Abstract
Nirmatrelvir/ritonavir (N/R) is one of the most effective antiviral drugs against SARS-CoV-2. The preclinical development, pharmacodynamics and pharmacokinetics of N/R are reviewed herein. Randomized clinical trials have been conducted exclusively with pre-Omicron variants of concern, but in vitro studies show that efficacy against all Omicron sublineages is preserved, as confirmed by post-marketing observational studies. Nevertheless, investigations of large viral genome repositories have shown that mutation in the main protease causing resistance to N/R are increasingly frequent. In addition, virological and clinical rebounds after N/R discontinuation have been reported in immunocompetent patients. This finding is of concern when translated to immunocompromised patients, in whom N/R efficacy has not been formally investigated in clinical trials. Economical sustainability and perspectives for this therapeutic arena are discussed.
Keywords: COVID-19, small-chemical antivirals, main protease, protease inhibitors, nirmatrelvir, ritonavir, Paxlovid®, Bexovid®, PF-00835231, PF-07321332
Introduction
Oral small-molecule antivirals against SARS-CoV-2 have been approved worldwide. These drugs simplify the management of infection and reduce hospitalization rates in patients with COVID-19 who are at risk for disease progression.
One class of small-molecule antivirals targets the SARS-CoV-2 main polyprotein protease (MPro), which is often referred to as 3C-like protease (3CLpro) or nonstructural protein 5 (Nsp5). MPro is a chymotrypsin-like cysteine protease, the catalytic site of which consists of H41 and C145 residues [1]. Homologous enzymes are found in most positive-sense, single-stranded RNA viruses [2]. Proteases have an indispensable role in the life cycle of a virus and this, combined with a high degree of conservation, renders Mpro an ideal target for drugs [3]. In this narrative review, the preclinical and clinical development, pharmacokinetics, and pharmacodynamics of N/R are discussed, with a focus on efficacy against the Omicron variant of concern (VOC). Also considered are two emerging phenomena, rebounds and resistance, as well as pharmacoeconomics and perspectives for MPro inhibitors.
Methods
On December 1, 2022, PubMed, medRxiv, bioRxiv, and ResearchSquare repositories were searched for English language manuscripts published after December 1, 2019 using the following queries: “nirmatrelvir AND resistance”, “nirmatrelvir AND (rebound OR relapse)”, “nirmatrelvir AND mutations”. Case reports, case series, and clinical trials were included; secondary research was excluded. Search results were manually assessed for relevance, and references in each suitable manuscript were further screened for additional sources.
Preclinical and clinical development
During the SARS outbreak in 2002, Pfizer launched a research program that led to an intravenous ketone-based covalent Mpro cysteine protease inhibitor, PF-00835231. As expected from the 96% overall homology and 100% catalytic domain homology between SARS-CoV-1 and SARS-CoV-2 MPro, PF-00835231 was effective against SARS-CoV-2 in vitro [3]. During the COVID-19 pandemic, Pfizer developed the phosphate prodrug, nirmatrelvir (PF-07321332), to be taken orally. A phase 1 clinical trial in healthy subjects (NCT04756531) investigated nirmatrelvir as either a single agent or in combination with the pharmacokinetic enhancer (CYP3A4 inhibitor), ritonavir. The combination (N/R) resulted in higher and prolonged nirmatrelvir serum levels. Consequently, Pfizer used N/R in the Evaluation of Protease Inhibition for COVID-19 (EPIC) series of clinical trials. In the phase 2/3 EPIC high-risk (EPIC-HR) randomized controlled trial (RCT), 2246 unvaccinated outpatients with COVID-19 at high risk of progression were treated within 3 days of symptom onset (NCT04960202). At the time, the predominant circulating lineage was the Delta VOC. Patients were randomized according to the protocol to receive N/R as 3 tablets (2 × 150 mg tablets of nirmatrelvir and 1 × 100 mg tablet of ritonavir), or placebo twice daily for 5 days. A total of 1120 patients were randomized to receive N/R and 1126 to receive placebo (twice daily for 5 days). On November 5, 2021, Pfizer announced the results of an interim analysis of the first 774 treated patients, showing that hospitalization was 0.77% (3 of 389) in the N/R arm and 7% in the placebo arm, which included 7 deaths by day 28 [4]. The final data were announced on December 14, 2021 [5] and published in NEJM on February 16, 2022: hospitalization rates through day 28 were 0.72% (5 of 697) in the N/R arm vs. 6.45% (44 of 682) with 9 deaths in the placebo arm by day 28. A similar ratio (0.77% vs 6.31%) was seen in those treated within 5 days (88.9% reduction in the relative risk of hospitalization: -5.81%) [6]. In what may be the fastest drug development project in modern pharmacology [7], Pfizer applied to the US Food and Drug Administration (FDA) for emergency use authorization (EUA) of nirmatrelvir tablets co-packaged with tablets of ritonavir (Paxlovid®/Bexovid®) on November 16, 2021, 11 days after the interim analysis results were made public. The treatment was indicated for use in COVID-19 outpatients aged over 12 years, and weighing more than 40 kg [8]. The EUA was granted on December 22, 2021 [9]. Regulatory agencies in other countries quickly followed suit, with N/R authorizations occurring after a further 4 days in Israel [10], 9 days in the UK [11], 35 days in Europe [12], and 50 days in China [13]. As of December 2022, N/R has been authorized in more than 50 countries [14].
On March 9, 2022, Pfizer initiated EPIC-PEDS, a phase 2/3 trial in 140 children aged 6-18 years that compared 300 mg vs. 150 mg nirmatrelvir within the N/R formulation. Pfizer is also working to develop a body weight-adjusted formulation in 3 additional cohorts below the age of 6 years [14].
In September 2021, Pfizer began the phase 2/3 study, EPIC-PEP (Post-Exposure Prophylaxis; NCT05047601) to evaluate the efficacy and safety of N/R in adult household contacts of COVID-19 patients within 3 days of exposure. On April 29, 2022, the company reported that the 5- or 10-day courses led to statistically nonsignificant reductions in infection of 32% and 37%, respectively [15].
In August 2021, Pfizer initiated the phase 2/3 trial, EPIC in Standard-Risk Patients (EPIC-SR; NCT05011513). On December 14, 2021, the company disclosed that the primary endpoint of symptom amelioration for 4 consecutive days was not met in an interim analysis of 954 patients. A 70% relative risk reduction in hospitalization or death (treatment: 3/428; placebo: 10/426) was reported, but this was not statistically significant [5]. Results from an updated analysis of 1153 patients, reported on June 14, 2022, showed a 51% relative risk reduction of hospitalization (treatment: 5/576; placebo: 10/569), but again this was not statistically significant. The trial was halted by the company [16]. On June 30, 2022, Pfizer announced the submission of a New Drug Application (NDA) to the FDA for high-risk patients aged over 12 years and weighing more than 40 kg [17].
Another emerging off-label usage for N/R is the treatment of post-acute sequelae of SARS-CoV-2 (PASC), a multifaceted entity colloquially called ‘long COVID’ [18,19] and sometimes related to persistent infection [20,21].
Pharmacokinetic interactions and dose adjustments
Pharmacokinetic interactions with N/R were reviewed recently [22,23]. Ritonavir substantially elevates blood levels of co-administered CYP3A-dependent drugs, with increases in area-under-the-curve (AUC) blood concentrations ranging from 1.8- to 20-fold. Consequently, N/R is contraindicated in patients receiving drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious reactions, including amiodarone, flecainide, propafenone, quinidine, colchicine, clozapine, lovastatin, simvastatin, sildenafil, triazolam, and midazolam. N/R is also contraindicated in patients treated with drugs that are potent CYP3A inducers, such as carbamazepine, phenobarbital, phenytoin, and rifampin, which lead to reduced N/R plasma concentrations. In a large US study, the cumulative prevalence of these contraindications among hospitalized patients was estimated to be 14.6%, with higher rates in men vs. women (18% vs. 11.3%), in older patients vs. younger patients (26.9% vs. 8.8%), and in those with comorbidities vs. those without comorbidities (>37% vs. 3.9%); notably, cumulative prevalence was 50.7% among those who died [24].
N/R should also be avoided in organ transplant patients receiving medications to prevent rejection, such as tacrolimus, cyclosporin, sirolimus and everolimus, unless blood levels of those drugs can be followed closely. This is particularly challenging for solid organ transplant recipients with COVID-19 who are on such drugs. These patients are at increased risk for complications of COVID-19 but frequent blood testing to measure drug levels during isolation is logistically difficult. An N/R dose reduction strategy to minimize drug-drug interactions has been studied. The manufacturer recommends dose adjustment to 150 mg nirmatrelvir and 100 mg ritonavir twice daily for 5 days for patients with moderate renal impairment (estimated glomerular filtration rate [eGFR] ≥30 to <60 mL/min). Lingscheld et al. administered N/R 150/100 mg twice daily to 4 patients with end-stage renal disease under hemodialysis and showed high nirmatrelvir blood concentrations that were still within the range known from patients without renal failure; no accumulation took place and levels declined to zero within a few days after the end of treatment [25]. Although not formally recommended, a dose of 300 mg nirmatrelvir (with 100 mg ritonavir) daily and after hemodialysis on dialysis days, is anticipated to provide effective blood concentrations for enzyme inhibition [26]. Brown et al. administered such a modified 5-day N/R regimen to 15 patients with COVID-19 and the treatment was well tolerated and effective, with only 1 patient manifesting rebound symptoms, which resolved in 2 days [27].
Efficacy against Omicron in vitro
All the commercially available anti-spike monoclonal antibodies have lost activity against recent Omicron sublineages [28], [29], [30]. In contrast, all the authorized small-molecule antivirals have thus far retained efficacy against the various Omicron sublineages. The common Mpro mutations in Omicron (P132H) do not affect the catalytic site of nirmatrelvir [31], and this drug has retained in vitro efficacy against the following VOC Omicron sublineages: BA.1 [31], [32], [33], [34], [35], [36], [37], [38], BA.1.1 [32], BA.2 [32,36,39], BA.2.12.1 [32], BA.4 [32], BA.5 [32], BA.2.75 [40], BQ.1.1 and XBB [29] (<2-fold increases in IC50).
Efficacy against Omicron in clinical trials
In addition to in vitro data, nirmatrelvir can restrict viral infection in the respiratory organs of hamsters infected with BA.2 [41]. However, a pharmacokinetic human-equivalent dose of N/R did not significantly reduce shed SARS-CoV-2 titers in ferrets and failed to block virus transmission to untreated direct-contact ferrets, whereas transmission was fully suppressed in a group of animals treated with a human-equivalent dose of molnupiravir. Prophylactic administration of molnupiravir to uninfected ferrets in direct contact with infected animals blocked productive SARS-CoV-2 transmission, whereas all contacts treated with prophylactic N/R became infected [42].
Hence, clinical data are needed. In a study of eligible Clalit Health Services patients, among the 42 819 aged over 65 years, the 2504 who were treated with N/R during the Omicron wave had 72% fewer hospitalizations and 81% less mortality, but no benefit was seen in the 40-65 years age group [43].
Considering the EPIC-SR RCT was halted, it is unlikely that any further RCT evidence will be forthcoming during the Omicron wave; therefore, any new information will have to come from cohort studies, which have intrinsic biases.
Among 1 072 004 non-hospitalized COVID-19 patients in Hong Kong during the BA.2.2 wave (March-April 2022), the 5663 who received N/R had a lower risk of mortality (hazard ratio [HR] 0.25) and hospitalization (-31%, HR 0.69) than those who did not receive N/R, regardless of vaccination status and age (dichotomized at 65 years) [44].
Similarly, among 6036 patients (87% vaccinated) prescribed N/R in Massachusetts and New Hampshire during the Omicron wave (Jan-May 2022), the overall risk of hospitalization within 14 days (<1% following an outpatient diagnosis) was 45% lower compared with in the 24 286 patients who did not take the protease inhibitor [45].
In a retrospective cohort of 5287 patients in the Kaiser Permanente Southern California (KPSC) healthcare network who received prescriptions for N/R from December 31, 2021 to May 26, 2022, 6 (0.11%) patients were hospitalized for symptoms consistent with COVID-19 during the 5–15 days after treatment was dispensed. All hospitalized patients were in groups at high risk for severe COVID-19 and 2 died [46].
In a retrospective cohort of 111, mostly vaccinated, patients in Italy treated with N/R between February and June 2022 (BA.1 and BA.2 waves), Gentile et al. reported only 1 (0.9%) hospitalization [47].
A propensity score-matched (PSM) study from the US-Optum dataset during the period December 22, 2021 to June 8, 2022 showed that the incidence of hospitalization within 30 days was 1.21% for 2808 patients in the N/R group and 6.94% for 10 849 patients in the non-N/R group, with an HR of 0.16 (84% relative risk reduction) [48].
In another PSM study from the USA at the time of BA.2/BA.2.12.1, 3614 N/R patients vs. 4835 untreated patients had lower all-cause hospitalization (0.9% vs. 1.3%), COVID-19-related hospitalization (adjusted odds ratio [aOR]: 0.42), 28-day all-cause mortality (aOR: 0.05), and 28-day emergency room (ER) visits (3.9% vs 4.2%) [49].
Schwartz et al. showed that among 8876 outpatients treated with N/R in Ontario, hospitalization or death within 30 days was lower compared with that in unexposed individuals (2.1% vs 3.7%). In the secondary analysis, the relative odds of death were significantly reduced (1.6% vs 3.3%). The number of patients that needed to be treated to prevent one case of severe COVID-19 was 62. Findings were similar across strata of age, vaccination status, and comorbidities [50].
Compared with untreated matched controls, 1587 N/R-treated patients in the Veterans Health Administration (VHA) had a lower 30-day risk of hospitalization (27.10/1000 vs. 41.06/1000, risk difference [RD] -13.97) and death (3.15/1000 vs. 14.86/1000, RD -11.71). Among individuals who were alive at day 31, there were no further significant reductions in 31-180-day incidence of hospitalization (sub-HR 1.07) or death (HR 0.61). A statistically significant difference in 30-day or 31-180-day risk of hospitalization or death was not observed between matched N/R- or molnupiravir-treated participants. Incidence of most post-COVID conditions was similar across groups [51].
Hospitalization is not the only efficacy endpoint. Among 9217 outpatients in the healthcare databases of the US Department of Veterans Affairs, treatment with N/R in March-June 2022 was associated with reduced risk of PASC (HR 0.74, absolute risk reduction [ARR] 2.32) compared with control, including reduced risk of 10 of 12 post-acute sequelae in the cardiovascular system (dysrhythmia and ischemic heart disease), coagulation and hematologic disorders (deep vein thrombosis, and pulmonary embolism), fatigue, liver disease, acute kidney disease, muscle pain, neurocognitive impairment, and shortness of breath. N/R was also associated with reduced risk of post-acute death (HR 0.52, ARR 0.28), and post-acute hospitalization (HR 0.70, ARR 1.09). N/R was associated with reduced risk of PASC in people who were unvaccinated, vaccinated, and boosted, and in people with primary SARS-CoV-2 infection and reinfection [50].
Resistance
As with any other antiviral, resistance to nirmatrelvir can be either basal or treatment-emergent.
Mutations in Mpro causing resistance to nirmatrelvir are already found in circulating SARS-CoV-2 viruses (Table 1 and Fig. 1 ). For example, in May 2022, the M49I mutation was found in 1883 genomes, with a slight uptick in late 2021 [52], while 6 different types of mutations at position 191 (nt 10625-10627) were found in 9262 sequences (https://coronavirus3d.org/#/drug). Hu et al. identified in GISAID sequences 66 prevalent Mpro mutations located at the nirmatrelvir binding site, 11 of which (including S144M/F/A/G/Y, M165T, E166Q, H172Q/F, and Q192T/S/V) showed <10-fold change in enzymatic activity and resistance to nirmatrelvir (K i > 10-fold increase) [53]. Sasi et al. identified 5 mutations (N142L, E166M, Q189E, Q189I, and Q192T) that reduce the potency of nirmatrelvir: in particular, the IC50 of nirmatrelvir was reduced by 24-fold against E166M [54]. Dias Noske et al. reported that N/R retained most of its in vitro activity against most of the 14 naturally occurring polymorphisms close to the binding site, with only G143S and Q189K linked to higher resistance. Of interest, ensitrelvir had a different resistance profile, driven by M49I, G143S and R188S, but not for Q189K [55]. Phylogenetic analyses indicate that nirmatrelvir-resistant variants pre-existed the introduction of nirmatrelvir into the human population and are transmissible [56].
Table 1.
Summary of main MPro variants associated with N/R resistance reported to date, with prevalence estimate in the GISAID databank.
| Variant [lineage] |
Variant grouping |
Ref |
Domain location |
Subsite location (as defined by [94]) |
Total mutational counts (specific mutation; total at that position) [via GISAID CoVsurver: https://www.gisaid.org/epiflu-applications/covsurver-mutations-app/] |
Effect of variant on proteolytic activity |
Nirmatrelvir inhibition |
|---|---|---|---|---|---|---|---|
| High frequency potential resistance mutations predicted by Coronavirus3D. Residues of interest are identified based on atomic distance from nirmatrelvir inhibitor and computational predictions of ligand-protein interactions. The emergence and dynamics of mutations at these positions observed in circulating virus are tracked via data from GISAID. [52] (https://coronavirus3d.org/) | |||||||
| M49I | T | [52] | D1 | S2 | 1898; 2062 | n.a. | n.a. |
| V186F | P | [52] | SBL | none | 1966; 3211 | n.a. | n.a. |
| R188K | P | [52] | SBL | S2 | 243; 408 | n.a. | n.a. |
| T190I | P | [52] | SBL | close to S4 | 1868; 1945 | n.a. | n.a. |
| A191V | P | [52] | SBL | close to S4 | 8492; 9320 | n.a. | n.a. |
| Experimentally characterized Mpro variants | |||||||
| G15S | |||||||
| [C.37 Lambda] | T | [53] | D1 | outside of binding site | 27289; 29511 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| [95] | kcat/Km = 16,500 S−1 M−1 (1.9-fold decrease); Crystal structure shows binding mode and Mpro conformation is not altered compared to WT complex | Ki = 4.07 nM* (4.3-fold increase, P = 0.0002*) | |||||
| [96] | kcat/Km = 15,000 S−1 M−1 (1.08-fold decrease) | Ki =10.3 nM (1.05-fold decrease) | |||||
| T21I [B.1.1.318] | T | [53] | D1 | outside of binding site | 15618;15890 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| [96] | kcat/Km = 10,000 S−1 M−1 (1.58-fold decrease) | IC50 =10.1 nM (1.11-fold increase) | |||||
| [59] | EC50 1.4-fold increase | ||||||
| H41M/T/Y | IA | [53] | D1 | S1 pocket/ catalytic residue | 84/26/19; 378 | Enzymatically inactive (H41 forms catalytic dyad with C145) | Inactive Mpro variant, unlikely to be tolerated in circulating variants |
| M49I/T/L/V | T | [53] | D1 | S2 pocket | 2059/78/71/54; 2346 | kcat/Km of M49I and M49L showed 1.69 and 1.74-fold increase | All remained sensitive to nirmatrelvir (< 3-fold change in IC50 value) |
| L50F | T | [59] | D1 | Close to S2 | 4532; 4821 | Interferes with dimerization (Kd = 2287 nM); 95.5% reduction in protease activity | 1.4-fold EC50 increase |
| L89F [B.1.2] | T | [53] | D1 | Outside of binding site | 153727; 153995 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| [96] | kcat/Km = 12,000 S−1 M−1 (1.33-fold decrease) | IC50 =10.5 nM (1.06-fold decrease) | |||||
| K90R [B.1.351 Beta] | T | [53] | D1 | Outside of binding site | 175764; 176349 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| [96] | kcat/Km = 9,000 S−1 M−1 (1.79-fold decrease) | IC50 = 12.7 nM (1.13-fold increase) | |||||
| [95] | kcat/Km = 28,300 S−1 M−1; (1.1-fold decrease); Crystal structure shows binding mode and Mpro conformation is not altered compared to WT complex | Ki = 1.05 nM (1.1-fold increase, n.s.) | |||||
| P108S [B.1.1.284] | T | [53,97] | D2 | Outside of binding site | 25774; 30707 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| P132H [B.1.1.529 Omicron] | T | [53] | D2 | Outside of binding site | 4255443; 4274244 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| [95] | kcat/Km = 20,800 S−1 M−11; (1.5-fold decrease); Crystal structure shows binding mode and Mpro conformation is not altered compared to WT complex | Ki = 0.635 nM (1.4-fold decrease, n.s.) | |||||
| [96] | kcat/Km = 23,000 S−1 M−11 (1.44-fold increase) | IC50 =12.2 nM (1.09-fold increase) | |||||
| [98] | kcat/Km =10,000 S−1 M−1 (1.1-fold increase); Tm=53.6 C, 2.6 C lower than WT | IC50= 32nM (1.23-fold increase) | |||||
| T135I | T | [53] | D2 | Outside of binding site | 1325; 1481 | Similar kcat/Km value | Remained sensitive inhibition (<2.9-fold change in Ki values |
| N142X | T | [53] | D2 | Close to S1 | 431 | All have similar enzymatic activity as the WT (<4.1-fold change in kcat/Km value) | All remained sensitive to nirmatrelvir (<3.5-fold change in IC50 values) |
| S144M/F/AG/Y | R | [53] | D2 | S1 pocket (forms oxyanion hole) | 15/14/9/2/2; 235 | kcat/Km values are comparable to WT (from 2.8 to 8.0-fold) | Ki increase 19.2-38.0-fold |
| H163W | IA | [53] | D2 | S1 pocket | 4656; 5032 | Enzymatically inactive, hydrogen bond with P1 is critical for substrate binding | Inactive Mpro variant, unlikely to be tolerated in circulating variants |
| H164N | T | [53] | D2 | S1 pocket (via hydrogen bond from main chain carboxyl) | 4664; 5031 | 4.2-fold lower kcat/Km value; is comparable to WT | Remained sensitive (<4.1-fold change in Ki values) |
| M165T | R | [53] | D2 | S2 | 7; 5180 | 8.3-fold decrease in kcat/Km value | Ki = 56.3 nM (29.9-fold increase) |
| E166Q/A/V | R | [53] | D2 | S1 | 4665; 5084 | E166Q: Same enzymatic activity as WT | E166Q: Ki= 22.0 nM (11.7-fold increase) |
| [59] | Interferes with dimerization (Kd = 235 nM); 86.4% reduction in protease activity | E166A: Ki= 230 nM (10-fold increase) | |||||
| [58] | n.a. | E166V: 267-fold increase in EC50 | |||||
| [57] | n.a. | E166V: EC50 = 14.08 uM (265-fold increase) | |||||
| L50F/E166V | R | [58] | n.a. | n.a. | n.a. | n.a. | 80-fold increase in EC50 |
| L167F | T | [59] | D2 | Close to S4 | 18; 442 | Interferes with dimerization (Kd = 127 nM); 83.6% reduction in protease activity | IC50 = 100 nM (4.4-fold change) |
| L50F/E166A/L167F | R | [59] | D1/D2/D2 | n.a. | n.a. | Interferes with dimerization (Kd = 966 nM); 94.7% reduction in protease activity | IC50 = 1600 nM (72-fold change) |
| P168S | [57] | D2 | None | 456; 778 | P168R: Scored as inactive | ||
| H172Q/F | R | [53] | D2 | Close to S1 | 12/5; 260 | H172Q: kcat/Km = 3.2-fold lower; H172F: kcat/Km = 9.9-fold lower H172Y: kcat/Km = 790 M-1S-1 (13.9-fold decrease) | Ki increase by more than 10-fold H172Y: Ki =275 nM (146.3-fold increase) |
| Q189X | T | [53] | SBL | Close to S2 | 1436 | All retained similar kcat/Km values (between 1.9- and 9.2-fold) Q189E: 20669 S−1 M−1 (1.88-fold increase) | No significant resistance for any variants (<3.1-fold change in IC50) |
| H172Y/Q189E | R | [53,99] | D2/SBL | n.a. | n.a | kcat/Km = 1009 S−1 M−1 (10.9-fold decrease) | Ki = 528 nM (281.1-fold increase) |
| Q192T/S/V | R | [53] | SBL | S4 | 181/27/6; 1462 | Q192T: kcat/Km = 9.2-fold lower; Q192S: kcat/Km = 8.9-fold lower; Q192V: kcat/Km = 9.0-fold lower | All showed inhibition resistance (Ki increase >22.2-fold) |
| L205V [P.2 Zeta] | T | [53] | D3 | Outside of binding site | 6013; 6256 | Activity similar to WT | no significant IC50 or Ki value shifts observed (< 2-fold) |
| [96] | kcat/Km =15 000 S−1 M−1 (1.08-fold decrease) | IC50 = 10.7 nM (1.05-fold decrease) | |||||
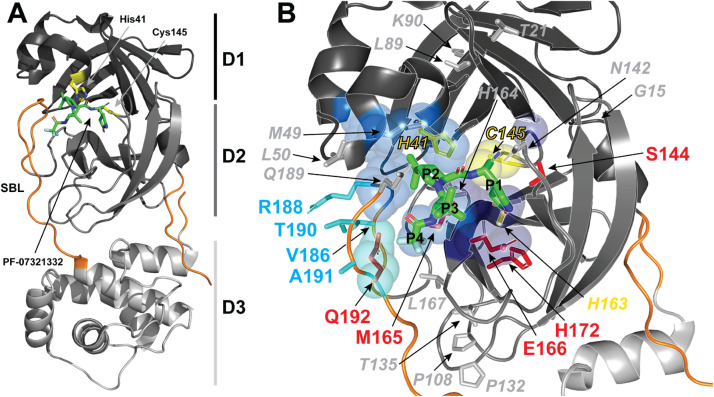
Fig. 1.
The structure of SARS-CoV-2 Mpro in complex with the PF-07321332 inhibitor (PBD 7VH8). A) The Mpro monomer is displayed in cartoon representation to illustrate the domain organization of the protease. Domain I-III (residues 10-99, 100-184, and 201-303, respectively) are colored black, dark grey and light grey, respectively. The substrate binding loop (SBL), amino- and carboxy-termini are colored orange, and PF-07321332 is displayed as green sticks. The catalytic triad (Cys145 and His41) is displayed as yellow sticks. B) Positions of frequent amino acid substitution in Mpro variants are mapped to a detailed view of the contacts between PF-07321332 and Mpro at the interface between Domain I and II. Substrate residue numbers are indicated in black and viral protease amino acids involved in each subsite are displayed as spheres, with S1, S2 and S4 subsites colored dark blue, marine blue and cyan, respectively. Amino acid positions with quantified resistance to nirmatrelvir inhibition, positions that can tolerate multiple mutations without affecting activity or inhibitor binding, positions that yield inactive Mpro, and positions with common variations that are predicted to impart nirmatrelvir resistance are displayed as sticks and colored red, grey, yellow, and cyan, respectively.
The widespread use of N/R could make even low probability events more likely. N/R-resistant variants selected in vitro harbored different sets of Mpro mutations, with the L50F and E166V combination driving an 80-fold increase in EC50 [57]. Of interest, the N/R-resistant variants have high fitness but, as expected, remain sensitive to remdesivir [58]. The L50F + E166A + L167F combination is instead associated with a >20-fold increase in EC50 values for nirmatrelvir [59]. In vitro passaging of SARS-CoV-2 in increasing concentrations of nirmatrelvir generated mutants harboring T21I, P252L, or T304I mutations in Mpro: E166V mutation lead to ∼300-fold reduction in nirmatrelvir activity, but resulted in a loss of viral replicative fitness compensated by L50F and T21I [60].
Although the short (5-days) treatment course reduces selective pressure for N/R resistance, widespread deployment as a monotherapy, including to immunosuppressed patients who could fail to clear the virus after the 5-day schedule, increases the probability of resistance emergence.
Relapses
Since April 2022, there have been multiple reports of Omicron-infected patients experiencing virological and clinical relapse within days after completion of the 5-day N/R regimen. A search of PubMed, ResearchSquare preprint server and Google News performed on August 3, 2022 retrieved 32 cases [61], [62], [63], [64], [65], [66], [67], the features of which are summarized in Supplementary Table 1. Most patients were vaccinated, and no patient died, but 6/31 (5%) required hospitalization. A few authors reported the isolation of infectious SARS-CoV-2 [63,64,66] and transmission during the rebounds [61]. Accordingly, the CDC recommended wearing a mask for 10 days in case of rebound [68]. All relapses resolved without additional antiviral treatment (with a single exception in the Chief Medical Advisor to the President of the United States [69]) within a couple of weeks. Sequencing in many patients indicated that relapse was not due to a treatment-emergent mutation or infection with a different viral strain [64].
It was soon realized that in the protocol of the RCT that led to drug approval, nasopharyngeal or nasal swabs were collected on day 1 (baseline) and days 3, 5, 10, and 14, but the final publication only reported outcomes on day 5 [6]. A look back at the Center for Drug Evaluation and Research (CDER) review identified that “Several subjects appeared to have a rebound in SARS-CoV-2 RNA levels around Day 10 or Day 14 [i.e. days 13 and 17 since onset of first symptoms], although this occurred among subjects with or without potential resistance-associated substitutions detected at Day 1 or Day 5”, but clinical symptoms were not reported, and the subset with available samples was very small [70]. In response to FDA inquiries, the EPIC-HR investigators conducted real-time polymerase chain reaction (PCR) and next-generation sequencing at days 10 and 14. The proportion of present/persistent rebounds was 1.73% (17/980) vs. 2.32% (23/990) and that of transient rebounds was 2.35% (23/980) vs. 4.65% (46/990) in placebo vs. N/R participants, respectively. The authors concluded that this was not associated with low nirmatrelvir exposure, hospitalization or death, severe symptom relapse, serological status, or Mpro gene treatment-emergent mutations [71]. Accordingly, in the placebo arm of the ACTIV-2/A5401 trial, 12% of participants had viral rebound (viral rebounders being older than non-rebounders), symptom rebound occurred in 27% of participants after initial symptom improvement and in 10% of participants after initial symptom resolution, and the combination of high-level viral rebound to ≥5.0 log10 RNA copies/mL and symptom rebound after initial improvement was observed in 1-2% of participants [72].
Explanations proposed so far for the rebound phenomenon include the shortness of the schedule, insufficient dosing in obese patients, pharmacokinetic interactions with concurrent medications lowering plasma levels of nirmatrelvir, and/or failure of the drug to eradicate the virus from as yet unidentified drug-inaccessible sanctuary tissues [73]. Fumagalli et al. used a mouse model of SARS-CoV-2 infection and showed that nirmatrelvir administration soon after infection blunts the development of SARS-CoV-2-specific antibody and T cell responses. Accordingly, upon secondary challenge, nirmatrelvir-treated mice recruited significantly fewer memory T and B cells to the infected lungs and to mediastinal lymph nodes, respectively [74].
Although the efficacy of N/R at preventing hospitalization [6] is clear, it is essential to establish the exact frequency of relapses (regardless of mechanism) with the currently circulating Omicron sublineages in fully vaccinated and boosted subjects. In a cohort of 483 high-risk COVID-19 patients treated with N/R, 2 patients (0.4%) required hospitalization by day 30; 4 (0.8%) experienced mild clinical relapses at a median of 9 days after treatment, and all resolved without additional COVID-19-directed therapy, but virological monitoring was not performed [75]. In a study of 11 270 patients treated with N/R and 2374 patients treated with molnupiravir in the USA, the 7-day and 30-day COVID-19 rebound rates after N/R treatment were 3.53% and 5.40% for COVID-19 infection, 2.31% and 5.87% for COVID-19 symptoms, and 0.44% and 0.77% for hospitalizations, respectively. The 7-day and 30-day COVID-19 rebound rates after molnupiravir treatment were 5.86% and 8.59% for COVID-19 infection, 3.75% and 8.21% for COVID-19 symptoms, and 0.84% and 1.39% for hospitalizations, respectively. This clearly shows the phenomenon is shared across antivirals. Patients with COVID-19 rebound had a significantly higher prevalence of underlying medical conditions than those without [76]. Hence, prospective observational studies reporting higher incidence of viral rebounds in 3-dose mRNA-vaccinated N/R-treated compared with N/R-untreated cohorts that are not PSM (e.g., in BA.2 [77]) have limited meaning.
In the largest dataset of untreated mRNA-vaccinated individuals infected with the Omicron variant, viral rebound (defined with PCR monitoring) occurred in 6% of 494 infections [78]. In a large, retrospective cohort study, the risks of both COVID-19 rebound infections and symptoms 2-8 days after N/R treatment were higher in the BA.5 cohort than in the PSM BA.2.12.1 cohort (HR 1.32) [79]. In the absence of biological rationales, a notoriety bias may explain this finding.
In a prospective cohort study based on 12 sequential quick antigen assays over 16 days conducted between August 4, 2022 and November 1, 2022 in California, viral rebound incidence was 14.2% in the N/R group (18/127) and 9.3% in the control group (4/43). The incidence of COVID-19 symptom rebound was higher in the N/R group (18.9%) than in the control group (7.0%) [80].
Considering the aforementioned findings, there is an urgent need to establish whether prolonging antiviral therapy can prevent the rebound phenomenon. Of interest, on June 30, 2022, Pfizer launched a triple-blind, phase 2 study (NCT05438602) in which immunocompromised patients were to be randomized to treatment with N/R for 5, 10 or 15 days and followed-up for 24 weeks.
Pharmacoeconomics
In the USA, a 5-day N/R course costs USD 529 (£410; €490) according to the independent US non-profit Institute for Clinical and Economic Review (ICER) [81]. This is estimated to correspond to an expenditure of USD 21 000 per hospital admission averted. By contrast, the per-patient hospitalization cost in the USA for COVID-19 is estimated at USD 24 826, without taking into consideration personal and societal costs [82]. In the post-vaccine Omicron era, the cost-benefit further worsened: the ARR dropped from 5.8% (in the original RCT that led to authorization) to 1.8% [43], causing an increase in the number needed to treat to prevent a single hospitalization from 19 to 56 patients. In other words, about 35 000 USD have to be spent to prevent a single hospital admission.
On March 15, 2022, the Drugs for Neglected Diseases initiative (DNDi) expressed concern, on behalf of a consortium of 26 African and global research bodies, that Pfizer would not provide access to N/R for testing in combination with other drugs at later disease stages as the company wanted to run those trials internally [83]. On March 17, the United Nations-backed Medicines Patent Pool (MPP) signed agreements with 35 manufacturers of generic drugs in Europe, Asia, and Central and South America to make N/R and supply it to 95 poorer countries.
Then, on March 22, 2022 Pfizer agreed with UNICEF to supply 4 million courses of treatment to 95 low- and middle-income countries, beginning in April 2022, pending authorization or approval [84]. Two days later, the Africa Centers for Disease Control and Prevention agreed to a memorandum of understanding with Pfizer to provide N/R for African countries [85]. However, availability in India of a locally made generic version of N/R (made by Hetero Labs and Optimus) has been delayed by a requirement of the country's Central Drugs Standard Control Organization for additional local clinical trials [86]. That situation remained unchanged as of June 14, 2022 [87]. An additional problem is the distribution of substandard and falsified medical products on the black market, of which inequality is a driving force [88].
Perspectives
The experience with monotherapy for HIV-1 and HCV shows that viral resistance may develop rapidly when using a single drug. Viral resistance that develops while on therapy has also been described for influenza virus infection, particularly in immunocompromised patients. The ability of SARS-CoV-2 to escape antiviral therapies has been amply demonstrated by the emergence of monoclonal-antibody-resistant variants in treated individuals [28], particularly those who are immunocompromised and cannot clear the infection. Given the historical precedents for resistance to other antiviral therapies, the fact that mutations associated with resistance to N/R are already found in circulating SARS-CoV-2 genomes, the enormous number of patients being treated, and the rebound phenomenon showing that not all SARS-CoV-2 virions are eradicated with 5 days of treatment, N/R resistance is likely to occur rapidly. One potentially effective strategy to reduce the likelihood of N/R resistance is to combine it with other small molecule antivirals or antibody-based therapies. However, considering that antibody-resistant SARS-CoV-2 variants emerged in immunocompromised individuals, who have reduced capacity to clear infection, perhaps this population should be targeted for combination therapy to reduce the probability that N/R-resistant variants emerge. Such individuals could be treated with a combination of other small-molecule antivirals (e.g., additive and synergic effects are expected when Mpro inhibitors are combined with RNA-dependent RNA polymerase [RdRp] inhibitors, such as remdesivir or molnupiravir [91,92]) or antibody-based therapies, which may increase the therapeutic benefit and preserve the efficacy of nirmatrelvir.
Novel oral Mpro inhibitors that do not require ritonavir boosting and are administered once daily, such as ensitrelvir/S-217622 (Xocova®, Shionogi) [93], are in advanced stages of development. These inhibitors would largely be free of pharmacokinetic interactions. The clinical pipeline also includes EDP-235 (Enanta) and PBI-0451 (Pardes Bio), whereas the preclinical pipeline includes boceprevir, STI-1558 (Sorrento), SH-879 (Sosei Heptares), EDDC-2214 (Everest Medicine), ASC-11 (Ascletis), GC376 (Anivive Lifesciences), and NLC-V-01 (Tollovir®, Todos Medical). Rupintrivir is selective for rhinoviruses but its derivative, Mpro-1, is also effective against SARS-CoV-2.
Conclusions
N/R preclinical development has been fast and furious and has contributed to alleviating the COVID-19 healthcare burden in 2022. This drug has proven to be remarkably effective, with preserved in vitro and clinical efficacy against Omicron. N/R has become the most prescribed antiviral in the world, generating $1.5 billion in sales in the first quarter of 2022. For example, in the USA more than 160 000 patients were prescribed this drug per week as of May 2022 [89], and in Italy, as of June 21, 2022, N/R had been prescribed to more than 17 000 unique patients [90]. Despite this, pharmacokinetic interactions are preventing deployment in frail and comorbid patients, which represent the residual burden of the pandemic. Furthermore, treatment-emergent resistance, early relapse, and economical sustainability represent clouds on the horizon that could undermine the long-term future of N/R. For such reasons, research on alternative MPro inhibitors should not be discontinued because of the success of N/R.
Acknowledgments
Funding
None.
Declaration of Competing Interests
A.C. is on the scientific advisory board of SAB Therapeutics and owns stock options. The other authors declare no conflict of interest related to this manuscript.
Ethical Approval
Not require.
Sequence Information
Not applicable.
Editor:Dr Jim Gray
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2022.106708.
Appendix. Supplementary materials
References
- 1.Dai W, Zhang B, Jiang XM, Su H, Li J, Zhao Y, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei J, Hilgenfeld R. RNA-virus proteases counteracting host innate immunity. FEBS Letters. 2017;591:3190–3210. doi: 10.1002/1873-3468.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 4.Pfizer's Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study. Accessed online at https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate on June 27, 2022.
- 5.Narasimhan M, Mahimainathan L, Araj E, Clark AE, Markantonis J, Green A, et al. Clinical evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays in infected, recovered, and vaccinated groups. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00388-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small Molecule of The Year, 2022. Accessed at https://drughunter.com/small-molecule-of-the-year-2021/?utm_source=rss&utm_medium=rss&utm_campaign=small-molecule-of-the-year-2021 on June 27, 2022.
- 8.Pfizer Seeks Emergency Use Authorization for Novel COVID-19 Oral Antiviral Candidate. Accessed at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-seeks-emergency-use-authorization-novel-covid-19 on June 27, 2022.
- 9.Pfizer Receives U.S. FDA Emergency Use Authorization for Novel COVID-19 Oral Antiviral Treatment. Accessed online at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-receives-us-fda-emergency-use-authorization-novel on June 27, 20022 2022.
- 10.Israel Authorizes Use of Pfizer Antiviral COVID Pill Paxlovid. Accessed online at https://www.haaretz.com/israel-news/israel-authorizes-use-of-pfizer-antiviral-covid-pill-paxlovid-1.10495082 on June 27, 2022.
- 11.Oral COVID-19 antiviral, Paxlovid, approved by UK regulator. Accessed online at https://www.gov.uk/government/news/oral-covid-19-antiviral-paxlovid-approved-by-uk-regulator on June 27, 2022.
- 12.COVID-19: EMA recommends conditional marketing authorisation for Paxlovid. Accessed online at https://www.ema.europa.eu/en/news/covid-19-ema-recommends-conditional-marketing-authorisation-paxlovid on June 27, 2022.
- 13.The State Food and Drug Administration approved Pfizer's new coronavirus treatment drug neimatevir tablet/ritonavir tablet combination package for import registration under emergency conditions [translated from Chinese]. Accessed online at https://www.nmpa.gov.cn/yaowen/ypjgyw/20220212085753142.html on June 27, 2022.
- 14.Pfizer Initiates Phase 2/3 Study of Novel COVID-19 Oral Treatment in Pediatric Participants. Accessed online at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-initiates-phase-23-study-novel-covid-19-oral on June 27, 2022.
- 15.Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use. Accessed at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-top-line-results-phase-23-epic-pep-study on June 27, 2022.
- 16.Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to U.S. FDA. Accessed at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting on June 27, 2022.
- 17.Pfizer Announces Submission of New Drug Application to the U.S. FDA for PAXLOVID™. Accessed online at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-submission-new-drug-application-us-fda on July 1, 2022.
- 18.Geng L, Bonilla H, Shafer R, Miglis M, Yang P. Case Report of Breakthrough Long COVID and the Use of Nirmatrelvir-Ritonavir. In: ResearchSquare, editor. 2022 [Google Scholar]
- 19.Peluso M, Anglin K, Durstenfeld MS, Martin J, Kelly J, Hsue P, et al. Effect of oral nirmatrelvir on Long COVID symptoms: 4 Cases and Rationale for Systematic Studies. Pathog Immun. 2022;7:95–103. doi: 10.20411/pai.v7i1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895. doi: 10.1016/j.cell.2022.01.014. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swank Z, Senussi Y, Alter G, Walt DR. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis. 2022:ciac722. doi: 10.1093/cid/ciac722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girardin F, Manuel O, Marzolini C, Buclin T. Evaluating the risk of drug-drug interactions with pharmacokinetic boosters: the case of ritonavir-enhanced nirmatrelvir to prevent severe COVID-19. Clin Microbiol Infect. 2022;28(8):1044–1046. doi: 10.1016/j.cmi.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heskin J, Pallett SJC, Mughal N, Davies GW, Moore LSP, Rayment M, et al. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet. 2022;399:21–22. doi: 10.1016/S0140-6736(21)02657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoertel N, Boulware DR, Sánchez-Rico M, Burgun A, Limosin F. Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.42140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lingscheid T, Kinzig M, Krüger A, Müller NB, Bölke G, Tober-Lau P, et al. Pharmacokinetics of nirmatrelvir and ritonavir in COVID-19 patients with end stage renal disease on intermittent haemodialysis. Antimicrob Agents Chemother. 2022;66 doi: 10.1128/aac.01229-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiremath S, McGuinty M, Argyropoulos C, Brimble KS, Brown P, Chagla Z, et al. Prescribing nirmatrelvir/ritonavir (Paxlovid) for COVID-19 in advanced CKD. Clin J Am Soc Nephrol. 2022;17(8):1247–1250. doi: 10.2215/CJN.05270522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown PA, McGuinty M, Argyropoulos C, Clark EG, Colantonio D, Giguere P, et al. Early experience with modified dose nirmatrelvir/ritonavir in dialysis patients with coronavirus. disease-2019. 2022 2022.05.18.22275234. [Google Scholar]
- 28.Focosi D, McConnell S, Casadevall A, Cappello E, Valdiserra G, Tuccori M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Infect Dis. 2022;22(11):e311–e326. doi: 10.1016/S1473-3099(22)00311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N Engl J Med. 2023;388(1):89–91. doi: 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2022 doi: 10.1016/j.cell.2022.12.018. S0092-8674(22)01531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greasley SE, Noell S, Plotnikova O, Ferre R, Liu W, Bolanos B, et al. Structural basis for the in vitro efficacy of nirmatrelvir against SARS-CoV-2 variants. J Biol Chem. 2022;298 doi: 10.1016/j.jbc.2022.101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387:468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabrowska A, Szczepanski A, Botwina P, Mazur-Panasiuk N, Jirincova H, Rabalski L, et al. Efficacy of antiviral drugs against the omicron variant of SARS-CoV-2. 2021 2021.12.21.473268. [Google Scholar]
- 34.Vangeel L, De Jonghe S, Maes P, Slechten B, Raymenants J, André E, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198 doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bojkova D, Widera M, Ciesek S, Wass MN, Michaelis M, Cinatl JN. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant SARS-CoV-2 isolates. Cell Res. 2022;32:319–321. doi: 10.1038/s41422-022-00619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi H, Hishiki T, Akazawa D, Kim KS, Woo J, Shionoya K, et al. Different efficacies of neutralizing antibodies and antiviral drugs on SARS-CoV-2 Omicron subvariants, BA.1 and BA.2. Antiviral Res Antiviral Res. 2022;205 doi: 10.1016/j.antiviral.2022.105372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai DK, Yurgelonis I, McMonagle P, Rothan HA, Hao L, Gribenko A, et al. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 Variants of Concern. 2022 2022.01.17.476644. [Google Scholar]
- 38.Rosales R, McGovern BL, Rodriguez ML, Rai DK, Cardin RD, Anderson AS, et al. Nirmatrelvir, Molnupiravir, and Remdesivir maintain potent in vitro activity against the SARS-CoV-2 Omicron variant. 2022 doi: 10.1016/j.antiviral.2024.105970. 2022.01.17.476685. [DOI] [PubMed] [Google Scholar]
- 39.Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron Subvariant BA.2. N Engl J Med. 2022;386:1475–1477. doi: 10.1056/NEJMc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito A, Tamura T, Zahradnik J, Deguchi S, Tabata K, Kimura I, et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2.75. Cell Host Microbe. 2022;30:1540–1555. doi: 10.1016/j.chom.2022.10.003. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uraki R, Kiso M, Iida S, Imai M, Takashita E, Kuroda M, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Nature. 2022;607:119–127. doi: 10.1038/s41586-022-04856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox R, Lieber CM, Wolf JD, Karimi A, Lieberman NAP, Sticher ZM, et al. Paxlovid-like nirmatrelvir/ritonavir fails to block SARS-CoV-2 transmission in ferrets. bioRxiv. 2022 2022.11.20.517271. [Google Scholar]
- 43.Arbel R, Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med. 2022;387(9):790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400:1213–1222. doi: 10.1016/S0140-6736(22)01586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dryden-Peterson S, Kim A, Kim AY, Caniglia EC, Lennes I, Patel R, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv 2022;2022.06.14.22276393.
- 46.Malden DE, Hong V, Lewin BJ, Ackerson BK, Lipsitch M, Lewnard JA, et al. Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment - California, December 2021-May 2022. MMWR. 2022;71:830–833. doi: 10.15585/mmwr.mm7125e2. [DOI] [PubMed] [Google Scholar]
- 47.Gentile I, Scotto R, Schiano Moriello N, Pinchera B, Villari R, Trucillo E, et al. Nirmatrelvir/ritonavir and molnuipiravir in the treatment of mild/moderate COVID-19: results of a real-life study. Vaccines. 2022;10:1731. doi: 10.3390/vaccines10101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X, Kelly SP, Liang C, Li L, Shen R, Leister-Tebbe H, et al. Real-World effectiveness of nirmatrelvir/ritonavir in preventing hospitalization among patients with COVID-19 at high risk for severe disease in the United States: A Nationwide Population-Based Cohort Study. 2022 2022.09.13.22279908. [Google Scholar]
- 49.Aggarwal NR, Molina K, Beaty L, Bennett TD, Carlson N, Ginde AA. Real-world use of nirmatrelvir-ritonavir in COVID-19 outpatients during the emergence of Omicron variants BA.2/BA2.12.1. 2022 2022.09.12.22279866. [Google Scholar]
- 50.Schwartz K.L., Wang J., Tadrous M., Langford B.J., Daneman N., Leung V., et al. Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada. 2022 doi: 10.1101/2022.11.03.22281881. [DOI] [Google Scholar]
- 51.Bajema KL, Berry K, Streja E, Rajeevan N, Li Y, Yan L, et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes. medRxiv. 2022 doi: 10.7326/M22-3565. 2022.12.05.22283134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sedova M, Jaroszewski L, Iyer M, Godzik A. Monitoring for SARS-CoV-2 drug resistance mutations in broad viral populations. 2022 2022.05.27.493798. [Google Scholar]
- 53.Hu Y, Lewandowski EM, Tan H, Morgan RT, Zhang X, Jacobs LM, et al. Naturally occurring mutations of SARS-CoV-2 main protease confer drug resistance to nirmatrelvir. 2022 doi: 10.1021/acscentsci.3c00538. 2022.06.28.497978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasi VM, Ullrich S, Ton J, Fry SE, Johansen-Leete J, Payne RJ, et al. Predicting antiviral resistance mutations in SARS-CoV-2 main protease with computational and experimental screening. Biochemistry. 2022;61:2495–2505. doi: 10.1021/acs.biochem.2c00489. [DOI] [PubMed] [Google Scholar]
- 55.Noske GD, Silva ES, Godoy MO, Dolci I, Fernandes RS, Guido RVC, et al. Structural basis of nirmatrelvir and ensitrelvir resistance profiles against SARS-CoV-2 Main Protease naturally occurring polymorphisms. 2022 2022.08.31.506107. [Google Scholar]
- 56.Moghadasi SA, Heilmann E, Moraes SN, Kearns FL, von Laer D, Amaro RE, et al. Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors. 2022 doi: 10.1126/sciadv.ade8778. 2022.08.07.503099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iketani S, Hong SJ, Sheng J, Bahari F, Culbertson B, Atanaki FF, et al. The functional landscape of SARS-CoV-2 3CL Protease. 2022 doi: 10.1016/j.chom.2022.08.003. 2022.06.23.497404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Gammeltoft KA, Ryberg LA, Pham LV, Fahnøe U, Binderup A, et al. Nirmatrelvir resistant SARS-CoV-2 variants with high fitness in vitro. 2022 doi: 10.1126/sciadv.add7197. 2022.06.06.494921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jochmans D, Liu C, Donckers K, Stoycheva A, Boland S, Stevens SK, et al. The substitutions L50F, E166A and L167F in SARS-CoV-2 3CLpro are selected by a protease inhibitor in vitro and confer resistance to nirmatrelvir. 2022 doi: 10.1128/mbio.02815-22. 2022.06.07.495116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iketani S, Mohri H, Culbertson B, Hong SJ, Duan Y, Luck MI, et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature. 2022 doi: 10.1038/s41586-022-05514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charness M, Gupta K, Stack G, Strymish J, Adams E, Lindy D, et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med. 2022;387:1045–1047. doi: 10.1056/NEJMc2206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonelli G, Focosi D, Turriziani O, Tuccori M, Brandi R, Fillo S, et al. Virological and clinical rebounds of COVID-19 soon after nirmatrelvir/ritonavir discontinuation. Clin Microb Infect. 2022;28:1657–1658. doi: 10.1016/j.cmi.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boucau J, Uddin R, Marino C, Regan J, Choudhary MC, Flynn JP, et al. Virologic characterization of symptom rebound following nirmatrelvir-ritonavir treatment for COVID-19. Clin Infect Dis. 2022:ciac512. doi: 10.1093/cid/ciac512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlin AF, Clark AE, Chaillon A, Garretson AF, Bray W, Porrachi M, et al. Virologic and immunologic characterization of COVID-19 recrudescence after nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2022:ciac496. doi: 10.1093/cid/ciac496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coulson JM, Adams A, Gray LA, Evans A. COVID-19 "Rebound" associated with nirmatrelvir/ritonavir pre-hospital therapy. J Infect. 2022;85(4):436–480. doi: 10.1016/j.jinf.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Epling BP, Rocco JM, Boswell KL, Laidlaw E, Galindo F, Kellogg A, et al. Clinical, virologic, and immunologic evaluation of symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment. Clin Infect Dis. 2022:ciac663. doi: 10.1093/cid/ciac663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alshanqeeti S, Bhargava A. COVID-19 rebound after Paxlovid treatment: A Case Series and Review of Literature Cureus. 2022;14:e26239. doi: 10.7759/cureus.26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.COVID-19 Rebound After Paxlovid Treatment. Accessed online at https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf on June 27, 2022.
- 69.The New York Times Fauci says he believes Paxlovid kept him out of the hospital, even though he tested positive again. 2022 June 29. [Google Scholar]
- 70.Center for Drug Evaluation and Research (CDER) Review. Emergency Use Authorization (EUA) for Paxlovid (nirmatrelvir tables co-packaged with ritonavir tablets). Accessed online at https://www.fda.gov/media/155194/download on May 1, 2022.
- 71.Soares H, Baniecki M, Cardin R, Leister-Tebbe H, Zhu Y, Guan S, et al. Viral load rebound in placebo and nirmatrelvir-ritonavir treated COVID-19 patients is not associated with recurrence of severe disease or mutations. Research Square. 2022.
- 72.Deo R, Choudhary MC, Moser C, Ritz J, Daar ES, Wohl DA, et al. Viral and symptom rebound in untreated COVID-19 Infection. 2022 2022.08.01.22278278. [Google Scholar]
- 73.Rubin R. From positive to negative to positive again-the mystery of why COVID-19 rebounds in some patients who take Paxlovid. JAMA. 2022;327(24):2380–2382. doi: 10.1001/jama.2022.9925. [DOI] [PubMed] [Google Scholar]
- 74.Fumagalli V, Di Lucia P, Ravà M, Marotta D, Bono E, Grassi S, et al. Nirmatrelvir treatment blunts the development of antiviral adaptive immune responses in SARS-CoV-2 infected mice. 2022 doi: 10.15252/emmm.202317580. 2022.11.22.517465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ranganath N, O'Horo JC, Challener DW, Tulledge-Scheitel SM, Pike ML, Michael O'Brien R, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of Coronavirus Disease-2019 in high-risk persons. Clin Infect Dis. 2022:ciac481. doi: 10.1093/cid/ciac481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, Berger NA, Davis PB, Kaelber DC, Volkow N, Xu R. COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. 2022 2022.06.21.22276724. [Google Scholar]
- 77.Dai EY, Lee KA, Nathanson AB, Leonelli AT, Petros BA, Brock-Fisher T, et al. Viral kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron infection in mRNA-vaccinated individuals treated and not treated with nirmatrelvir-ritonavir. 2022 2022.08.04.22278378. [Google Scholar]
- 78.Hay JA, Kissler SM, Fauver JR, Mack C, Tai CG, Samant RM, et al. Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: A retrospective cohort study. eLife. 2022:e81849. doi: 10.7554/eLife.81849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu R, Wang L, Volkow ND, Davis PB, Berger NA, Kaelber DC. COVID-19 rebound after Paxlovid treatment during Omicron BA.5 vs BA.2.12.1 subvariant predominance period. 2022 2022.08.04.22278450. [Google Scholar]
- 80.Pandit JA, Radin JM, Chiang D, Spencer EG, Pawelek JB, Diwan M, et al. The Paxlovid Rebound Study: A prospective cohort study to evaluate viral and symptom rebound differences between Paxlovid and untreated COVID-19 Participants. 2022:2022.11.14.22282195.
- 81.Institute for Clinicala and Econimic Review (ICER). Special Assessment of Outpatient Treatments for COVID-19. Accessed online at https://icer.org/wp-content/uploads/2021/08/ICER_COVID_19_Draft_Evidence_Report_020322.pdf on June 27, 2022.
- 82.Shrestha SS, Kompaniyets L, Grosse SD, Harris AM, Baggs J, Sircar K, et al. Estimation of Coronavirus Disease 2019 Hospitalization Costs From a Large Electronic Administrative Discharge Database, March 2020-July 2021. Open forum infectious diseases. 2021;8:ofab561. doi: 10.1093/ofid/ofab561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drugs for Neglected Diseases initiative. Pfizer blocking research to generate evidence on optimal use of novel antiviral for COVID-19 patients in low- and middle-income countries. 15 Mar 2022. https://dndi.org/press-releases/2022/pfizer-blocking-research-to-generate-evidence-optimal-use-novel-antiviral-covid19-low-and-middle-income-countries/.
- 84.Pfizer to Supply UNICEF up to 4 Million Treatment Courses of Novel COVID-19 Oral Treatment for Low- and Middle-Income Countries. Accessed online at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-supply-unicef-4-million-treatment-courses-novel on June 27, 2022.
- 85.Africa CDC has MOU with Pfizer for supplies of COVID-19 pill. Accessed online at https://www.reuters.com/world/africa/africa-cdc-has-mou-with-pfizer-supplies-covid-19-pill-2022-03-10/on June 27, 2022.
- 86.Nod for Paxlovid's Indian generic version to take longer. Accessed online at https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/nod-for-paxlovids-indian-generic-version-to-take-longer/articleshow/89672841.cms on June 27, 2022.
- 87.Drugmakers seem to be in no hurry to launch Covid antiviral pill Paxlovid. Accessed online at https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/drugmakers-seem-to-be-in-no-hurry-to-launch-covid-antiviral-pill-paxlovid/articleshow/92190063.cms on June 27, 2022.
- 88.Plata GG. The black market for covid-19 antiviral drugs. BMJ. 2022;377:o1282. doi: 10.1136/bmj.o1282. [DOI] [PubMed] [Google Scholar]
- 89.Gold J, Kelleher J, Magid J, Jackson B, Pennini M, Kushner D, et al. Dispensing of oral antiviral drugs for treatment of COVID-19 by Zip Code–Level Social Vulnerability — United States, December 23, 2021–May 21, 2022. MMWR. 2022;72:825–829. doi: 10.15585/mmwr.mm7125e1. [DOI] [PubMed] [Google Scholar]
- 90.AIFA Report n.13 dati settimanali 16-22 giugno (Lagevrio-Paxlovid). Accessed online at aifa.gov.it on June 29. 2022 [Google Scholar]
- 91.Shen Y, Ai J, Lin N, Zhang H, Li Y, Wang H, et al. An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants. Emerg Microbes Infect. 2022;11:1518–1523. doi: 10.1080/22221751.2022.2078230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gidari A, Sabbatini S, Schiaroli E, Bastianelli S, Pierucci S, Busti C, et al. The combination of molnupiravir with nirmatrelvir or GC376 has a synergic role in the inhibition of SARS-CoV-2 replication in vitro. Microorganisms. 2022;10:1475. doi: 10.3390/microorganisms10071475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukae H, Yotsuyanagi H, Ohmagari N, Doi Y, Imamura T, Sonoyama T, et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: Results of the Phase 2a Part. Antimicrob Agents Chemother. 2022;66 doi: 10.1128/aac.00697-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Y, Fang C, Zhang Q, Zhang R, Zhao X, Duan Y, et al. Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332. Protein Cell. 2022;13:689–693. doi: 10.1007/s13238-021-00883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greasley SE, Noell S, Plotnikova O, Ferre RA, Liu W, Bolanos B, et al. Structural basis for Nirmatrelvir in vitro efficacy against the Omicron variant of SARS-CoV-2. 2022 doi: 10.1016/j.jbc.2022.101972. 2022.01.17.476556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ullrich S, Ekanayake KB, Otting G, Nitsche C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg Med Chem Lett. 2022;62 doi: 10.1016/j.bmcl.2022.128629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abe K, Kabe Y, Uchiyama S, Iwasaki YW, Ishizu H, Uwamino Y, et al. Pro108Ser mutation of SARS-CoV-2 3CLpro reduces the enzyme activity and ameliorates the clinical severity of COVID-19. Sci Rep. 2022;12:1299. doi: 10.1038/s41598-022-05424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sacco MD, Hu Y, Gongora MV, Meilleur F, Kemp MT, Zhang X, et al. The P132H mutation in the main protease of Omicron SARS-CoV-2 decreases thermal stability without compromising catalysis or small-molecule drug inhibition. Cell Res. 2022;32:498–500. doi: 10.1038/s41422-022-00640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Oliveira VM, Ibrahim MF, Sun X, Hilgenfeld R, Shen J. H172Y mutation perturbs the S1 pocket and nirmatrelvir binding of SARS CoV-2 main protease through a nonnative hydrogen bond. 2022 2022.07.31.502215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.