Abstract
Purpose:
High-quality treatment for intact cervical cancer requires external radiation therapy, brachytherapy, and chemotherapy, carefully sequenced and completed without delays. We sought to determine how frequently current treatment meets quality benchmarks and whether new technologies have influenced patterns of care.
Methods and Materials:
By searching diagnosis and procedure claims in MarketScan, an employment-based health care claims database, we identified 1508 patients with nonmetastatic, intact cervical cancer treated from 1999 to 2011, who were <65 years of age and received >10 fractions of radiation. Treatments received were identified using procedure codes and compared with 3 quality benchmarks: receipt of brachytherapy, receipt of chemotherapy, and radiation treatment duration not exceeding 63 days. The Cochran-Armitage test was used to evaluate temporal trends.
Results:
Seventy-eight percent of patients (n=1182) received brachytherapy, with brachytherapy receipt stable over time (Cochran-Armitage Ptrend=.15). Among patients who received brachytherapy, 66% had high–dose rate and 34% had low–dose rate treatment, although use of high–dose rate brachytherapy steadily increased to 75% by 2011 (Ptrend<.001). Eighteen percent of patients (n=278) received intensity modulated radiation therapy (IMRT), and IMRT receipt increased to 37% by 2011 (Ptrend<.001). Only 2.5% of patients (n=38) received IMRT in the setting of brachytherapy omission. Overall, 79% of patients (n=1185) received chemotherapy, and chemotherapy receipt increased to 84% by 2011 (Ptrend<.001). Median radiation treatment duration was 56 days (interquartile range, 47-65 days); however, duration exceeded 63 days in 36% of patients (n=543). Although 98% of patients received at least 1 benchmark treatment, only 44% received treatment that met all 3 benchmarks. With more stringent indicators (brachytherapy, ≥4 chemotherapy cycles, and duration not exceeding 56 days), only 25% of patients received treatment that met all benchmarks.
Conclusion:
In this cohort, most cervical cancer patients received treatment that did not comply with all 3 benchmarks for quality treatment. In contrast to increasing receipt of newer radiation technologies, there was little improvement in receipt of essential treatment benchmarks.
Introduction
External beam radiation therapy combined with brachytherapy has been considered the backbone of curative therapy for stages IB2-IVA cervical cancers for more than 50 years (1–4). Level 1 data demonstrate that the addition of concurrent cisplatin-based chemotherapy to radiation therapy significantly improves local control and overall survival (5-7). Retrospective studies further demonstrate the importance of delivering treatment within 8 to 9 weeks to optimize local control and disease-free survival (8-13). These 3 elements, external beam radiation therapy to target the tumor and regional lymph nodes, carefully sequenced with brachytherapy; weekly concurrent chemotherapy; and treatment duration of 56 days or less, can be viewed as benchmarks for quality treatment of intact cervical cancer (14).
However, this multimodal treatment is complex, posing serious logistical challenges to physicians who may treat few cases per year. Several studies have raised concern that in the United States, many women with cervical cancer did not receive treatment that met these benchmarks. For example, estimates of rates of brachytherapy use ranged from 58% to 88% in recent US studies (15, 16), and another study suggested that use of brachytherapy has actually been decreasing (16). Experts have questioned whether this trend was triggered by the emergence of newer radiation techniques and modalities, such as intensity modulated radiation therapy (IMRT) and stereotactic ablative radiation therapy (SABR), which provide more treatment options but may add complexity to treatment delivery (17). Further evidence that treatment does not meet quality benchmarks comes from a recent study that documented that 43% of cervical cancer patients in surveyed US facilities had treatment protracted beyond 60 days (16, 18). Lesser contemporary evidence is available regarding the adequacy of concurrent chemotherapy delivery, particularly for care delivered in usual community settings in the United States. Moreover, no previous study documented the frequency with which US patients completed treatment that met all 3 benchmarks. Such data are critical for informing future strategies to improve the quality of cervical cancer treatment.
To better understand the frequency and nature of lapses in cervical cancer treatment, we conducted a study of practice patterns in a large cohort of US cervical cancer patients identified using MarketScan Commercial Claims and Encounters database (Truven Health Analytics), an employment-based health care claims database. We sought to characterize the frequency with which treatment met the individual benchmarks for quality treatment, including delivery of brachytherapy, delivery of chemotherapy, and radiation treatment duration, as well as the frequency with which all 3 benchmarks were met.
Methods and Materials
Data
This study was approved by the Institutional Review Board. Patient data used for this study were extracted from the MarketScan de-identified health care claims database that includes employees and spouse and dependent beneficiaries. Beneficiaries originated from 45 US employers and corresponded with approximately 100 payers. Medicare beneficiaries (patients ≥65 years of age) were not included in this dataset. The parent database from which our sample was derived is a convenience sample of 28 million insured US lives, with data obtained from employers, health plans, and state Medicaid agencies. Comprehensive, adjudicated service-level inpatient and outpatient claims are included.
Cohort selection
We identified women 18 to 64 years of age with a diagnosis claim of cervical cancer on the same date as the procedure claim for radiation therapy between 1999 and 2011 and who were continuously enrolled from 12 months prior to 12 weeks after the first radiation therapy date (n=3275 subjects). From within this group, we identified patients with intact cervical cancer treated using radiation with definitive intent as indicated by diagnosis and procedure claims. Specifically, we sequentially excluded patients with metastatic disease (n=369), those who underwent cervical surgery within the preceding 12 months (n=972), who received previous treatment for cervical cancer (n=164), with no claim to indicate completion of a radiation simulation (n=84), and who received ≤10 radiation treatment fractions (n=178). This yielded a final sample of 1508 patients.
Radiation therapy
We defined radiation treatment delivered within 12 weeks of the index date (earliest radiation procedure claim) as part of the primary treatment course. We specified external beam radiation treatment, including indicators of IMRT or SABR treatment using Common Procedural Terminology and International Classification of Diseases, version 9, procedure codes. We also specified brachytherapy modality (high-dose rate [HDR] vs low-dose rate). Unique claims dates defined the number of brachytherapy treatments. We calculated total duration of radiation treatment based on the earliest and latest dates of delivery claims for external beam RT and/or brachytherapy and evaluated whether treatment was delivered within 8 weeks (56 days), 9 weeks (63 days), or 10 weeks (70 days).
We examined use of newer external beam technologies, including IMRT and SABR, during any part of the radiation treatment course. To determine whether IMRT or SABR may have been used as a replacement for brachytherapy, we identified a group of patients whose treatment met the following criteria: (1) >25 external radiation treatment fractions; (2) no brachytherapy; and (3) ≥1 fraction of IMRT or SABR after the 25th radiation treatment fraction. We refer to these patients as treated with “IMRT in the setting of brachytherapy omission” or “SABR in the setting of brachytherapy omission” for the remainder of the article.
For coding RT as well as chemotherapy, we chose to classify receipt of treatments on the basis of any claim submitted for that treatment, regardless of final charges or reimbursements. Our intent was to ensure the most sensitive capture of treatments delivered, regardless of, for example, possible reimbursement contributions from secondary insurance sources.
Chemotherapy
We used procedure codes to identify chemotherapy delivered during the primary treatment course. Unique chemotherapy delivery claims dates defined the number of concurrent cycles. We classified receipt of treatments on the basis of any claim submitted for that treatment, for the reasons outlined in the preceding paragraph.
Compliance with quality benchmarks
We assigned each patient a score indicating whether the patient’s treatment met 0, 1, 2, or 3 of the following benchmark treatment elements in addition to their external beam radiation course: (1) brachytherapy delivered; (2) at least 1 cycle of concurrent chemotherapy delivered; and (3) treatment completed within 63 days (9 weeks). We also assigned scores using a second, more stringent definition in which the chemotherapy benchmark was receipt of at least 4 cycles and the treatment duration benchmark was treatment completed within 8 weeks (56 days). Finally, we assigned scores (ranging from 0-2) using a third, more lenient definition in which treatment duration was not considered and score was based only on the receipt of any brachytherapy and any chemotherapy.
Other covariates
From the MarketScan enrollment file, we determined age; geographic region; employee versus spouse/dependent status; and insurance type during the treatment period (health maintenance organization vs other insurance, including comprehensive, point of service, or preferred provider organization). From inpatient and/or outpatient claims, we determined year of treatment, regional lymph node involvement, and whether the patient presented with hydronephrosis. Presentation with hydronephrosis was coded on the basis of either a diagnosis code indicating this condition or a procedure code indicating placement or removal of a ureteral stent or nephrostomy tube from 8 weeks prior to 6 weeks after the start of radiation therapy (Table E2; available online at www.redjournal.com).
To determine socioeconomic variables, we linked files to the Area Health Resource File (AHRF) (19) according to patients’ county and health service areas (HSA) of residence during the year of their treatment. The National Center for Health Statistics defines HSA as a single county or a cluster of contiguous counties that are relatively selfcontained with respect to hospital care (20). From the AHRF, we determined county-level population density as well as HSA-level radiation oncologist density. The AHRF does not contain specific variables for the density of medical oncologists and gynecologic oncologists. Therefore, as proxy variables for these variables of interest, we used HSA-level density of internal medicine subspecialists and gynecologist subspecialists.
Statistical analysis
We calculated frequency and temporal trends in compliance of treatment with individual quality elements as well as quality indicator scores for multiple treatment elements. We examined temporal trends in treatments delivered between 2000 and 2011 by using the Cochran-Armitage test.
We used logistic regression models to identify independent predictors of treatment use, including predictors of receiving: (1) brachytherapy; (2) HDR brachytherapy (326 patients who never received brachytherapy were excluded from this model); (3) IMRT (53 patients treated before 2003 were excluded from this model because use of IMRT was extremely infrequent before 2003); and (4) chemotherapy. We also modeled predictors of (5) achieving a score of 3 of 3 benchmarks (47 patients with a score of 0 or who were treated in 1999, before concurrent chemotherapy was the established standard of care, were excluded).
To select covariates in logistic models, we used χ2 tests to identify unadjusted associations with P values of <.25 along with clinically significant covariates that did not meet this threshold for significance. Model fit was assessed using the Hosmer and Lemeshow test. In multivariate models, we excluded 34 patients with missing AHRF variables. All analyses were conducted using SAS version 9.2 software (SAS, Cary, NC) and assumed a 2-tailed alpha value of .05.
Results
Patient characteristics
In 1508 patients, median age was 51 years (interquartile range [IQR], 44-57 years of age). Other patient characteristics are listed in Table 1.
Table 1.
Patient characteristics (N=1508)
| Characteristic | % of sample (no. of patients) |
|---|---|
| Median age (y) (interquartile range) | 51 (44-57 years) |
| Year of diagnosis | |
| 1999 | 0.4 (6) |
| 2000 | 1.2 (18) |
| 2001 | 2.0 (30) |
| 2002 | 3.5 (53) |
| 2003 | 5.8 (87) |
| 2004 | 6.9 (104) |
| 2005 | 8.8 (133) |
| 2006 | 7.2 (109) |
| 2007 | 9.7 (146) |
| 2008 | 13.1 (197) |
| 2009 | 12.7 (192) |
| 2010 | 16.6 (251) |
| 2011 | 12.1 (182) |
| Pelvic/para-aortic node involvement | 15.0 (226) |
| Hydronephrosis | 13.7 (207) |
| Type of insurance* | |
| HMO/capitation | 13.9 (209) |
| PPO/other | 86.1 (1299) |
| Beneficiary role | |
| Employee | 66.4 (1001) |
| Spouse/dependent | 33.6 (507) |
| Geographic region | |
| Northeast | 13.0 (191) |
| Midwest | 25.4 (375) |
| South | 49.0 (722) |
| West | 12.7 (186) |
| Rural/urban population density (persons per county) | |
| ≤20,000 | 6.2 (91) |
| >20,001-250,000 | 42.1 (620) |
| >250,001-1,000,000 | 31.7 (467) |
| >1,000,000-2,000,000 | 9.6 (141) |
| >2,000,000 | 10.5 (155) |
| No. of radiation oncologists per HSA | |
| 0 | 26.9 (397) |
| 1-5 | 30.5 (450) |
| 6-10 | 9.4 (139) |
| 10-20 | 11.9 (162) |
| 20-50 | 14.8 (218) |
| >50 | 7.3 (108) |
| No. of internal medicine subspecialists per HSA | |
| 0 | 3.3 (48) |
| 1-50 | 14.9 (220) |
| 51-100 | 27.9 (411) |
| 101-200 | 36.6 (539) |
| >200 | 17.4 (256) |
| No. of gynecologist subspecialists per HSA | |
| 0 | 14.2 (209) |
| 1-5 | 7.2 (106) |
| 6-10 | 14.0 (206) |
| 10-20 | 45.7 (674) |
| >20 | 18.9 (279) |
Abbreviations: HSA = health service area; HMO = health maintenance organization; PPO = preferred provider organization.
For variables derived from the Area Health Resource File, values are based on the 1474 patients linked to this data source.
Radiation therapy
Patients received a median of 27 (IQR, 25-30) fractions of external beam radiation. Ninety-seven percent of the patients received 20 or more fractions, and 85% received 25 or more fractions; fewer than 1% of patients received 11 to 14 fractions, and 2% received 15 to 19 fractions (Fig. E1; available online at www.redjournal.com). Seventy-eight percent of all patients (n=1182) received brachytherapy. The frequency of brachytherapy did not change significantly over time (Cochran-Armitage Ptrend=.15); 78% of patients received brachytherapy in 2000, compared to 80% in 2011. Of the 1182 patients who received brachytherapy, 66% (n=777) received HDR brachytherapy. Rates of HDR use among patients treated with brachytherapy rose steadily and significantly over time (Ptrend <.001), from 21% in 2000 to 75% in 2011 (Fig. 1). Patients were most frequently treated with 5 HDR treatments (Fig. 2).
Fig. 1.
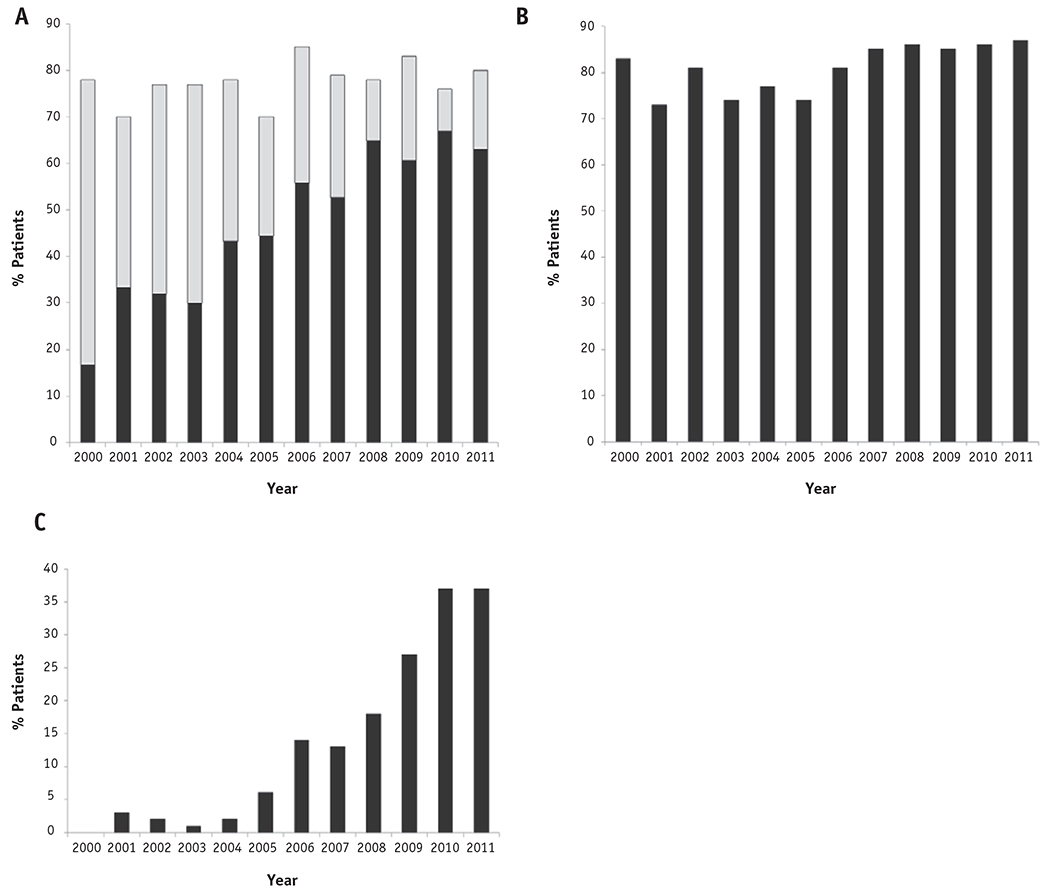
Temporal trends in the use of (a) any brachytherapy (total bar) and high-dose rate brachytherapy (dark bar), (b) chemotherapy, and (c) intensity modulated radiation therapy.
Fig. 2.
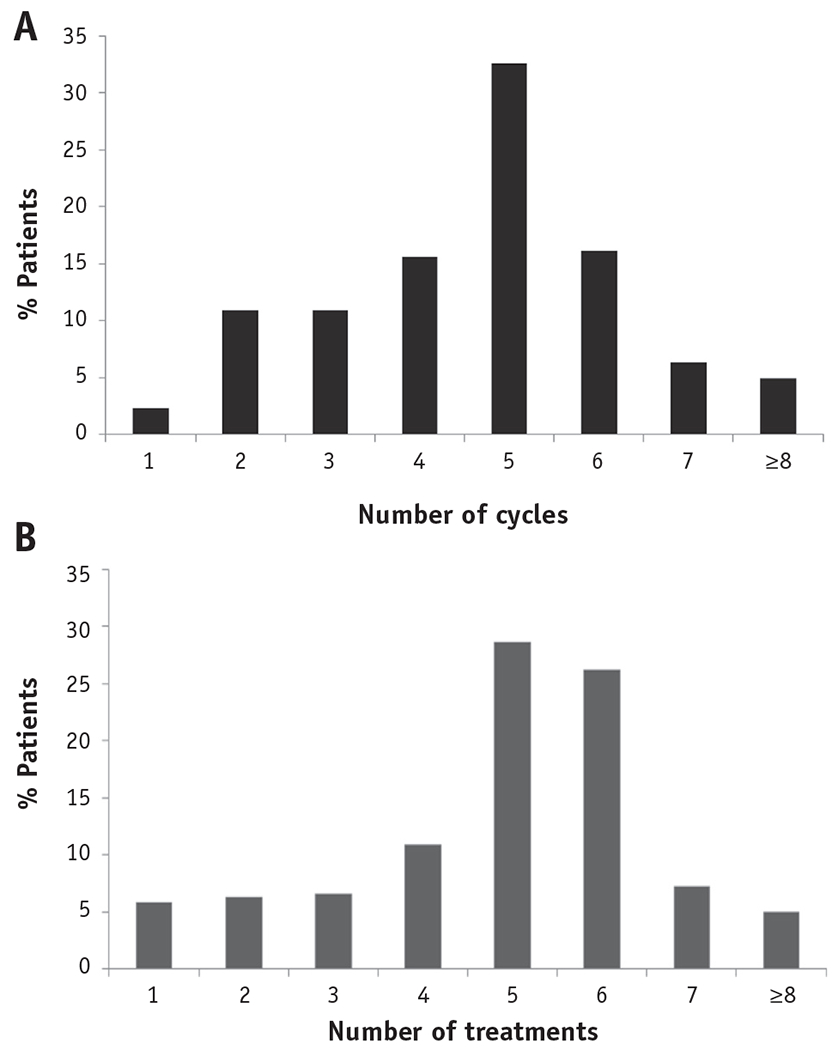
Distributions of (a) number of chemotherapy cycles and (b) number of high-dose rate treatments.
IMRT constituted a portion of treatment in 18% of all patients (n=278); rates of IMRT use increased from 0% in 2000 to 37% in 2011 (Ptrend<.001) (Fig. 1). Seventy-six percent of patients who received IMRT also received brachytherapy, compared to 79% of patients who did not receive IMRT (P=.23). Furthermore, only 2.5% of patients (n=38) met our predefined criterion of having received IMRT in the setting of brachytherapy omission. SABR was used in only 6 patients (0.4%), and of these, 3 patients (0.2%) received SABR in the setting of brachytherapy omission.
Chemotherapy
Seventy-nine percent of all patients (n=1185) received concurrent chemotherapy. Rates of concurrent chemotherapy use increased over time, from 78% in 2000 to 84% in 2011 (Ptrend<.001) (Fig. 1). Of the patients who received chemotherapy, 81% received at least 4 cycles, and 90% received a platinum-based agent.
Independent predictors of use of brachytherapy, HDR brachytherapy, IMRT, and chemotherapy are shown in Table E1 (available online at www.redjournal.com). Notably, patients who were older were less likely to receive any brachytherapy or chemotherapy. Patients who received brachytherapy were also more likely to have been treated using IMRT and concurrent chemotherapy.
Radiation treatment duration
The median duration of radiation therapy was 56 days (IQR: 47-65 days) for the entire sample of 1508 patients. For the 1285 patients treated with brachytherapy, the median duration was 57 days (IQR: 50-66 days). Duration of radiation treatment exceeded 56 days for 50% of patients, 63 days for 36% of patients, and 70 days for 15% of patients (Fig. 3).
Fig. 3.
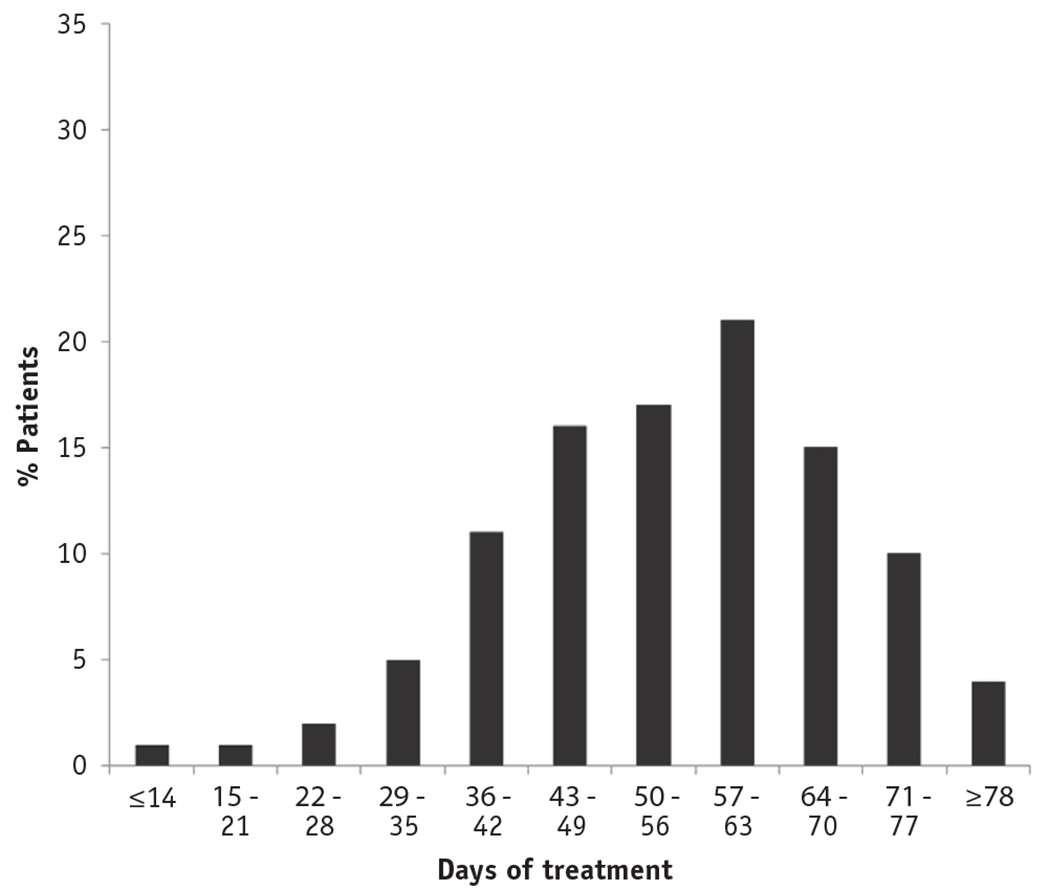
Distribution of radiation treatment duration.
Compliance of treatment with quality benchmarks
Although 98% of patients had treatment that met at least 1 of the 3 quality benchmarks (receipt of brachytherapy, receipt of chemotherapy, or radiation treatment duration of ≤63 days), only 65% of patients had treatment that met both the brachytherapy and chemotherapy benchmarks, and only 44% of patients had treatment that met all 3 quality benchmarks. Little improvement was seen over time: only 47% of patients treated between 2007 and 2011 had treatment that met all 3 benchmarks. Patients were more likely to have treatment that met all 3 benchmarks if they were younger (odds ratio [OR]=1.02 with each year of younger age; 95% confidence interval [CI]: 1.01-1.04, P<.001) or if they received their cancer treatment in a geographic location with a higher density of practicing radiation oncologists (OR = 1.47 for >10 vs ≤10 radiation oncologists practicing in the HSA; 95% CI: 1.11-1.96, P=.008) (Table 2).
Table 2.
Predictors of receiving treatment meeting all 3 quality benchmarks
| Treatment that met all 3 quality benchmarks (Hosmer-Lemeshow P=.19) |
||||
|---|---|---|---|---|
| Variable | Adjusted OR | 95% CI | P | |
| Patient attributes | ||||
| Age (per each y younger age) | 1.02 | 1.01 | 1.04 | <.001 |
| Pelvic/para-aortic node involved | 0.96 | 0.71 | 1.31 | .80 |
| Hydronephrosis | 0.68 | 0.50 | 0.94 | .02 |
| Treatment attributes | ||||
| Year of treatment | .24 | |||
| 2000 | 0.91 | 0.33 | 1.51 | .86 |
| 2001 | 0.64 | 0.27 | 1.67 | .30 |
| 2002 | 0.86 | 0.45 | 1.15 | .66 |
| 2003 | 0.66 | 0.37 | 1.37 | .14 |
| 2004 | 0.81 | 0.48 | 0.81 | .42 |
| 2005 | 0.49 | 0.29 | 1.33 | .01 |
| 2006 | 0.80 | 0.48 | 1.28 | .39 |
| 2007 | 0.79 | 0.49 | 1.55 | .34 |
| 2008 | 0.99 | 0.64 | 1.45 | .98 |
| 2009 | 0.89 | 0.57 | 1.38 | .60 |
| 2010 | 1.03 | 0.68 | 1.55 | .90 |
| 2011 (referent) | - | |||
| Received IMRT | 1.18 | 0.88 | 1.58 | .27 |
| Received HDR brachytherapy | 1.77 | 1.41 | 2.22 | <.001 |
| Sociodemographic attributes | ||||
| HMO vs other insurance | 1.05 | 0.76 | 1.44 | .78 |
| Employee vs other | 0.89 | 0.72 | 1.14 | .41 |
| Geographic region | .34 | |||
| Northeast | 1.04 | 0.74 | 1.47 | .82 |
| Midwest | 0.94 | 0.72 | 1.23 | .65 |
| South (referent) | - | |||
| West | 0.72 | 0.50 | 1.04 | .08 |
| Population, persons per county >1,000,000 vs ≤1,000,000 | 0.91 | 0.68 | 1.23 | .55 |
| No. of radiation oncologists per HSA >10 vs ≤10 | 1.47 | 1.11 | 1.96 | .008 |
| No. of internal medicine subspecialists per HSA >100 vs ≤100 | 0.88 | 0.66 | 1.19 | .48 |
| No. of gynecologist subspecialists per HSA >10 vs ≤10 | 0.80 | 0.60 | 1.06 | .16 |
Abbreviations: HDR = high-dose rate; HMO = health maintenance organization; HSA = health services area; IMRT = intensity modulated radiation therapy; OR = odds ratio.
Data show predictors of receiving treatment meeting all 3 of the quality benchmarks receipt of brachytherapy, receipt of chemotherapy, and radiation treatment lasting 63 days or less.
With more stringent indicators (brachytherapy, ≥4 cycles of chemotherapy, and radiation treatment duration of ≤56 days), only 25% of the entire sample had treatment that met all 3 benchmarks, and only 26% of patients treated between 2007 and 2011 had treatment that met all 3 benchmarks.
Discussion
Although our study of US women undergoing radiation therapy for intact cervical cancer demonstrated that each individual quality benchmark was met in most cases, the proportion of patients in whom all 3 quality benchmarks were met was disappointingly low. In 2011, 80% of patients received brachytherapy, 86% received chemotherapy, and 64% completed radiation treatment within 63 days; however, only 44% of patients received treatment that met all 3 essential, benchmarked standards. Our results suggest that curative treatment is being compromised in many US women with cervical cancer. These results are particularly sobering because our cohort of comparatively younger, insured women represents a relatively advantaged subset of patients. Many US patients are socioeconomically disadvantaged, uninsured, or underinsured and may therefore face greater barriers to health care access and treatment than those in our cohort (21, 22). Therefore, our results could represent the most optimistic scenario of current US cervical cancer care and imply pervasive fracturing of multidisciplinary, multimodal cervical cancer treatment delivery.
Rates of compliance with individual benchmarks in our study were consistent with previous analyses. In another study of 261 US cervical cancer patients treated between 2005 and 2007, 88% of patients received brachytherapy, but this rate of brachytherapy omission was still double the rate of brachytherapy omission in the previous decade (18). A Surveillance, Epidemiology, and End Results database study suggested even lower use of brachytherapy, showing that rates of cervical brachytherapy had fallen to as low as 43% to 58% through 2009 (13, 22), although it was unclear whether these low rates of reported brachytherapy use could have been biased by underreporting (15, 16, 23).
Use of concurrent chemotherapy has been more rapidly disseminated as standard practice since the publication of prospective randomized trials establishing its efficacy (5-7). Before these trials, rates of concurrent chemotherapy ranged from 9% to 35% but dramatically increased to 67% to 80% in the setting of a curative radiation treatment course (24). Our study, which showed similar contemporary chemotherapy use rates, validates these previous findings. The expected fraction would be low for our study patients, whose treatment failed the chemotherapy benchmark due to ineligibility for treatment at presentation (for example, due to poor performance status or older age), given that our sample included only women younger than 65 years of age. Our results also highlight the concern that many patients treated with chemotherapy still failed to receive brachytherapy, undermining the curative potential of treatment.
Treatment prolongation remains a serious problem for US cervical cancer patients. In previous studies, many patients experienced significant treatment prolongations, and up to 43% of patients had a radiation treatment course exceeding 60 days (15, 19). In our study reported here, the radiation treatment duration benchmark was the benchmark achieved least often. Our results warrant further investigation to identify characteristics of facilities that successfully avoided consistent treatment prolongations. We reviewed our institutional data and found that of 388 consecutive patients treated at our institution with chemoradiation during the years 2000 through 2004, 99.5% completed brachytherapy, 73% completed treatment within 56 days, and 92% completed treatment within 63 days. Failure to complete timely treatment likely reflects not only the challenges associated with delivery of chemotherapy or brachytherapy but also the challenges of coordinating complex treatment.
Lapses in high-quality treatment delivery have an important influence on patient outcome. Omission of brachytherapy diminishes long-term overall survival and cause-specific survival by as much as one-third (16). In this study, we were unable to evaluate the quality and dose of brachytherapy delivered, but these factors may further influence disease control (4). Omission of chemotherapy diminishes long-term overall survival by one-third and recurrence-free survival by one-half (20). Each additional day of treatment prolongation beyond 56 days is estimated to reduce the rate of local disease control by 0.5% to 1% per day (8). We can only hope to achieve the highest possible cure rates when treatment includes all of the essential elements.
While our study helps to identify the magnitude of gaps in cervical cancer care, we can only hypothesize regarding the underlying causal mechanisms. Tanderup et al (17) recently conjectured that newer technologies such as IMRT and SABR may be increasingly replacing brachytherapy in community practice, despite ongoing concerns that externalbeam techniques do not permit delivery of an adequate central tumor dose. However, our data did not strongly support the hypothesis that new technology was the dominating factor propelling brachytherapy omission. Although we did not have detailed information about the treatment fields, very few patients in our cohort received either IMRT or SABR in the setting of brachytherapy omission.
Patients in our study were also more likely to receive multiple components of care if they received their cancer treatment in a geographic location with a higher density of practicing radiation oncologists. This variable may have served as a surrogate marker of physicians’ access to the multidisciplinary resources needed to coordinate all components of complex, multimodal treatment. Physician resources are required to communicate and plan treatment along with gynecologic oncologists and/or medical oncologists, hospitals, operating suites, or other radiation facilities equipped with a brachytherapy-compatible suite, and anesthesiologists, all to complete multiple brachytherapy sessions and chemotherapy in a timely manner. Eroding time for clinical care remains a leading universal challenge to US physicians and pressures to increase efficiency and control costs are rising (25). Similar challenges may challenge the complex physician communication and coordination required to deliver multiple components of cervical cancer treatment. Studies are still needed to clarify how these physician or physician-network factors interact with other downstream barriers to cervical cancer treatment delivery.
Cervical cancer patients represent a small percentage of all cancer patients in the United States. However, our results highlight concerns that may have implications beyond patients with cervical cancer. Multimodal treatment approaches increasingly serve as the cornerstone of curative strategies in patients with a large variety of cancer types, including lung, brain, pediatric, prostate, and rectal cancers. Therefore, identifying barriers to multimodal treatment is a critical and timely oncologic public health concern.
Our study has several limitations. First, our results will require validation in older patients and in uninsured and underinsured patients. Second, MarketScan claims could underestimate treatment use because of secondary insurance coverage. However, our reported frequencies of compliance with individual benchmarks are consistent with and therefore externally validated by frequencies of treatment reported in previously published studies. In studies of treatment patterns for other cancer sites using MarketScan data, frequencies of treatment use demonstrate external validity. Finally, claims-based variables indicating hydronephrosis and regional nodal involvement are expected to be specific but not sensitive and therefore are not intended to replace actual staging.
Conclusions
Most patients in this cohort of US women undergoing curative RT received treatment that did not meet all the essential benchmarks for high-quality treatment. Substantial barriers exist to coordinating and delivering complex oncologic treatment, rendering a large portion of patients vulnerable to substandard care.
Supplementary Material
Summary.
We benchmarked 3 measures of quality treatment for intact cervical cancer by analyzing national health insurance claims data. In 1508 patients treated from 1999 to 2011, only 44% received treatment that met all 3 quality benchmarks: delivery of brachytherapy (received by 78% of patients), delivery of concurrent chemotherapy (received by 79% of patients), and radiation treatment duration not exceeding 63 days (achieved in 64% of patients).
Conflict of interest:
Dr Meyer has received sponsor-paid travel from Astra Zeneca.
This work was supported by Cancer Prevention and Research Institute of Texas (S.H.G., RP101207), Duncan Family Institute, a philanthropic gift from Ann and Clarence Cazalot, and National Institutes of Health National Cancer Institute award P30CA016672 (Biostatistics Resource Group), and the Biostatistics Resource Group.
Footnotes
Supplementary material for this article can be found at www.redjournal.org.
Dr Eifel holds a patent issued for an adaptive intracavitary brachytherapy applicator in conjunction with Nucletron/M D Anderson Cancer Center.
References
- 1.Corn BW, Hanlon AL, Pajak TF, et al. Technically accurate intracavitary insertions improve pelvic control and survival among patients with locally advanced carcinoma of the uterine cervix. Gynecol Oncol 1994;53:294–300. [DOI] [PubMed] [Google Scholar]
- 2.Hanks GE, Herring DF, Kramer S. Patterns of care outcome studies. Results of the national practice in cancer of the cervix. Cancer 1983; 51:959–967. [DOI] [PubMed] [Google Scholar]
- 3.Lanciano RM, Won M, Coia LR, et al. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: A final report of the 1973 and 1978 patterns of care studies. Int J Radiat Oncol Biol Phys 1991;20:667–676. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan AN, Moughan J, Small W Jr., et al. The quality of cervical cancer brachytherapy implantation and the impact on local recurrence and disease-free survival in radiation therapy oncology group prospective trials 0116 and 0128. Int J Gynecol Cancer 2012; 22:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 2004;22:872–880. [DOI] [PubMed] [Google Scholar]
- 6.Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol 2002;20:966–972. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Guan QL, Wang K, et al. Radiochemotherapy versus radiotherapy in locally advanced cervical cancer: A meta-analysis. Arch Gynecol Obstet 2011;283:103–108. [DOI] [PubMed] [Google Scholar]
- 8.Fyles A, Keane TJ, Barton M, et al. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol 1992;25:273–279. [DOI] [PubMed] [Google Scholar]
- 9.Girinsky T, Rey A, Roche B, et al. Overall treatment time in advanced cervical carcinomas: A critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys 1993;27:1051–1056. [DOI] [PubMed] [Google Scholar]
- 10.Lanciano RM, Pajak TF, Martz K, et al. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: A patterns-of-care study. Int J Radiat Oncol Biol Phys 1993;25:391–397. [DOI] [PubMed] [Google Scholar]
- 11.Perez CA, Grigsby PW, Castro-Vita H, et al. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys 1995;32:1275–1288. [DOI] [PubMed] [Google Scholar]
- 12.Shaverdian N, Gondi V, Sklenar KL, et al. Effects of treatment duration during concomitant chemoradiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys 2013;86:562–568. [DOI] [PubMed] [Google Scholar]
- 13.Song S, Rudra S, Hasselle MD, et al. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer 2013;119:325–331. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan AN, Thomadsen B. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: General principles. Brachytherapy 2012;11:33–46. [DOI] [PubMed] [Google Scholar]
- 15.Smith GL, Eifel PJ. Trends in the utilization of brachytherapy in cervical cancer in the United States. In regard to Han et al. Int J Radiat Oncol Biol Phys 2014;88:459–460. [DOI] [PubMed] [Google Scholar]
- 16.Han K, Milosevic M, Fyles A, et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013;87:111–119. [DOI] [PubMed] [Google Scholar]
- 17.Tanderup K, Eifel PJ, Yashar CM, et al. Curative radiation therapy for locally advanced cervical cancer: Brachytherapy is NOT optional. Int J Radiat Oncol Biol Phys 2014;88:537–539. [DOI] [PubMed] [Google Scholar]
- 18.Eifel PJ, Ho A, Khalid N, et al. Patterns of radiation therapy practice for patients treated for intact cervical cancer in 2005 to 2007: A quality research in radiation oncology study. Int J Radiat Oncol Biol Phys 2014;89:249–256. [DOI] [PubMed] [Google Scholar]
- 19.Eifel PJ, Moughan J, Erickson B, et al. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: A patterns of care study. Int J Radiat Oncol Biol Phys 2004;60:1144–1153. [DOI] [PubMed] [Google Scholar]
- 20.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137–1143. [DOI] [PubMed] [Google Scholar]
- 21.Ashing-Giwa K, Rosales M. Evaluation of therapeutic care delay among Latina- and European-American cervical cancer survivors. Gynecol Oncol 2013;128:160–165. [DOI] [PubMed] [Google Scholar]
- 22.Ashing-Giwa KT, Gonzalez P, Lim JW, et al. Diagnostic and therapeutic delays among a multiethnic sample of breast and cervical cancer survivors. Cancer 2010;116:3195–3204. [DOI] [PubMed] [Google Scholar]
- 23.Han K, Milosevic M, Fyles A, et al. In reply to Smith and Eifel. Int J Radiat Oncol Biol Phys 2014;88:460–461. [DOI] [PubMed] [Google Scholar]
- 24.Barbera L, Paszat L, Thomas G, et al. The rapid uptake of concurrent chemotherapy for cervix cancer patients treated with curative radiation. Int J Radiat Oncol Biol Phys 2006;64:1389–1394. [DOI] [PubMed] [Google Scholar]
- 25.Wolters Kluwer Health 2013. Physician Outlook Survey. Available at: http://www.wolterskluwerhealth.com/News/Documents/White%20Papers/Wolters%20Kluwer%20Health%20Physician%20Study%20Executive%20Summary.pdf. Accessed May 5, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


