Abstract
Two forms of hydrophobic vitamin E (VE), α-tocopherol (Toc) and α-tocotrienol (Toc3), have been proposed to be effective against Alzheimer’s disease (AD), the etiology of which is thought to involve endoplasmic reticulum (ER) stress. However, previous studies reported conflicting effects of Toc and Toc3 on the risk of AD. We prepared liposomes mimicking the phase separation of the ER membrane (solid-ordered/liquid-disordered phase separation) and studied how VE can influence the interaction between amyloid-β (Aβ) and the ER membrane. We found that Toc could inhibit the formation of the solid-ordered phase more significantly than Toc3. Furthermore, Aβ protofibril adsorption on ER stress-mimicking membranes was more strongly suppressed by Toc compared with Toc3. Therefore, we concluded that VE can relieve ER stress by destabilizing the solid-ordered phase of the ER membrane and subsequently reducing the amount of Aβ adsorbed on the membrane. Moreover, Toc exerted a stronger effect than Toc3.
Vitamin E (VE) was discovered as a fat-soluble vitamin that could prevent infertility.1 α-Tocopherol (Toc), the most abundant form of VE, and α-tocotrienol (Toc3), another form of VE, share chroman rings in the same methylation state. Toc and Toc3 have different hydrocarbon side chains attached to the chroman ring; Toc has a phytyl group, while Toc3 has a farnesyl group. Unlike the phytyl group, the farnesyl group has three trans double bonds, making Toc3 more hydrophilic.2 Negative correlations have been reported between plasma Toc and Toc3 levels and the development of Alzheimer’s disease (AD), although several reports have contradicted these findings.3−5 It is not well understood how Toc and Toc3 inhibit AD.
Endoplasmic reticulum (ER) stress resulting from abnormalities in protein folding is believed to be a major factor involved in the etiology of AD.6 To reduce ER stress, the unfolded protein response (UPR) is induced to cope with misfolded proteins by increasing protein folding ability, decreasing protein synthesis, and promoting ER-associated degradation.7,8 If ER stress cannot be alleviated, the UPR triggers cell death. Amyloid-β (Aβ) is prone to misfolding, and the accumulation of Aβ causes AD due to neuronal cell death induced by ER stress.9,10 The toxicity of Aβ varies depending on its degree of polymerization, with intermediates to the fibrillar structure being highly toxic.10,11 Previous studies have shown that Aβ oligomers and protofibrils, which are highly toxic Aβ species, have a greater impact on membrane dynamics than monomers and fibrils, which are less toxic species.12,13 Furthermore, we found that highly toxic Aβ oligomers and protofibrils tend to accumulate on the solid-ordered (So) phase of membranes, which is rich in saturated lipids, rather than the liquid-disordered (Ld) phase, which is rich in unsaturated lipids, or the liquid-ordered (Lo) phase, which is rich in saturated lipids and cholesterol (Chol), by using liposomes.14−16 The ER membrane is known to have low Chol content.17,18 Saturation of ER membrane lipids is known as ER stress.19,20 When long-chain fatty acids, such as palmitate, are fed, saturated lipids are synthesized, forming the So phase on the ER membrane, leading to cell death.21 Therefore, we considered that the formation of the So phase is related to ER stress. Biomimetic membranes can form a variety of phase states such as the So, Ld, and Lo phases.22 Therefore, we prepared a Chol-free biomimetic membrane that forms the So phase as a model for the ER stress membrane.
Recently, Toc, but not Toc3, was reported to reduce the cytotoxicity caused by 24S-hydroxycholesterol (24S-OHC), which is associated with AD.23 Toc and Toc3 are present in biological membranes and have different effects on membrane properties.24−26 In this study, we clarify the effects of Toc and Toc3 on the adsorption of Aβ on ER stress-mimicking membranes.
To assess the effects of Toc and Toc3 on ER stress-mimicking membranes, we observed phase separation in liposomes by fluorescence microscopy at room temperature. Liposomes were prepared by the natural swelling method. Liposomes were composed of dipalmitoylphosphocholine (DPPC) and dioleoylphosphocholine (DOPC) with and without VE (Toc and Toc3). The chemical structures of DOPC, DPPC, Toc, and Toc3 are shown in Figure S1. Chol is often used to observe the phase behavior of lipid membranes;22 however, because we focused on the ER membrane with low Chol levels, we did not use Chol. VE was added from 0 to 10% (0, 3, 5, 8, and 10%) with a fixed ratio of DOPC:DPPC = 50:50. As a fluorescent probe, rhodamine B 1,2-dihexadecanoyl-sn-glycerol-3-phosphoethanolamine, triethylammonium salt (Rhod-DHPE), which localizes to the DOPC-rich phase, was used. The details of the experimental method can be found in the Supporting Information.
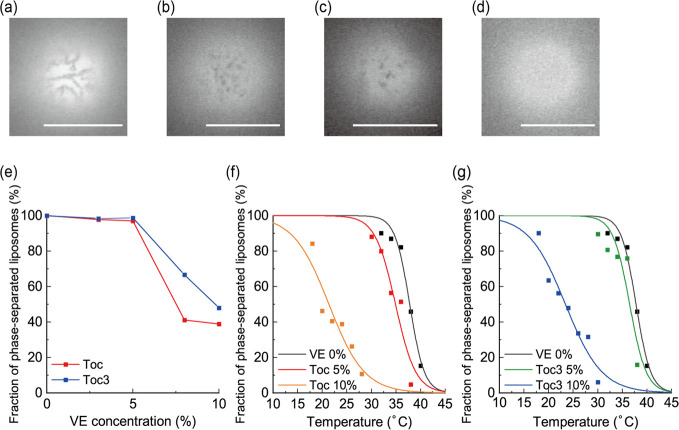
At DOPC:DPPC = 50:50 without VE, phase separation was observed (Figure 1a). The dark and bright regions were the DPPC-rich So phase and DOPC-rich Ld phase, respectively. We added VE to this binary lipid mixture. Although So/Ld phase separation was also observed at 5% VE, the domain size became smaller (Figure 1b,c). After adding more VEs, phase separation was suppressed and the homogeneous phase appeared (Figure 1d). The fractions of phase-separated liposomes are summarized in Figure 1e. The important difference between Toc and Toc3 was that Toc strongly suppressed phase separation compared with Toc3.
Figure 1.
(a) Microscopic images of So/Ld phase separation at DOPC/DPPC = 50:50 (0% VE), (b) at DOPC/DPPC/Toc = 47.5:47.5:5 (5% Toc), and (c) at DOPC/DPPC/Toc3 = 47.5:47.5:5 (5% Toc3). (d) Microscopic image of homogeneous liposomes at DOPC/DPPC/Toc = 45:45:10 (10% Toc). Scale bars: 10 μm. (e) Fraction of phase-separated liposomes as a function of VE concentration. (f, g) Fraction of phase-separated liposomes containing Toc and Toc3, respectively, as a function of temperature.
Next, to estimate the effects of Toc and Toc3 on the thermal stability of So/Ld phase separation, we measured the miscibility temperature (Tmix) of the phase-separated domain, which was defined as the temperature at which the fraction of phase separation was 50%. Tmix reflects the thermal stability of phase-separated structures. We measured the fraction of phase-separated liposomes at each temperature, and the obtained experimental results were fitted by Boltzmann’s sigmoid function (eq S1) to obtain Tmix. The measured Tmix values were 37.8 °C (control), 34.9 °C (5% Toc), 21.4 °C (10% Toc), 36.5 °C (5% Toc3), and 23.4 °C (10% Toc3) (Figure 1f,g). The values of Tmix decreased as VE concentration increased. In addition, the tendency for a reduction of Tmix can be seen more clearly for Toc than for Toc3. These data suggest that Toc and Toc3 disturb So/Ld phase separation.
The chain melting transition temperatures (main transition) of DPPC membranes containing Toc and Toc3 were investigated using differential scanning calorimetry (DSC). The details of DSC can be found in the Supporting Information. The thermograph for the DPPC single-component membrane showed two peaks at 37.2 and 42 °C, corresponding to pretransition and main transition, respectively (Figure 2a,b), which was consistent with a previous report.27 Because pretransition disappeared by adding VE, we focused on the shift of the main transition temperature. As the concentration of VE increased, the peak shifted toward a lower temperature and became broader, indicating that VE inhibited the strong attraction between DPPC molecules and reduced phase transition cooperativity. Moreover, the peak shape became asymmetric. We assumed that this asymmetric peak was composed of two symmetric peaks, and we plotted the changes in the positions of these two peaks (Figure 2c). The details of peak deconvolution and peak changes are shown in Figures S2 and S3. The sharp peak at higher temperatures and the broad peak at lower temperatures corresponded to the DPPC-rich So phase and VE-rich phase, respectively. These two peaks shifted toward a lower temperature as the concentration of VE increased. As previously reported, Toc showed a concentration-dependent decrease in the phase transition temperature of DPPC.28 In this study, we found that Toc3 slightly destabilized these phases compared with Toc. Therefore, VE reduced the thermal stability of both phases.
Figure 2.
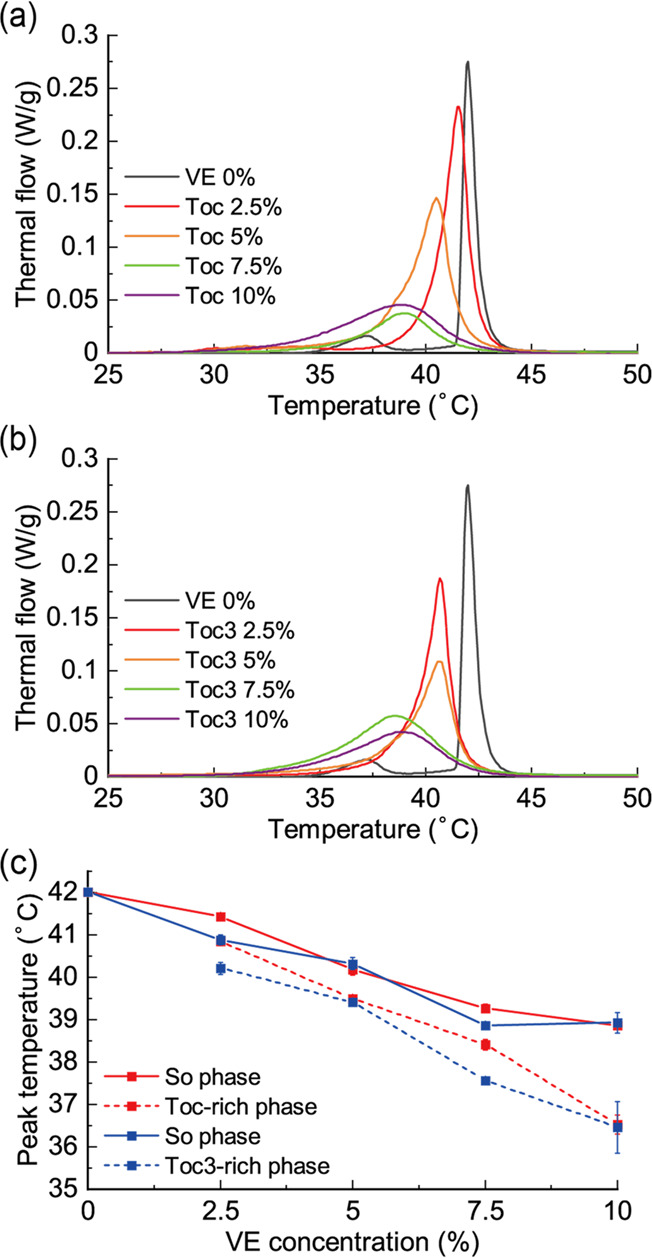
DSC thermograph of membranes composed of (a) DPPC/Toc and (b) DPPC/Toc3. (c) Peak temperature changes of deconvoluted DSC thermographs as a function of VE concentration.
Because Toc and Toc3 both decreased the thermal stability of DPPC membranes representing the So phase in DSC, they may be mainly partitioned into the Ld phase in So/Ld phase-separated membranes. However, some amount of VE was incorporated into the DPPC-rich So phase because of mixing entropy. We considered that the amount of Toc in the So phase was larger than that of Toc3 because Toc3 destabilized DPPC membranes compared with Toc according to DSC. On the other hand, Toc destabilized the phase-separated structures at room temperature and decreased Tmix compared with Toc3. VE incorporated into the So phase diluted the favorable interactions between DPPC molecules and decreased Tmix.29 The destabilization effect of Toc was stronger than that of Toc3 because the amount of Toc in the So phase was probably larger. In the future, it will be important to measure the exact amount of VE in the So phase, for example, by nuclear magnetic resonance.
The differences of partitioning between Toc and Toc3 arose from differences in their chemical structures (Figure S1). From normal-phase high-performance liquid chromatography, it was reported that Toc3, with its unsaturated side chain, is more polar than Toc2. In other words, Toc3 is more hydrophilic than Toc. The DOPC-rich Ld phase is more loosely packed than the DPPC-rich So phase, making it easier to incorporate water molecules into the Ld phase. Therefore, Toc3 is more likely to be partitioned into the hydrophilic-Ld phase than Toc. In contrast, the amount of Toc in the So phase was larger than that of Toc3. Similar behavior has been observed with the addition of fatty acids to lipid membranes, and a saturated fatty acid (palmitic acid) has higher affinity to the So phase than a trans-fatty acid (elaidic acid).30
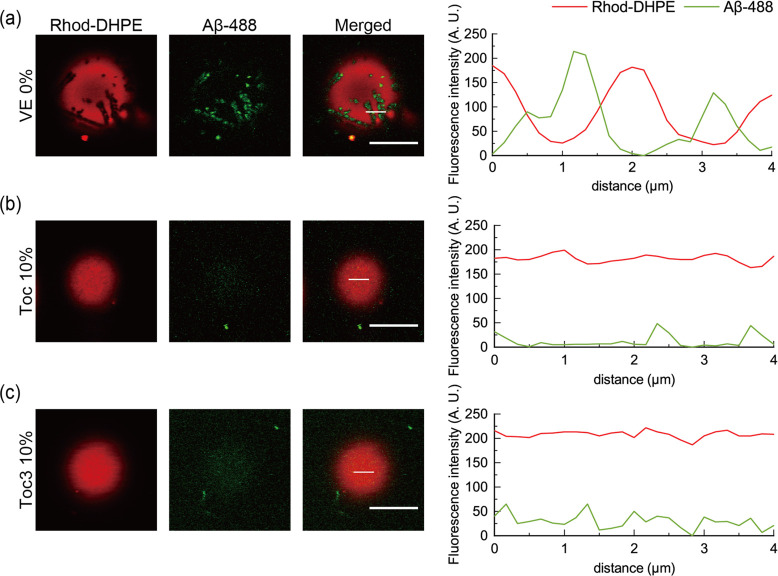
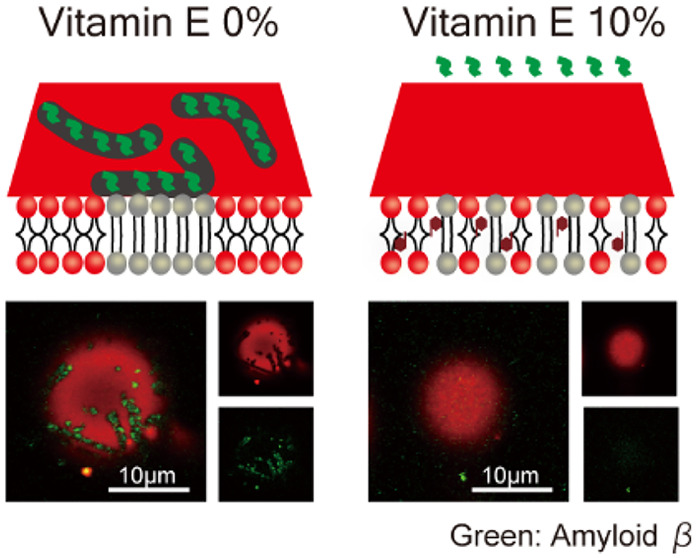
Next, we added Aβ protofibrils to liposomes containing Toc and Toc3 and observed them at room temperature by confocal laser scanning microscopy. We used Aβ peptide with 42 amino acid residues and fluorescently labeled Aβ (HiLyte Fluor 488, Aβ-488) and mixed them at a ratio of 2:1. The mixed Aβ was incubated for 12 h to aggregate spontaneously into protofibrils.16 Details of sample preparation can be found in the Supporting Information. At 0% VE, So/Ld phase-separated liposomes were observed, and the regions with higher and lower Rhod-DHPE fluorescence intensities corresponded to the Ld and So phases, respectively (Figure 3a). The fluorescence intensity of Aβ-488 was higher in the So phase, indicating that Aβ protofibrils are selectively adsorbed onto the So phase, which is consistent with previous studies.15,16 At 5% VE, most of the liposomes were phase-separated into the So and Ld phases. Similarly, Aβ protofibrils were selectively adsorbed onto the So phase (Figure S4). On the other hand, at 10% VE, more than 50% of liposomes exhibited a homogeneous phase, as already shown in Figure 1e. In such liposomes, we could not observe Aβ adsorption onto the surface of liposomes (Figure 3b,c). We concluded that Aβ adsorption correlated to So phase formation. Because Toc strongly suppressed the formation of the So phase compared with Toc3 from microscopic observations (Figure 1), Toc prevented Aβ adsorption onto the ER stress-mimicking membranes compared with Toc3.
Figure 3.
Microscopic images obtained by confocal laser scanning microscopy and fluorescence intensity profiles along the white lines in the merged images. Aβ protofibril adsorption on liposomes containing (a) 0% VE, (b) 10% Toc, and (c) 10% Toc3. Red and green fluorescence represent Rhod-DHPE and Aβ-488, respectively. Red and green lines in the fluorescence intensity profiles indicate the intensities of Rhod-DHPE and Aβ-488, respectively. Scale bars: 10 μm.
There are various conflicting reports regarding Toc and Toc3.31,32 The chroman ring structure responsible for their antioxidant effect is the same, and only the hydrocarbon chains are different between them. This may result in their different localization in organelle membranes and membrane subdomains and then cause different effects on diseases. In this study, we found that Toc inhibited So phase formation more strongly than Toc3, suggesting that local lipid composition affects the localization of Toc and Toc3. We have shown that 25S-OHC promotes ER stress-induced cell death via changes in membrane properties.33 24S-OHC, which has a similar structure to 25S-OHC, also induces neuronal cell death via ER stress.34 Toc can inhibit this 24S-OHC-induced cell death; however, the mechanism by which Toc inhibits cytotoxicity by 24S-OHC is poorly understood. 24S-OHC is esterified by acyl-coenzyme A:cholesterol acyltransferases residing in ER membrane proteins to form 24S-OHC-ester, which causes the loss of ER membrane integrity.34 Our findings suggests that Toc has a greater effect on ER membrane properties than Toc3 in the ER stress state and that the Toc-induced changes in ER membrane properties may contribute to the mitigation of the toxicity of 24S-OHC ester.
The ER is one of the most important organelles for lipid metabolism, and the ER membrane changes its lipidome in response to the surrounding environment. Supplementation of cultured cells with saturated fatty acids promotes the synthesis of saturated lipids in the ER, leading to the formation of the So phase.21 We hypothesized that the accumulation of Aβ on the So phase causes ER stress. Thus, the higher the saturated lipid content of the ER membrane, the more misfolded proteins may be adsorbed to the ER membrane. Eventually, the UPR fails to adequately alleviate ER stress, and cell death is induced.
VE is expected to be effective in inhibiting the onset of AD,3,4,35 however, it is not clear whether Toc or Toc3 is more effective in inhibiting AD. One of the reasons for this is that there are multiple mechanisms involved in the pathogenesis of AD. Using ER stress-mimicking membranes, our results have clearly show that by inhibiting the membrane adsorption of Aβ protofibrils, Toc is more effective than Toc3. This is because Toc destabilizes the So domain more strongly than Toc3, and a similar mechanism may apply to other molecules such as docosahexaenoic acid and eicosatetraenoic acid, which are thought to prevent AD.29−31
The involvement of ER stress has been reported in diseases other than AD, such as diabetes.39 VE and docosahexaenoic acid are thought to have inhibitory roles in the development of type 2 diabetes.40,41 Saturated fatty acid intake is a known risk factor for diabetes.42 Docosahexaenoic acid (DHA) alters the cell lipidome and increases the levels of polyunsaturated lipids.43 In ternary model membranes (DOPC, DPPC, and Chol), the disappearance of So/Ld phase separation occurs when the ratio of DOPC to DPPC increases.22 However, increasing the unsaturation in the low-Tm lipid in mixtures with desaturated phospholipid in binary model membranes has been shown to increase So thermostability.44 An increased concentration of polyunsaturated free fatty acids in the cell may compete with palmitate for the synthesis of phospholipids in the ER, as shown for the unsaturated oleic acid and DHA.21,45 Therefore, the effects in ER and model membranes may differ. Thus, preventing the saturation of ER membrane lipids can avoid the induction of the UPR and cell death and alleviate these diseases.
In this study, we investigated the phase behavior and thermal stability of the phase-separated structures in ER stress-mimicking membranes containing Toc and Toc3. From microscopic observations, Toc suppressed So phase formation in ER stress-mimicking membranes more strongly than Toc3. On the other hand, DSC showed that the DPPC-rich So phase was destabilized more by Toc3 than by Toc. It was also demonstrated that the amount of Toc in the So phase was probably larger than that of Toc3, and Toc inhibited the formation of the So phase more strongly than Toc3. In addition, Aβ adsorption onto ER stress-mimicking membranes was also reduced by VE, especially Toc, due to the suppression of So phase formation. It is considered that the suppression of So phase formation by adding Toc has more pronounced effects on the reduction of ER stress-denatured proteins compared with the addition of Toc3.
Acknowledgments
We acknowledge support from Grants-in-Aid for Scientific Research (B) (Grant No. JP26289311) and from the Japan Society for the Promotion of Science (JSPS) and JST SPRING (Grant No. JPMJSP2102).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.2c03098.
The authors declare no competing financial interest.
Supplementary Material
References
- Evans H. M.; Bishop K. S. On the Existence of a Hitherto Unrecognized Dietary Factor Essential for Reproduction. Science 1922, 56, 650–651. 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A.; Gorgen S.; Pettersson J.; Lampi A.-M. Normal-Phase High-Performance Liquid Chromatography of Tocopherols and Tocotrienols Comparison of Different Chromatographic Columns. J. Chromatogr. A 2000, 881, 217–227. 10.1016/S0021-9673(99)01346-1. [DOI] [PubMed] [Google Scholar]
- Mangialasche F.; Kivipelto M.; Mecocci P.; Rizzuto D.; Palmer K.; Winblad B.; Fratiglioni L. High Plasma Levels of Vitamin E Forms and Reduced Alzheimer’s Disease Risk in Advanced Age. JAD 2010, 20, 1029–1037. 10.3233/JAD-2010-091450. [DOI] [PubMed] [Google Scholar]
- Browne D.; McGuinness B.; Woodside J. V.; McKay G. J. Vitamin E and Alzheimer’s Disease: What Do We Know so Far?. CIA 2019, 14, 1303–1317. 10.2147/CIA.S186760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangialasche F.; Xu W.; Kivipelto M.; Costanzi E.; Ercolani S.; Pigliautile M.; Cecchetti R.; Baglioni M.; Simmons A.; Soininen H.; Tsolaki M.; Kloszewska I.; Vellas B.; Lovestone S.; Mecocci P. Tocopherols and Tocotrienols Plasma Levels Are Associated with Cognitive Impairment. Neurobiology of Aging 2012, 33, 2282–2290. 10.1016/j.neurobiolaging.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Hashimoto S.; Saido T. C. Critical Review: Involvement of Endoplasmic Reticulum Stress in the Aetiology of Alzheimer’s Disease. Open Biol. 2018, 8, 180024–180038. 10.1098/rsob.180024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I.; Ron D. Integrating the Mechanisms of Apoptosis Induced by Endoplasmic Reticulum Stress. Nat. Cell Biol. 2011, 13, 184–190. 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E.; Logue S. E.; Gorman A. M.; Samali A. Mediators of Endoplasmic Reticulum Stress-induced Apoptosis. EMBO Rep 2006, 7, 880–885. 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T.; Zhu H.; Morishima N.; Li E.; Xu J.; Yankner B. A.; Yuan J.. Caspase-12 Mediates Endoplasmic- Reticulum-Specific Apoptosis and Cytotoxicity by Amyloid-b. 2000, 403, 98–103. [DOI] [PubMed] [Google Scholar]
- Umeda T.; Tomiyama T.; Sakama N.; Tanaka S.; Lambert M. P.; Klein W. L.; Mori H. Intraneuronal Amyloid β Oligomers Cause Cell Death via Endoplasmic Reticulum Stress, Endosomal/Lysosomal Leakage, and Mitochondrial Dysfunction in Vivo. J. Neurosci. Res. 2011, 89, 1031–1042. 10.1002/jnr.22640. [DOI] [PubMed] [Google Scholar]
- Taneja V.; Verma M.; Vats A. Toxic Species in Amyloid Disorders: Oligomers or Mature Fibrils. Ann. Indian Acad. Neurol 2015, 18, 138–145. 10.4103/0972-2327.144284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshavn K. J.; Satriano C.; Lin Y.; Zhang R.; Dulchavsky M.; Bhunia A.; Ivanova M. I.; Lee Y.-H.; La Rosa C.; Lim M. H.; Ramamoorthy A. Reduced Lipid Bilayer Thickness Regulates the Aggregation and Cytotoxicity of Amyloid-β. J. Biol. Chem. 2017, 292, 4638–4650. 10.1074/jbc.M116.764092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca M. F. M.; Kotler S. A.; Brender J. R.; Chen J.; Lee D.; Ramamoorthy A. Two-Step Mechanism of Membrane Disruption by Aβ through Membrane Fragmentation and Pore Formation. Biophys. J. 2012, 103, 702–710. 10.1016/j.bpj.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T.; Morita M.; Kishimoto Y.; Komatsu Y.; Vestergaard M.; Takagi M. Biomimetic Microdroplet Membrane Interface: Detection of the Lateral Localization of Amyloid Beta Peptides. J. Phys. Chem. Lett. 2010, 1, 170–173. 10.1021/jz900106z. [DOI] [Google Scholar]
- Phan H. T. T.; Vestergaard M. C.; Baek K.; Shimokawa N.; Takagi M. Localization of Amyloid Beta (Aβ1–42) Protofibrils in Membrane Lateral Compartments: Effect of Cholesterol and 7-Ketocholesterol. FEBS Lett. 2014, 588, 3483–3490. 10.1016/j.febslet.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Morita M.; Hamada T.; Tendo Y.; Hata T.; Vestergaard M. C.; Takagi M. Selective Localization of Alzheimer’s Amyloid Beta in Membrane Lateral Compartments. Soft Matter 2012, 8, 2816–2819. 10.1039/c2sm07185a. [DOI] [Google Scholar]
- Jacquemyn J.; Cascalho A.; Goodchild R. E. The Ins and Outs of Endoplasmic Reticulum-controlled Lipid Biosynthesis. EMBO Rep 2017, 18, 1905–1921. 10.15252/embr.201643426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G.; de Kroon A. I. P. M. Lipid Map of the Mammalian Cell. Journal of Cell Science 2011, 124, 5–8. 10.1242/jcs.071233. [DOI] [PubMed] [Google Scholar]
- Volmer R.; van der Ploeg K.; Ron D. Membrane Lipid Saturation Activates Endoplasmic Reticulum Unfolded Protein Response Transducers through Their Transmembrane Domains. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 4628–4633. 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.; Stanzione F.; Oak A.; Kim G. H.; Yerneni S.; Qi L.; Sum A. K.; Chan C. Intrinsic Structural Features of the Human IRE1α Transmembrane Domain Sense Membrane Lipid Saturation. Cell Reports 2019, 27, 307. 10.1016/j.celrep.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.; Zhao Z.; Zhang L.; Shi L.; Shahriar S.; Chan R. B.; Di Paolo G.; Min W. Metabolic Activity Induces Membrane Phase Separation in Endoplasmic Reticulum. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 13394–13399. 10.1073/pnas.1712555114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L.; Keller S. L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y.; Asa M.; Urano Y.; Saito Y.; Nishikawa K.; Noguchi N. Tocopherol Suppresses 24(S)-Hydroxycholesterol-Induced Cell Death via Inhibition of CaMKII Phosphorylation. Biochimie 2018, 153, 203–209. 10.1016/j.biochi.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Yoshida Y.; Niki E.; Noguchi N. Comparative Study on the Action of Tocopherols and Tocotrienols as Antioxidant: Chemical and Physical Effects. Chem. Phys. Lipids 2003, 123, 63–75. 10.1016/S0009-3084(02)00164-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. J.; Tsuchiya M.; Wassall S. R.; Choo Y. M.; Govil G.; Kagan V. E.; Packer L. Structural and Dynamic Membrane Properties of.Alpha.-Tocopherol and.Alpha.-Tocotrienol: Implication to the Molecular Mechanism of Their Antioxidant Potency. Biochemistry 1993, 32, 10692–10699. 10.1021/bi00091a020. [DOI] [PubMed] [Google Scholar]
- Saito Y.; Yoshida Y.; Nishio K.; Hayakawa M.; Niki E. Characterization of Cellular Uptake and Distribution of Vitamin E. Ann. N. Y. Acad. Sci. 2004, 1031, 368–375. 10.1196/annals.1331.047. [DOI] [PubMed] [Google Scholar]
- Sugahara K.; Shimokawa N.; Takagi M. Thermal Stability of Phase-Separated Domains in Multicomponent Lipid Membranes with Local Anesthetics. Membranes 2017, 7, 33–47. 10.3390/membranes7030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunert G.; Tomaszewska-Gras J.; Siejak P.; Pietralik Z.; Kozak M.; Polewski K. Disruptive Effect of Tocopherol Oxalate on DPPC Liposome Structure: DSC, SAXS, and Fluorescence Anisotropy Studies. Chem. Phys. Lipids 2018, 216, 104–113. 10.1016/j.chemphyslip.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Allender D. W.; Schick M. The Effect of Solutes on the Temperature of Miscibility Transitions in Multicomponent Membranes. Biophys. J. 2017, 113, 1814–1821. 10.1016/j.bpj.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa N.; Mukai R.; Nagata M.; Takagi M. Formation of Modulated Phases and Domain Rigidification in Fatty Acid-Containing Lipid Membranes. Phys. Chem. Chem. Phys. 2017, 19, 13252–13263. 10.1039/C7CP01201B. [DOI] [PubMed] [Google Scholar]
- Boccardi V.; Baroni M.; Mangialasche F.; Mecocci P. Vitamin E Family: Role in the Pathogenesis and Treatment of Alzheimer’s Disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2016, 2, 182–191. 10.1016/j.trci.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen C. K.; Khanna S.; Roy S. Tocotrienols: Vitamin E beyond Tocopherols. Life Sciences 2006, 78, 2088–2098. 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.; Baek K.; Phan H. T. T.; Shimokawa N.; Takagi M. Glycosyl Chains and 25-Hydroxycholesterol Contribute to the Intracellular Transport of Amyloid Beta (Aβ-42) in Jurkat T Cells. FEBS Open Bio 2017, 7, 865–876. 10.1002/2211-5463.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano Y.; Ho Vo D.-K.; Hirofumi A.; Noguchi N. 24(S)-Hydroxycholesterol Induces ER Dysfunction-Mediated Unconventional Cell Death. Cell Death Discov. 2019, 5, 113–125. 10.1038/s41420-019-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y.; Ito S.; Ohtsuki S.; Yamamoto N.; Takahashi T.; Iwata N.; Jishage K.; Yamada H.; Sasaguri H.; Yokota S.; Piao W.; Tomimitsu H.; Saido T. C.; Yanagisawa K.; Terasaki T.; Mizusawa H.; Yokota T. Depletion of Vitamin E Increases Amyloid β Accumulation by Decreasing Its Clearances from Brain and Blood in a Mouse Model of Alzheimer Disease. J. Biol. Chem. 2009, 284, 33400–33408. 10.1074/jbc.M109.054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquer J. A.; Tierney M. C.; Zecevic J.; Bettger W. J.; Fisher R. H. Fatty Acid Analysis of Blood Plasma of Patients with Alzheimer’s Disease, Other Types of Dementia, and Cognitive Impairment. Lipids 2000, 35, 1305–1312. 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- Yanai H. Effects of N-3 Polyunsaturated Fatty Acids on Dementia. J. Clin Med. Res. 2017, 9, 1–9. 10.14740/jocmr2815w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo van Lent D.; Egert S.; Wolfsgruber S.; Kleineidam L.; Weinhold L.; Wagner-Thelen H.; Maier W.; Jessen F.; Ramirez A.; Schmid M.; Scherer M.; Riedel-Heller S. G.; Wagner M. Eicosapentaenoic Acid Is Associated with Decreased Incidence of Alzheimer’s Dementia in the Oldest Old. Nutrients 2021, 13, 461–476. 10.3390/nu13020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; Lin C.; Haataja L.; Gurlo T.; Butler A. E.; Rizza R. A.; Butler P. C. High Expression Rates of Human Islet Amyloid Polypeptide Induce Endoplasmic Reticulum Stress–Mediated β-Cell Apoptosis, a Characteristic of Humans With Type 2 but Not Type 1 Diabetes. Diabetes 2007, 56, 2016–2027. 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- De Caterina R.; Madonna R.; Bertolotto A.; Schmidt E. B. N-3 Fatty Acids in the Treatment of Diabetic Patients: Biological Rationale and Clinical Data. Diabetes Care 2007, 30, 1012–1026. 10.2337/dc06-1332. [DOI] [PubMed] [Google Scholar]
- Badawi A. Vitamins D, C, and E in the Prevention of Type 2 Diabetes Mellitus: Modulation of Inflammation and Oxidative Stress. BTT 2011, 5, 7–19. 10.2147/BTT.S14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risérus U.; Willett W. C.; Hu F. B. Dietary Fats and Prevention of Type 2 Diabetes. Prog. Lipid Res. 2009, 48, 44–51. 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K. R.; Malmberg E.; Symons J. L.; Fan Y.-Y.; Chapkin R. S.; Ernst R.; Levental I. Lipidomic and Biophysical Homeostasis of Mammalian Membranes Counteracts Dietary Lipid Perturbations to Maintain Cellular Fitness. Nat. Commun. 2020, 11, 1339–1351. 10.1038/s41467-020-15203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg A.; Ekholm O. O.; Slotte J. P. Miscibility of Sphingomyelins and Phosphatidylcholines in Unsaturated Phosphatidylcholine Bilayers. Biophys. J. 2015, 109, 1907–1916. 10.1016/j.bpj.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile N. M.; Han X.; Harp J. D.; Gale S. E.; Ory D. S.; Schaffer J. E. Disruption of Endoplasmic Reticulum Structure and Integrity in Lipotoxic Cell Death. J. Lipid Res. 2006, 47, 2726–2737. 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.