Abstract
Introduction
The prognostic significance of the thrombin generation assay (TGA) with a thrombomodulin (TM) challenge in patients entering hospital with severe COVID-19 is uncertain.
Methods
We prospectively evaluated an automated TGA (aTGA) using the ST-ThromboScreen® assay and ST-Genesia® analyser in 179 patients with severe COVID-19 during their admission to 2 university hospitals. The primary outcome was early survival at Day 28 (D28). Secondary outcomes were late survival at Day 90 (D90), later transfer to an intensive care unit (ICU), and occurrence of any thrombotic complications during hospitalisation.
Results
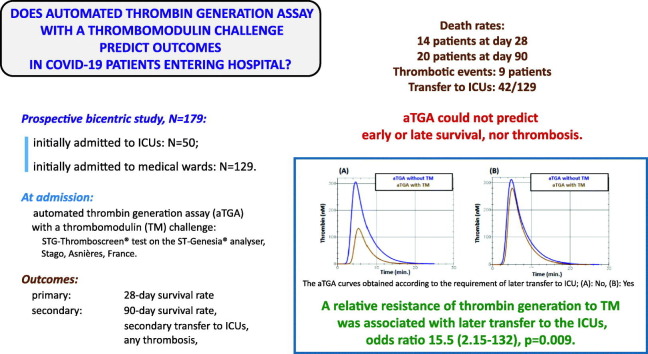
Among the 174 patients, 50 were initially admitted to ICUs. Forty-two were transferred to ICUs before D28. Fourteen patients, all in ICUs, died before D28, and 20 before D90, all but 1 in ICUs. None of the aTGA-derived results were associated with vital status either at D28 or D90. Nine patients had a thrombotic event with no association with the aTGA results. Later transfer to the ICU was associated with higher velocity index, thrombin peak height and endogenous thrombin potential (ETP) values of the aTGA performed with TM, and mainly with a lower TM-induced decrease in ETP (odds ratio 15.5 (2.15–132), p = 0.009).
Conclusions
aTGA, a global assay supposed to evidence coagulopathy, could predict neither early or late survival, nor thrombotic events, in hospitalised COVID-19 patients. Its clinical justification in that setting is thus unlikely. A relative resistance of the ETP to TM was associated with later transfer to the ICU and deserves further investigation.
Keywords: SARS-CoV-2, COVID-19, Thrombin generation, Survival, Coagulopathy
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leading to the coronavirus disease 2019 (COVID-19) is associated with a multifactorial coagulopathy related to inflammation resulting from the cytokine storm [1], [2]. Excessive amounts of thrombin are generated with a subsequent hypercoagulability state leading to a high prevalence of thrombotic manifestations, many with fatal outcomes [3], [4], [5]. This quickly led teams managing patients hospitalised with severe COVID-19 to raise the question of the informativity of initially performed innovative coagulation tests describing coagulation as a whole on key clinical outcomes.
Thrombin generation assay (TGA) is a global dynamic assay which measures thrombin generation and inhibition continuously and simultaneously [6], [7]. A number of clinical uses have been proposed for TGA and evaluated, including for patients with viral infections and COVID-19 [8]. Adding purified thrombomodulin to the system reveals thrombin-mediated activation of the protein C system, limiting thrombin generation itself, and allows certain thrombotic phenotypes to be recognised [9]. The initial laboratory techniques supporting the use of this test were manual, making it difficult to apply to routine patient management. Recently, automated instruments and commercially available reagents, providing the normalization of thrombin generation parameters based on a reference plasma [10], have introduced a convenient opportunity for the individual annotation of patients.
We prospectively studied the prognostic value of a fully automated thrombin generation assay (aTGA), performed among COVID-19 patients entering hospital, for predicting death and major clinical outcomes.
2. Patients and methods
2.1. Study design
This was a multicentric, observational prospective study in severe COVID-19 patients entering hospital through the Emergency Departments of the University Hospitals of Nîmes, Montpellier, Bordeaux and Limoges, France, from April 6th, 2020 to March 29th, 2021. After an initial evaluation and urgent stabilisation, patients were admitted to the intensive care units (ICUs) dedicated to COVID-19 or to general medical wards, depending on their care requirements.
2.2. Objectives and outcomes
The objective of the study was to evaluate the ability of the quantitative parameters describing the results of the automated thrombin generation assay (aTGA) used to predict primary and secondary outcomes.
The primary outcome was the 28-day survival rate, defined during hospitalisation and evaluated 28 days after admission.
Secondary outcomes were the 3-month survival rate, 90 days after admission; transfer to an ICU during hospitalisation for patients admitted to medicine wards, and the occurrence of any thrombotic complications during hospitalisation.
2.3. Study population
2.3.1. Definition, eligibility criteria
Suspected COVID-19 patients were screened. Patients of both sexes, aged 18 years or over, were evaluated for the main inclusion criteria: symptomatology / severity requiring hospital admission and treatment. SRAS-CoV-2 infection was assessed by real-time reverse-transcriptase polymerase chain reaction. Consecutive positive patients who did not meet the exclusion criteria below, but who finally gave free informed consent and signed the consent form (patient or carer) and were beneficiaries of a health insurance plan, were included.
The exclusion criteria included “impossibility to give the patient clear information, patients under safeguard of justice or state guardianship, pregnant or breastfeeding women, any long-term anticoagulant treatment, any long-term anti-platelet therapy, any symptomatic venous or arterial thrombotic event associated with the onset of SARS-CoV-2 infection before hospital admission and any inclusion in a concomitant therapeutic trial”. Also excluded from the trial were patients with known pre-existing haemostatic diseases including haemorragic diseases (thrombocytopenia, platelet function deficiency, von Willebrand's disease, haemophilia, coagulation factor deficiency, hyperfibrinolysis) and thrombotic risk factors (antithrombin, protein C or protein S deficiency, positive F5 rs6025 or F2 rs1799963polymorphisms, antiphospholipid antibodies).
All final inclusions were made within 24 h of entering the emergency department, when the initial, trial-specific clinical and biological assessment was made. Fig. 1 shows the flowchart for inclusions. The study was recorded on the ClinicalTrials.gov database under the reference: NCT04356950.
Fig. 1.
Flow-diagram showing selection and inclusion of patients and their subsequent evolutions. ICU: intensive care unit.
2.3.2. Sample-size estimation
We designed our study using the hypothesis (based on previous observations) that 12 % of hospitalised patients would die 28 days after admission. According to this hypothesis, 175 patients (corresponding to 21 expected deaths) had to be included in the cohort to be able to demonstrate a difference between non-survivors and survivors with a large size effect (Cohen's D value = 0.8 corresponding to an area under the receiver operator characteristics ROC curve AUC = 0.71) [11] using a bilateral α risk of 5 % and a power 1-β of 90 %.
2.4. Laboratory investigations
All laboratory data were produced by laboratory workers blinded to the origin of patients and the outcomes of the study.
2.4.1. Blood sampling for coagulation assays and plasma preparation
Blood was drawn at inclusion, before prescribing any antithrombotic treatment that might falsify the laboratory tests under study, from an antecubital vein with a light tourniquet and a 21-G needle. The blood was collected in tubes containing 0.109 mol/L citrate (Beckton Dickinson SA, Le Pont de Claix, France), at a 9:1 vol:vol ratio, after discarding the first few millilitres for other laboratory tests. Platelet-poor plasma (PPP) was prepared by double centrifugation at 2500g for 15 min within 1 h of blood collection. Samples were stored (−80 °C) and transferred on dry ice to the Biological Resource Centre at Nîmes University Hospital (NF S96–900, ISO 9001/ISO 20387 certified, number 210230/1285F) before being used in 2021. Before use, plasma samples were thawed in a water bath at 37 °C for 5 min, then gently homogenised.
2.4.2. Routine tests
Complete blood cell count (CBC) with white blood cell differential, aPTT, PT (INR), fibrinogen, ferritin, creatinine, albumin, LDH, CPK, bilirubin, ASAT, ALAT levels, and blood gas tests were performed by routine laboratory techniques.
2.4.3. Automated thrombin generation assay (aTGA)
The STG-Thromboscreen® assay was performed on the ST-Genesia® analyser, Stago, Asnières, France. It uses a fluorogenic substrate for thrombin, is fully automated, and allows standardised thrombin generation (TG) measurements to be obtained using a dedicated calibrator, reagents, internal quality controls, and reference plasma samples. More specifically, the calibration curve is obtained with a fixed amount of human purified thrombin (STG-Thrombical®) in buffer solution in the presence of Z-Gly-Gly-Arg-AMC (the substrate) plus Ca++ (STG-Fluostart®), in a cuvette warmed to exactly 37 °C. The fluorescence increase (AMC) from thrombin-mediated substrate cleavage is measured every 15 s at 377 nm excitation / 450 nm emission wavelengths. Once the calibration is validated, individual plasma samples are run in duplicate. In the plasma samples, a known amount of AMC (STG-Fluoset®) allows the calibration curve to be adjusted by correcting the plasma colour, and rectifying the inner effect filter. For this study, TGAs were performed in the absence and presence of thrombomodulin TM using the dedicated STG-ThromboScreen® reagent containing a mixture of phospholipids and a medium picomolar concentration of human Tissue Factor TF chosen by the manufacturer to obtain a 50 % decrease of the endogenous thrombin potential in presence of purified TM. The final TM concentration is not disclosed by Stago. Briefly, 80 μL of plasma was dispensed by the analyser in plastic cuvettes in presence of 20 μL of the initiating reagent (STG-ThromboScreen® with/without TM) and 20 μL of STG-Fluostart®. The incubation rotor and measuring plate were heated to 37 °C, and the temperature was strictly maintained during the whole reaction. The thrombin measured over time is calculated by the software built-in in the ST®-Genesia using the modified Thrombinoscope software (Diagnostica Stago, Asnières-sur-Seine, France). TG parameters were automatically calculated, namely: lag time (LT), thrombin peak height (PH), time to peak (TTP), endogenous thrombin potential (ETP), velocity index (V), and start tail (ST). ETP was considered as the main parameter as it represents the total amount of thrombin work that a plasma sample can generate under specific experimental conditions. The accuracy and precision of the instrument and reagent were monitored with quality controls at low, normal and high levels. Despite low analytical variability (coefficient of variation <10 %), we normalised results in the absence of TM with the reference plasma, by dividing each patient‘s values by the reference plasma's corresponding value. The TM effect on the assay was quantified dividing the ETP value obtained in the presence of TM, by the ETP value obtained in the absence of TM.
2.4.4. Fibrin generation marker
D-dimers were assayed by an automated enzyme linked fluorescent assay: Vidas® D-dimers Exclusion™ II, BioMérieux, Marcy l'Etoile, France.
2.5. Data recorded for the study
Clinical and laboratory parameters and variables were recorded at inclusion by senior investigators, for each patient included. This included demographic data and each patient's history, ongoing treatments, clinical, radiological and laboratory characteristics at inclusion and treatments prescribed upon entry to the wards.
2.6. Ethics approval and consent to participate
All patients included had given their informed consent to take part in the study, which was approved by the “CPP Île-de-France I-Paris-Hôtel Dieu” ethics committee on April 24th, 2020, ref. #CPPIDF1–2020-ND48 cat.2. The study was performed in accordance with the 1996 revised version of the 1975 declaration of Helsinki and its later amendments, and with the French law on clinical research: ref. ANSM (“Agence National de Sécurité des Médicaments et des produits de Santé) #: 1641233, registered under the number ID-RCB: 2020-AO1068–31 and ClinicalTrials.gov Identifier, NCT04356950.
2.7. Statistical analysis
First of all, the demographic and clinical characteristics of the patients included were statistically described. Quantitative variables were expressed as means and standard deviations. Qualitative variables were expressed as numbers and percentages. Patient characteristics were compared according to vital status. For quantitative variables, we used a Student's test or a Wilcoxon-Mann-Whitney test when distribution of the variable was skewed. For qualitative variables was used a Chi2 test or Fisher's exact test.
The values of the different quantitative parameters describing the aTGA profiles obtained were described and compared according to vital status at 28 days for the primary outcome, using a Wilcoxon-Mann-Whitney test. The prognostic value of these parameters was evaluated using ROC curves, with discriminatory ability quantified by the area under the curve (AUC) based on Hanley's method, and then compared with the value of 0.5. We finally computed the crude odds ratio (OR) and two-sided 95 % confidence intervals (95%CI) using a univariate logistics regression model. The same statistical methodology was used for the other outcomes. For later transfer to the intensive care unit due to worsening during the hospital stay, the analysis was based on the 129 patients who had been admitted to medical wards.
No specific treatment or procedure was applied to missing data. Patients lost to follow-up for a given outcome were excluded from the analysis.
The statistical analysis was conducted using R 4.1.2 (Foundation for Statistical Computing, Vienna, Austria) and SAS® software (SAS 2002–2017 Institute Inc.). P values of 0.05 or less were considered significant.
2.8. Funding
The study was funded by an exceptional call for tender launched by Nîmes University Hospital to support clinical research on COVID-19: ref.# NIMAO 2020 COVID-19. The funder had no role in the study design, interpretation of results or writing the report.
3. Results
We included 179 patients with severe COVID-19 entering the Emergency Departments of the University Hospitals of Nîmes (N = 144, 28 of them with an initial admission to the central ICU) and Montpellier (N = 35, 22 to the ICU) from April 6th, 2020 to March 29th, 2021 (Fig. 1). The University Hospitals of Bordeaux and Limoges did not include any patients in the study. Most of the severe COVID-19 patients during this period were included in therapeutic trials, thus limiting eligibilities for this study.
3.1. Patient characteristics
Table 1 describes patients at inclusion.
Table 1.
Characteristics of the 179 patients at inclusion, then by vital status at Day 28 (D28) and at Day 90 (D90).
Quantitative variables are expressed as means with standard deviations (SD), or medians with inter-quartile ranges (IQR). Qualitative variables are expressed as numbers (N) and percentages (%).
| Variable [missing values, N] |
All (N = 179) |
Survival, D28 (N = 179) |
Survival, D90 (N = 174) |
||||
|---|---|---|---|---|---|---|---|
| Demographic data and patient's history | Yes, 165a | No, 14a | Pb | Yes, 154a | No, 20a | Pb | |
| Age at inclusion, years [0] | 64.8 (14.3) | 63.9 (14.5) | 75.4(6.0) | 0.001 | 63.8(14.4) | 73.0(9.7) | 0.002 |
| Sex [0] | |||||||
| Female | 69 (39 %) | 66 | 3 | 0.2 | 61 | 5 | 0.2 |
| Male | 110 (61 %) | 99 | 11 | 93 | 15 | ||
| Body mass index, kg.m−2 [0] | 28.0 (6,2) | 27.9(6.1) | 29.5(6.4) | 0.4 | 28.0(6.1) | 28.8(6.6) | 0.8 |
| Active smoking [6] | 12 (6.9 %) | 10 | 2 | 0.3 | 9 | 3 | 0.2 |
| Alcohol consumption [8] | 22 (13 %) | 20 | 2 | 0.7 | 16 | 5 | 0.07 |
| Diabetes mellitus [28] | 41 (27.1 %) | 37 | 4 | > 0.9 | 33 | 6 | 0.8 |
| Cardiac diseases [28] | 21 (14.6 %) | 20 | 1 | 0.7 | 18 | 2 | > 0.9 |
| Symptomatic coronary insufficiency | 5 (3.3 %) | 4 | 1 | 0.3 | 4 | 1 | 0.4 |
| Rhythmic heart disease | 10 (6,6 %) | 10 | 0 | 0,6 | 9 | 0 | 0.6 |
| Heart failure | 6 (4.0 %) | 6 | 0 | > 0.9 | 5 | 1 | 0.6 |
| Symptomatic cerebrovascular insufficiency [28] | 4 (2.6 %) | 3 | 1 | 0.3 | 3 | 1 | 0.4 |
| Symptomatic peripheral arterial disease [28] | 4 (2.6 %) | 4 | 0 | > 0.9 | 4 | 0 | > 0.9 |
| Venous thromboembolic disease [28] | 8 (5.3 %) | 8 | 0 | 0.6 | 7 | 1 | > 0.9 |
| Deep vein thrombosis | 7 (4.6 %) | 7 | 0 | > 0.9 | 7 | 0 | > 0.9 |
| Pulmonary embolism | 1 (0.8 %) | 1 | 0 | > 0.9 | 1 | 0 | > 0.9 |
| Superficial vein thrombosis | 2 (1.7 %) | 2 | 0 | > 0.9 | 1 | 1 | > 0.9 |
| Chronic obstructive pulmonary disease [28] | 12 (9.9 %) | 10 | 2 | 0.3 | 10 | 2 | 0.7 |
| Chronic kidney disease / insufficiency [28] | 14 (12 %) | 12 | 2 | 0.6 | 11 | 2 | 0.7 |
| Chronic hepatic disease /insufficiency [28] | 5 (4.1 %) | 5 | 1 | 0.4 | 4 | 1 | 0.5 |
| Chronic psychiatric disease [28] | 12 (7.9 %) | 11 | 1 | > 0.9 | 11 | 1 | > 0.9 |
| Chronic inflammatory disease [28] | 6 (4.0 %) | 5 | 1 | 0.5 | 5 | 1 | 0.6 |
| Auto-immune diseases [28] | 6 (4.0 %) | 6 | 0 | > 0.9 | 6 | 0 | > 0.9 |
| Cancers [28] | 23 (15.2 %) | 20 | 3 | 0.7 | 20 | 3 | > 0.9 |
| Solid cancer | 20 (13.3 %) | 17 | 3 | 0.4 | 17 | 3 | 0.7 |
| Haematological cancer | 3 (2.0 %) | 3 | 0 | > 0.9 | 3 | 0 | > 0.9 |
| Ongoing treatments [0] | 99 (55 %) | 91 | 8 | 0.9 | 86 | 11 | > 0.9 |
| Antihypertensive drugs | 62 (63 %) | 57 | 5 | > 0.9 | 53 | 7 | > 0.9 |
| Angiotensin-converting enzyme inhibitors | 15 (15 %) | 14 | 1 | > 0.9 | 14 | 1 | > 0.9 |
| Non-inhaled corticosteroids | 13 (13 %) | 13 | 0 | 0.6 | 12 | 0 | 0.3 |
| Inhaled corticosteroids | 14 (14 %) | 12 | 2 | 0.3 | 12 | 2 | 0,7 |
| Non-steroidal anti-inflammatory drugs | 7 (7.1 %) | 4 | 3 | 0.011 | 3 | 3 | 0.018 |
| Anti-inflammatory biotherapy | 1 (1.0 %) | 1 | 0 | > 0.9 | 0 | 0 | > 0.9 |
| Immunosuppressants | 4 (4.0 %) | 4 | 0 | > 0.9 | 4 | 0 | > 0.9 |
| Hypocholesterolemic drugs: statins | 34 (34 %) | 30 | 4 | 0.4 | 27 | 7 | 0.047 |
| Chemotherapy drugs | 3 (3.0 %) | 3 | 0 | > 0.9 | 3 | 0 | > 0.9 |
| Serotonin reuptake inhibitors. | 3 (3.0 %) | 3 | 0 | > 0.9 | 3 | 0 | > 0.9 |
| Clinical, radiological, laboratory characteristics at inclusion | |||||||
| Admission in ICU [0] | 50 (28 %) | 45 | 5 | 0.5 | 42 | 8 | 0.2 |
| General condition | |||||||
| Tiredness [0] | 61 (34 %) | 58 | 3 | 0.4 | 54 | 6 | 0.7 |
| Fever ≥38.5 °C [3] | 65 (37 %) | 57 | 8 | 0.1 | 53 | 11 | 0.09 |
| Myalgias [0] | 12 (6.7 %) | 11 | 1 | > 0.9 | 9 | 2 | 0.6 |
| Anosmia [0] | 15 (8.4 %) | 15 | 0 | 0.6 | 12 | 3 | 0.4 |
| SOFA score [42] | 4.1 (2.8) | 3.8(2.6) | 5.8(2.9) | 0.026 | 3.6(2.4) | 6.1(3.4) | 0.005 |
| Simplified SOFA score [44] | 0.9 (0.69) | 1.3(0.5) | 1.14(0.4) | 0.2 | 1.25(0.4) | 1.3(0.65) | 0.18 |
| Lungs | |||||||
| Cough [0] | 68 (38 %) | 63 | 5 | 0.9 | 63 | 5 | 0.2 |
| Productive cough [1] | 8 (4.5 %) | 8 | 0 | > 0.9 | 8 | 0 | 0.6 |
| Dyspnoea [0] | 110 (61 %) | 104 | 6 | 0.14 | 98 | 10 | 0.2 |
| Respiratory frequency, per min. [5] | 23.8 (5.2) | 23.6(5.3) | 25.2(4.2) | 0.3 | 23.6(5.3) | 24.8(4.7) | 0.3 |
| Acute respiratory insufficiency [1] | 25 (14 %) | 23 | 2 | > 0.9 | 21 | 4 | 0.5 |
| Acute respiratory distress syndrome [0] | 16 (8.9 %) | 14 | 2 | 0.4 | 12 | 4 | 0.09 |
| Blood oxygen saturation level, % [4] | 94.6 (3.4) | 95.0(3.2) | 91.1(3.9) | < 0.001 | 94.9(3.3) | 92.0(3.8) | < 0.001 |
| Percentage of lung involvement after chest CT scan [12] | 40.9 (18.4) | 39.7(17.9) | 53.1(19.4) | 0.018 | 39.6(17.7) | 50.6(19.8) | 0.019 |
| Pulmonary condensation syndrome [7] | 71 (56 %) | 69 | 2 | 0.011 | 65 | 4 | 0.006 |
| Pulmonary ground glass opacities [5] | 71 (56 %) | 113 | 10 | 0.4 | 103 | 15 | 0.5 |
| Heart | |||||||
| Heart beating frequency, per min. [7] | 83.8 (16.1) | 83.2(15.0) | 90.9(25.5) | 0.2 | 83.6(14.1) | 90.5(24.7) | 0.2 |
| diastolic arterial pressure, mm Hg [5] | 74.3 (13.0) | 74.6(12.8) | 71.0(14.8) | 0.2 | 75.1(12.7) | 69.8(14.2) | 0.04 |
| systolic arterial pressure, mm Hg [5] | 129.7 (19.5) | 128.9(19.0) | 138.2(24.1) | 0.088 | 128.7(18.2) | 139.2(26.7) | 0.022 |
| Gut | |||||||
| Diarrhoea [0] | 33 (18 %) | 32 | 1 | 0.5 | 28 | 5 | 0.5 |
| Nausea and vomiting [0] | 9 (5.0 %) | 8 | 1 | 0.5 | 6 | 2 | 0.2 |
| Treatments prescribed on entry to the wards | |||||||
| Antibiotics [7] | 101 (58.7 %) | 89 | 12 | 0.074 | 86 | 15 | 0.008 |
| Antiviral drugs [7] | 2 (1.2 %) | 1 | 1 | 0.2 | 1 | 1 | 0.2 |
| Methylprednisolone [7] | 143 (83.1 %) | 130 | 13 | > 0.9 | 122 | 16 | 0.5 |
| Oxygen therapy [0] | 179(100 %) | 165 | 14 | > 0.9 | 161 | 18 | 0.6 |
| Nasal cannula [0] | 100 (55.9 %) | 98 | 2 | < 0.001 | 93 | 4 | < 0.001 |
| Non-invasive mechanical ventilation[0] | 29 (16.2 %) | 24 | 5 | 0.005 | 19 | 8 | 0.01 |
| Invasive mechanical ventilation [0] | 50 (27.9 %) | 43 | 7 | 0.025 | 42 | 8 | 0.02 |
| Haemodialysis [0] | 1 (0.6 %) | 1 | 0 | > 0.9 | 0 | 1 | 0.12 |
| Unfractionated heparin UFH [9] | 40 (23.5 %) | 35 | 5 | 0.3 | 32 | 7 | 0.3 |
| Low-molecular weight heparin LMWH [9] | 130 (76.5 %) | 121 | 9 | 0.7 | 113 | 13 | 0.3 |
| Antiplatelet agents [9] | 7 (4.1 %) | 5 | 2 | 0.11 | 4 | 3 | 0.037 |
| Laboratory data | |||||||
| Complete blood cell count and differential | |||||||
| haemoglobin, g.L−1 [2] | 135 (18) | 135(18) | 136(18) | > 0.9 | 135(18) | 136(17) | 0.8 |
| Haematocrit, % [2] | 40.7 (5.0) | 40.7(5.0) | 41.1(5.1) | 0.8 | 40.8(5.0) | 41.0(4.9) | 0.9 |
| Erythrocyte mean corpuscular haemoglobin [15] | 29.8 (2.3) | 29.8(2.3) | 30.6(1.8) | 0.4 | 29.7(2.3) | 30.2(2.6) | 0.3 |
| White blood cells, 109.L−1 [4] | 7.8 (3.9) | 7.5(3.4) | 11.1(6.7) | 0.014 | 7.5(3.3) | 10.3(6.3) | 0.029 |
| Neutrophils, 109.L−1 [15] | 6.3 (3.8) | 6.1(3.4) | 9.2(7.4) | 0.13 | 6.1(3.3) | 8.5(6.6) | 0.2 |
| Monocytes, 109.L−1 [15] | 0.5 (0.5) | 0.5(0.2) | 1.1(1.7) | 0.7 | 0.5(0.2) | 0.8(1.3) | 0.8 |
| Lymphocytes, 109.L−1 [15] | 1.1 (1.1) | 1.1(1.2) | 0.9(0.6) | 0.4 | 1.1(1.2) | 0.9(0.5) | 0.7 |
| Eosinophils, 109.L−1 [15] | 0.0 (0.1) | 0.0(0.1) | 0.0(0.0) | 0.6 | 0.0(0.1) | 0.0(0.0) | 0.5 |
| Platelets, 109.L−1 [3] | 227.0 (81.4) | 226.3 (81.0) | 234.6 (88.5) | 0.4 | 228.2(82.6) | 221.7(81.4) | > 0.9 |
| Coagulation | |||||||
| aPTT ratio [31] | 1.1 (0.2) | 1.1(0.2) | 1.2(0.3) | 0.2 | 1.1(0.2) | 1.2(0.3) | 0.4 |
| PT ratio [29] | 1.1 (0.2) | 1.1(0.2) | 1.1(0.1) | 0.3 | 1.1(0.2) | 1.1(0.1) | 0.5 |
| INR [29] | 1.1 (0.3) | 1.2(0.3) | 1.1(0.2) | 0.7 | 1.2(0.3) | 1.1(0.2) | 0.7 |
| D-dimers, μg.L−1 [15] | 1550.0 (1502.4) | 1536.1 (1528.4) |
1712.7 (1207.8) |
0.3 | 1569.8 (1568.2) | 1534.8 (1038.0) |
0.4 |
| Fibrinogen, g.L−1 [23] | 6.8 (1.7) | 6.7(1.7) | 7.2(1.8) | 0.4 | 6.7(1.7) | 7.0(1.6) | 0.7 |
| Inflammation and metabolisms | |||||||
| C reactive protein, mg.L−1 [10] | 109.3 (79.8) |
105.1 (76.8) |
163.7 (100.4) |
0.036 | 106.8 (77.9) | 142.9 (91.4) |
0.09 |
| Albumin, g.L−1 [26] | 36.5 (4.6) | 36.6(4.7) | 35.3 (3.0) | 0.2 | 36.6(4.6) | 35.4(4.2) | 0.4 |
| Ferritin, μg.L−1 [38] | 2784.1 (7752.6) | 2820.7 (8271.7) |
2520.8 (1495.9) | 0.14 | 2820.7 (8271.7) | 2520.8 (1495.9) | 0.14 |
| LDH, I.U.L−1 [38] | 392.9 (148.1) |
384.0 (147.3) |
473.8 (137.4) |
0.071 | 391.0 (146.4) |
446.0 (153.2) |
0.3 |
| CPK, I.U.L−1 [38] | 206.0 (255.1) |
202.3 (261.3) |
274.4 (179.8) |
0.14 | 202.3 (268.1) | 256.8 (160.3) |
0.040 |
| Bilirubin, μmol.L−1 [38] | 10.0 (6.4) | 9.7(6.0) | 13.2(9.4) | 0.5 | 9.9(6.2) | 12.1(8.0) | 0.4 |
| ALAT, I.U.L−1 [24] | 47.3 (46.8) | 47.0(48.0) | 49.6 (32.2) | 0.5 | 47.1(48.9) | 45.5(27.9) | 0.7 |
| Creatinine, μmol.L−1 [0] | 89.8(46.7) | 88.7(46.5) | 102.6(49.1) | 0.2 | 86.6(38.0) | 117.2(88.0) | 0.038 |
| Blood gas test results | |||||||
| pH [18] | 7.5 (0.1) | 7.5(0.1) | 7.5(0.1) | 0.2 | 7.5(0.1) | 7.4(0.1) | 0.08 |
| PaO2, mm Hg [18] | 80.6 (31.4) | 81.0(31.2) | 77.0(34.3) | 0.3 | 81.8(32.0) | 74.3(30.0) | 0.12 |
| PaCO2, mm Hg [18] | 34.9 (5.9) | 34.6(6.0) | 37.2(5.2) | 0.044 | 34.4(5.8) | 37.7(6.9) | 0.023 |
P values in bold characters are statistically significant
Quantitative values: mean(SD).
Quantitative values: Wilcoxon rank sum test.
Mean age was around 65 years and the majority of patients were men (61 %). The mean body mass index (BMI) values indicated overweight and various general comorbidities were common. Anti-hypertensive drugs were taken by roughly 6 patients out of ten, angiotensin-converting enzyme inhibitors by 15 % and statins by one third. The mean oxygen blood saturation level was 94.6 %. Fifty patients (28 %) entered the intensive care unit (ICU) directly, with systematic mechanical ventilation. Methylprednisolone and oxygen were the main treatments prescribed and, among antithrombotic regimens, a prophylactic-dose of low-molecular weight heparin (LMWH) was given to 83 % of the patients. Mean values of haemoglobin, platelets and white blood cell counts were normal, as were aPTT and PT values. A sustained inflammatory reaction was generally found (high fibrinogen, CRP, ferritin and low albumin values), but renal function was globally not impaired and liver enzymes were normal. Strikingly, high D-dimer plasma concentrations were confirmed. TGA parameters showed a variable percentage of missing data: 9.5 % in the absence of TM and 15 % in the presence of TM. The overall description of the aTGA parameters is displayed in Table 2 . The aTGA after supplementation of samples by purified thrombomodulin (TM) evidenced decreased endogenous thrombin potential (ETP) values to 60 % of the initial values: thus corresponding to the inhibition of 40 % of the initial activities.
Table 2.
Values of aTGA parameters at inclusion in the whole population of patients, then categorised according to the vital status at Day 28 (D28).
AUC: area under the receiver operating characteristic curve. OR: odds ratio.
| Survival, D28 |
|||||||
|---|---|---|---|---|---|---|---|
| THROMBIN GENERATION ASSAY TGA, parameters | Overall (N = 179) | Yes: N=165a | No: N=14a | Pb | AUC | OR, 95 % C.I. | Pc |
| Without thrombomodulin, TM- | |||||||
| Lag time (LT_TM-), min. | 4.0 (2.4) | 3.9 (2.4) | 4.9 (2.6) | 0.07 | 0.645 (0.496–0.794) | 1.11 (0.93–1.32) | 0.2 |
| Normalised LT_TM- | 1.9 (1.2) | 1.9 (1.2) | 2.4 (1.2) | 0.13 | 0.629 (0.454–0.803) | 1.22 (0.83–1.74) | 0.2 |
| Velocity index (V_TM-), nmol.min.−1 | 193.6 (121.8) | 194.8 (122.4) | 180.7 (118.5) | 0.8 | 0.517 (0.339–0.696) | 1.00 (0.99–1.00) | 0.7 |
| Normalised V_TM- | 179.5 (113.4) | 181.2 (114.0) | 162.0 (109.4) | 0.7 | 0.536 (0.344–0.728) | 1.00 (0.99–1.00) | 0.6 |
| Thrombin peak height (PH_TM-), nmol.L−1 | 277.5 (103.9) | 279.6 (102.2) | 254.9 (123.0) | 0.7 | 0.529 (0.353–0.706) | 1.00 (0.99–1.00) | 0.4 |
| Normalised PH_TM- | 132.4 (50.3) | 133.9 (49.5) | 117.1 (58.4) | 0.5 | 0.560 (0.379–0.741) | 0.99 (0.98–1.00) | 0.3 |
| Time to peak (TTP_TM-), min. | 6.2 (3.1) | 6.1 (2.9) | 7.6 (4.4) | 0.2 | 0.597 (0.425–0.768) | 1.10 (0.96–1.25) | 0.14 |
| Normalised TTP_TM- | 1.4 (0.7) | 1.4 (0.7) | 1.7 (1.0) | 0.2 | 0.598 (0.414–0.782) | 1.45 (0.79–2.58) | 0.2 |
| Start tail (ST_TM-), min. | 19.7 (6.2) | 19.4 (5.5) | 22.6 (11.2) | 0.3 | 0.587 (0.420–0.755) | 1.06 (0.98–1.12) | 0.09 |
| Normalised ST_TM- | 1.0 (0.3) | 1.0 (0.3) | 1.2(0.6) | 0.2 | 0.609 (0.430–0.789) | 2.78 (0.77–9.21) | 0.084 |
| Endogenous thrombin potential (ETP_TM-), nmol.min. | 1421.1 (392.1) | 1431.0 (377.2) | 1316.7 (530.9) | 0.6 | 0.545 (0.358–0.732) | 1.00 (1.00–1.00) | 0.3 |
| Normalised ETP_TM- | 109.9 (30.5) | 110.9 (29.2) | 99.6 (41.3) | 0.4 | 0.574 (0.370–0.778) | 0.99 (0.97–1.01) | 0.2 |
| With thrombomodulin, TM+ | |||||||
| Lag time (LT_TM+), min. | 4.6 (2.2) | 4.6 (2.2) | 5.2 (2.3) | 0.2 | 0.613 (0.456–0.739) | 1.11 (0.87–1.36) | 0.3 |
| Velocity index (V_TM+), nmol.min.−1 | 176.5 (128.6) | 175.6 (129.5) | 187.6 (123.0) | 0.7 | 0.464 (0.287–0.641) | 1.00 (1.00–1.00) | 0.8 |
| Thrombin peak height (PH_TM+), nmol.L−1 | 216.3 (114.1) | 215.2 (114.4) | 229.1 (114.1) | 0.6 | 0.549 (0.372–0.725) | 1.00 (1.00–1.01) | 0.7 |
| Time to peak (TTP_TM+), min. | 6.5 (2.6) | 6.5 (2.6) | 7.1 (2.6) | 0.3 | 0.582 (0.409–0.755) | 1.16 (0.86–1.29) | 0.5 |
| Start tail (ST_TM+),min. | 16.1 (2.9) | 16.1 (3.0) | 16.9 (2.2) | 0.08 | 0.653 (0.478–0.828) | 1.12 (0.89–1.26) | 0.4 |
| Endogenous thrombin potential (ETP_TM+), nmol.min. | 874.9 (428.2) | 868.4 (424.7) | 951.0 (481.4) | 0.5 | 0.558 (0.371–0.745) | 1.00 (1.00–1.00) | 0.5 |
| Thrombomodulin effect on TGA | |||||||
| (ETP_TM+: ETP_TM-) ratio | 0.6 (0.2) | 0.6 (0.2) | 0.6 (0.2) | 0.5 | 0.558 (0.377–0.739) | 2.33 (0.17–40.3) | 0.5 |
Mean (SD).
Wilcoxon rank sum test.
Wald test for OR = 1.
3.2. Clinical outcomes
Forty-eight patients among the 129 initially admitted to medical wards had to be transferred to ICUs before Day 28 due to clinical worsening (Fig. 1), but none between Days 28 and 90.
Among the 92 patients treated in ICUs, fourteen died before D28. Five patients among those treated in ICUs at D28 died before D90. Among the 87 patients still alive in medical wards at D28, 5 left the hospital before D90 and were subsequently lost to follow-up, and 1 died before D90. To summarise, 14 patients died before D28, all in ICUs; and 20 patients died before D90, with all but 1 in ICUs.
Nine patients had thrombotic events: 5 had venous thromboembolism (VTE) and 4 had a thrombotic event strictly related to extracorporeal membrane oxygenation treatment.
3.3. Primary outcome
Demographic, clinical and laboratory data at inclusion were compared according to vital status at D28 in Table 1: non-survivors were older, with a higher rate of patients taking non-steroidal anti-inflammatory drugs, with higher sequential organ failure assessment (SOFA) score values, lower blood oxygen saturation levels, higher percentages of lung involvement revealed by chest CT scan, higher rates of mechanical ventilation and higher values of white blood cells, C reactive protein and PaCO2.
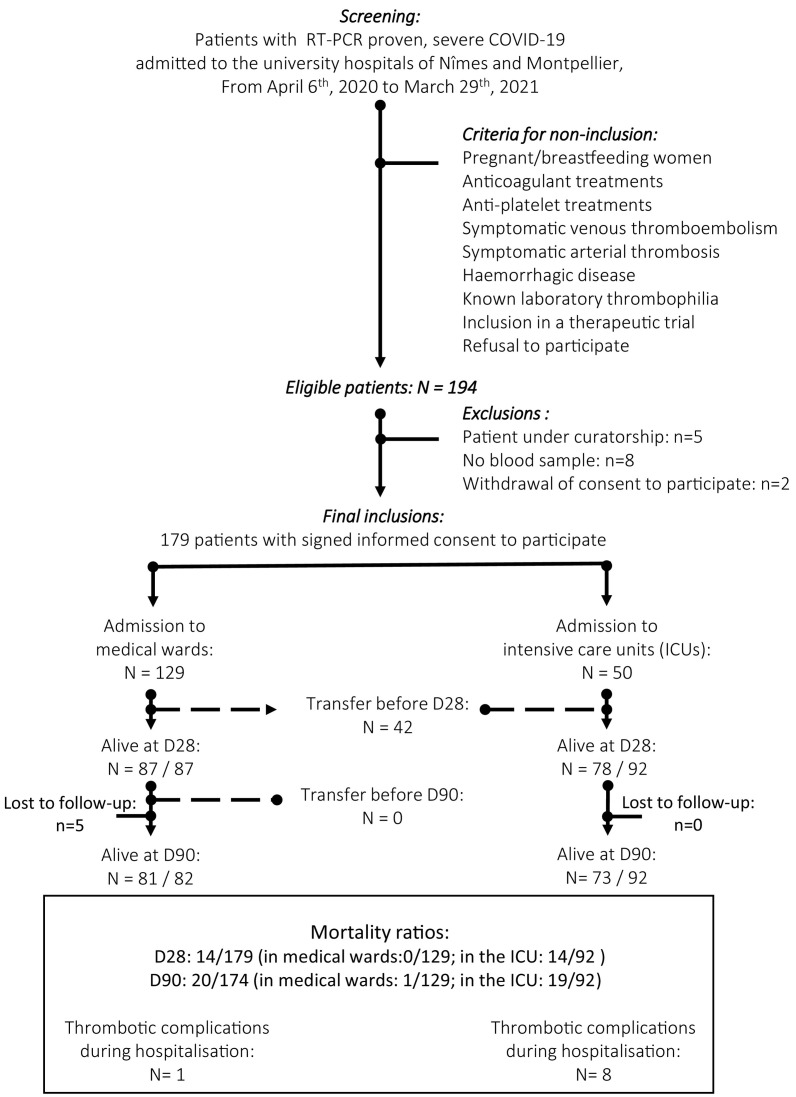
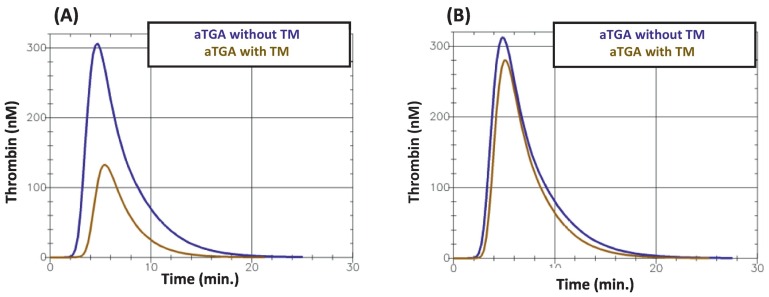
Fig. 2 gives an example of the obtained thrombin generation curves.
Fig. 2.
Two examples of the aTGA curves obtained (A) in a patient who survived at D28 and (B) a patient who did not. TM: thrombomodulin.
Table 2 analyses the values of the aTGA parameters at inclusion categorised according to the vital status at D28. None of them were significantly different between survivors and non-survivors. Of note, we observed a non-significant 115 nM.min decrease of the ETP values in the non-survivor group compared to the survivor-group: this may reflect a degree of consumption coagulopathy hampering thrombin generation.
3.4. Secondary outcomes
Among the demographic, clinical and laboratory data at inclusion (Table 1), non-survivors at D90 had higher age values, a higher rate of patients taking non-steroidal anti-inflammatory drugs or statins, higher SOFA score values, lower blood oxygen saturation levels, higher percentages of lung involvement according to the chest CT scan, lower diastolic blood pressure but higher systolic blood pressure values, a higher rate of patients taking antibiotics, a higher rate of mechanical ventilation, a higher rate of treatment by an antiplatelet agent, higher values for white blood cells, creatinine, CPK and PaCO2.
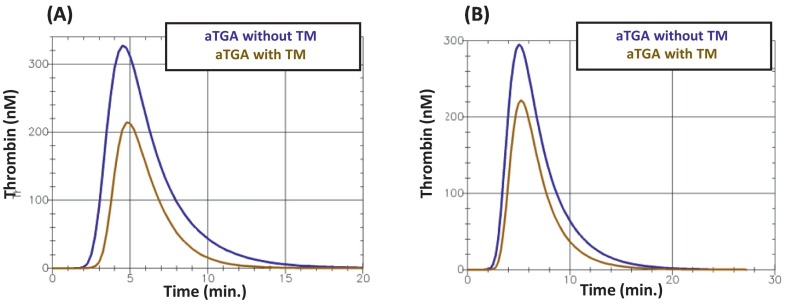
Fig. 3 gives an example of the obtained thrombin generation curves.
Fig. 3.
Two examples of the aTGA curves obtained (A) in a patient who survived at D90 and (B) a patient who did not. TM: thrombomodulin.
None of the aTGA parameters at inclusion were significantly associated with the vital status at D90 either (Table 3 ). Here also, we observed a non-significant 100 nM.min decrease of the ETP values in the non-survivor group compared to the survivor-group which may denote a relative degree of consumption coagulopathy.
Table 3.
Values of aTGA parameters according to vital status at Day 90 (D90). AUC: area under the receiver operating characteristic curve. OR: odds ratio.
| Survival, D90 |
||||||
|---|---|---|---|---|---|---|
| THROMBIN GENERATION ASSAY TGA, parameters | Yes: N=154a | No: N=20a | Pb | AUC | OR, 95 % C.I. | Pc |
| Without thrombomodulin, TM- | ||||||
| Lag time (LT_TM-), min. | 3.9 (2.4) | 4.6 (2.4) | 0.3 | 0.579 (0.438–0.719) | 1.08 (0.90–1.27) | 0.3 |
| Normalised LT_TM- | 1.9 (1.2) | 2.2 (1.1) | 0.4 | 0.559 (0.404–0.715) | 1.15 (0.78–1.60) | 0,4 |
| Velocity index (V_TM-), nmol.min.−1 | 190.9 (116.5) | 216.1 (166.1) | 0.8 | 0.479 (0.312–0.647) | 1.00 (1.00–1.01) | 0.4 |
| Normalised V_TM- | 177.4 (109.3) | 192.9 (147.4) | 0.9 | 0.489 (0.313–0.665) | 1.00 (1.00–1.00) | 0.6 |
| Thrombin peak height (PH_TM-), nmol.L−1 | 278.1 (100.7) | 273.6 (136.0) | > 0.9 | 0.491 (0.323–0.660) | 1.00 (1.00–1.00) | 0.9 |
| Normalised PH_TM-, | 133.0 (48.8) | 126.7 (63.5)) | 0.9 | 0.511 (0.338–0.684) | 1.00 (0.99–1.01) | 0.6 |
| Time to peak (TTP_TM-), min. | 6.2 (2.9) | 7.0 (4.0) | 0.7 | 0.530 (0.373–0.687) | 1.06 (0.96–1.10) | 0.3 |
| Normalised TTP_TM, | 1.4 (0.7) | 1.6 (0.9) | 0.7 | 0.528 (0.364–0.692) | 1.28 (0.69–2.18) | 0.3 |
| Start tail (ST_TM-), min. | 19.6 (5.6) | 21.2 (10.1) | 0.8 | 0.517 (0.361–0.672) | 1.03 (0.96–1.10) | 0.3 |
| Normalised ST_TM- | 1.0(0.3) | 1.1 (0.5) | 0.6 | 0.565 (0.395–0.736) | 1.91 (0.52–5.88) | 0.3 |
| Endogenous thrombin potential (ETP_TM-), nmol.min. | 1437.5 (379.5) | 1325.5 (496.3) | 0.5 | 0.452 (0.292–0.612) | 1.00 (1.00–1.00) | 0.2 |
| Normalised ETP_TM- | 111.2 (29.5) | 101.3 (38.1) | 0.4 | 0.537 (0.375–0.700) | 0.99 (0.97–1.01) | 0.2 |
| With thrombomodulin, TM+ | ||||||
| Lag time (LT_TM+), min. | 4.6 (2.2) | 5.2 (2.5) | 0.3 | 0.576 (0.388–0.719) | 1.11 (0.89–1.33) | 0.3 |
| Velocity index (V_TM+), nmol.min.−1 | 173.3 (124.7) | 211.3 (166.3) | 0.5 | 0.550 (0.383–0.717) | 1.00 (1.00–1.01) | 0.3 |
| Thrombin peak height (PH_TM+), nmol.L−1 | 215.2 (112.8) | 235.0 (135.3) | 0.5 | 0.553 (0.387–0.718) | 1.00 (1.00–1.01) | 0.5 |
| Time to peak (TTP_TM+), min. | 6.5 (2.5) | 7.1 (3.1) | 0.6 | 0.542 (0.385–0.699) | 1.08 (0.89–1.33) | 0.3 |
| Start tail (ST_TM+), min. | 16.1 (2.9) | 16.8 (3.3) | 0.3 | 0.576 (0.413–0.739) | 1.07 (0.90–1.23) | 0.4 |
| Endogenous thrombin potential (ETP_TM+), nmol.min. | 874.6 (424.6) | 926.1 (499.7) | 0.6 | 0.542 (0.376–0.708) | 1.00 (1.00–1.00) | 0.6 |
| Thrombomodulin effect on TGA | ||||||
| (ETP_TM+: ETP_TM-) ratio | 0.6 (0.2) | 0.6 (0.3) | 0.5 | 0.552 (0.388–0.715) | 1.84 (0.20–19.3) | 0.6 |
Mean (SD).
Wilcoxon rank sum test.
Wald test for OR = 1.
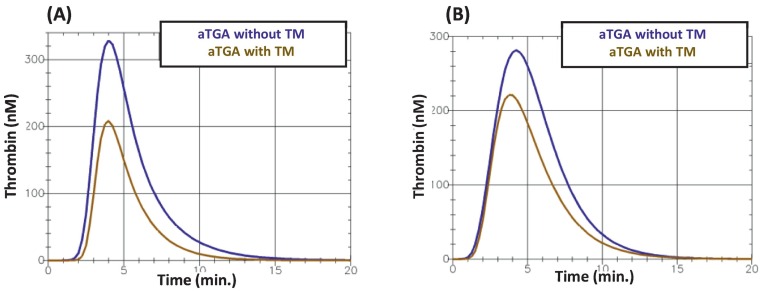
In the analysis of the aTGA parameter values categorised according to whether the patient had required later transfer to an ICU (Table 4), the velocity index and thrombin peak height values, with and without a TM challenge, were higher in transferred patients than in non-transferred patients, as was the endogenous thrombin potential obtained after TM supplementation, the TM-induced decrease in ETP being consequently significantly lower in non-survivors. This relative resistance of ETP to the addition of TM was the most significant finding associated with secondary transfer to ICU, and the corresponding average odds ratio reached 15.5 (95%CI, 2.15–132, p = 0.009). The corresponding mean AUC value ranged from 0.6 to 0.8 (0.660, 95%CI 0.541–0.799), thus indicating good (although not very good) strength of agreement. Supplementary Table 1 shows the patients' characteristics according to the occurrence of later transfer to an ICU.
Table 4.
Values of aTGA parameters in patients initially admitted to a medical ward, according to the need for later transfer to an ICU due to clinical worsening. AUC: area under the receiver operating characteristic curve. OR: odds ratio.
| Transfer to ICU after an initial admision into a medicine ward |
||||||
|---|---|---|---|---|---|---|
| THROMBIN GENERATION ASSAY TGA, parameters | No, N=87a | Yes, N=42a | Pb | AUC | OR, 95 % C.I. | Pc |
| Without thrombomodulin, TM- | ||||||
| Lag time (LT_TM-), min. | 3.8 (2.8) | 4.1 (2.0) | 0.2 | 0.576 (0.464–0.689) | 1.04 (0.88–1.22) | 0.6 |
| Normalised LT_TM- | 1.9 (1.5) | 2.0 (0.9) | 0.2 | 0.571 (0.448–0.694) | 1.07 (0.76–1.50) | 0.7 |
| Velocity index (V_TM-), nmol.min.−1 | 167.6 (105.1) | 217.4 (135.9) | 0.040 | 0.620 (0.498–0.742) | 1.00 (1.00–1.01) | 0.05 |
| Normalised V_TM- | 159.5 (107.4) | 193.2 (119.6) | 0.086 | 0.605 (0.478–0.732) | 1.00 (1.00–1.01) | 0.2 |
| Thrombin peak height (PH_TM-), nmol.L−1 | 260.3 (94.9) | 288.65(112.9) | 0.050 | 0.615 (0.491–0.739) | 1.00 (1.00–1.01) | 0.2 |
| Normalised PH_TM- | 125.9 (48.2) | 134.9 (53.6) | 0.15 | 0.589 (0.462–0.715) | 1.00 (1.00–1.01) | 0.4 |
| Time to peak (TTP_TM-), min. | 6.2 (3.4) | 6.4 (3.3) | 0.8 | 0.518 (0.397–0.640) | 1.02 (0.89–1.15) | 0.7 |
| Normalised TTP_TM- | 1.4 (0.9) | 1.5 (0.7) | > 0.9 | 0.504 (0.375–0.633) | 1.09 (0.62–1.84) | 0.7 |
| Start tail (ST_TM-), min. | 19.9 (5.9) | 20.0 (8.8) | 0.2 | 0.573 (0.452–0.694) | 1.00 (0.94–1.06) | > 0.9 |
| Normalised ST_TM- | 1.1 (0.3) | 1.1 (0.5) | 0.5 | 0.545 (0.418–0.673) | 1.19 (0.38–3.44) | 0.7 |
| Endogenous thrombin potential (ETP_TM-), nmol.min. | 1407.9 (389.0) | 1388.9 (401.4) | > 0.9 | 0.504 (0.389–0.618) | 1.00 (1.00–1.00) | 0.8 |
| Normalised ETP_TM- | 108.9 (29.7) | 107.6 (31.5) | > 0.9 | 0.499 (0.376–0.621) | 0.98 (0.97–1.00) | 0.8 |
| With thrombomodulin, TM+ | ||||||
| Lag time (LT_TM+), min. | 4.4 (2.2) | 4.6 (1.7) | 0.2 | 0.573 (0.456–0.689) | 1.17 (0.89–1.49) | 0.7 |
| Velocity index (V_TM+), nmol.min.−1 | 141.2 (108.2) | 208.8 (132.6) | 0.007 | 0.665 (0.546–0.783) | 1.00 (1.00–1.01) | 0.06 |
| Thrombin peak height (PH_TM+), nmol.L−1 | 182.9 (100.9) | 245.8 (110.0) | 0.003 | 0.680 (0.562–0.798) | 1.00 (1.00–1.01) | 0.05 |
| Time to peak (TTP_TM+), min. | 6.4 (2.5) | 6.3 (2.0) | 0.8 | 0.487 (0.364–0.609) | 1.00 (0.82–1.18) | > 0.9 |
| Start tail (ST_TM+), min. | 15.9 (2.8) | 15.5 (1.9) | 0.7 | 0.480 (0.363–0.597) | 1.00 (0.76–1.11) | 0.5 |
| Endogenous thrombin potential (ETP_TM+), nmol.min. | 752.4 (386.0) | 952.0 (392.7) | 0.008 | 0.663 (0.542–0.784) | 1.00 (1.00–1.00) | 0.05 |
| Thrombomodulin effect on TGA | ||||||
| (ETP_TM+: ETP_TM-) ratio | 0.5 (0.2) | 0.6 (0.2) | 0.009 | 0.660 (0.541–0.799) | 15.5 (2.15–132) | 0.009 |
Mean (SD).
Wilcoxon rank sum test.
Wald test for OR = 1.
Fig. 4 gives an example of the obtained thrombin generation curves.
Fig. 4.
Two examples of the aTGA curves obtained (A) in a patient who did not require later transfer to ICU and (B) a patient who required a later transfer to ICU. TM: thrombomodulin.
None of the aTGA parameters were significantly associated with the occurrence of a thrombotic event during the patients' hospital stay (Table 5 ).
Table 5.
Values of aTGA parameters in patients according to the occurrence of any thrombotic event diagnosed during hospital stay.
AUC: area under the receiver operating characteristic curve. OR: odds ratio.
| Thrombotic event during hospital stay |
||||||
|---|---|---|---|---|---|---|
| THROMBIN GENERATION ASSAY TGA, parameters | No, N=170a | Yes, N=9a | Pb | AUC | OR, 95 % C.I. | pc |
| Without thrombomodulin, TM- | ||||||
| Lag time (LT_TM-), min. | 4.0 (2.4) | 4.3 (1.7) | 0.4 | 0.609 (0.393–0.825) | 1.05 (0.98, 1.27) | 0.7 |
| Normalised LT_TM- | 1.9 (1.2) | 2.1 (0.8) | 0.5 | 0.590 (0.349–0.831) | 1.08 (0.43, 1.59) | 0.8 |
| Velocity index (V_TM-), nmol.min.−1 | 191.9 (120.8) | 236.7 (151.8) | 0.4 | 0.592 (0.266–0.918) | 1.00 (1.00, 1.01) | 0.4 |
| Normalised V_TM- | 178.2 (113.0)) | 211.6 (128.3) | 0.5 | 0.591 (0.268–0.914) | 1.00 (1.00, 1.01) | 0.6 |
| Thrombin peak height (PH_TM-), nmol.L−1 | 275.8 (102.4) | 319.2(142.6) | 0.3 | 0.627 (0.291–0.963) | 1.00 (1.00,1.01) | 0.3 |
| Normalised PH_TM- | 131.7 (49.8) | 148.8 (64.3) | 0.3 | 0.626 (0.288–0.965 | 1.01 (0.99, 1.02) | 0.4 |
| Time to peak (TTP_TM-), min. | 6.2 (3.1) | 6.5 (2.5) | 0.9 | 0.479 (0.210–0.748) | 1.03 (0.71, 1.20) | 0.8 |
| Normalised TTP_TM- | 1.4(0.7) | 1.5(0.6) | 0.9 | 0.492 (0.218–0.765) | 1.11 (0.23, 2.16) | 0.8 |
| Start tail (ST_TM-), min. | 19.7 (6.3) | 19.7 (6.3) | 0.9 | 0.519 (0.232–0.806) | 1.00 (0.84, 1.10) | > 0.9 |
| Normalised ST_TM- | 1.0 (0.3) | 1.1(0.4) | 0.8 | 0.530 (0.236–0.823) | 1.11 (0.004, 5.85) | > 0.9 |
| Endogenous thrombin potential (ETP_TM-), nmol.min. | 1416.5 (392.6) | 1541.7 (392.5) | 0.3 | 0.620 (0.359–0.880) | 1.00 (1.00, 1.00) | 0.4 |
| Normalised ETP_TM- | 109.5 (30.6) | 118.1 (28.3) | 0.4 | 0.611 (0.354–0.868) | 0.01 (0.98, 1.04) | 0.5 |
| With thrombomodulin, TM+ | ||||||
| Lag time (LT_TM+), min. | 4.6 (2.2) | 6.2 (3.5) | 0.2 | 0.651 (0.407–0.894) | 1.11 (0.89, 1.33) | 0.3 |
| Velocity index (V_TM+), nmol.min.−1 | 175.7 (127.9) | 196.2 (156.2) | 0.7 | 0.550 (0.215–0.886) | 1.00 (1.00, 1.01) | 0.3 |
| Thrombin peak height (PH_TM+), nmol.L−1 | 215.7 (112.3) | 230.6 (163.9) | 0.6 | 0.558 (0.218–0.898) | 1.00 (1.00, 1.01) | 0.5 |
| Time to peak (TTP_TM+), min. | 6.4 (2.5) | 8.4 (4.5) | 0.4 | 0.613 (0.355–0.871) | 1.08 (0.89, 1.26) | 0.4 |
| Start tail (ST_TM+), min. | 16.0 (2.8) | 18.8 (4.7) | 0.11 | 0.694 (0.44–0.944) | 1.07 (0.90, 1.23) | 0.4 |
| Endogenous thrombin potential (ETP_TM+), nmol.min. | 871.8 (418.2) | 949.9 (678.4) | 0.6 | 0.556 (0.210–0.902) | 1.00 (1.00, 1.00) | 0.6 |
| Thrombomodulin effect on TGA | ||||||
| (ETP_TM+: ETP_TM-) ratio | 0.6 (0.2) | 0.6 (0.4) | >0.9 | 0.515 (0.172–0.858) | 0.64 (0.02–26.6) | 0.8 |
Mean (SD).
Wilcoxon rank sum test.
Wald test for OR = 1.
4. Discussion
This is the first multicentric prospective work evaluating an automated thrombin generation assay, which includes a thrombomodulin challenge, performed in patients entering hospital for the management of severe COVID-19, in its capacity to predict strong clinical outcomes: early survival at Day 28 and late survival at Day 90, later transfer to an ICU before Day 90, and the occurrence of a thrombotic event. The aTGA was unable to predict early or late in-hospital mortality. Neither could the thrombotic events we observed. Only values of the thrombin peak height and of the endogenous thrombin potential after TM challenge, and more than all the TM-mediated lowering of the ETP value, were associated with the likelihood of later transfer to an ICU due to clinical worsening. These data were consistent with a relative resistance of tissue factor-mediated thrombin generation to TM in patients whose health declined towards a need for resuscitation.
This relative resistance of tissue factor-mediated thrombin generation to TM in COVID-19 has also been detected in a Spanish study on 42 patients on admission [12] and was associated with the occurrence of an adverse event. This new putative marker for clinical worsening may help for risk stratification on admission. TM is a type-I transmembrane protein that is mainly expressed on endothelial cells, but also on macrophages, monocytes, neutrophils and platelets, and that has multiple biological functions, among which anti-inflammatory, anticoagulant and fibrinolysis functions: it can be released in the circulation [13]. Elevated plasma levels of soluble TM is a component of the so-called COVID-19 coagulopathy and two recent findings suggest that soluble TM is highly correlated with survival among COVID-19 patient [14], [15], soluble TM levels being considered as a surrogate marker for the degree of endothelial injury. The impairment of the anticoagulant response to extrinsically added TM in our aTGA, i.e. the relative TM resistance, could mean an alteration in the reserve anticoagulant adjustment capacity of patients which could contribute to their unfavourable evolution in an evolving clinical situation of increased demand on the haemostasis system. In this view, the links and correlation between increased soluble TM concentrations and increased resistance of thrombin generation to TM deserves to be investigated, as the impact on the actors of the TM effect on thrombin generation, like protein C, S, and factors V and VIII.
A recent review paper on the clinical use of thrombin generation assays dedicated a sub-section to available results on viral infections and COVID-19 [8] and deplored the analysis of thrombograms in patients receiving heparin and the lack of systematic documentation on assay details. Our work does not fall under this criticism. We also used an automated TGA methodology (standardised as far as possible), which uses commercially-available reagents, to reduce the variability of the assay to acceptable limits [9], [10], as is mandatory for clinical applications.
The same laboratory method that we used was chosen by White and coll. [16] to perform a transversal study between noncritical (n = 34) and critical (n = 75) patients with COVID-19: disease severity did not increase thrombin generation but their prognosis was not studied. It was also used by Benati and coll. [17] in a limited study comparing 16 COVID-19 cases with 19 healthy controls. Their results suggested some degrees of coagulation exhaustion in patients, at least at the stage when intensive care was required. Blasi and coll. [18], again with the same technical option, studied 23 COVID-19 patients and found normal in vitro thrombin generation which persisted on anticoagulation. On the other hand, using the same laboratory tests, Campello and coll. [19] studied 89 patients, 30 of whom had been admitted to the ICU, and found increased thrombin generation at diagnosis which was reduced to levels of healthy controls under standard thromboprophylaxis. An increased thrombin potential with high ETP values over the first week after ICU admission was also observed by Hardy and coll. in a small, longitudinal, observational study [20].
A similar TGA, but manually performed using local reagents, was tested by von Meijenfeldt and coll. [21] in 102 hospitalised patients with COVID-19 receiving various levels of respiratory support: patients with higher respiratory support exhibited substantial ex vivo thrombin generation. As did de Laat and coll. [22] on a cohort of 519 consecutive patients with a suspected SARS-CoV-2 infection: 26 % were positive and had higher lag times and lower ETP inhibition by TM, without any significant differences in patients with thrombosis. De la Morena-Barrio and coll. [3] applied a similar methodology to 127 patients with confirmed COVID-19 hospitalised at a single centre: patients with more prolonged lag time and decreased ETP had more severe diseases, defined according to both vascular events and death, the D-dimer/ETP ratio being associated with in-hospital mortality. Billoir and coll. [4] prospectively tested a manual TGA test without any TM challenge on 85 patients with COVID-19, including 33 critically ill patients, without finding any difference between the 2 severity groups. By lowering the TF concentration to 1 pM in their manually performed TGA test, the same team [5] studied the in-hospital clinical evolution of 99 COVID-19 patients and found that a model containing thrombin peak value among 5 parameters could indicate a risk of worsening, transfer to an ICU or death.
Our study has limitations. Obtaining a perfect blood sample for haemostasis studies in not-so-young patients entering hospital with an acute, distressing disease in which dyspnoea and, sometimes, a sense of impending death increases their anxiety, is always a challenge even for highly trained nurses and doctors. It is not impossible that some of the results are de facto impacted and do not reflect the impact of the disease itself. We also have a significant amount of missing data, which is common when working on emergencies, but the depletion of medical resources and their lack of capacity in the face of the pandemic has made the collection of data more difficult than usual. We also observed few thrombotic events, which probably reduced our chances of finding an association with the aTGT results, if any, due to lack of statistical power. We only tested patients at admission and therefore had no means of knowing whether modifications in the aTGA results day after day might indicate a negative clinical course. There are certain limitations to the generalizability of results as they obviously depend on the spontaneous distribution of patients requiring resuscitation or not upon hospital admission, a balance highly submitted to variations, depending on the number of vaccinated people, the type of SARS-CoV-2 variant, mean age of the local population, their comorbidities etc… The value of a single test may also have limitations, because the tests have to be more comprehensive of the complex situation of thrombogenesis: for instance the mutual behaviour of TGA and D-dimers may need to be taken into account. None of the aTGA-derived variables were significantly correlated with D-dimer values, except the TM-induced modification of ETP values (weak correlation: Spearman's ρ coefficient = 0.16, p < 0.05): they do not therefore provide the same information. However, by taking into account all the TGA parameters with and without TM as well as the shape of the curves, there are a lot of information regarding the coagulable state of the patient. The aTGA is a global coagulation test, sensitive to all changes in protein levels involved in the coagulation cascade, so it can better reflect bleeding and thrombotic risk compared with clotting-time based assays. Another point concerns the circulating half-life of the markers: the relatively long half-life of D-dimers (6–8 h) may provide an advantage in predicting non-immediate clinical events, while aTGA may be subject to more rapid variations. D-dimers are not specific and are increased in many situations, so difficult to use. TGA is on the other hand more comprehensive compared to all other tests measuring one single point estimate. Focusing on thrombotic complications, currently felt as consequences of complex modifications of the whole vascular biology system, the values of a single parameter, aTGA, should probably be interpreted as relative. Looking for a haemostasis-derived prognostic score, which was not our purpose, will probably be better solved with a battery of tests including markers related to the different branches of vascular biology.
Our study also has certain strengths. It was a prospective, bi-centric study and the single entry point of patients through the emergency medicine departments facilitated the systematic screening of consecutive patients. We could rely on a certified hospital ward dedicated to the management of biological resources for clinical research purposes. The tests under focus were centralised in a single laboratory and performed blinded to the clinical outcomes. The overall mortality rates were finally very close to what we had estimated to calculate the number of patients required for inclusion (11.4 % vs. 12 %) and the outcomes were strong, easy-to-confirm clinical ones.
Early reports emphasized the rate of thrombotic complications in COVID-19 patients and described the COVID-19 coagulopathy and endotheliopathy which became a central component of the disease [23], [24]. It was also shown that patients with COVID-19 had increased thrombin generation potential, despite prophylactic anticoagulation, whereas patients with sepsis did not, thus pointing to a particularity [25]. It was thus tempting to imagine that TGA with a TM challenge, currently one of the most promising studied global coagulation assays in many diseases, performed using a technical option for limited variability, might have the capacity to indicate the survival prognosis. However, the results show no or limited legitimacy in that setting. This might suggest, particularly the ETP response to TM evoking relative resistance, a high risk of later transfer to an ICU due to clinical worsening, but the strength of agreement in that setting appears to be too limited to support a clinically relevant decision by itself alone. This particular point will need to be further analysed, incorporating all the cofactors and covariates likely to modulate the predictive capacity. A specific score could be developed that would allow us to offer maximum medical care, earlier on.
The following is the supplementary data related to this article.
Demographic, clinical and laboratory data according to the need for later transfer to an ICU.
Funding
This work was supported by an exceptional internal grant from Nîmes University Hospital dedicated to clinical research projects on COVID-19 and SARS-CoV-2 infection. The funder had no role in the study design, interpretation of results or writing the report.
Ethics approval
All included patients had given informed consent to participate in the study, which was approved by the Ethics Committee “CPP Île-de-France I-Paris-Hôtel Dieu”, on April 24th, 2020, ref. # CPPIDF1–2020-ND48 cat.2. The study was performed in accordance with the 1996 revised version of the 1975 Declaration of Helsinki and its later amendments, and with the French law on clinical research: ref. ANSM (“Agence National de Sécurité des Médicaments et des produits de Santé) #: 1641233, study registration number ID-RCB: 2020-AO1068–31.
ClinicalTrials.gov Identifier: NCT04356950.
CRediT authorship contribution statement
Jean-Christophe Gris conceived and designed the study, contributed to the data analysis and wrote the paper.
Paul Loubet, Didier Laureillard, Albert Sotto, Laurent Muller, Saber Davide Barbar, Claire Roger, Jean-Yves Lefrant, Boris Jung, Kada Klouche, Isabelle Quéré and Antonia Perez-Martin managed the patients.
Taissa Pereira dos Santos and Thibault Mura designed the study methodology and performed the statistical analysis.
Florence Guillotin and Mathias Chéa performed the laboratory work.
All authors contributed to the writing of the paper and approved its final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to thank all the study participants and colleagues who supported us.
We also wish to thank the following key members of the research team at the “Direction de la Recherche Clinique et de l'Innovation”, Nîmes University Hospital: S. Clément, C. Meyzonnier, N. Best, A. Megzari, E. Dupeyron, S. Granier, B. Lafont, C. Masseguin, O. Albert, S. Guerrero, P. Rataboul and M.P. Francheschi.
We are grateful to all the staff at the BESPIM (Département de Biostatistique, Epidémiologie, Santé Publique et Innovation en Methodologie) at Nîmes University Hospital under the direction of T. Mura and P. Fabbro-Peray. We thank Sophie de Boüard for her administrative help and data management. Our thanks also go to Teresa Sawyers for her expert editorial assistance.
We are also grateful to the members of the Laboratory of Haematology of the University Hospital of Montpellier, who managed the reception and preparation of the blood samples of the patients included locally.
We are particularly indebted to Dr. Éva Cochery-Nouvellon, Pharm.D., Ph.D., who efficiently managed the coordination of the laboratory work.
Data availability
The dataset supporting the conclusions of this article are available in the clinical data repository of Nîmes University Hospital, Place du Pr. Robert Debré, 30,029 Nîmes cedex 9, France.
References
- 1.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit. Care. 2020;24(1):360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leentjens J., van Haaps T.F., Wessels P.F., Schutgens R.E.G., Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8(7):e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Morena-Barrio M.E., Bravo-Pérez C., Miñano A., de la Morena-Barrio B., Fernandez-Perez M.P., Bernal E., Gómez-Verdu J.M., Herranz M.T., Vicente V., Corral J., Lozano M.L. Prognostic value of thrombin generation parameters in hospitalized COVID-19 patients. Sci. Rep. 2021;11(1):7792. doi: 10.1038/s41598-021-85906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billoir P., Leprêtre P., Thill C., Bellien J., Le Cam Duchez V., Selim J., Tamion F., Clavier T., Besnier E. Routine and advanced laboratory tests for hemostasis disorders in COVID-19 patients: a prospective cohort study. J. Clin. Med. 2022;11(5):1383. doi: 10.3390/jcm11051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billoir P., Alexandre K., Duflot T., Roger M., Miranda S., Goria O., Joly L.M., Demeyere M., Feugray G., Brunel V., Etienne M., Duchez V.Le Cam. Investigation of coagulation biomarkers to assess clinical deterioration in SARS-CoV-2 infection. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.670694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemker H.C., Giesen P., Al Dieri R., Regnault V., de Smedt E., Wagenvoord R., Lecompte T., S. Béguin S Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 7.Hemker H.C., Giesen P., Al Dieri R., Regnault V., de Smed E., Wagenvoord R., Lecompte T., S. Béguin S The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol. Haemost. Thromb. 2002;32(5–6):249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 8.Binder N.B., Depasse F., Mueller J., Wissel T., Schwers S., Germer M., Hermes B., Turecek P.L. Clinical use of thrombin generation assays. J. Thromb. Haemost. 2021;19(12):2918–2929. doi: 10.1111/jth.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dargaud Y., Trzeciak M.C., Bordet J.C., Ninet J., Negrier C. Use of calibrated automated thrombinography +/- thrombomodulin to recognise the prothrombotic phenotype. Thromb. Haemost. 2006;96(5):562–567. [PubMed] [Google Scholar]
- 10.Calzavarini S., Brodard J., Quarroz C., Maire L., Nützi R., Jankovic J., Rotondo L.C., Giabbani E., Fiedler G.M., Nagler M., Angelillo-Scherrer A. Thrombin generation measurement using the ST genesia thrombin generation system in a cohort of healthy adults: Normal values and variability. Res. Pract. Thromb. Haemost. 2019;3(4):758–768. doi: 10.1002/rth2.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice M.E., Harris G.T. Comparing effect sizes in follow-up studies: ROC area, Cohen’s d, and r. Law Hum. Behav. 2005;29(5):615–620. doi: 10.1007/s10979-005-6832-7. [DOI] [PubMed] [Google Scholar]
- 12.Montano A., Marco A., Jaime F.L.Lopez, Bello I.Fernandez, Gomez I.Marquez, Iglesias J.M.Reguera, Perez M.I.Munoz, Marco P. Thrombomodulin resistance: a new prothrombtic pathway in COVID-19. HemaSphere. 2022;6(S3):2971. doi: 10.1097/01.HS9.0000849628.12518.1d. [DOI] [Google Scholar]
- 13.Boron M., Hauzer-Martin T., Keil J., Sun X.L. Circulating thrombomodulin: release mechanismsMeasurements, and Levels in Diseases and Medical Procedures. TH Open. 2022;11(6):e194–e212. doi: 10.1055/a-1801-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.X Jin Y Duan T Bao J Gu Y Chen Y Li S Mao Y Chen W Xie n.d. The values of coagulation function in COVID-19 patients, PLoS One 15 (10) e0241329, doi:10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed]
- 16.White D., MacDonald S., Edwards T., Bridgeman C., Hayman M., Sharp M., Cox-Morton S., Duff E., Mahajan S., Moore C., Kirk M., Williams R., Besser M., Thomas W. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int. J. Lab. Hematol. 2021;43(1):123–130. doi: 10.1111/ijlh.13329. [DOI] [PubMed] [Google Scholar]
- 17.Benati M., Salvagno G.L., Nitto S., Gelati M., Lavorgna B., Fava C., Minuz P., Lippi G. Thrombin generation in patients with coronavirus disease 2019. Semin. Thromb. Hemost. 2021;47(4):447–450. doi: 10.1055/s-0041-1722844. [DOI] [PubMed] [Google Scholar]
- 18.Blasi A., von Meijenfeldt F.A., Adelmeijer J., Calvo A., Ibañez C., Perdomo J., Reverter J.C., Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J. Thromb. Haemost. 2020;18(10):2646–2653. doi: 10.1111/jth.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campello E., Bulato C., Spiezia L., Boscolo A., Poletto F., Cola M., Gavasso S., Simion C., Radu C.M., Cattelan A., Tiberio I., Vettor R., Navalesi P., Simioni P. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. 2021;59(7):1323–1330. doi: 10.1515/cclm-2021-0108. [DOI] [PubMed] [Google Scholar]
- 20.Hardy M., Michaux I., Lessire S., Douxfils J., Dogné J.M., Bareille M., Horlait G., Bulpa P., Chapelle C., Laporte S., Testa S., Jacqmin H., Lecompte T., Dive A., Mullier F. Prothrombotic disturbances of hemostasis of patients with severe COVID-19: a prospective longitudinal observational study. Thromb. Res. 2021;197(197):20–23. doi: 10.1016/j.thromres.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundström A., Rudberg A.S., Magnusson M., Mackman N., Thalin C., Lisman T. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality. Res. Pract. Thromb. Haemost. 2020;5(1):132–141. doi: 10.1002/rth2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Laat B., Traets M.J.M., De Laat-Kremers R.W.M., Verweij S.P., Ninivaggi M., Jong E., Huskens D., Blok B.A., Remme G.C.P., Miszta A., Nijhuis R.H.T., Herder G.J.M., Fijnheer R., Roest M., Fiolet A.T.L., Remijn J.A. Haemostatic differences between SARS-CoV-2 PCR-positive and negative patients at the time of hospital admission. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0267605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iba T., Warkentin T.E., Thachil J., Levi M., Levy J.H. Proposal of the definition for COVID-19-associated coagulopathy. J. Clin. Med. 2021;10(2):191. doi: 10.3390/jcm10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouck E.G., Denorme F., Holle L.A., Middelton E.A., Blair A.M., de Laat B., Schiffman J.D., Yost C.C., Rondina M.T., Wolberg A.S., Campbell R.A. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic, clinical and laboratory data according to the need for later transfer to an ICU.
Data Availability Statement
The dataset supporting the conclusions of this article are available in the clinical data repository of Nîmes University Hospital, Place du Pr. Robert Debré, 30,029 Nîmes cedex 9, France.