Abstract
The index 2020 ISASS Guideline Statement “Intraosseous Ablation of the Basivertebral Nerve for the Relief of Chronic Low Back Pain” was generated in response to growing requests for background, supporting literature, evidence, as well as proper coding for intraosseous basivertebral nerve ablation. Since the guideline was published, the American Medical Association has added Current Procedural Terminology category I codes for basivertebral nerve ablation: 64628 and 64629. Additionally, the has recognized a need for greater specificity in differentiating various types of low back pain and has designatedthe International Classification of Diseases, 10th revision, Clinical Modification code M54.51, vertebrogenic low back pain, to ensure correct diagnosis. The timing of these additions provides an opportunity to refresh the ISASS Guideline to ensure proper diagnosis and procedural coding and to update the supporting literature and evidence.
Keywords: intraosseous ablation, basivertebral nerve, chronic low back pain, vertebrogenic pain
Introduction
Low back pain (LBP) is the most expensive occupational disorder in the United States, and according to the 2019 Global Burden of Disease data, LBP is now the leading condition for disability worldwide.1 An estimated 49.5 million adults in the United States suffer from chronic LBP (CLBP) with 11% of their life years living with disability.2,3 CLBP is defined as pain that has persisted for more than 12 weeks. The National Institutes of Health Pain Consortium adds that CLBP also is present on at least half of the days in the past 6 months.4 Recent longitudinal studies have shown that higher levels of CLBP pain and disability levels were statistically significantly related to poorer health-related quality of life, societal impact, and health care costs, with disability from CLBP having a stronger association than pain.5
CLBP direct cost estimates have risen sharply over the past couple of decades from approximately $96 million (for a 12-month period reported in a 2008 claims analysis)6 to an estimated $134.5 billion for low back and neck pain spending in 2016; this paid-claims analysis concentrated on adjusted ages from 20 to 64 years, with 57.2% covered by private insurance, 33.7% covered by public insurance, and 9.2% covered by out-of-pocket payments.7 As is the case with many medical conditions, a minority of CLBP patients consume most health care resources. Analyses of commercial payer and Medicare claims databases reveal that 15% of CLBP patients account for 75% of health care costs (MarketScan, Truven Health Analytics, October 2011 to September 2016).
Clinicians treating axial CLBP have historically been challenged with limited objective differentiators for pain sources, as well as poor effect sizes and a lack of high-quality evidence for existing treatments.8 A lack of validated diagnostic reference standards or specific imaging biomarkers for the various sources of pain leads to a diagnosis of “nonspecific” LBP in 85% of patients. This lack of differentiation in pain sources resulted in large variations in treatment, including overtreatment, with poorly validated, nonspecific therapies (Table 1), and in refractory cases, surgical interventions may be recommended, which further drives up the high cost of CLBP treatment.9,10
Table 1.
Care management options often used for treating chronic low back pain.
| 1. Avoidance of activities that aggravate pain |
| 2. Trial of chiropractic manipulation |
| 3. Trial of physical therapy |
| 4. Cognitive support and recovery reassurance |
| 5. Spine biomechanics education |
| 6. Specific lumbar exercise program |
| 7. Home use of heat/cold modalities |
| 8. Low-impact aerobic exercise as tolerated |
| 9. Pharmacotherapy (eg, non-narcotic analgesics and nonsteroidal anti-inflammatory drugs) |
| 10. Spinal injections (eg, epidural steroid injections, medial branch blocks, and facet injections) and/or facet ablations |
While the disc has been the target for many CLBP treatments, the adjacent vertebral endplates (VEPs) have historically been ignored. A significant body of evidence has accumulated over the past 25 years demonstrating that the VEPs are a significant and underappreciated source of CLBP. These studies confirm the presence of pain fibers (nociceptors) in the VEPs that trace back to the basivertebral nerve (BVN) located within the vertebral body and that proliferate with endplate damage.11–18
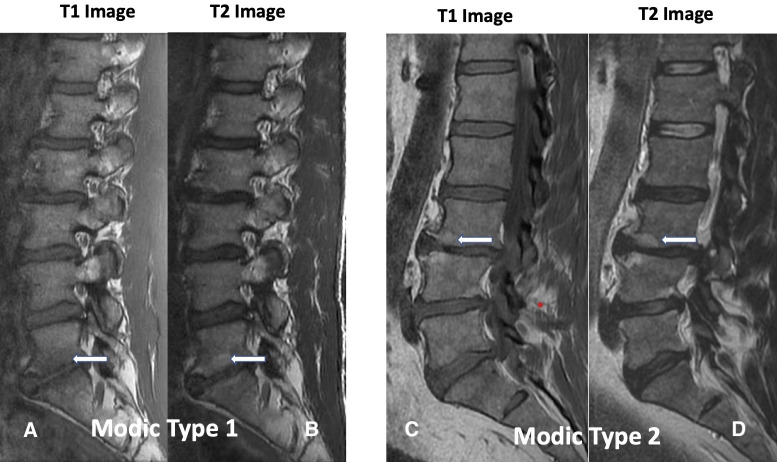
Damaged VEPs with resulting chronic inflammation are readily visible as type 1 and/or type 2 Modic changes on routine magnetic resonance imaging (Figure 1), a specific biomarker for CLPB.19–21 Type 1 and type 2 Modic changes have been associated with more severe CLBP, higher levels of disability, and worse outcomes from conservative care, leading to higher costs of treatment.22,23
Figure 1.
Modic change 1 (MC1) and Modic change 2 (MC2). Images A and B demonstrate decreased signal intensity of T1-weighted images and increased signal intensity on T2-weighted images, respectively (white arrows), corresponding to MC1 at the L5-S1 disc space. Images C and D correspond to L3-L4 MC2 characterized by increased endplate signal intensity on T1-weighted images and on T2-weighted images, respectively (white arrows). There are similar changes at the L4-L5 disc space (no arrows).
Vertebral endplate pain was recently validated by the Centers for Disease Control and Prevention with International Classification of Diseases 10th Revision code M54-51, vertebrogenic pain, allowing for a specific diagnosis. It is estimated that 15% of CLBP patients suffer from primary vertebrogenic pain.
Patients with vertebrogenic pain at the L3-S1 levels describe typical anterior column symptoms with midline low lumbar pain, with or without radiation to the paraspinal region, and infrequently to the gluteal regions. Pain is exacerbated with sitting, bending (forward flexion), and physical activity.24 This is in contrast to posterior column pain that is associated with primary paraspinal tenderness25 and is exacerbated with spinal extension (eg, lumbosacral facet joint pain)25 or pain with provocation maneuvers, which place sheer, rotational, and/or compressive forces on the sacroiliac joint,26–28 and is associated with buttock and posterior thigh pain depending on the age of the patient.26,29
Interruption of pain transmission from the VEPs via destruction of the intraosseous BVN using radiofrequency ablation energy is a treatment option for patients with vertebrogenic pain. The American Medical Association Current Procedural Terminology Editorial Panel recently recognized the evidence for this minimally invasive outpatient treatment by approving Current Procedural Terminology Category 1 codes for thermal destruction of intraosseous BVN: 64628 and 64629. The addition of these codes provides an opportunity to refresh the 2020 ISASS guideline30 to ensure proper diagnosis and procedural coding and to update the supporting literature and evidence.
The BVN ablation (BVNA) treatment arm of the SMART trial was followed prospectively at 2 and 5 years posttreatment. Of the 128 patients in the per protocol treatment arm of the original SMART trial, 106 (83%) were available for 2-year follow-up.31 Clinical improvements in the Oswestry Disability Index (ODI), visual analog scale (VAS), and the short form 36 (SF-36) physical component summary were statistically significant compared with the baseline at all follow-up timepoints through 2 years (3, 6, 9, 12, 18, and 24 months). Patients treated with BVNA for CLBP exhibited sustained clinical benefits in ODI and VAS and maintained high responder rates through 2 years following treatment.
The US patients from the original SMART trial were followed for a minimum of 5 years following BVNA.32 Of the 117 US-treated patients in the original SMART trial, 100 (85%) were available for review with a mean follow-up of 6.4 years (5.4–7.8 years). The mean ODI scores improved from 42.81 to 16.86 at 5-year follow-up and a reduction of 25.95 points (P < 0.001). The mean reduction in VAS pain score was 4.38 points (baseline of 6.74, P < 0.001). In total, 66% of patients reported a >50% reduction in pain, 47% reported a >75% reduction in pain, and 34% reported complete pain resolution. The composite responder rate for BVNA using thresholds of ≥15-point ODI and ≥2-point VAS for function and pain at 5 years was 75%. At baseline, of 100 patients, 30 were actively taking opioids at least once per week; at 5 years, only 8 were actively taking opioids, for a 73% reduction. Additionally, at baseline, 59 of 100 patients had received an injection in the prior 12 months to having BVNA; at 5 years, 4 of 100 had received an injection in the prior 12 months, and only 1 had an injection in the region of the BVNA.
INTRACEPT Randomized Clinical Trial With Prospective, Single-Arm, 1- and 2-Year Follow-up
The INTRACEPT study33,34 was a prospective, open-label, randomized clinical trial (RCT) comparing intraosseous BVNA to the current standard of care for patients with chronic vertebrogenic LBP. A total of 140 patients with CLBP of at least 6 months duration with Modic type 1 or 2 vertebral endplate changes between L3 and S1, were randomized 1:1 using computer-generated permuted blocks of 6 to undergo either BVNA or continue standard care. Treatment at up to 4 vertebral bodies was allowed, and 12% of patients had prior discectomy.
At the time of a prespecified interim analysis,33 140 subjects were randomized (66 BVNA and 74 standard care) at 20 study sites with 104 subjects (n = 51 BVNA and n = 53 standard care) having completed their 3-month primary endpoint visit. Baseline characteristics for all randomized subjects showed a mean age of 49.7 years, a mean ODI of 45.9 (severe impact), a mean VAS of 6.79 (moderate to severe pain), and a percentage of subjects with LBP symptoms ≥5 years of 71.4%. Over 70% of subjects had previously undergone at least 1 trial of physical therapy or a formal exercise program; 42% had received chiropractic care; and 70% had undergone spinal injections, with 16% having undergone prior radiofrequency ablation of a facet or sacroiliac joint(s). Baseline characteristics were similar between the 2 arms, with no significant differences requiring an adjustment in the analysis.
The prespecified interim analysis showed clear statistical superiority (P < 0.001) for all primary and secondary patient-reported outcome measures in the BVNA arm compared with the ongoing standard care control arm.33 This resulted in an independent Data Management Committee recommendation to halt enrollment in the study and offer early crossover to active treatment for the control arm. Comparing the BVNA treatment arm with the standard care control arm, the mean changes in ODI at 3 months were −25.3 points vs −4.4 points, respectively, resulting in an adjusted difference of 20.9 points (P < 0.001). Changes in mean VAS were −3.46 for BVNA vs −1.02 for standard care control, an adjusted difference of 2.44 cm (P < 0.001).
The 12-month INTRACEPT publication reported the outcomes of the fully randomized cohort of 140 patients.34 The publication included the between-arm differences at 3 and 6 months (the point of early cross to active treatment) and the 6-month posttreatment outcomes for the control crossover group who demonstrated a nearly identical statistical improvement as the original BVNA arm for the 3- and 6-month timepoints. Results from BVNA (n = 66) remained superior to standard care (n = 74), with BVNA demonstrating a 25.7-point reduction in mean ODI (P < 0.001) and a 3.8 cm VAS reduction (P < 0.001) from baseline. Sixty-four percent of patients receiving BVNA treatment reported a ≥50% reduction in VAS, and 29% were pain-free. Functional outcomes measured via SF-36 and EQ-5D-5L were also significantly reduced at all timepoints through 12 months from baseline for the BVNA arm. Similarly, the former standard care patients who elected to cross to active treatment with BVNA (92%) demonstrated a 25.9 point mean ODI reduction (P < 0.001) from re-baseline at 6 months post-BVNA.
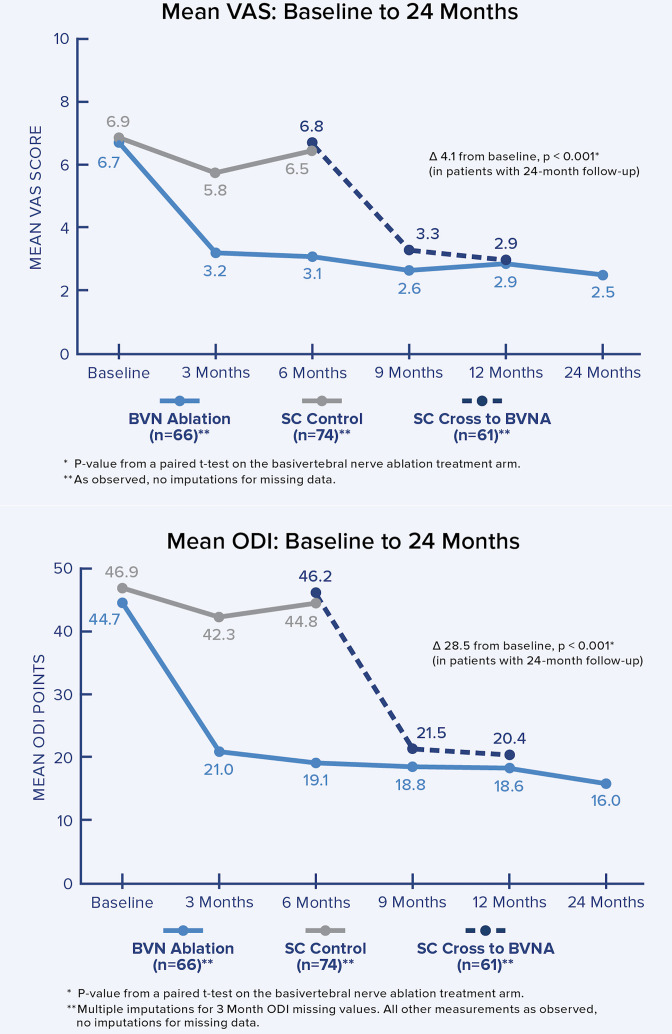
The 2-year follow-up of the original INTRACEPT trial reported on 58 of the original 66 BVNA randomized patients who completed a 24-month visit (88% retention rate).35 Improvements in ODI, VAS, SF-36 physical component summary, and EQ-5D-5L were statistically significant at all timepoints through 2 years. At 24 months, ODI and VAS improved 28.5 points (from baseline 44.5; P < 0.001) and 4.1 cm (from baseline 6.6; P < 0.001), respectively. A combined responder rate of ODI ≥15 and VAS ≥2 was 73.7%. A ≥50% reduction in pain was reported in 72.4% of treatment arm patients, and 31.0% were pain-free at 2 years. At 24 months, only 3 (5%) of patients had steroid injections at the same level as the BVNA, and 62% fewer patients were actively taking opioids. There were no serious device or device-procedure-related adverse events for BVNA reported through 24 months of follow-up. See Figure 2 for the mean ODI and VAS for each study timepoint through 24 months of follow-up.
Figure 2.
Mean ODI and mean VAS over time. These graphs depict the mean ODI and mean VAS at each study follow-up for each arm. A statistically significant and clinically meaningful difference between arms in mean ODI and VAS improvement was demonstrated at 6 months as well as from baseline/re-baseline for each timepoint in patients treated with BVNA, including in control patients that crossed to active treatment. BVNA, basivertebral nerve ablation; ODI, Oswestry Disability Index; SC, standard care; VAS, visual analog scale.
Multicenter, Prospective, Single-Arm Cohort Study
A single-arm prospective, multicenter, open-label cohort study to evaluate the effectiveness of intraosseous BVNA for the treatment of primary vertebrogenic-related CLBP (identified by clinical assessment and type 1 or type 2 Modic changes at L3 to S1) was conducted in 2 typical spine practice settings with more permissive inclusion of typical CLBP patients (such as patients who have had a prior discectomy and users of extended-release opioids).36,37 The primary endpoint was a patient-reported change in ODI from baseline to 3 months postprocedure. Secondary outcome measures included changes in LBP pain VAS, SF-36, EQ-5D-5L, and response rates.
An interim analysis was conducted with approximately 60% of the treated patients who completed their 3-month primary endpoint visit.36 The median age of the n = 28 interim analysis population was 45 years, and baseline values for ODI and VAS were 48.5 and 6.36 cm (on a 0–10 cm scale), respectively, demonstrating a severe level of disability and pain within this interim analysis population. Seventy-five percent of the study patients reported LBP symptoms for ≥5 years, with 25% actively using opioids and 61% previously treated with injections. Clinically meaningful and statistically significant improvements were demonstrated in all outcome measures at the 3-month primary endpoint. The mean reduction in ODI from baseline at 3 months posttreatment was −30.07 ± 14.52 points (P < 0.0001). The mean reduction in VAS pain score from baseline was −3.50 ± 2.33 (P < 0.0001). Using a minimal clinically important difference (MCID) of ≥10-point improvement in ODI, 93% of patients were responders; using an MCID of a ≥20-point improvement in ODI, 75% were responders. Likewise, VAS MCID of a ≥2.0 cm reduction was achieved in 75% of patients. Importantly, in this population of working-aged individuals, 83% reported improvement in work function.
A 1-year follow-up of the full cohort for this single-arm prospective study, including 45 of 47 patients (retention rate of 96%), has been published.37 Patients demonstrated a mean reduction in ODI of 32.31 (P < 0.001) with 88.89% (40/45) patients reporting a ≥15-point ODI decrease at 12 months. The mean VAS pain score decreased by 4.31 at 12 months (P < 0.001) and more than 69% reported a 50% reduction in the VAS pain scale. Similarly, SF-36 and EQ-SD-5L scores improved to 26.27 and 0.22, respectively (each P < 0.001).
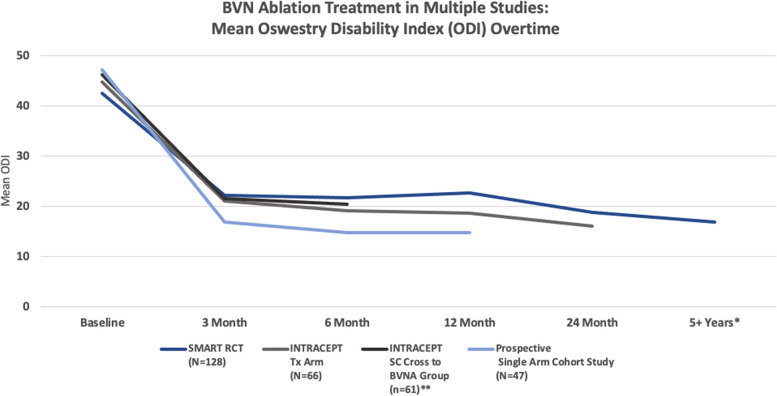
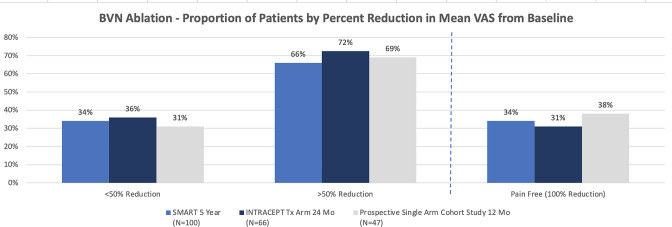
Pain and functional improvements post-BVNA have been demonstrated as both reproducible and durable in the above 2 RCTs and are further confirmed in the prospective single-arm cohort study of approximately 50 typical spine patients from 2 community spine practices (see Figures 3 and 4).
Figure 3.
Multistudy comparison of functional improvement. Comparison of mean ODI over time for the 2 level I RCTs and the chronic low back pain single-arm study.33,34,36,38 *SMART RCT US per protocol treatment arm at mean of 6.4 years. **Standard arm re-baselined and offered active treatment at a median of 5.8 months. BVN, basivertebral nerve; ODI, Oswestry Disability Index; RCT, randomized clincial trial; SC, standard care.
Figure 4.
Comparison of proportion of patients by percent reduction in VAS for the 2 level I RCTs and the chronic low back pain single arm study.34,36,38 BVN, basivertebral nerve; VAS, visual analog scale.
DeVivo et al – Prospective, Single-Arm Study (2020)
This independent study assessed the feasibility and safety of percutaneous computed tomography-guided BVNA.39 BVNA was performed in 56 consecutive patients presenting with vertebrogenic chronic LBP using an articulating bipolar radiofrequency electrode (STAR Tumor Ablation System Merit) off-label. Patients were evaluated at 3 and 12 months posttreatment using a composite endpoint of clinical success, defined as an improvement in VAS ≥2 cm and an improvement in ODI ≥10 points.
The computed tomography-assisted technique was determined to be successful in all patients after assessment of the ablation zone. At 3- and 12-month follow-ups, VAS and ODI scores decreased significantly compared with baseline. Clinical success was reached in 54/56 patients (96.5%) for pain and 54/56 patients (96.5%) for disability. The mean VAS decreased 4.3 cm (range 1–7.5), with ODI decreasing 32.4 (range 6–42) points.
Fishchenko et al – Single Group Observational Study (2021)
This independent prospective study evaluated the outcomes of 19 patients selected for BVNA based upon >6 months of LBP, Modic I and II from L3 to S1, no improvement following drug treatment, and an ODI >30 and VAS >4.40 These subjects were followed for a minimum of 12 months. After 12 months, patients were observed to have a mean decrease in VAS of 5.2 cm from a baseline of 7.6 cm (68% reduction) and a mean improvement in ODI of 27.5 points from a baseline of 49.2 (56% reduction).
Conger et al – Systematic Review (2021)
A systematic review of BVNA was published in 2021.41 Of the 725 publications screened for the analysis, 7 publications with 321 participants were ultimately included. The reported 3-month success rate for ≥50% pain reduction ranged from 45% to 63%. Rates of functional improvement (≥10-point ODI improvement threshold) ranged from 75% to 93%. For comparison to sham treatment, the relative risk of treatment success defined by ≥50% pain reduction and ≥10-point ODI improvement was 1.25 (95% CI: .88–1.77) and 1.38 (95% CI: 1.10–1.73), respectively. For comparison to continued standard care treatment, the relative risk of treatment success defined by ≥50% pain reduction and ≥10-point ODI improvement was 4.16 (95% CI: 2.12–8.14) and 2.32 (95% CI: 1.52–3.55), respectively.
The systematic review concluded there is moderate-quality evidence that suggests this procedure is effective in reducing pain and disability in patients with CLBP who are selected based on type 1 or 2 Modic changes, among other inclusion and exclusion criteria used in the published literature to date. The success of the procedure appears to be dependent on the effective targeting of the BVN.
Conger et al have recently had an updated systematic review of the literature with a single-arm meta-analysis accepted for publication.42 Of the 856 unique records screened, 12 publications met inclusion criteria, representing 6 unique study populations, with 414 participants allocated to receive BVNA. Single-arm meta-analysis showed a success rate of 65% (95% CI 51%–78%) and 64% (95% CI 43%–82%), for ≥50% pain relief at 6 and 12 months, respectively. The rates of ≥15-point ODI improvement were 75% (95%CI 63%–86%) and 75% (95% CI 63%–85%) at 6 and 12 months, respectively.
Procedure Safety
In the studies originating from the United States and listed with https://ClinicalTrials.gov (including the Smart trial, the Intracept trial, and the Multi-center, Prospective, Single Arm Cohort Study36,37), safety data have been collected in 473 clinical trial patients. There has been 1 serious device procedure-related event reported (0.2%): a vertebral compression fracture in a sham-control crossover patient with osteopenia who was taking hormone therapy. The fracture healed spontaneously by 8 weeks. There have been 26 nonserious device procedure-related event reports (5.5%). The most common was an increase in back pain and the onset of leg pain (radiculitis/radiculopathy). All nonserious events were transient in nature with a median time to resolution of 66.5 days and were typically treated with oral medication (written communication, Relievant Medsystems, Inc, March 2022).41,42
In the review of serious adverse events in the MAUDE database, 1 incidence of procedure-related retroperitoneal hematoma and 1 incidence of postprocedure endplate fracture were reported since commercialization of the device in 2018.43
Evidence and Literature Conclusion
Intraosseous ablation of the BVN is supported by a basic and clinical evidence foundation, including a systematic review; a level I, sham-controlled RCT, a second level I RCT against standard conservative management, 3 single group prospective studies and a post hoc secondary analysis. Outcomes data >5 years (mean 6.4 years) following a single BVNA procedure suggest the durability of the treatment effect.
Evidence from the level I studies also indicates that BVNA may assist in decreasing the need for opioids to manage axial LBP. Additionally, it appears that successful BVNA decreases the need for additional treatment (spinal injections and future invasive surgery) in the region where BVNA was performed (Table 2).
Table 2.
Supporting literature and evidence.
| Author and Year | Design | Study Size | Inclusion Criteria | Age of Participants, y | Participant Duration of Pain | Targeting Success | Adverse Events |
| Becker 201744 | SGOS | 16 | CLBP >6 mo Modic 1 or 2 changes L3 to S1 or positive discography | Mean 48.0 (range 34–66) |
Not reported | 91% | n = 4: lumbar pain, buttock pain, dysesthesia, and transient numbness resolved with pain medications. |
| Fischgrund 201838 | RCT | 225 randomized, 147 received BVNA, 128 PP (87%) at 12-mo of follow-up |
CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 46.9 (range 26–69) | 6–12 mo – 4%, 1–2 y – 10%, 2–3 y – 7%, 3–5 y – 12%, >5 y – 67% |
95% | 1 nerve root injury (sham group), 1 vertebral compression fracture (sham group), 1 retroperitoneal hemorrhage (sham group), 7 lumbar radiculitis, and transient motor or sensory deficits all resolved with supportive care. |
| Fischgrund 201931 | SGOS | 106 of 128 PP BVNA (83%) at 24 mo of follow-up | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 47.4 (range 27–69) | 6–12 mo – 5%, 1–2 y – 11%, 2–3 y – 6%, 3–5 y – 14%, >5 y – 64% |
89% | Previously discussed. No additional serious or related adverse events reported through 24 mo of follow-up. |
| Fischgrund 202032 | SGOS | 100 of 117 PP BVNA US population (85%) at 5+ y of follow-up | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 47.2 (range 26–69) | 6–12 mo – 4%, 1–2 y – 11%, 2–3 y – 4%, 3–5 y – 12%, >5 y – 69% |
89% | Previously discussed. No additional serious or related adverse events reported through a mean of 6.4 y of follow-up. |
| Khalil 201933 | RCT | 140 total randomized 51 of 66 randomized to BVNA treatment arm with a 3-mo primary endpoint visit completed (interim analysis population) |
CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 50.0 (range 26–70) | 6–12 mo – 8%, 1–2 y – 6%, 2–3 y – 10%, 3–5 y – 14%, >5 y – 63% |
96% | Interim analysis reported events; n = 15: incisional pain, leg pain/paresthesia, back pain in a new location, urinary retention, and lateral femoral cutaneous neurapraxia. All resolved. |
| Smuck 202134 | RCT | All BVNA treated (at 12 months): 61 of 66 BVNA treatment arm at 12 mo of follow-up (92%) 61 of 74 standard care controls that crossed to active treatment (82% crossover rate) |
CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | BVNA mean 49.4 (range 30–68); Crossover mean 49.5 (range 26–70) |
6–12 mo – 6%, 1–2 y – 6%, 2–3 y – 9%, 3–5 y – 15%, >5 y – 64% 6–12 mo – 3%, 1–2 y – 0%, 2–3 y – 10%, 3–5 y – 7%, >5 y – 80% |
97% | Full cohort events through 12 mo of follow-up; n = 21: 1 incisional pain, 1 nausea, and 1 inability to complete the procedure related to anesthesia, 1 urinary retention, 1 incision infection, 4 back pain related to procedure positioning, 13 leg pain/paresthesia (resolved median 43 d with oral medication). |
| Koreckij 202135 | SGOS | 58 of 66 BVNA treatment arm at 24 months of follow-up (88%) | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 50.4 (range 30–68) | 6–12 mo – 3%, 1–2 y – 5%, 2–3 y – 9%, 3–5 y – 16%, >5 y – 67% |
98% | Previously discussed. No additional serious or related events through 24 mo of follow-up. |
| Truumees 201936 | SGOS | 28 of 48 BVNA single arm with 3-mo primary endpoint visit (interim analysis population) | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 45.2 (SD 8.89) | 6–12 mo – 0%, 1–2 y – 11%, 2–3 y – 14%, 3–5 y – 0%, >5 y – 75% |
97% | n = 3: 1 aborted procedure due to inability to access and 2 leg pain events due to pedicle breach, resolved with oral medication. |
| Macadaeg 202037 | SGOS | 45 of 48 BVNA (full cohort) with 12-mo visit (94%) | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Median 45.0 (range 25–66) | 1–2 y – 14.9%, 2–3 y – 10.6%, 3–5 y – 2.1%, >5 y – 72.3% |
96% | Full cohort through 12 mo of follow-up adverse events; n = 5: 1 aborted procedure due to inability to access, 3 radiculitis associated with potential pedicle breach resolved with oral medications, 1 corneal abrasion, 1 skin reaction to surgical prep. |
| DeVivo 2020 | SGOS | 56 | CLBP >6 mo despite >6 wk treatment, with Modic 1 or 2 changes L3 to S1 | Median 43.0 (range 38–52) | Not reported | 100% | None. |
| Fishchenko 202140 | SGOS | 19 | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 52.6 (SD 6.9) | 1–2 y 73.7%, >5 y 26.3% | Not reported | n = 1: arterial injury of the “lumbalis sinistra” causing a hematoma within the iliopsoas with associated plexitis, treated with endovascular embolization. |
| Markman 201945 | PSA | 225 randomized, 147 received BVNA, 128 PP | CLBP >6 mo despite treatment with Modic 1 or 2 changes L3 to S1, minimum ODI 30, VAS 4 | Mean 46.9 (range 26–69) | 6–12 mo – 4%, 1–2 y – 10%, 2–3 y – 7%, 3–5 y – 12%, >5 y – 67% |
95% | Not reported. |
Abbreviations: BVNA, basivertebral nerve ablation; CLBP, chronic low back pain; ODI, Oswestry Disability Index; PP, per protocol; PSA, prospective single arm; RCT, randomized clinical trial; SGOS, single group observational study; VAS, visual analog scale.
Based on post-BVNA magnetic resonance imaging.
Collectively, the studies reviewed demonstrate that BVNA provides clinically meaningful improvements in pain and function at 5+ years with an excellent safety profile. This evidence supports BVNA as a treatment option for a well-defined subpopulation of CLBP patients.
Indications/Limitations of Coverage
Intraosseous ablation of the BVN from the L3 through S1 vertebrae may be considered medically indicated for individuals with CLBP when all the following criteria are met:
CLBP of at least 6 months duration.
Failure to respond to at least 6 months of nonsurgical management.
Magnetic resonance imaging-demonstrated* MC1 or MC2 in at least 1 vertebral endplate at 1 or more levels from L3 to S1. (*Endplate changes, inflammation, edema, disruption, and/or fissuring.)
Fibrovascular bone marrow changes (hypointense signal for Modic type 1).
Fatty bone marrow changes (hyperintense signal for Modic type 2).
BVNA is NOT indicated in the following:
Patients with severe cardiac or pulmonary compromise.
Presence of implanted pulse generator(s) (eg, pacemaker and defibrillator)/electronic implants except for circumstances where a specific patient safety precaution may be implemented.
Co-existence of other obvious radiographic etiology for patient’s axial CLBP requiring a medically necessary surgical intervention.
Active or chronic infection—systemic or local.
Patients who are pregnant.
Skeletally immature patients (generally age <18 years).
Current or post-trauma, tumor, infection, or poor bone quality compromising vertebral pedicle/body.
Cauda equina syndrome defined as neural compression causing neurogenic bowel or bladder dysfunction.
Radiographic confirmation of gross spinal instability including angular or translatory instability (grade 2 or greater spondylolisthesis) at index level(s).
Morbid obesity precluding satisfactory procedural imaging.
Targeted ablation zone is <10 mm away from a sensitive structure not intended for ablation.
Situation where unintended tissue damage may result based on the clinical assessment by the physician.
Application with electrosurgical instruments NOT tested and specified for use with the current US Food and Drug Administration clearance for the relievant Requests for Designation.
Coding and Coverage History
Intraosseous ablation of the BVN is a procedure commercially performed since 2018. Since 1 January 2022, the procedure is reported with American Medical Association Current Procedural Terminology codes 64628 and 64629:
64628: Thermal destruction of intraosseous BVN, including all imaging guidance; first 2 vertebral bodies, lumbar or sacral.
64629: Each additional vertebral body, lumbar or sacral (list separately in addition to code for primary procedure).
Patients indicated for the procedure may be described diagnostically by the International Classification of Diseases, 10th Revision, codes for medical necessity are as follows:
M47.816: Spondylosis without myelopathy or radiculopathy, lumbar region.
M47.817: Spondylosis without myelopathy or radiculopathy, lumbosacral region.
M51.36: Other intervertebral disc degeneration, lumbar.
M51.37: Other intervertebral disc degeneration, lumbosacral.
M54.50: LBP.
M54.51: Vertebrogenic LBP.
Physician Qualifications
Intraosseous BVNA is a surgical procedure that may be performed by physicians with spinal expertise and advanced training in pedicular access.
Such spinal specialists have successfully completed a residency/fellowship in their specialty and have participated in a specialized training course under the supervision of a physician experienced in the procedure using specimens that permit hands-on experience with the surgical technique.
At this time, the procedure should be performed in either the hospital outpatient setting or ambulatory surgical center where either general anesthesia or moderate conscious sedation is available.
Coverage/Conclusion
The utilization of intraosseous BVNA to address vertebrogenic LBP has become a recognized safe, predictable, and durable surgical method for the management of chronic axial LBP identified using well-established clinical and magnetic resonance imaging findings, Modic type 1 and/or type 2 changes. The procedure is supported by level I evidence including a systematic review and 2 RCTs demonstrating a statistically significant decrease in pain and an improvement in function with outcomes sustained >5 years after a single treatment. These results were seen in a patient population that is one of the most expensive and difficult to provide care for. In this era of rising health care costs and increasing need for therapies to reduce the use of opioids, BVNA may provide a treatment option to fill the treatment gap paradigm for patients that fail nonsurgical treatment.
The ISASS policy does not endorse any specific system to perform the procedure and has made its recommendation that vertebrogenic LBP is most successfully addressed by intraosseous ablation of the BVN. This was based upon the analysis of peer-reviewed publications (see Table 2), including 2 international studies (DeVivo et al39 and Fishchenko et al40). However, at this time, there is only 1 FDA 510(k) system cleared for performing intraosseous BVNA in the United States (K153272 510[k]: Intracept Intraosseous Nerve Ablation System, 2016, and K190504 510[k]: Intracept Intraosseous Nerve Ablation System, 2019).
References
- 1. Institute for Health Metrics and Evaluation (IHME) . United States Profile. IHME, University of Washington; 2018. http://www.healthdata.org/node/5300. August 2021. [Google Scholar]
- 2. Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the global burden of disease study 2017. Ann Transl Med. 2020;8(6):299. 10.21037/atm.2020.02.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S. Lancet low back pain series working group. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 4. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH task force on research standards for chronic low back pain. J Pain. 2014;15(6):569–585. 10.1016/j.jpain.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mutubuki EN, Beljon Y, Maas ET, et al. The longitudinal relationships between pain severity and disability versus health-related quality of life and costs among chronic low back pain patients. Qual Life Res. 2020;29(1):275–287. 10.1007/s11136-019-02302-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehra M, Hill K, Nicholl D, Schadrack J. The burden of chronic low back pain with and without a neuropathic component: a healthcare resource use and cost analysis. J Med Econ. 2012;15(2):245–252. 10.3111/13696998.2011.642090 [DOI] [PubMed] [Google Scholar]
- 7. Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863–884. 10.1001/jama.2020.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keller A, Hayden J, Bombardier C, van Tulder M. Effect sizes of non-surgical treatments of non-specific low-back pain. Eur Spine J. 2007;16(11):1776–1788. 10.1007/s00586-007-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62–68. 10.3122/jabfm.2009.01.080102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173(17):1573–1581. 10.1001/jamainternmed.2013.8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79(1):147–153. 10.1302/0301-620x.79b1.6814 [DOI] [PubMed] [Google Scholar]
- 12. Antonacci MD, Mody DR, Heggeness MH. Innervation of the human vertebral body: a histologic study. J Spinal Disord. 1998;11(6):526–531. [PubMed] [Google Scholar]
- 13. Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003;28(23):2570–2576. 10.1097/01.BRS.0000096942.29660.B1 [DOI] [PubMed] [Google Scholar]
- 14. Fras C, Kravetz P, Mody DR, Heggeness MH. Substance P-containing nerves within the human vertebral body. An immunohistochemical study of the basivertebral nerve. Spine J. 2003;3(1):63–67. 10.1016/s1529-9430(02)00455-2 [DOI] [PubMed] [Google Scholar]
- 15. Bailey JF, Liebenberg E, Degmetich S, Lotz JC. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat. 2011;218(3):263–270. 10.1111/j.1469-7580.2010.01332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lotz JC, Fields AJ, Liebenberg EC. The role of the vertebral end plate in low back pain. Global Spine J. 2013;3(3):153–164. 10.1055/s-0033-1347298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14(3):513–521. 10.1016/j.spinee.2013.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Degmetich S, Bailey JF, Liebenberg E, Lotz JC. Neural innervation patterns in the sacral vertebral body. Eur Spine J. 2016;25(6):1932–1938. 10.1007/s00586-015-4037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 20. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of modic changes. Eur Spine J. 2016;25(11):3723–3734. 10.1007/s00586-016-4459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dudli S, Sing DC, Hu SS, et al. ISSLS Peize in basic Science 2017: intervertebral disc/bone marrow cross-talk with modic changes. Eur Spine J. 2017;26(5):1362–1373. 10.1007/s00586-017-4955-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15(9):1312–1319. 10.1007/s00586-006-0185-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailey JF, Fields AJ, Ballatori A, et al. The relationship between endplate pathology and patient-reported symptoms for chronic low back pain depends on lumbar paraspinal muscle quality. Spine (Phila Pa 1976). 2019;44(14):1010–1017. 10.1097/BRS.0000000000003035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCormick ZL, Sperry BP, Boody BS, et al. Pain location and exacerbating activities associated with treatment success following basivertebral nerve ablation: an aggregated cohort study of multicenter prospective clinical trial data. Pain Med. 2022;23(Suppl 2):S14–S33. 10.1093/pm/pnac069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen SP, Bhaskar A, Bhatia A, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med. 2020;45(6):424–467. 10.1136/rapm-2019-101243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DePalma MJ, Ketchum JM, Saullo TR. Multivariable analyses of the relationships between age, gender, and body mass index and the source of chronic low back pain. Pain Med. 2012;13(4):498–506. 10.1111/j.1526-4637.2012.01339.x [DOI] [PubMed] [Google Scholar]
- 27. Depalma MJ, Ketchum JM, Trussell BS, Saullo TR, Slipman CW. Does the location of low back pain predict its source? PM R. 2011;3(1):33–39. 10.1016/j.pmrj.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 28. Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16(10):1539–1550. 10.1007/s00586-007-0391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12(2):224–233. 10.1111/j.1526-4637.2010.01045.x [DOI] [PubMed] [Google Scholar]
- 30. Lorio M, Clerk-Lamalice O, Beall DP, Julien T. International society for the Advancement of Spine Surgery guideline-intraosseous ablation of the basivertebral nerve for the relief of chronic low back pain. Int J Spine Surg. 2020;14(1):18–25. 10.14444/7002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 2-year results from a prospective randomized double-blind sham-controlled multicenter study. Int J Spine Surg. 2019;13(2):110–119. 10.14444/6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischgrund JS, Rhyne A, Macadaeg K, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2020;29(8):1925–1934. 10.1007/s00586-020-06448-x [DOI] [PubMed] [Google Scholar]
- 33. Khalil JG, Smuck M, Koreckij T, et al. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019;19(10):1620–1632. 10.1016/j.spinee.2019.05.598 [DOI] [PubMed] [Google Scholar]
- 34. Smuck M, Khalil J, Barrette K, et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. Reg Anesth Pain Med. 2021;46(8):683–693. 10.1136/rapm-2020-102259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koreckij T, Kreiner S, Khalil JG, et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-month treatment arm results. N Am Spine Soc J. 2021;8:100089. 10.1016/j.xnsj.2021.100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Truumees E, Macadaeg K, Pena E, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Eur Spine J. 2019;28(7):1594–1602. 10.1007/s00586-019-05995-2 [DOI] [PubMed] [Google Scholar]
- 37. Macadaeg K, Truumees E, Boody B, et al. A prospective, single arm study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. N Am Spine Soc J. 2020;3:100030. 10.1016/j.xnsj.2020.100030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2018;27(5):1146–1156. 10.1007/s00586-018-5496-1 [DOI] [PubMed] [Google Scholar]
- 39. De Vivo AE, D’Agostino G, D’Anna G, et al. Intra-osseous basivertebral nerve radiofrequency ablation (BVA) for the treatment of vertebrogenic chronic low back pain. Neuroradiology. 2021;63(5):809–815. 10.1007/s00234-020-02577-8 [DOI] [PubMed] [Google Scholar]
- 40. Fishchenko IV, Garmish AR, Kravchuk LD, Saponenko AI. Radiofrequency ablation of the basivertebral nerve in the treatment of chronic low back pain: analysis of a small clinical series. Hir Pozvonoč. 2021;18(3):61–67. 10.14531/ss2021.3.61-67 [DOI] [Google Scholar]
- 41. Conger A, Schuster NM, Cheng DS, et al. The effectiveness of intraosseous basivertebral nerve radiofrequency neurotomy for the treatment of chronic low back pain in patients with modic changes: a systematic review. Pain Med. 2021;22(5):1039–1054. 10.1093/pm/pnab040 [DOI] [PubMed] [Google Scholar]
- 42. Conger A, Burnham TR, Clark T, Teramoto M, McCormick ZL. The effectiveness of intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebrogenic low back pain: an updated systematic review with single-arm meta-analysis. Pain Med. 2022;23(Suppl 2):S50–S62. 10.1093/pm/pnac070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Medical Device Reports for FDA MAUDE online database . https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. 30 March 2022.
- 44. Becker S, Hadjipavlou A, Heggeness MH. Ablation of the basivertebral nerve for treatment of back pain: a clinical study. Spine J. 2017;17(2):218–223. 10.1016/j.spinee.2016.08.032 [DOI] [PubMed] [Google Scholar]
- 45. Markman JD, Rhyne AL, Sasso RC, et al. Association between opioid use and patient-reported outcomes in a randomized trial evaluating basivertebral nerve ablation for the relief of chronic low back pain. Neurosurgery. 2020;86(3):343–347. 10.1093/neuros/nyz093 [DOI] [PubMed] [Google Scholar]