 42.0 years), while diabetes duration was similar with 15.0 and 16.0 years, respectively. Percentages of missing data varied from 0% in age and sex up to 66.5% in glucose-lowering treatment among female type 1 patients. Median BMI was comparable in women and men (25.3 vs. 25.0 kg/m2), (mean BMI 25.3 vs. 25.6 kg/m2). Blood pressure was measured equally in men and women, with higher median blood pressure values in men compared to women (131/77 vs. 126/75 mmHg), (mean blood pressure 132/77 vs. 128/74 mmHg). Women had higher HDL cholesterol levels of 1.7 mmol/l against 1.4 mmol/l in men. Males had slightly higher creatinine values than females with 77.0 and 65.0 µmol/l, respectively, and equal levels of albuminuria. HbA1c levels were similar between both sexes, with 60.7 mmol/mol in men and women, (mean HbA1c 63.2 mmol/mol in both sexes). The distribution of insulin use and oral glucose-lowering treatment were equal between both groups. Percentages of missing values on glucose-lowering treatment varied between male and female patients (17.3 vs. 30.3% missings). In Table 1 of the supplementary materials the effect of COVID-19 on the follow-up of adult type 1 patients is made visible. Patients visiting the outpatient clinic during the COVID pandemic were older (47.0 vs. 36.0 years, p < 0.001), had shorter diabetes duration (9.0 vs. 17.0 years, p < 0.001). In contrast, BMI was comparable between both years (25.0 vs. 25.3, p = 0.137).
Table 1.
Study characteristics of type 1 patients included in DPARD from 2016 up to 2021 by sex
| All | Male | Female | p value | |
|---|---|---|---|---|
| (n = 4,655) | (n = 2,489) | (n = 2,166) | ||
| Age (years) | 40.0 (18.0–97.0) | 42.0 (18.0–90.0) | 37.0 (18.0–97.0) | 0.001 |
| Male sex (%) | 53.5 | < 0.001 | ||
| Diabetes duration (years) | 15.0 (0.0–78.0) | 16.0 (0.0–78.0) | 15.0 (0.0–77.0) | 0.320 |
| unknown (%) | 1.7 | 2.9 | 0.4 | < 0.001 |
| Smoking status | ||||
| smoker (%) | 10.5 | 12.7 | 8 | < 0.001 |
| non-smoker (%) | 51.4 | 51.4 | 51.3 | 0.993 |
| unknown (%) | 38.1 | 35.9 | 40.7 | 0.028 |
| BMI (kg/m2) | 25.2 (10.0–47.1) | 25.3 (10.0–44.5) | 25.0 (10.0–47.1) | 0.724 |
| < 20 (%) | 5.7 | 5.4 | 6 | 0.423 |
| 20–24 (%) | 26.8 | 26.5 | 27.1 | 0.778 |
| 25–29 (%) | 25.3 | 28.9 | 21.2 | < 0.001 |
| ≥ 30 (%) | 9.6 | 7.4 | 12.2 | < 0.001 |
| unknown (%) | 32.6 | 31.8 | 33.5 | 0.384 |
| Blood pressure | ||||
| systolic (mmHg) | 129 (65–215) | 131 (82–211) | 126 (65–215) | < 0.001 |
| diastolic (mmHg) | 76 (40–118) | 77 (49–114) | 75 (40–118) | < 0.001 |
| unknown (%) | 45 | 44.6 | 45.5 | 0.546 |
| Cholesterol | ||||
| HDL-c (mmol/l) | 1.5 (0.4–4.0) | 1.4 (0.4–3.9) | 1.7 (0.6–4.0) | < 0.001 |
| unknown (%) | 33.8 | 31.9 | 36.1 | 0.003 |
| LDL-c (mmol/l) | 2.6 (0.4–6.5) | 2.6 (0.4–5.7) | 2.6 (0.4–6.5) | 0.959 |
| unknown (%) | 36.1 | 34.2 | 38.2 | 0.006 |
| Kidney function | ||||
| creatinine (µmol/l) | 72.0 (26.0–789.0) | 77.0 (35.0–789.0) | 65.0 (26.0–719.0) | < 0.001 |
| unknown (%) | 29.1 | 27.3 | 31.3 | 0.003 |
| albuminuria (mg/l) | 6.0 (0.1–5555.0) | 7.0 (0.1–5555.0) | 6.0 (0.2–3406.0) | 0.051 |
| < 20 (mg/l) | 35.8 | 36.4 | 35.2 | < 0.001 |
| 20–200 (mg/l) | 7.7 | 8.6 | 6.6 | 0.022 |
| > 200 (mg/l) | 1.8 | 2.0 | 1.6 | 0.438 |
| unknown (%) | 54.7 | 53.0 | 56.6 | 0.014 |
| HbA1c (mmol/mol) | 60.6 (25.0 –149.0) | 60.7 (26.0–148.0) | 60.7 (25.0–149.0) | 0.921 |
| unknown (%) | 4.3 | 4.7 | 3.9 | 0.192 |
| Diabetes treatment | ||||
| insulin only (%) | 32.4 | 33 | 31.7 | 0.529 |
| oral agents only (%) | 0 | 0 | 0 | NA |
| insulin + oral agents (%) | 2.2 | 2.5 | 1.8 | 0.140 |
| unknown (%) | 65.4 | 64.5 | 66.5 | 0.537 |
| Insulin pump therapy (%) | 23.4 | 17.3 | 30.3 | < 0.001 |
| unknown (%) | 52 | 55.7 | 47.7 | 0.002 |
Absolute numbers are presented as median (range) or percentages (%). NA = not applicable
Table 2 shows the parameters performed in the diagnostic process and follow-up of all 4,655 male and female patients with type 1 diabetes mellitus included in DPARD. Lifestyle advice, lipid testing, and kidney function were performed more often in men, and auto-antibody testing was assessed more in women before adjustment for age and BMI. Laboratory examination was the most frequently performed process measurement (96.9% in men and 97.4% in women) among which HbA1c levels were assessed most often (95.3% in men vs. 96.1% in women). MODY testing was the process parameter performed the least among all process parameters on which hospitals have provided data, with 1.4% in men and 1.6% in women. Before adjusting for age and BMI, the relative risk difference for assessing total cholesterol, HDL, LDL, triglycerides, cholesterol ratio, creatinine, and albumin in urine varied between 6.0 and 7.7% in favor of males. After adjustment, none of the gender differences in process measurements reached statistical significance: discussed lifestyle advice (OR 1.04 (95% CI 0.91–1.18), p = 0.59); BMI measurement (OR 0.99 (95% CI 0.90–1.09), p = 0.81); systolic blood pressure assessment (OR 0.94 (95% CI 0.85–1.03), p = 0.18); diastolic blood pressure assessment (OR 0.94 (95% CI 0.86–1.03), p = 0.20); foot examination (OR 0.95 (95% CI 0.83–1.02), p = 0.12); eye examination (OR 0.92 (95% CI 0.84–1.00), p = 0.06); HbA1c measurement (OR 0.87 (95% CI 0.68–1.12), p = 0.28); lipid testing (OR 0.87 (95% CI 0.71–1.07), p = 0.19); total cholesterol (OR 1.04 (95% CI 0.94–1.15), p = 0.45); HDL cholesterol OR 1.02 (95% CI 0.91–1.14), p = 0.72); LDL-cholesterol (OR 1.02 (95% CI 0.91–1.13), p = 0.73), triglycerides (OR 1.01 (95% CI 0.91–1.13), p = 0.80); cholesterol ratio (OR 1.02 (95% CI 0.92–1.13); p = 0.66); creatinine (OR 0.98 (95% CI 0.87–1.12), p = 0.81); albuminuria (OR 1.06 (95% CI 0.97–1.16), p = 0.19); ICA/GAD/IA2 antibodies (OR 0.94 (95% CI 0.79–1.13), p = 0.51); TPO antibodies (OR 0.91 (95% CI 0.76–1.09), p = 0.30), celiac disease antibodies (OR 0.89 (95% CI 0.73–1.09), p = 0.25); MODY testing (OR 0.98 (95% CI 0.68–1.42), p = 0.91). In the adjusted model, men showed a 17% higher odds of having a BMI below 30 kg/m2 and a 23% lower odds of a blood pressure controlled ≤ 130/80 mmHg compared to women. No gender differences were found in HbA1c control (≤ 53 mmol/mol), lipid control (LDL-cholesterol < 2.6 mmol/l) or kidney function (eGFR ≥ 60 ml/min).
Table 2.
Process parameters performed in adult patients with type 1 diabetes mellitus up to 2021 by sex
| All | Male | Female | Unadjusted | Adjusted * | |||
|---|---|---|---|---|---|---|---|
| (n = 4,655) | (n = 2,489) | (n = 2,166) | OR (95% CI) | p value | OR (95% CI) | p value | |
| Process parameters performed | |||||||
| Lifestyle advice given/risk factors known | 72.5 | 74.7 | 70.0 | 1.18 (1.08–1.29) | < 0.001 | 1.04 (0.91–1.18) | 0.593 |
| Physical examination | |||||||
| BMI measurement | 67.4 | 68.2 | 66.5 | 1.06 (0.97–1.15) | 0.207 | 0.99 (0.90–1.09) | 0.809 |
| Systolic blood pressure | 55.0 | 55.4 | 54.6 | 1.02 (0.94–1.11) | 0.569 | 0.94 (0.85–1.03) | 0.177 |
| Diastolic blood pressure | 55.0 | 55.4 | 54.5 | 1.03 (0.95–1.11) | 0.527 | 0.94 (0.86–1.03) | 0.200 |
| Foot examination | 60.2 | 60.9 | 59.4 | 1.05 (0.96–1.14) | 0.286 | 0.95 (0.83–1.02) | 0.124 |
| Eye examination | 42.3 | 41.9 | 42.8 | 0.98 (0.90–1.06) | 0.557 | 0.92 (0.84–1.00) | 0.058 |
| Laboratory testing | 97.1 | 96.9 | 97.4 | 0.87 (0.68–1.11) | 0.265 | 0.87 (0.68–1.12) | 0.280 |
| HbA1c measurement | 95.7 | 95.3 | 96.1 | 0.87 (0.71–1.06) | 0.169 | 0.87 (0.71–1.07) | 0.193 |
| Lipid measurement | |||||||
| Total cholesterol | 62.0 | 64.1 | 59.6 | 1.14 (1.05–1.24) | 0.002 | 1.04 (0.94–1.15) | 0.452 |
| HDL cholesterol | 66.2 | 68.1 | 63.9 | 1.14 (1.05–1.25) | 0.002 | 1.02 (0.91–1.14) | 0.719 |
| LDL-cholesterol | 63.9 | 65.8 | 61.8 | 1.13 (1.04–1.23) | 0.005 | 1.02 (0.91–1.13) | 0.734 |
| Triglycerides | 66.3 | 68.2 | 64.1 | 1.14 (1.05–1.24) | 0.003 | 1.01 (0.91–1.13) | 0.795 |
| Total/HDL-c ratio | 61.5 | 63.4 | 59.4 | 1.13 (1.04–1.23) | 0.005 | 1.02 (0.92–1.13) | 0.655 |
| Kidney function | |||||||
| Creatinine | 70.9 | 72.7 | 68.7 | 1.15 (1.05–1.25) | 0.003 | 0.98 (0.87–1.12) | 0.814 |
| Albuminuria | 45.3 | 47.0 | 43.4 | 1.11 (1.02–1.20) | 0.013 | 1.06 (0.97–1.16) | 0.185 |
| ICA/GAD/IA2 antibodies | 21.6 | 19.2 | 24.3 | 0.81 (0.73–0.89) | < 0.001 | 0.94 (0.79–1.13) | 0.508 |
| TPO antibody | 21.8 | 19.2 | 24.7 | 0.80 (0.72–0.88) | < 0.001 | 0.91 (0.76–1.09) | 0.301 |
| Celiac disease antibody | 21.4 | 18.8 | 24.5 | 0.79 (0.71–0.87) | < 0.001 | 0.89 (0.73–1.09) | 0.253 |
| MODY testing | 1.5 | 1.4 | 1.6 | 0.92 (0.66–1.29) | 0.645 | 0.98 (0.68–1.42) | 0.914 |
| Intermediate outcomes | |||||||
| BMI < 30 | 57.8 | 60.8 | 54.3 | 1.21 (1.11–1.31) | < 0.001 | 1.17 (1.07–1.28) | < 0.001 |
| BP ≤ 130/80 | 25.8 | 22.9 | 29.1 | 0.80 (0.73–0.87) | < 0.001 | 0.77 (0.69–0.85) | < 0.001 |
| HbA1c ≤ 53 (7%) | 24.4 | 25.2 | 23.5 | 1.06 (0.97–1.17) | 0.215 | 1.05 (0.96–1.16) | 0.278 |
| HbA1c ≤ 64 (8%) | 59.3 | 58.5 | 60.2 | 0.95 (0.88–1.04) | 0.262 | 0.94 (0.86–1.02) | 0.141 |
| HbA1c ≤ 86 (10%) | 88.9 | 88.5 | 89.3 | 1.00 (0.85–1.17) | 0.997 | 1.04 (0.88–1.23) | 0.620 |
| LDL-cholesterol < 2.6 | 31.3 | 32.0 | 30.6 | 1.05 (0.96–1.15) | 0.285 | 1.00 (0.91–1.10) | 0.988 |
| eGFR ≥ 60 | 39.6 | 39.8 | 39.5 | 1.01 (0.93–1.10) | 0.834 | 0.95 (0.87–1.04) | 0.260 |
Absolute numbers are expressed as percentages (%). OR = odds ratio. BMI is stated in kg/m2, BP in mmHg, HbA1c in mmol/mol, LDL-cholesterol in mmol/l, eGFR in ml/min. * Adjusted for age and body mass index, odds ratios for BMI measurement, MODY testing, and BMI < 30 were adjusted for age only
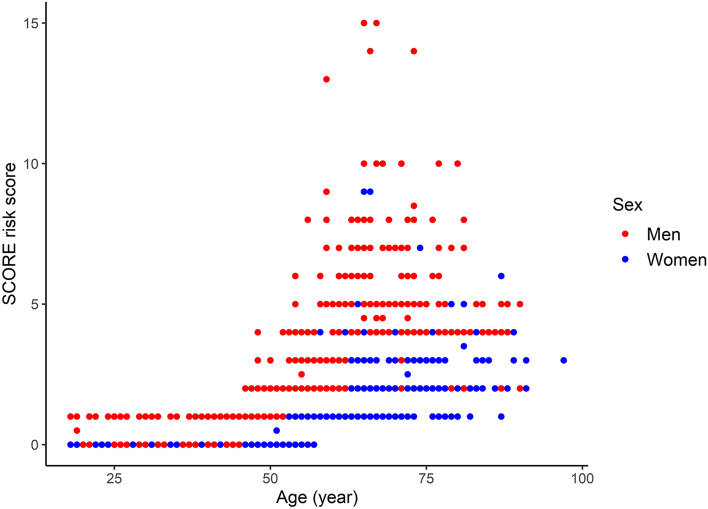
Figure 1 shows the cardiovascular risk of adult patients with type 1 diabetes mellitus according to sex. SCORE-risk scores ranged from 0 to 15, corresponding with an estimated 10-year risk of fatal cardiovascular disease (CVD) ranging from < 1% to a minimal 15%. Among both sexes, the median SCORE-risk score was 0 (estimated 10-year risk of deadly CVD < 1%). Risk scores were higher in men than in women, with scores up to 15 in men, comparable with a total 10-year risk for fatal cardiovascular disease of minimal 15%. In women, the maximum risk score was 9, indicating a 10-year risk for death by cardiovascular disease of 5–9%. A total of 5.8% of the male patients had a SCORE ≥ 5% compared to 0.6% of women.
Fig. 1.
Cardiovascular risk score according to sex in adults with type 1 diabetes mellitus
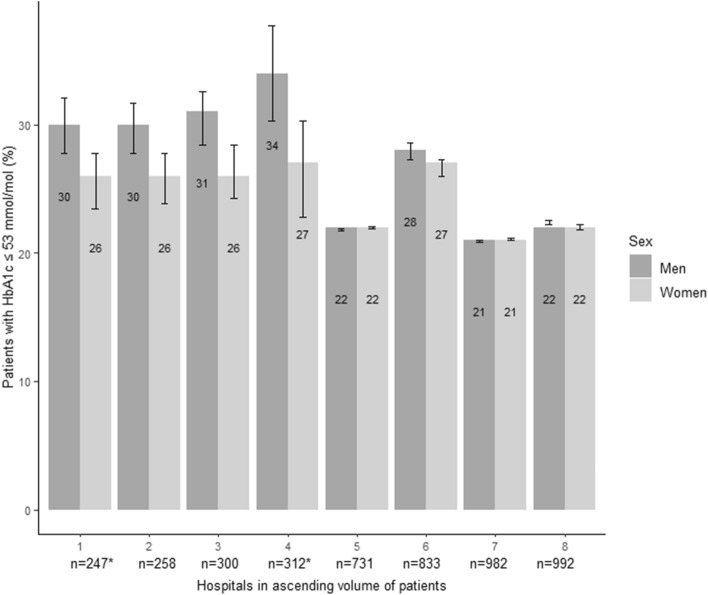
Figure 2 shows the hospital variation in achieving an HbA1c of 53 mmol/mol or lower by sex. Baseline characteristics by type of hospital (academic and non-academic) are shown in the supplementary materials Table 2. In men and women combined, the percentage of patients with an HbA1c of 53 mmol/mol or less varied from 21.0 to 30.1% across the eight medical centers included in DPARD. In six out of eight hospitals, more men than women reached the target of 53 mmol/mol. The proportion achieving a target HbA1c of 53 mmol/mol ranged among medical centers from 20.9 to 34.0% in men and 21.1 to 26.6% in women. The difference between men and women in reaching a 53 mmol/mol target ranged from 0 to 7% between the healthcare centers; in the four hospitals with a smaller volume up to 312 persons, this gender difference varied from 4–7%. In hospitals with a larger volume starting from 731 patients, gender disparities in HbA1c target achievement were lower, with a maximum difference of 1% between sexes. Among the 4,096 patients treated in secondary care, 24.3% of men and 23.1% of women had an HbA1c of 53 mmol/mol, whereas HbA1c target achievement was 32.2 and 26.1%, respectively, in 559 patients treated in academic care. The gender difference between academic and non-academic care in reaching an HbA1c target of 53 mmol/mol did not reach statistical significance (unadjusted OR 0.94, 95% CI 0.74–1.19; adjusted OR 0.88, 95% CI 0.69–1.13). Among all medical centers in DPARD, median HbA1c in mmol/mol ranged from 58 to 63 mmol/mol in men and women combined, and 57–63 mmol/mol in men versus 58–62 mmol/mol in women.
Fig. 2.
Hospital variation in the achievement of HbA1c of 53 mmol/mol and lower in adults with type 1 diabetes mellitus by sex,* Tertiary care hospitals
Discussion
This population-based cohort study investigated gender differences in care provided to type 1 outpatients and their outcomes, in order to determine whether gender-skewed treatment patterns can explain variation in clinical outcomes between sexes. Our study showed a higher odds of reaching weight control and a lower chance of achieving blood pressure targets in men than women. Among both sexes, the predicted 10-year risk of cardiovascular mortality using the SCORE equation was below 1%. Male patients had a higher estimated 10-year risk of cardiovascular death than women (ranging up to a minimum of 15% in men versus a maximum of 5–9% in women). Gender disparities in outcomes and cardiovascular risk appear not to be caused by gender-biased attitudes in healthcare professionals, as no gender differences were found in the performance of process measurements in type 1 diabetes care. Moreover, gender differences in achieving target HbA1c of 53 mmol/mol seem to vary among hospitals.
Our study showed gender disparities in blood pressure and BMI control in type 1 diabetes patients prior to adjustment. The poorer blood pressure control in men than women among patients with and without antihypertensive treatment is consistent with previous literature [17]. Several studies have proposed reasons for gender differences in blood hypertension control; higher treatment rates of antihypertensive drugs in women, gender-based prescription patterns, lower awareness in men biological, behavioral, and physiological factors have been shown to impact blood pressure control [17, 18]. Nevertheless, information about antihypertensive treatment is not yet available in DPARD and therefore gender differences in pharmacological treatment or patient compliance could not be studied. Furthermore, disparities in weight control were seen; BMI targets below 30 mg/kg2 were found less frequently in women (54.3%) than in men (60.8%), which is confirmed by existing literature given the higher prevalence of obesity in adult women [4].
We found a median estimated 10-year fatal CVD event rate based on the SCORE risk chart was below 1% among our type 1 diabetes patients. These risk scores are most likely underestimated, given the fact that the SCORE equation chart is based on the general population, and diabetes mellitus is not considered in this equation.[16]. At the same time, mortality due to CVD in type 1 patients is approximately three times higher than in the general population [19]. In contrast, for type 2 diabetes patients, the SCORE risk equation has been shown to overestimate the risk of fatal CVD by 18% with an absolute risk of 8.7% compared to the observed 7.4% event rate in the UK Prospective Diabetes Study (UKPDS). However, the SCORE risk evaluation is validated also in diabetes patients, and rather than calculating absolute risks, our goal was to assess risk differences between men and women. Male patients had a higher estimated 10-year CVD mortality risk, ranging up to a minimum of 15% versus a maximum of 5–9% in women. A high-risk score of SCORE ≥ 5% was found in 5.8% of men and 0.6% of women. Another study in the Netherlands also observed a 9–10 time difference in high-risk scores between sexes, with 8.5% of the male and 0.8% of the female patients having a SCORE ≥ 5% [20]. Other studies confirmed that more men than women were categorized in the high CVD risk category. The higher risk of cardiovascular death in men is consistent with the literature [21]. However, in the SCORE model, male sex is added as a risk factor leading to higher risk scores in men; therefore, finding a gender difference is a self-fulfilling prophecy [16]. On the other hand, the SCORE risk chart is calibrated on existing datasets, which confirms persistent gender differences in cardiovascular mortality risk since gender differences are even embedded in these risk scores. In addition, the SCORE risk chart very likely underestimated the actual cardiovascular risk in our population. However, we believe the gender differences to be a true reflection of reality since this risk chart has been validated in a population that included diabetes patients.
Before adjustment for BMI and age, significant differences in the performance of the process measures for lipid profile, kidney function and lifestyle advice were found in favor of men; after adjustment no differences were found. This could be mainly explained by the significantly higher age in men included in our study. Since we found it highly unlikely that data would be entered differently in electronic health records for men and women, we believe these numbers to reflect the extent to which these variables are measured among both sexes. Moreover, we expect gender differences in type 1 diabetes care not to be affected by gender-related health-seeking behavior that may play a considerable role in type 2 diabetes. Studies assessing gender disparities in the diabetes care process are limited. Consistent with our findings, a study from Italy found no gender differences in the performance of process measures in type 1 diabetes [4]. In contrast to type 1 diabetes, several studies showed gender disparities in assessing process measures in type 2 diabetes; however, it was inconsistent in which of both sexes process parameters were performed more frequently [22, 23]. Gender differences in weight control, blood pressure control, and cardiovascular mortality risk are unlikely to be explained by physician attitudes toward gender, since no disparities between sexes were found in the performance of process measures. However, pharmacological treatment and doctor-patient interaction were not studied. As previously mentioned, a male disadvantage in antihypertensive treatment was shown in the literature. In addition, effective communication between healthcare professionals and patients increases patient satisfaction, improves adherence to treatment plans, and leads to better health outcomes [24]. Therefore, gender-skewed communication patterns in physicians could lead to variation in treatment and outcomes between sexes. Furthermore, behavioral, biological, and physiological gender differences not taken into account in our study may also partially explain the disparities in outcomes, as shown in previous studies [10]. The absence of differences in process parameter performance between sexes makes our study findings just as interesting and insightful on diabetes care as when we would have found differences. In the knowledge that gender-skewed associations in diabetes and cardiovascular care are scattered throughout the scientific landscape, the absence of gender-biased behavior of healthcare professionals is an important finding, since our ultimate goal is not to make women equal to men but to deliver excellent care to every type 1 diabetes patient for whom each gender is honored and justified.
Although no gender differences were shown in HbA1c control in the total type 1 diabetes population in DPARD, there are indications for gender differences among hospitals in the proportion of patients achieving an HbA1c of 53 mmol/mol. In six out of eight hospitals, men were more likely to reach this HbA1c target than women. This gender difference in target achievement was more pronounced in hospitals with a smaller volume compared to hospitals with a larger treatment volume of type 1 patients. This association between hospital volume of patients and gender-skewed treatment goals may possibly be explained by more protocolled care, more multidisciplinary consultation meetings promoting inter-specialty collaboration, and more protocolled care in high-volume centers.[25] However, in low-volume centers the proportion of patients with an HbA1c of 53 mmol/mol was higher, which possibly indicates that volume does not necessarily reflect quality of care. Moreover, the interhospital variation could also be (partially) caused by the patient- and disease characteristics differing between hospitals, as well as biological and behavioral patterns between the sexes. Studies investigating the effect of treatment volumes in diabetes care focus on primary care, and the effect of treatment volume on HbA1c control varies between these studies [26]. In the surgical field in the Netherlands, national clinical quality registries have shown that providing feedback information to healthcare professionals about the quality of delivered clinical care improves outcomes and lowers hospital variation [27]. Furthermore, we found no significant difference between academic and non-academic care, indicating that referral patterns for diabetes are not affected by gender bias. However, for a comprehensive and reliable view of interhospital variation, measurements of intermediate outcomes should be done for a more extended time period to exclude the probability of variation by chance, and casemix correction should be applied to correct for interhospital variation in patient- and disease characteristics. For this reason, in the near future a casemix model will be developed for DPARD, and the hospital variation of intermediate outcomes will be assessed again with the adjustment for casemix factors.
The evaluation of the performance of a large set of different process parameters suggests that major improvements in the care provided to adults with type 1 diabetes are needed in both sexes. Of all patients, almost two-thirds had a BMI below 30 kg/m2, approximately a quarter had blood pressure control, and less than a quarter had an HbA1c ≤ 53 mmol/mol. The Diabetes Control and Complications Trial (DCCT), the UKPDS, and subsequent trials showed that weight loss, glycemic control, and blood pressure control are associated with reduced microvascular complications in diabetes patients [28]. Therefore, guidelines in diabetes care recommend periodic screening and control of risk factors and HbA1c. However, blood pressure, BMI, and HbA1c were recorded annually in only, respectively, 55.0%, 67.4%, and 95.7% of all adult type 1 patients. Intensified performance of process parameters is essential for target achievement in diabetes care since adherence to guideline-recommended testing frequencies has shown to improve outcomes in diabetes care [29]. Gaining insight into the delivered diabetes care is crucial to monitor process measurement performance. Data from our national diabetes registry DPARD can be used to benchmark process measures and outcomes between hospitals and identify areas in need of improvement to enhance diabetes care in the Netherlands. While DPARD provides valuable information about diabetes care process, not all hospitals in the Netherland are included yet. This number will increase in the following years due to obligatory participation. In addition, to shed light on the effect of gender bias on treatment patterns and outcomes, more information is needed about treatment and comorbidities, which is expected to follow over the next years.
We provided a comprehensive overview of the diabetes care process fully viewed in the light of gender differences. Some limitations should be noted; study data are derived from electronic health records, which are not primarily designed for study purposes, possibly leading to underreporting of process parameter measurements [30]. In addition, DPARD is a relatively young registry, and as data quality in quality registries improves over time, this is also the case for DPARD. Moreover, there were missings in diabetes classification, which are most likely missing at random since a relation between entering classification data in electronic health records and diabetes type in highly unlikely. There is no presumed effect on the distribution and reliability of the data. In addition, given the high prevalence of clinical outcomes, the odds ratios may have slightly overestimated associations found between sex and outcomes, yet this will not have influenced its statistical significance. Furthermore, data on complications and comorbidity will be available in future times and therefore were currently not considered when calculating cardiovascular risk scores. Finally, DPARD has not reached national coverage yet, which will be effectuated in the years to come.
In conclusion, no gender disparities were shown in the process of diabetes care; a difference between sexes of 17% in achieving target BMI and 23% in the achievement of target blood pressure remains most likely due to multifactorial causes. In addition, there are indications for interhospital variation in gender differences, especially among hospitals with smaller treatment volumes. To fully gain a complete overview of gender differences in quality of care in the light of interhospital variation, further research is warranted with correction for casemix. Our ultimate goal is to deliver excellent care to every type 1 diabetes patient irrespective of sex. More frequent performance of process parameters and benchmark process measures may aid the aim for equal target achievement among both sexes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the diabetologists, pediatric endocrinologists, hospital quality assurance employees, and IT-employees from local hospitals across the Netherlands participating in DPARD. In addition, we want to thank Nordin Hanssen for his contribution to our study design.
Authors’ Contributions
All authors contributed to the study conception and design. The idea for the article was provided by CV and MK. Writing of the manuscript and statistical analysis were performed by JB and CV. JB, CV, and ES drafted the article. CV, ES, MK, MN, and HV commented on previous versions of the manuscript. All authors read and approved the final manuscript. MK and CV are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by the Dutch Association of Medical Specialists, the Dutch Diabetes Foundation, and the Netherlands Association of Internal Medicine. MN is supported by a ZONMW-VICI grant 2020 [09150182010020]. Neither organization was involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Data availability
The datasets generated and analyzed during the current study are not publicly available, as hospitals delivering data remain ownership of their data. Furthermore, DPARD-data contain information that could compromise research participant privacy but may be available from the corresponding author on reasonable request.
Declarations
Conflict of interest
None declared.
Ethics approval and consent to participate
In accordance with Dutch and European Privacy Protection Laws, ethical approval from a named institutional or licensing committee was not required for this study, since different regulations apply to data from national quality registries provided that data are completely untraceable to individual patients. For the same reasons, informed consent of subjects both under and above 18 years was not required according to Dutch and European Privacy and Protection Laws.
Consent for publication
Not required according to the Dutch and European Privacy Protection Laws.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation (2019) IDF Diabetes Atlas 9th edition
- 2.Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206. doi: 10.1016/S2213-8587(14)70248-7. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–76. doi: 10.1136/BMJ.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicardi V, Russo G, Napoli A, et al. Gender-disparities in adults with type 1 diabetes: more than a quality of care issue. A cross-sectional observational study from the AMD annals initiative. PLOS ONE. 2016;11(10):e0162960. doi: 10.1371/journal.pone.0162960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou HT, Lee TY, Li CY, et al. Incidence of diabetes-related complications in Chinese patients with type 1 diabetes: a population-based longitudinal cohort study in Taiwan. BMJ Open. 2017 doi: 10.1136/bmjopen-2016-015117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healy B. The yentl syndrome. N Engl J Med. 1991;325:274–276. doi: 10.1056/nejm199107253250408. [DOI] [PubMed] [Google Scholar]
- 7.Gulati M. Yentl’s bikini: sex differences in STEMI. J Am Heart Assoc. 2019;8:e012873–e012873. doi: 10.1161/JAHA.119.012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyun KK, Redfern J, Patel A, et al. Gender inequalities in cardiovascular risk factor assessment and management in primary healthcare. Heart. 2017;103:492–498. doi: 10.1136/HEARTJNL-2016-310216. [DOI] [PubMed] [Google Scholar]
- 9.Koopman C, Vaartjes I, Heintjes EM, et al. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J. 2013;34:3198–3205. doi: 10.1093/EURHEARTJ/EHT368. [DOI] [PubMed] [Google Scholar]
- 10.Rossi MC, Cristofaro MR, Gentile S, et al. Sex disparities in the quality of diabetes care: Biological and cultural factors may play a different role for different outcomes: a cross-sectional observational study from the amd annals initiative. Diabetes Care. 2013;36:3162–3168. doi: 10.2337/dc13-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JS, Bishu K, Dismuke CE, Egede LE. Sex differences in healthcare expenditures among adults with diabetes: evidence from the medical expenditure panel survey, 2002–2011. BMC Health Serv Res. 2017;17:259. doi: 10.1186/s12913-017-2178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bak JCG, Mul D, Serné EH, et al. DPARD: rationale, design and initial results from the dutch national diabetes registry. BMC Endocr Disord. 2021 doi: 10.1186/S12902-021-00782-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Management (2020) MMRD Home | MRDM. https://mrdm.nl/. Accessed 20 Mar 2020
- 14.Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD clinical practice consensus guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19:7–19. doi: 10.1111/pedi.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association AD. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 16.Conroy R. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Europ Heart J. 2003;24(11):987–1003. doi: 10.1016/S0195-668X(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Carrillo-Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1/ATTACHMENT/647D2630-0ABA-4985-B5C7-D4CA0A7F09E4/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoenes M, Neuberger HR, Volpe M, et al. (2009) Antihypertensive drug therapy and blood pressure control in men and women: an international perspective. J Hum Hypertens. 2010;245(24):336–344. doi: 10.1038/JHH.2009.76. [DOI] [PubMed] [Google Scholar]
- 19.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–1418. doi: 10.1056/NEJMOA1608664/SUPPL_FILE/NEJMOA1608664_DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- 20.Van DI, Kromhout D, Geleijnse JM, et al. Evaluation of cardiovascular risk predicted by different SCORE equations: the Netherlands as an example. Eur J Cardiovasc Prev Rehabil. 2010;17:244–249. doi: 10.1097/HJR.0B013E328337CCA2. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola TS, Gissler M, Merikukka M, et al. Sex differences in age-related cardiovascular mortality. PLoS One. 2013 doi: 10.1371/JOURNAL.PONE.0063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamidi ML, Wikström K, Inglin L, et al. Trends in the process and outcome indicators of type 2 diabetes care: a cohort study from Eastern Finland, 2012–2017. BMC Fam Pract. 2020;21:1–11. doi: 10.1186/S12875-020-01324-5/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juutilainen A, Kortelainen S, Lehto S, et al. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27:2898–2904. doi: 10.2337/DIACARE.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 24.Beverly EA, Ganda OP, Ritholz MD, et al. Look who’s (not) talking. Diabetes Care. 2012;35:1466–1472. doi: 10.2337/DC11-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung A, Stukel TA, Alter DA, et al. Primary care physician volume and quality of diabetes care: a population-based cohort study. Ann Intern Med. 2017;166:240–247. doi: 10.7326/M16-1056. [DOI] [PubMed] [Google Scholar]
- 26.Coombs LJ, Burston B, Liu D. Importance of an alternative approach to measuring quality in a volume-to-value world: a case study of diabetes care. BMJ Open Qual. 2017;6:e000216. doi: 10.1136/bmjoq-2017-000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voeten DM, Gisbertz SS, Ruurda JP, et al. Overall volume trends in esophageal cancer surgery results from the dutch upper gastrointestinal cancer audit. Ann Surg. 2021;274:449–458. doi: 10.1097/SLA.0000000000004985. [DOI] [PubMed] [Google Scholar]
- 28.Group TDC and CTR The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 29.Imai C, Li L, Hardie RA, Georgiou A. Adherence to guideline-recommended HbA1c testing frequency and better outcomes in patients with type 2 diabetes: a 5-year retrospective cohort study in Australian general practice. BMJ Qual Saf. 2021;30:706–714. doi: 10.1136/bmjqs-2020-012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Z, Melton GB, Arsoniadis EG, et al. Strategies for handling missing clinical data for automated surgical site infection detection from the electronic health record. J Biomed Inform. 2017;68:112–120. doi: 10.1016/j.jbi.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available, as hospitals delivering data remain ownership of their data. Furthermore, DPARD-data contain information that could compromise research participant privacy but may be available from the corresponding author on reasonable request.