Abstract
Background
During the initial surge of coronavirus disease 2019 (COVID-19), health-care utilization fluctuated dramatically, straining acute hospital capacity across the USA and potentially contributing to excess mortality.
Methods
This was an observational retrospective study of patients with COVID-19 admitted to a large US urban academic medical center during a 12-week COVID-19 surge in the Spring of 2020. We describe patterns in length of stay (LOS) over time. Our outcome of interest was prolonged LOS (PLOS), which we defined as 7 or more days. We performed univariate analyses of patient characteristics, clinical outcomes and discharge disposition to evaluate the association of each variable with PLOS and developed a final multivariate model via backward elimination, wherein all variables with a P-value above 0.05 were eliminated in a stepwise fashion.
Results
The cohort included 1366 patients, of whom 13% died and 29% were readmitted within 30 days. The LOS (mean: 12.6) fell over time (P < 0.0001). Predictors of PLOS included discharge to a post–acute care (PAC) facility (odds ratio [OR]: 11.9, 95% confidence interval [CI] 2.6–54.0), uninsured status (OR 3.2, CI 1.1–9.1) and requiring intensive care and intubation (OR 18.4, CI 11.5–29.6). Patients had a higher readmission rate if discharged to PAC facilities (40%) or home with home health agency (HHA) services (38%) as compared to patients discharged home without HHA services (26%) (P < 0.0001).
Conclusion
Patients hospitalized with COVID-19 during a US COVID-19 surge had a PLOS and high readmission rate. Lack of insurance, an intensive care unit stay and a decision to discharge to a PAC facility were associated with a PLOS. Efforts to decrease LOS and optimize hospital capacity during COVID-19 surges may benefit from focusing on increasing PAC and HHA capacity and resources.
Keywords: COVID-19, length of stay, post–acute care, readmissions, bed capacity
Introduction
During the initial surge of coronavirus disease 2019 (COVID-19), the US health-care system faced unprecedented challenges in providing patients access to medical care. The COVID-19 pandemic has strained acute hospital capacity across the country, and capacity limitations have contributed to excess mortality in this already devastating pandemic [1, 2]. Acute hospital length of stay (LOS) is a key indicator of hospital resource utilization and is central to capacity planning. Identifying reasons for discharge delays and prolonged LOS (PLOS) can enable health-care systems to develop strategies to improve hospital throughput and ensure patients receive the appropriate care in the optimal setting. Therefore, dedicated analysis of patterns in PLOS at US medical centers during COVID-19 surges may be useful for decision makers to plan for capacity challenges in this ongoing pandemic.
While several studies, including a large systematic review [3–7], have evaluated patterns in LOS and predictors of PLOS among patients with COVID-19 in Europe and Asia, few studies [8–10] have examined LOS patterns and predictors for patients admitted to US tertiary-care hospitals over the course of a COVID-19 surge. Furthermore, while these studies did explore the predictors of LOS, none examined both the impact of pre-admission disposition and discharge disposition, which are important factors in LOS. Specifically, the need for post–acute care (PAC) after discharge may prolong LOS if not readily available during the pandemic [11]. Given the relatively high rates of PAC utilization in the US as compared to other high-income countries [12] and limited understanding of LOS drivers to date, we aimed to evaluate how PAC needs in addition to clinical and non-clinical factors could impact LOS.
Objective
We described predictors of PLOS among adult patients admitted with COVID-19 at a single Massachusetts tertiary-care hospital during an initial COVID-19 surge. We defined PLOS as 7 or more days as this was our mean hospital LOS prior to the COVID-19 pandemic [13]. We performed univariate analyses of patient characteristics, including admission week, clinical outcomes and discharge disposition, to evaluate the association of each variable with PLOS and then used these results to develop a multivariate model for predictors of acute care hospital PLOS among this cohort.
Methods
Study design, setting and participants
This observational retrospective study was performed at a tertiary-care, 1043 bed academic medical center in Boston, Massachusetts, that serves as a regional referral center for New England and has ∼48 000 inpatient admissions annually. All patients 18 years and older with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction test admitted during the 12-week Spring 2020 Massachusetts surge (11 March 2020–3 June 2020) were included. Patients who remained hospitalized after 1 September 2020 were excluded due to data availability.
Data source
We used data from the Massachusetts General Hospital (MGH) COVID-19 Data Registry [14], a registry of confirmed SARS-CoV-2-infected patients hospitalized at MGH that is based on manual chart reviews and data extraction from electronic health records by trained reviewers. We obtained PAC referral information from the 4Next database, a web-based case management application developed by Mass General Brigham. We extracted Charlson Comorbidity Index (CCI), 30-day same-network readmissions and payer information from our electronic medical record. The CCI, a scoring system of conditions shown to predict 10-year mortality, was used as a measure of comorbidities among admitted patients [15]. Thirty-day same-network readmissions included readmissions to any hospital within our health-care system network, which includes two tertiary-care academic medical centers in Boston, Massachusetts and seven community hospitals in New England.
Statistical analysis and study outcome
We performed descriptive analyses of patient characteristics, clinical outcomes and discharge disposition. The ages were separated into quintiles. Intensive care unit (ICU) stay and intubation were merged into a categorical variable of ICU stay with intubation, ICU stay without intubation or no ICU stay. Comparisons of categorical variables were performed with Fisher’s exact tests whenever possible, and non-normally distributed two-group comparisons were performed by the Kruskal–Wallis rank test.
Our main outcome of interest was PLOS, which we defined as greater than the mean hospital LOS prior to the COVID-19 pandemic of 7 days. We defined LOS as the length of time between the placement of an inpatient admission order and a discharge order. We used a Kruskal–Wallis rank test to evaluate trends in LOS over time as the distribution was non-normal. We fit univariate logistic regression models to evaluate the association of each variable including patient characteristics, clinical outcomes, admission week as a temporal time trend and discharge disposition with PLOS. All covariates with statistically significant associations (P ≥ 0.05) in these univariate analyses were included in an initial multivariate logistic regression model. We then developed a final model via backward elimination, wherein all variables with a P-value >0.05 were eliminated in a stepwise fashion. We also assessed for effect modification between covariates with an a priori observation that age affects discharge disposition after an acute hospitalization [16]. All analyses were performed in STATA IC 14.2 (STATA Corp., College Station, TX, USA).
Results
Patient characteristics
A total of 1366 patients were included in this cohort. Patient characteristics, clinical outcomes and discharge disposition are summarized in Table 1. The mean age was 60 years. Most patients self-identified as White (44%) or Hispanic (38%) and 43% were non-English-speaking. While the majority were admitted from private homes (77%), a significant portion (19%) was presented from assisted living facilities (ALFs) and PAC facilities, which included skilled nursing facilities (SNFs), long-term acute care facilities and inpatient rehabs. Nearly one-third (31%) of the patients were admitted to the ICU, and 82% of patients in the ICU were intubated.
Table 1.
Characteristics, clinical outcomes and discharge disposition for patients admitted with COVID-19
| All patients | No PLOS (LOS ≤ 7 days) | PLOS (LOS > 7 days) | ||
|---|---|---|---|---|
| (n = 1366) | (n = 720; 52.7%) | (n = 646; 47.3%) | P-value | |
| Age, mean ± SD(years) | 60 ± 18 | 57.5 ± 19.1 | 62.1 ± 16.4 | <0.001 |
| Age quintile | <0.001 | |||
| 18–43 | 281 (20.6) | 194 (26.9) | 87 (13.5) | |
| 43–56 | 273 (20.0) | 142 (19.7) | 131 (20.3) | |
| 56–65 | 265 (19.4) | 125 (17.4) | 140 (21.7) | |
| 65–77 | 274 (20.1) | 123 (17.1) | 151 (23.4) | |
| ≥77 | 273 (20.0) | 136 (18.9) | 137 (21.2) | |
| Female sex | 585 (43) | 327 (45.5) | 258 (39.9) | 0.023 |
| Race/Ethnicity | 0.189 | |||
| White | 545 (43.5) | 289 (43.3) | 256 (43.8) | |
| African American/Black | 147 (11.7) | 88 (13.2) | 59 (10.1) | |
| Hispanic | 478 (38.2) | 253 (37.9) | 225 (38.5) | |
| Other | 82 (6.6) | 37 (5.6) | 45 (7.7) | |
| Primary language | 0.181 | |||
| English | 781 (58.7) | 422 (60.5) | 359 (56.8) | |
| Non-English | 549 (41.3) | 276 (39.5) | 273 (43.2) | |
| Housing prior to admission | <0.001 | |||
| Private home | 999 (77.1) | 512 (74.9) | 487 (79.7) | |
| ALF/PAC facility | 250(19.3) | 133 (19.4) | 117 (19.2) | |
| Undomiciled/Shelter | 46 (3.6) | 39 (5.7) | 7 (1.2) | |
| Primary insurance | 0.051 | |||
| Medicare/Commercial/VA/Self | 956 (71.4) | 486 (69.0) | 470 (74.0) | |
| Medicaid | 364 (27.2) | 210 (29.8) | 154 (24.3) | |
| Uninsured | 19 (1.4) | 8 (1.1) | 11 (1.7) | |
| CCI | <0.001 | |||
| CCI 0 | 419 (30.7) | 265 (36.8) | 154 (23.8) | |
| CCI 1 + 2 | 522 (38.2) | 267 (37.1) | 255 (39.5) | |
| CCI 3+ | 425 (31.1) | 188 (26.1) | 237 (36.7) | |
| ICU and intubation | <0.001 | |||
| ICU stay without intubation | 72 (5.3) | 24 (3.3) | 48 (7.4) | |
| ICU stay with intubation | 349 (25.6) | 31 (4.3) | 318 (49.2) | |
| Discharge disposition | <0.001 | |||
| Home without HHA | 566 (41.9) | 417 (58.5) | 149 (23.3) | |
| Home with HHA | 187 (13.8) | 187 (13.8) | 85 (13.3) | |
| PAC facility | 425 (31.1) | 131 (18.2) | 294 (45.5) | |
| Psychiatric hospital | 7 (0.5) | 5 (0.7) | 2 (0.3) | |
| Hospice | 7 (0.5) | 2 (0.3) | 5 (0.8) | |
| Deceased | 174 (12.9) | 63 (8.8) | 111 (17.2) | |
| 30-day readmission (1 or more) | 396 (29.0) | 188 (26.1) | 208 (32.3) | 0.014 |
| LOS median and IQR (days) | 7 (4; 15) | 4 (3; 7) | 15 (10; 29) | <0.001 |
| LOS mean ± SD (days) | 12.6 ± 15.0 | 4.0 ± 1.8 | 22.1 ± 17.4 |
Abbreviations: ALF = assisted living facility; CCI = Charlson comorbidity index; HHA = home health agency; ICU = intensive care unit; IQR = interquartile range; IRF = inpatient rehabilitation facility; LOS = length of stay; PAC = post acute care; PLOS = prolonged length of stay; SD = standard deviation; VA = Veterans Affairs.
Discharge disposition and clinical outcomes
A large portion of patients (41%) were discharged home without home health agency (HHA) services, and 14% were discharged home with HHA services. Moreover, 13% of the patients died during admission. Of the 1195 patients discharged alive, excluding those discharged to hospice (n = 7), 29% had one or more readmissions to the same health-care network within 30 days of discharge. Patients discharged to PAC facilities or home with HHA experienced high readmission rates of 40% (n = 170) and 38% (n = 71), respectively, which as a combined group had a statistically significant difference compared to patients discharged home (P < 0.001). Patients discharged home without HHA (26%, n = 147) were less likely to be readmitted, compared to all other alive discharges (P < 0.001).
LOS and multivariate analysis
The median LOS was 7 days, and the mean LOS was 12.6 days. The mean weekly LOS (Figure 1) fell over the course of the surge from a mean LOS of 13 days in the first 6 weeks to 11 days in the last 6 weeks of the12-week surge (P for trend = 0.0001). There were statistically significant differences between patients with a PLOS and those without it in the distributions of age, sex, CCI, ICU, intubation status and discharge disposition (see Table 1).
Figure 1.
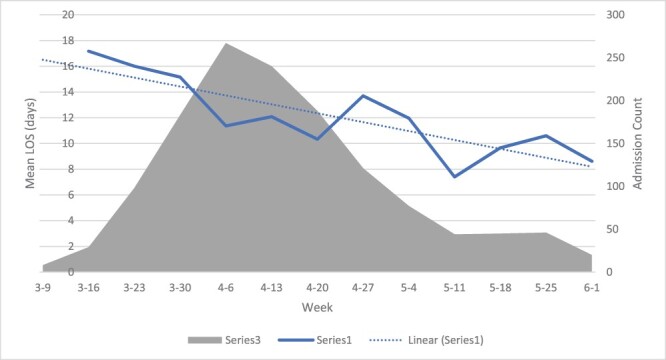
The mean weekly LOS and admission count for patients admitted with COVID-19. The mean weekly LOS fell over the course of the surge from a mean LOS of 13 days in the first 6 weeks to 11 days in the last 6 weeks of the 12-week surge (P for trend = 0.0001).
The final multivariate model (Table 2) included six covariates: age quintile, discharge disposition, primary insurance type, ICU and intubation status and housing prior to admission, as well as an interaction term between age quintile and discharge disposition that accounted for the effect modification of the discharge disposition by age category in excess of the individual contribution of these variables. Generally, increased age correlated with an increased likelihood of PLOS, though for patients >77, this increase is not significant. Age quintiles 56–66 (odds ratio [OR] 2.5, 95% confidence interval [CI] 1.5–4.5) and 65–77 (OR 2.5, 95% CI 1.4–4.5) had the greatest likelihood of PLOS as compared to the reference group of 18–43.
Table 2.
Multivariate logistic regression analysis for predictors of a prolonged length of stay
| Covariate | OR | 95% CI | P-value |
|---|---|---|---|
| Age quintile (in years) | |||
| 18–43 | Reference | ||
| 43–56 | 1.71 | 1.04, 2.8 | 0.03 |
| 56–65 | 2.53 | 1.5, 4.2 | <0.01 |
| 65–77 | 2.51 | 1.4, 4.5 | <0.01 |
| ≥77 | 1.73 | 0.83, 3.60 | 0.14 |
| Discharge disposition | |||
| Home (with or without HHA) | Reference | ||
| PAC facility | 11.92 | 2.63, 53.94 | <0.01 |
| Psychiatric hospital | 3.09 | 0.30, 31.66 | 0.34 |
| Hospice | 3.99 | 0.50, 31.66 | 0.19 |
| Deceased | 1.4 | 0.15, 12.82 | 0.76 |
| Interaction age quintile/discharge disposition | |||
| 43–56#Hospice | Reference | ||
| 43–56#PAC | 0.3 | 0.05, 1.71 | 0.18 |
| 43–56#Deceased | 1.22 | 0.07, 20.41 | 0.9 |
| 56–65#Hospice | Reference | ||
| 56–65#PAC | 0.18 | 0.03, 0.92 | 0.04 |
| 56–65#Deceased | 0.41 | 0.04, 4.86 | 0.48 |
| 65–77#Hospice | Reference | ||
| 65–77#PAC | 0.18 | 0.04, 0.94 | 0.04 |
| 65–77#Deceased | 0.61 | 0.06, 6.34 | 0.67 |
| ≥77#Hospice | Reference | ||
| ≥77#PAC | 0.35 | 0.07, 1.85 | 0.22 |
| ≥77#Deceased | 1.58 | 0.15, 16.69 | 0.7 |
| Housing | |||
| Private home | Reference | ||
| ALF/PAC facility | 0.6 | 0.39, 0.91 | 0.02 |
| Undomiciled/shelter | 0.24 | 0.10, 0.59 | <0.01 |
| Primary insurance | |||
| Medicare/Commercial/VA/Self | Reference | ||
| Medicaid | 1.08 | 0.75, 1.55 | 0.7 |
| Uninsured/COVID-19 fund | 3.2 | 1.13, 9.08 | 0.03 |
| ICU and intubation | |||
| No ICU stay, no intubation | Reference | ||
| ICU stay without intubation | 4.07 | 2.37, 6.99 | <0.01 |
| ICU stay with intubation | 18.41 | 11.46, 29.58 | <0.01 |
Abbreviations: ALF = assisted living facility; CI = confidence interval; HHA = home health agency; ICU = intensive care unit; PAC = post acute care; VA = Veterans Affairs.
In the final model, discharge to a PAC facility (OR 11.9, 95% CI 2.6–53.9) was an important predictor of PLOS. Only 1% of the patients within the cohort were uninsured. This small group was more likely to have PLOS (OR 3.18, 95% CI 1.12–8.97) as compared to patients covered by private insurance and Medicare. Both ICU stays with and without intubation predicted PLOS, though patients with an ICU stay with intubation were more likely to have a PLOS (OR 18.41, 95% CI 11.46–29.58) compared to those in the ICU without intubation (OR 4.07, 95% CI 2.37–6.99). Finally, patients presenting from ALFs or PAC facilities (OR 0.60, 95% CI 0.39–0.91) or who were undomiciled (OR 0.24, 95% CI 0.10–0.59) were less likely to have a PLOS compared to those who presented from a private home.
Discussion
Statement of principal findings
Among our large cohort of patients with COVID-19 hospitalized during a 12-week surge, hospital LOS was prolonged at 12.6 days compared to our institution’s pre-pandemic average of 7 days for all patients [13]. LOS was shortened over the course of the surge. The key predictors of PLOS within our multivariate model were discharge to a PAC facility, ICU stay, particularly ICU stay with intubation, and uninsured status. Protective factors against PLOS were pre-admission housing at a PAC facility, an ALF or being undomiciled.
In an unadjusted analysis, our cohort of patients also experienced a high readmission rate. Thirty-day readmissions were more likely to occur among those discharged to a PAC facility or a home with HHA as compared to a home without services. Readmissions may have also increased due to limited access to primary care as clinics transitioned to telemedicine care perhaps impacting capabilities for managing higher complexity patients [17].
Interpretation within the context of the wider literature
The median hospital LOS for patients with COVID-19 in the USA ranges from 7 to 8 days [8–10] and mirrors the median LOS of 7 days in our cohort. In a US study of 1643 adults admitted with severe COVID-19, only 41% of the patients admitted had a LOS >9 days [18], which is similar to our cohort where 46% of the patients had a LOS of >9 days. There has been wide international variation in LOS reported for patients with COVID-19, which has been attributed in part to differences in admission and discharge criteria as well as timing within the pandemic [3]. In a systematic review of 46 studies reporting LOS for patients hospitalized with COVID-19 in China, the median LOS was 14 days, whereas eight studies from outside of China reported a median LOS of only 5 days [3]. Several large differences between the health-care systems in the USA and China limit comparison, however, in outcomes such as LOS [19].15
In our cohort, mean LOS decreased over the course of the surge. Similarly, this may have resulted from changes in admission and discharge criteria over time. Clinicians likely became more comfortable discharging patients with the development of institutional guidelines on the management and discharge criteria of patients with COVID-19 [20], and access to PAC services also improved over the course of the surge as SNFs re-opened and alternative care sites were created locally [11].
The importance of PAC discharge access was demonstrated in our multivariate model in which discharge to a PAC facility was a strong predictor of PLOS with an OR approaching 12. Coordination between acute care hospitals and PAC facilities has been a well-recognized challenge prior to the COVID-19 pandemic, affecting 56% of delayed patients and causing on average 9 days of delay [21]. PAC capacity was severely limited early in the pandemic due to staffing shortages and challenges with infection control [22, 23]. Availability was improved as dedicated COVID-19 PAC facilities and HHA resources opened [11].
Interestingly, in our model, patients admitted from ALFs and PAC facilities had lower likelihoods of PLOS. This perhaps occurred because patients admitted from PAC facilities were subjected to a bed-hold policy under Medicare and Medicaid [24] and could promptly return to their prior facility when medically ready rather than having to undergo a new PAC referral process.
Implications for policy, practice and research
When planning for future pandemic surges, focus must be placed not only on hospital capacity but also on PAC capacity. Additionally, a robust system of communication should be developed between acute hospitals and PAC facilities to prevent a bottleneck in patient flow at the time of discharge. For example, our hospital established daily communication with a subset of SNFs about PAC bed availability for COVID and non-COVID patients. Hospitals and public entities may need to provide additional support to PAC agencies and facilities to optimize their operational capacity such as personal protective equipment, infection control resources and help with staffing to mitigate challenges together.
The strongest predictor of PLOS was an ICU stay with intubation. Several studies have noted that among patients hospitalized with COVID-19, ICU stays are associated with a longer hospital LOS [3–9]. Our analysis identified ICU with intubation to be a much greater driver of LOS than the ICU stay without intubation. A number of factors likely contributed to this relative increase in LOS including greater illness severity among intubated patients as well as greater rates of sedation use and delirium, which have been observed frequently in intubated patients with COVID-19 [25]. Of note, while CCI was included to adjust for clinical complexity, it was not included in the final multivariate model due to a lack of significance. In addition to PLOS, the high clinical complexity may have also resulted in higher readmission rates, which were seen for all patients but especially for those requiring PAC after hospitalization. Of the patients discharged alive following an ICU stay and intubation, nearly all ultimately required PAC facility discharges. For these patients, early case management involvement may reduce LOS if PAC potential barriers are identified early on, such as a lack of insurance or underinsurance for PAC services. Forecasting tools for acute hospital bed needs have been implemented to assist health leaders’ resource planning [26]. A similar approach could be developed to forecast short-term PAC capacity needs for a health system based on real-time ICU bed utilization.
Finally, the uninsured status was associated with PLOS. It is difficult to interpret this trend due to the small number of uninsured patients. Prior to the COVID-19 pandemic, the uninsured status has been associated with a reduced LOS among patients admitted to the ICU [27]. This has been attributed to the increased rates of patient-directed discharges observed among this group [21]. Our observation of PLOS among uninsured patients may reflect PAC facilities being less willing to accept uninsured patient referrals, despite federal coverage for acute and post-acute COVID-19 care for these patients [28]. Conversely, the few patients in our cohort experiencing homelessness prior to admission were less likely to have PLOS. This is counter to the observation that patients without stable housing typically have longer LOS [29]. This difference may be in part due to the establishment of respite and quarantine locations for this population at the height of the pandemic and a robust and integrated healthcare system in Boston for patients experiencing homelessness [30].
Strengths and limitations
This study has limitations. First, our data capture the first Massachusetts COVID-19 surge prior to the routine use of COVID-19 therapies including antivirals and immunosuppressive medications, which have been shown to impact hospital LOS [31, 32]. It took place at a tertiary-care academic medical center, limiting its generalizability to other care settings. In the development of our model, we did not account for certain factors known to contribute to LOS including provider factors (e.g. team structure and regionalization), system factors, and other patient factors, and did not adjust for multiple comparisons. We also noted wider CIs for certain covariates in our final model, which may have resulted from heterogeneity in these groups and/or a smaller number of events. Finally, the outcome data other than PLOS, including readmissions, were unadjusted. While 30-day readmission rates were high particularly for patients discharged to PAC facilities, they may be undercounted as only same-network readmissions were included.
Conclusion
This study is unique because it evaluates patterns in LOS over the course of a US COVID-19 surge and identifies key predictors of PLOS. These results may be used by health systems for surge planning and to develop targeted interventions to decrease LOS. For example, institutions could provide early enhanced discharge planning for patients requiring ICU care or PAC facility discharge or who lack insurance. Institutions may also explore strategies to increase PAC access, which continues to be limited by ongoing COVID-19 outbreaks, by providing additional resources to PAC facilities such as help with infection control and staffing resources. Addressing capacity constraints and reducing LOS while still delivering optimal care are of critical importance as hospitals need to simultaneously continue COVID-19- and non-COVID-19-related care.
Acknowledgements
The authors thank the data acquisition team including chart reviewers and data managers for the MGH COVID Registry.
Contributor Information
Jessica C O’neil, Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA.
Benjamin P Geisler, Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA; Institute for Medical Information Processing, Biometry and Epidemiology, Marchioninistr, 15, München 81377, Germany.
Donna Rusinak, Performance Analysis and Improvement, Massachusetts General Hospital, 125 Nashua Street, Boston, MA 02114, USA.
Ingrid V Bassett, Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA.
Virginia A Triant, Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA.
Rachael Mckenzie, Department of Case Management, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA.
Melissa L Mattison, Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA.
Amy W Baughman, Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA; Department of Case Management, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA.
Data availability statement
The data for this study are available on reasonable request from the corresponding author (A.W.B.). The data from the MGH COVID Registry are not publicly available because of confidentiality concerns around patient information.
Funding
Dr Bassett is supported by the Weissman Family MGH Scholar Award (R01 AI042006-24S1) and the National Institutes of Health (NIH award K24AI141036). Dr Baughman is supported by the Winickoff Scholars Program.
References
- 1. Janke AT, Mei H, Rothenberg C et al. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med 2021;16:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. French G, Hulse M, Nguyen D et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic—United States, July 2020-July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rees EM, Nightingale ES, Jafari Y et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med 2020. Sep 3;18:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shryane N, Pampaka M, Aparicio-Castro A et al. Length of stay in ICU of Covid-19 patients in England, March–May 2020. Int J Popul Data Sci 2021;5:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li K, Zhang C, Qin L et al. A nomogram prediction of length of hospital stay in patients with COVID-19 pneumonia: a retrospective cohort study. Dis Markers 2021;2021:5598824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong Y, Wu X, Qu J et al. Clinical characteristics of coronavirus disease 2019 and development of a prediction model for prolonged hospital length of stay. Ann Transl Med 2020;8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Z, Liu Y, Wei L et al. What are the risk factors of hospital length of stay in the novel coronavirus pneumonia (COVID-19) patients? A survival analysis in southwest China. PLoS One 2022;17:e0261216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebinger J, Wells M, Ouyang D et al. A machine learning algorithm predicts duration of hospitalization in COVID-19 patients. Intell Based Med 2021;5:100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallipattu SK, Jawa R, Moffitt R et al. Geospatial distribution and predictors of mortality in hospitalized patients with COVID-19: a cohort study. Open Forum Infect Dis 2020;7:ofaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tevald MA, Clancy MJ, Butler K et al. Activity measure for post-acute care “6-clicks” for the prediction of short-term clinical outcomes in individuals hospitalized with COVID-19: a retrospective cohort study. Arch Phys Med Rehabil 2021;102:2300–2308.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Neil JC, Geisler BP, Rusinak D et al. Case management in a COVID-19 surge: a single-institution study of disposition and access to post-acute care. J Am Geriatr Soc 2022;70:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figueroa JF, Papanicolas I, Riley K et al. International comparison of health spending and utilization among people with complex multimorbidity. Health Serv Res 2021;56:1317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Hospital Directory . Massachusetts General Hospital (220071)—Free Profile. https://www.ahd.com/free_profile/220071/Massachusetts_General_Hospital/Boston/Massachusetts/ (25 July 2021, date last accessed).
- 14. Bassett IV, Triant VA, Bunda BA et al. Massachusetts General Hospital Covid-19 registry reveals two distinct populations of hospitalized patients by race and ethnicity. PLoS One 2020;15:e0244270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 16. Doctoroff L, Herzig SJ. Predicting patients at risk for prolonged hospital stays. Med Care 2020;58:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexander GC, Tajanlangit M, Heyward J et al. Use and content of primary care office-based vs telemedicine care visits during the COVID-19 pandemic in the US. JAMA Netw Open 2020;3:e2021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. Pharmacoecon Open 2021;5:129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US-China Institute . Health Care in the U.S. and China. China.usc.edu. Published December 3, 2020. https://China.usc.edu/health-care-us-and-China (21 March 2021, date last accessed).
- 20. Greysen SR, Auerbach AD, Mitchell MD et al. Discharge practices for COVID-19 patients: rapid review of published guidance and synthesis of documents and practices at 22 US academic medical centers. J Gen Intern Med 2021;36:1715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao EJ, Yeluru A, Manjunath L et al. A long wait: barriers to discharge for long length of stay patients. Postgrad Med J 2018;94:546–50. [DOI] [PubMed] [Google Scholar]
- 22. Brown KA, Jones A, Daneman N et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. JAMA Intern Med 2021;181:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu H, Intrator O, Bowblis JR. Shortages of staff in nursing homes during the COVID-19 pandemic: what are the driving factors? J Am Med Dir Assoc 2020;21:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. CMS . Regulations & Guidance. https://www.cms.gov/Regulations-and-Guidance/Regulations-and-Guidance (17 March 2022, date last accessed).
- 25. Mao L, Jin H, Wang M et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castro LA, Shelley CD, Osthus D et al. How New Mexico leveraged a COVID-19 case forecasting model to preemptively address the health care needs of the state: quantitative analysis. JMIR Public Health Surveill 2021;7:e27888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Z, Min J, Bian J et al. Risk of health morbidity for the uninsured: 10-year evidence from a large hospital center in Boston, Massachusetts. Int J Qual Health Care 2019;31:325–30. [DOI] [PubMed] [Google Scholar]
- 28. Covid-19 Coverage Assistance Fund . Official web site of the U.S. Health Resources & Services Administration. Published March 17 2022. https://www.hrsa.gov/covid19-coverage-assistance?msclkid=8933a06fa97a11ecaf15b8d3178db055 (21 March 2022, date last accessed).
- 29. Hwang SW, Weaver J, Aubry T et al. Hospital costs and length of stay among homeless patients admitted to medical, surgical, and psychiatric services. Med Care 2011;49:350–4. [DOI] [PubMed] [Google Scholar]
- 30. Hsu YT, Lan FY, Wei CF et al. Comparison of COVID-19 mitigation and decompression strategies among homeless shelters: a prospective cohort study. Ann Epidemiol 2021;64:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. RECOVERY Collaborative Group, Horby P, Lim WS et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohl ME, Miller DR, Lund BC et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw Open. 2021;4:e2114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are available on reasonable request from the corresponding author (A.W.B.). The data from the MGH COVID Registry are not publicly available because of confidentiality concerns around patient information.


