Abstract
Background
We sought to compare cardiovascular outcomes, renal function, and diuresis in patients receiving standard diuretic therapy for acute heart failure (AHF) with or without the addition of SGLT2i.
Methods and results
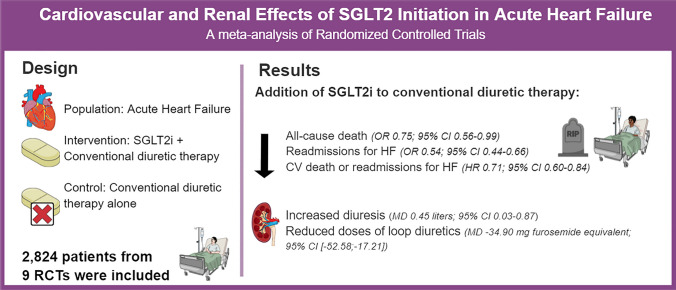
Systematic search of three electronic databases identified nine eligible randomized controlled trials involving 2,824 patients. The addition of SGLT2i to conventional therapy for AHF reduced all-cause death (odds ratio [OR] 0.75; 95% CI 0.56–0.99; p = 0.049), readmissions for heart failure (HF) (OR 0.54; 95% CI 0.44–0.66; p < 0.001), and the composite of cardiovascular death and readmissions for HF (hazard ratio 0.71; 95% CI 0.60–0.84; p < 0.001). Furthermore, SGLT2i increased mean daily urinary output in liters (mean difference [MD] 0.45; 95% CI 0.03–0.87; p = 0.035) and decreased mean daily doses of loop diuretics in mg of furosemide equivalent (MD -34.90; 95% CI [− 52.58, − 17.21]; p < 0.001) without increasing the incidence worsening renal function (OR 0.75; 95% CI 0.43–1.29; p = 0.290).
Conclusion
SGLT2i addition to conventional diuretic therapy reduced all-cause death, readmissions for HF, and the composite of cardiovascular death or readmissions for HF. Moreover, SGLT2i was associated with a higher volume of diuresis with a lower dose of loop diuretics.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-022-02148-2.
Keywords: Acute heart failure, Sodium-glucose cotransporter-2 (SGLT2) inhibitors, Renal function, Diuresis
Introduction
Acute heart failure (AHF) is the leading cause of unplanned hospitalization in those over 65 years and is characterized by new-onset or worsening symptoms of heart failure (HF) [1, 2]. Most patients with AHF are admitted with evidence of fluid overload and are generally treated with escalating doses of intravenous loop diuretics to improve symptoms and reduce morbidity [3, 4]. However, this diuretic regimen is limited by worsening renal function and many patients do not obtain adequate decongestion during the hospital stay for AHF [5–7]. In fact, current treatment for AHF has not changed significantly in decades. Unsurprisingly, post-discharge outcomes have remained poor with 30-day readmission and 1-year mortality rates being as high as 20–30% [8–14]. Therefore, there is an unmet need for new therapeutic strategies in this population.
In recent years, sodium-glucose cotransporter 2 inhibitors (SGLT2i) have been proved to be effective in the treatment of HF for reducing cardiovascular mortality and hospitalizations, regardless of diabetes or ejection fraction status [4, 15–17]. SGLT2i also reduce the composite outcome of cardiovascular death or deterioration of renal function in patients with chronic kidney disease (CKD) [18]. However, less is known about the safety and efficacy of adding SGLT2i to conventional diuretic therapy in patients admitted with AHF.
A prior meta-analysis examining this issue found a reduction in rehospitalizations for HF in patients treated with SGLT2i. However, there was no significant decrease in mortality with SGLT2i, which may have been related to limited power [19]. Therefore, we aimed to perform an updated systematic review and meta-analysis of randomized controlled trials (RCTs) comparing conventional diuretic therapy with or without concomitant SGLT2i for cardiovascular and renal endpoints in patients with AHF.
Methods
This systematic review with meta-analysis was registered in the international prospective register of systematic reviews (PROSPERO) under protocol CRD42022351714. This study was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline [20].
Study eligibility
We included studies that met the following eligibility criteria: (1) peer-reviewed RCTs; (2) comparing conventional diuretic therapy with or without SGLT2i; (3) initiated on hospitalization or within 30 days of hospitalization for AHF; (4) regardless of diabetes or ejection fraction status; and (5) reporting at least one of the clinical outcomes of interest. We excluded studies with (1) patients already taking SGLT2i at the time of admission with AHF; (2) no outcomes of interest; and (3) an overlapping patient population with a larger trial. There were no restrictions concerning the date or language of publication.
Search strategy and data extraction
MEDLINE, Cochrane, and Embase databases were systematically searched on August 27, 2022. The search strategy was as follows: (“acute heart failure” OR “decompensated heart failure” OR “worsening heart failure”) AND (SGLT2 OR ?gliflozin OR empagliflozin OR canagliflozin OR sotagliflozin OR dapagliflozin OR ertugliflozin). We extracted data for (1) all-cause death; (2) readmission for HF defined as rehospitalization or urgent visits for HF; (3) cardiovascular death; (4) the composite endpoint of cardiovascular (CV) death or readmissions for HF; (5) incidence of worsening renal function (WRF); (6) daily urinary output; and (7) daily need of loop diuretic. Incidences of urinary tract infections, ketoacidosis, hypotension, hypoglycemia, and amputations were also accessed for safety evaluation. Endpoint definitions of WRF and readmissions for HF for each included study are shown in Supplementary Table 11 in the Appendix. All identified articles were systematically assessed using the inclusion and exclusion criteria. Article selection and data extraction were undertaken independently by at least two reviewers (between P.C., T.V., and C.D.). Disagreements were resolved by consensus.
Quality assessment
The cochrane tool for assessing risk of bias in randomized trials (RoB 2) was utilized for quality assessment of randomized studies [21]. The risk of bias evaluation was performed independently by two authors (P.C., T.V.) with disagreements resolved by consensus. Publication bias was assessed with funnel-plot analysis and Egger’s test of the all-cause death endpoint to evaluate the symmetric distribution of trials with similar weights.
Sensitivity analysis
We performed a prespecified sensitivity analysis for all-cause death and HF readmissions including only studies with SGLT2i initiation in-hospital setting. In addition, we performed a metaregression analysis for all-cause death to assess for any interaction with the following characteristics: prevalence of diabetes mellitus and the proportion of patients admitted with new-onset HF (de novo HF). We also performed sensitivity analyses restricted to (1) studies with placebo control; (2) a follow-up period of 1 to 12 months; and (3) time-to-event statistical analyses reported as hazard ratios. Concerning the heterogeneous definitions of WRF across studies, we performed leave-one-out sensitivity analyses to ensure the results were not dependent on a single study. We also evaluated the Baujat plot to identify studies that had high contributions to the heterogeneity. Finally, we performed a subgroup analysis of studies with the same definitions of WRF.
Data analysis
Treatment effects for binary endpoints were compared using pooled odds ratios (OR) or hazard ratios (HR) with 95% confidence intervals, whereas continuous endpoints were compared using mean difference (MD) with 95% confidence intervals. We adopted the Mantel–Haenszel test in all binary endpoints and the inverse-variance for continuous endpoints. Heterogeneity was examined with Cochran’s Q test, I2 statistics, and Tau-square using the restricted maximum-likelihood estimator. Heterogeneity was reported as low (I2 = 0–25%), moderate (I2 = 26–50%), or high (I2 > 50%). The fixed-effects model was used for outcomes with low heterogeneity (I2 < 25%) and the random-effects model for studies with moderate to high heterogeneity (I2 > 25%). All statistical analyses were performed using R statistical software, version 4.2.1 (R Foundation for Statistical Computing).
Results
Study selection and baseline characteristics
Our systematic search yielded 596 potential articles, as detailed in Fig. 1. After removing duplicate records and studies with an exclusion criterion based on title/abstract review, 37 remained and were thoroughly reviewed for inclusion and exclusion criteria. Ultimately, 9 RCTs were included, with a total of 2,824 patients, of whom 1,411 patients were assigned to SGLT2i plus conventional diuretic therapy and 1,413 patients were assigned to conventional diuretic therapy alone. Study characteristics are present in Table 1, with additional information in the Supplementary Tables 2 and 3.
Fig. 1.
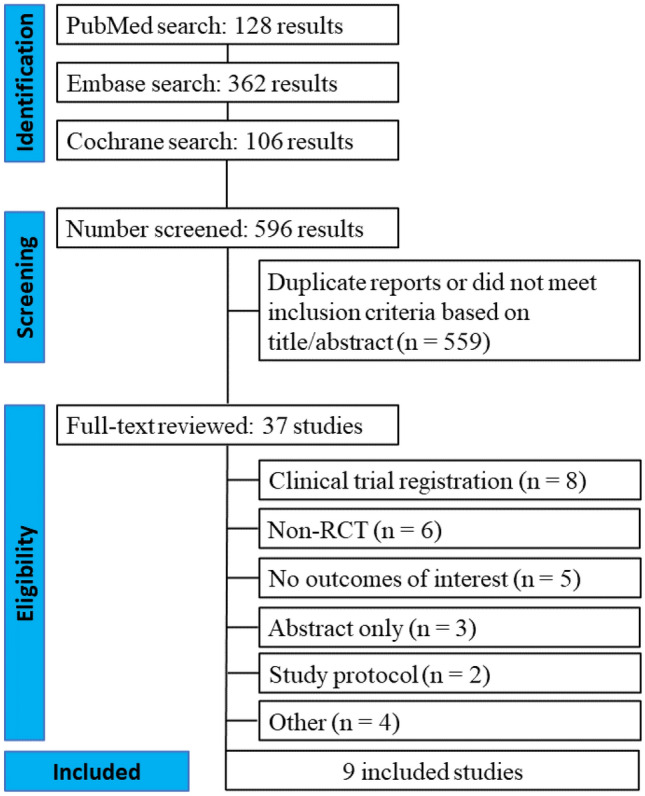
PRISMA flow diagram of study screening and selection. PRISMA flow diagram of study screening and selection. The search strategy in Embase, MEDLINE, and Cochrane yielded 596 studies, of which 37 were fully reviewed for inclusion and exclusion criteria. Nine studies were included in the meta-analysis
Table 1.
Baseline characteristics of included studies
| Charaya et al. 2022 [22] | DELIVER 2022 [23] | EMPA-RESPONSE-AHF 2021 [24, 25] | EMPAG-HF 2022 [26] | EMPULSE 2022 [27] | Ibrahim et al. 2020 [28] | SOLOIST-WHF 2020 [29] | Tamaki et al.a 2021 [30] | Thiele et al.a 2022 [31] | |
|---|---|---|---|---|---|---|---|---|---|
|
Patients (n) SGLT2i/control |
50/52 | 328/326 | 40/39 | 30/29 | 265/265 | 50/50 | 608/614 | 30/29 | 10/9 |
|
Age (Years) SGLT2i/control |
72.6 ± 12.2/74.2 ± 11.3 | 71.9 ± 9.1/71.9 ± 9.2 | 79 (73–83)/73 (61–83) | 72.9 ± 11.2/76.5 ± 8.3 | 71 (62–78)/70 (59–78) | 62.02 ± 8.8/60.64 ± 9.9/ | 69 (63–76)/70 (64–76) | 80 (77–83)/82 (75–84) | 71.8 ± 13.4/72.3 ± 9.9 |
|
% Male SGLT2i/control |
58/52 | 50.6/52.8 | 60/74 | 63.3/58.6 | 67.5/64.9 | 56/52 | 67.4/65.1 | 60/62 | 60/33.3 |
|
%De novo HF SGLT2i/control |
34/38 | 0/0 | 48/46 | 60/48 | 33.2/32.8 | 0/0 | 0/0 | NA | NA |
|
%DM2 SGLT2i/control |
30/30 | 50.9/46.6 | 38/28 | 43.3/34.5 | 46.8/43.8 | 100/100 | 100/100 | 100/100 | 40/11 |
|
%Hypertension SGLT2i/control |
92/92 | NA | 68/56 | 90/86.2 | 77.4/83.4 | 56/62 | NA | 97/93 | NA |
| NTproBNP baseline levels | 5333 (2029–9902)/4381 (2313–11,514) |
1316 (766, 2377), 1256 (732, 2458) |
4406 (2873–6979)/6168 (3180–10,489) | 4726 ± 4516/4823 ± 4995 | 3299 (1843–6130)/3106 (1588–6013) | NA | 1817 (855–3659)/1741 (843–3582) | 2750 (1660–4710)/3950 (2200–8870) | 3562 ± 2527/3996 ± 6293 |
|
LVEF SGLT2i/control |
45.6 ± 15.7/44.4 ± 13.6 | 52.2 ± 7.9/52.8 ± 8.5 | 36 ± 17/37 ± 14 | 45 ± 16/44 ± 14 | 31.0 (23.0–45.0)/32.0 (22.5–49.0) | 32.54 ± 2.99/32.23 ± 2.49 | 35 (28–47)/35 (28–45) | 44 (32–61)/39 (32–51) | 34 ± 11/38 ± 11 |
| SBP at mm Hg | 132.9 ± 17.9/130.1 ± 21.1 | 127.0 ± 15.2/127.4 ± 14.9 | 127 ± 22/121 ± 25 | 139 ± 25/132 ± 21 | 120 (109.0–135.0)/122 (110.0–138.0) | 110.74 ± 12.51/113.08 ± 14.97 | 122 (111–135)/ 122 (112–133) | 120 ± 18/115 ± 16 | 139 ± 23/126 ± 24 |
|
eGFR baseline SGLT2i/control |
55.65 ± 18.17/52.7 ± 17.34 | 57.4 ± 19.0/58.0 ± 18.7 | 55 ± 18/55 ± 18 | 58.2 ± 19.3/62.2 ± 18.2 | 50.0 (36.0–65.0)/54.0 (39.0–70.0) | NA | 49.2 (39.5–61.2)/50.5 (40.5–64.6) | 40 (31–54)/35 (23–54) | 60 ± 20/56 ± 16 |
| SGLT2i drug |
Dapagliflozin 10 mg per day |
Dapagliflozin 10 mg per day |
Empagliflozin 10 mg per day |
Empagliflozin 25 mg per day |
Empagliflozin 10 mg per day |
Dapagliflozin 10 mg per day |
Sotagliflozin 200-400 mg per day |
Empagliflozin 10 mg per day |
Empagliflozin 10 mg per day |
| Follow-up period | 30 days | Median of 2.3 years | 60 days | 30 days | 90 days | Length of hospital stay | Median of 9.2 months | 7 days | 30 days |
| Placebo-controlled | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| The time between hospital admission and randomization to SGLT2i | Until 24 h according to inclusion criteria | 13.76% in-hospital, 22.48% 1 to 7 days after discharge, 63.76% 8 to 30 days after discharge | Until 24 h according to inclusion criteria | Until 12 h according to inclusion criteria | The median time from hospital admission to randomization was 3 days | SGLT2i was initiated during hospitalization without specifying a time | 48.8% in hospital, 51.2% within a median of 2 days after discharge | Until 96 h according to inclusion criteria | The median time between hospital admission and randomization was 72 h |
Values are mean ± SD or median (interquartile range)
DM2, Diabetes mellitus type 2; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal probrain natriuretic peptide; SBP, systolic blood pressure
aThese studies were prematurely terminated due to the COVID-19 pandemic
Cardiovascular endpoints
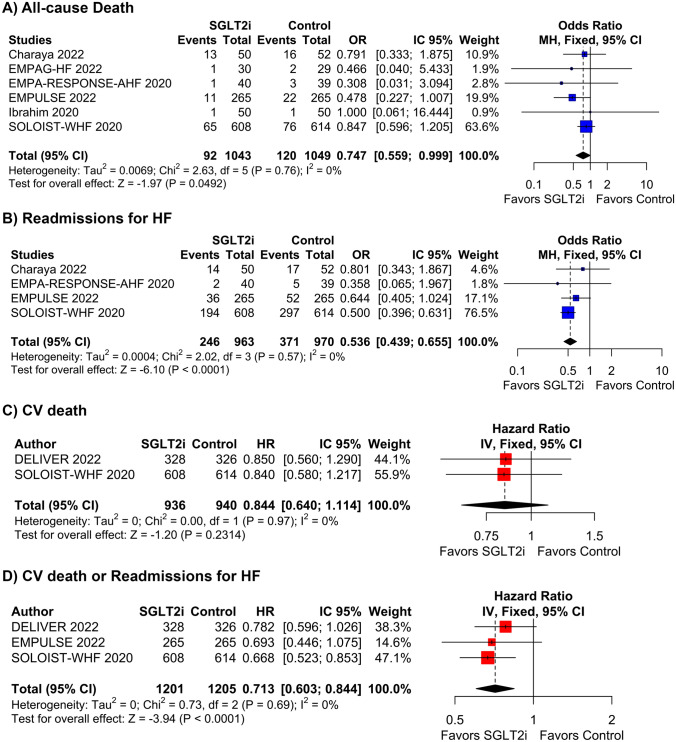
Starting SGLT2i during hospitalization for AHF or shortly after hospital discharge reduced the rate of all-cause deaths (OR 0.75; 95% CI 0.56–0.99; p = 0.049; I2 = 0%; Fig. 2A), readmissions for HF (OR 0.54; 95% CI 0.44–0.66; p < 0.001; I2 = 0%; Fig. 2B), and the composite of CV death or readmissions for HF (HR 0.71; 95% CI 0.60–0.84; p < 0.001; I2 = 0%; Fig. 2D). There was no difference in CV deaths (HR 0.84; 95% CI 0.64–1.11; p = 0.231; I2 = 0%; Fig. 2C) between groups. SOLOIST-WHF had the highest weight for these endpoints, and 51.2% of its population was assigned to SGLT2i treatment only after hospital discharge. All other studies for these endpoints had SGLT2i initiated in the hospital. Thus, we performed a sensitivity analysis withdrawing SOLOIST-WHF from the pooled analysis to address the effects of SGLT2i initiation before hospital discharge. In this analysis, similar results were found in all-cause deaths (OR 0.57; 95% CI 0.34–0.96; p = 0.035; I2 = 0%; Fig. 3A) and readmissions for HF (OR 0.65; 95% CI 0.44–0.97; p = 0.034; I2 = 0%; Fig. 3B).
Fig. 2.
Forrest plot of cardiovascular endpoints with or without SGLT2i in AHF
Fig. 3.
Subgroup analysis of studies with in-hospital treatment initiation with or without SGLT2i
Prespecified metaregressions were performed showing no significant interaction between all-cause death and covariants of (1) diabetes mellitus prevalence and (2) the proportion of patients admitted with new-onset HF (de novo HF). These results are available in Figs. S1–S2 in the supplementary appendix.
In the sensitivity analysis, the beneficial effect of SGLT2i was preserved after restricting follow-up periods to 1–12 months. All-cause deaths (OR 0.75; 95% CI 0.56–0.99; p = 0.048; I2 = 0%), readmissions for HF (OR 0.54; 95% CI 0.44–0.66; p < 0.001; I2 = 0%), and the composite of CV death and readmission for HF (HR 0.67; 95% CI 0.54–0.83; p < 0.001; I2 = 0%) were significantly lower with SGLT2i relative to control. Due to insufficient data, the analysis of CV death in this setting was not performed. These results are available in Fig. S3 in the supplementary appendix.
Additionally, a time-to-event sensitivity analysis calculating HR found similar results to the pooled analysis. CV deaths and the composite of CV death or readmission for HF had already been calculated as HR for the effect estimate because only these data were available for such outcomes. The sensitivity analysis for readmissions for HF (HR 0.69; 95% CI 0.56–0.84; p < 0.001; I2 = 0%) also demonstrated a benefit in favor of SGLT2 inhibitors. There was no difference between the groups regarding all-cause deaths (HR 0.89; 95% CI 0.71–1.12; p = 0.317; I2 = 0%) in the time-to-event analysis, albeit this was likely due to reduced power, as only two studies reported this outcome. These results are available in Fig. S4 in the Supplementary Appendix.
We also performed a subgroup analysis restricted to placebo-controlled studies. Results were similar to those found in the overall pooled analysis of all studies. Readmissions for HF (OR 0.52; 95% CI 0.43–0.64; p < 0.001; I2 = 0%) and the composite of CV death or readmissions for HF (HR 0.71; 95% CI 0.60–0.84; p < 0.001; I2 = 0%) were reduced with SGTL2 inhibitors. There was no difference in the occurrence of CV deaths (0.84; 95% CI 0.64–1.11; p = 0.231; I2 = 0%) between groups. There was also a trend toward reduced all-cause deaths with SGLT2 inhibitor use relative to placebo (OR 0.74; 95% CI 0.54–1.01; p = 0.056; I2 = 0%), though power was also reduced (4 studies). These results are available in Fig. S5 in the supplementary Appendix.
Renal function assessment
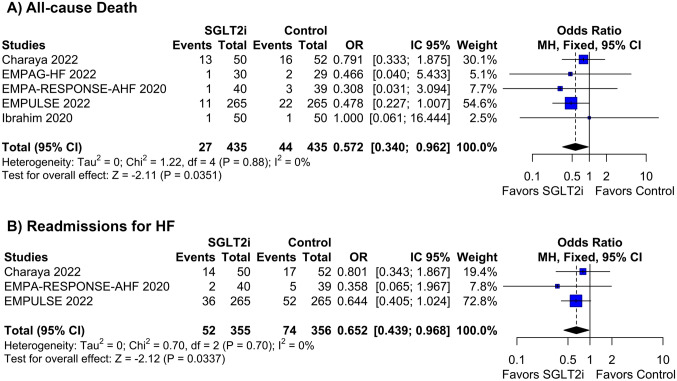
There was no difference in the occurrence of WRF between patients treated with or without SGLT2i (OR 0.75; 95% CI 0.43–1.29; p = 0.290; I2 = 48%; Fig. 4B). The definition of WRF in each study is reported in Table S1 of the Supplementary Appendix. Due to high heterogeneity, we performed a leave-one-out sensitivity analysis by iteratively removing one study at a time to ensure the results were not dependent on a single study. The removal of each study from the pooled analysis did not affect the WRF endpoint, except for the study from Charaya et al. [22]. The withdrawal of this study reduced the frequency of WRF in the SGLT2i group compared with the control group and eliminated the heterogeneity in the endpoint (OR 0.64; 95% CI 0.43–0.95; p = 0.029; I2 = 0%; Fig. S6 in the Supplementary Appendix). The Baujat plot also confirmed the heterogeneity in this endpoint was predominantly from Charaya et al. (Fig. S7 in the Supplementary Appendix).
Fig. 4.
The incidence of worsening renal function was similar with or without SGLT2i in AHF
We also performed a sensitivity analysis joining only studies with the same definition of WRF (an increase ≥ 0.3 mg/dL of serum creatinine level). This analysis found a reduced frequency of WRF in the SGLT2i group compared with the control group (OR 0.43; 95% CI 0.21–0.88; p = 0.021; I2 = 0%; Fig. S8 in the Supplementary Appendix). Moreover, a subgroup analysis including only placebo-controlled trials showed similar results in the frequency of WRF (OR 0.70; 95% CI 0.47–1.06; p = 0.094; I2 = 7%; Fig. S9 in the Supplementary Appendix).
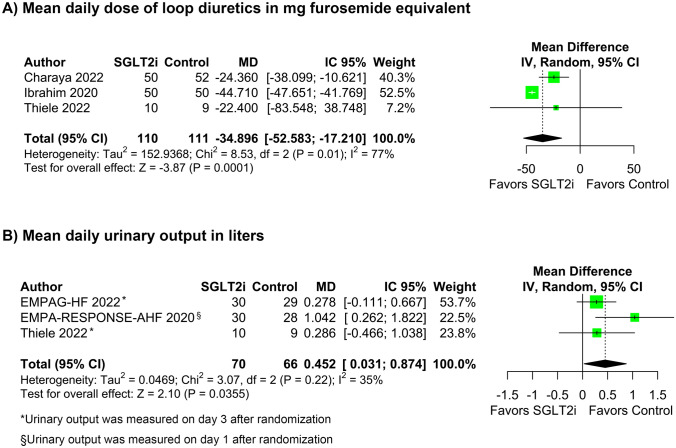
Diuresis parameters
SGLT2i therapy improved diuresis parameters. The mean daily doses of loop diuretics in mg of furosemide equivalent was lower with SGLT2i (MD − 34.90; 95% CI [− 52.58, − 17.21]; p < 0.001; I2 = 77%; Fig. 5A) compared with control group, whereas the mean daily urinary output, in liters, significantly increased (MD 0.45; 95% CI 0.03–0.87; p = 0.035; I2 = 35%; Fig. 5B).
Fig. 5.
The mean daily dose of loop diuretics in milligrams furosemide equivalent was significantly lower with SGLT2i therapy in AHF (A). Daily urinary output in liters was significantly higher with SGLT2i added to conventional diuretic therapy compared with diuretic therapy alone (B)
Safety outcomes
There was no difference between groups in the incidence of urinary tract infection (OR 0.99; 95% CI 0.70–1.42; p = 0.969; I2 = 22%), hypotension (OR 1.16; 95% CI 0.73–1.84; p = 0.526; I2 = 0%), ketoacidosis (OR 0.67; 95% CI 0.19–2.36; p = 0.528; I2 = 0%), amputations (OR 2.35; 95% CI 0.61–9.10; p = 0.217; I2 = 0%). In contrast, the incidence of hypoglycemia was higher in the SGLT2i group (OR 4.27; 95% CI 1.07–17.02; p = 0.039; I2 = 0%). These results are plotted in Figure S10 of the supplementary appendix.
Quality assessment
The risk of Bias 2 (RoB 2) tool was used for quality assessment [21]. No studies were considered at high risk of bias as described in figure S11 in the supplementary appendix. On funnel plot analysis, studies occupied symmetrical distribution according to weight and converged toward the pooled effect as the weight increased. Egger’s test also indicates no evidence of publication bias (p = 0.26; Fig. S12 in the Supplementary Appendix).
Discussion
In this systematic review and meta-analysis of 9 RCTs including 2824 patients, the addition of SGLT2i to conventional therapy was compared to conventional diuretic therapy alone in patients admitted with AHF. The main findings from the pooled analysis were: (1) SGLT2i reduced all-cause death, readmissions for HF, and the composite of CV death or readmissions for HF compared with conventional diuretic therapy alone; (2) SGLT2i was associated with lower daily doses of loop diuretics and higher mean daily urinary output in liters as compared with conventional diuretic therapy alone without SGLT2i; and (3) the incidence of WRF was not significantly different with versus without the addition of SGLT2i to conventional diuretic therapy.
It is well established that loop diuretics are the first-line therapy for volume overload in patients with AHF aiming to produce natriuresis and a negative fluid balance. However, the resulting volume depletion triggers sodium retention due to activation of the renin–angiotensin–aldosterone system and sympathetic nervous system, especially in tubular sites not targeted by loop diuretics [1, 32]. Therefore, one strategy to overcome diuretic resistance is the combination of two different classes of diuretics.
SGLT2i may be ideal to combine with loop diuretics in patients with AHF. These agents reduce glucose and sodium reabsorption in the proximal tubule. Considering that a large amount of sodium is reabsorbed in the proximal tubule, SGLT2i probably helps to overcome diuretic resistance by increasing natriuresis. Therefore, a higher urinary output is found in the SGLT2i group, in addition to lower requirements of loop diuretics, likely due to the natriuretic effect of SGLT2i [33, 34]. Indeed, higher urinary sodium concentration has been associated with better in-hospital and post-discharge outcomes in patients with AHF [35–38]. Recent studies in patients with AHF have shown greater urinary sodium excretion and lower natriuretic peptides in the SGLT2i-treated individuals [26, 30, 39, 40]. In addition, increased glucosuria promotes osmotic diuresis, which may be a pivotal mechanism responsible for increased diuresis with SGLT2i, as observed in the EMPA-RESPONSE-AHF trial [25, 41]. SGLT2i are also more effective in eliminating fluid from interstitial space rather than intravascular space by excreting more electrolyte-free water. [34]
Once tubular content of glucose and sodium are high with SGLT2i use, there is a decrease in the tubuloglomerular feedback, which, in turn, lowers the glomerular capillary pressure, the renal transport work, and oxygen consumption. This mechanism may explain the acute reduction in eGFR observed after initiation of SGLT2i and the long-term preservation of eGFR observed in patients with CKD, for example [33]. In patients with AHF, individual studies found a mild reduction in eGFR with the initiation of SGLT2i. Our study showed that this initial reduction in eGFR is transient, as the incidence of WRF was not significantly different with versus without the addition of SGLT2i to conventional diuretic therapy. These data corroborate findings of diuretic therapy in AHF, wherein the acute decline in eGFR is not associated with death or hospitalization for HF if there is concomitant evidence of decongestion [42, 43].
Observational studies comparing diuretic therapy with or without SGLT2i in patients with AHF have found a reduction in the composite endpoint of mortality and hospitalization for HF with SGLT2i, as well as in HF rehospitalization rates [40, 44]. In contrast, RCTs have shown conflicting results about the use of SGLT2i in this population. The recently published Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial found no evidence of benefit from dapagliflozin with regards to the primary endpoint of worsening HF or cardiovascular death in the patients enrolled during or following hospitalization [23]. The Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial, however, found a benefit in the same endpoint for patients who were recently hospitalized with worsening HF and were randomized to receive sotagliflozin as compared with placebo [29].
None of these studies, whether observational or randomized, showed a benefit in all-cause or cardiovascular mortality, as individual endpoints, for SGLT2i use in this scenario. To the best of our knowledge, this is the first meta-analysis to find a significant difference in all-cause mortality with SGLT2i in patients with AHF. Our meta-analysis expands on prior findings by also showing a significant reduction in the incidence of readmissions for HF, as well as the composite of CV death or readmission for HF in patients with AHF treated with SGLT2i compared with conventional diuretic therapy alone.
Our study has some limitations. First, there were slight differences between the RCTs included in terms of the patient population, control group (placebo and no treatment), and time of follow-up. However, the absence of heterogeneity in the pooled analysis of cardiovascular outcomes suggests that the studies are similar enough in terms of the relative efficacy of SGLT2i vs. control to be pooled in this meta-analysis. Moreover, we performed sensitivity analyses to address these issues, which largely showed similar findings to the pooled data. Second, our findings may not apply to all patients with AHF. In general, the selected trials excluded patients with AHF triggered by acute coronary syndrome, as well as those with end-stage renal disease and advanced liver failure. Third, a large proportion of the cohort was enrolled after discharge; however, our sensitivity analysis restricted to in-hospital initiation of SGLT2i found similar results for the endpoints of all-cause death and for HF hospitalization. Fourth, we included patients with acute-on-chronic HF, as well as de novo HF, which may have somewhat different prognosis [9]. Given the lack of individual data, it was not possible to perform analyses to stratify these two populations. However, we performed a metaregression addressing the influence of de novo HF in all-cause death and found no statistical significance. Fifth, the outcomes of mean daily urinary output and mean daily dose of loop diuretics were based on small samples, as these outcomes were not reported in all studies, with broad confidence intervals and high heterogeneity. And, finally, the endpoint of WRF had elevated heterogeneity, identified as originating from Charaya et al. [22]. This study found a non-statistically significant increase in WRF with SGLT2i relative to no SGLT2i therapy. However, SGLT2i treatment was initiated within 24 h of admission and without necessarily achieving stable diuretic doses. Removal of this study from WRF analysis demonstrated a significant reduction in WRF with SGLT2i initiation for AHF.
Conclusions
In patients admitted with AHF, the addition of SGLT2i to conventional diuretic therapy was associated with lower all-cause death, readmissions for HF, and the composite of CV death or readmissions for HF. Moreover, SGLT2i increased the daily urinary output and reduced the mean daily doses of loop diuretics during hospitalization, without a significant increase in WRF. These findings suggest that SGLT2i should be considered in the treatment of patients with AHF.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No funding support.
Declarations
Conflict of interest
All authors report no relationships that could be construed as a conflict of interest. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Registration
Registered in PROSPERO Database (CRD42022351714).
References
- 1.Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers. 2020 doi: 10.1038/S41572-020-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinnenberg L, Givertz MM. Acute heart failure. Trends Cardiovasc Med. 2020;30:104–112. doi: 10.1016/J.TCM.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Testani JM, ter Maaten JM. Decongestion in acute heart failure: does the end justify the means? JACC Heart Fail. 2016;4:589–590. doi: 10.1016/J.JCHF.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:E895–1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Gracia J, Demissei BG, ter Maaten JM, Cleland JG, O’Connor CM, Metra M, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;258:185–191. doi: 10.1016/J.IJCARD.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 6.Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF) Circ Heart Fail. 2015;8:741–748. doi: 10.1161/CIRCHEARTFAILURE.114.001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felker GM, O’Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health. 2020;8:e411–e422. doi: 10.1016/S2214-109X(20)30004-8/ATTACHMENT/1802E068-4F97-416B-9C39-478CB776A7F6/MMC1.PDF. [DOI] [PubMed] [Google Scholar]
- 9.Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) Intensive Care Med. 2011;37:619–626. doi: 10.1007/S00134-010-2113-0. [DOI] [PubMed] [Google Scholar]
- 10.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/JAMA.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, et al. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2019;21:1338–1352. doi: 10.1002/EJHF.1492. [DOI] [PubMed] [Google Scholar]
- 12.Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SLT, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. doi: 10.1001/JAMA.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harikrishnan S, Sanjay G, Anees T, Viswanathan S, Vijayaraghavan G, Bahuleyan CG, et al. Clinical presentation, management, in-hospital and 90-day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17:794–800. doi: 10.1002/EJHF.283. [DOI] [PubMed] [Google Scholar]
- 14.Altibi AM, Prousi G, Agarwal M, Shah M, Tripathi B, Ram P, et al. Readmission-free period and in-hospital mortality at the time of first readmission in acute heart failure patients-NRD-based analysis of 40,000 heart failure readmissions. Heart Fail Rev. 2021;26:57–64. doi: 10.1007/S10741-019-09912-Z. [DOI] [PubMed] [Google Scholar]
- 15.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMOA1911303. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMOA2022190/SUPPL_FILE/NEJMOA2022190_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 17.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMOA2107038/SUPPL_FILE/NEJMOA2107038_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 18.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMOA2024816/SUPPL_FILE/NEJMOA2024816_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 19.Salah HM, Al’Aref SJ, Khan MS, Al-Hawwas M, Vallurupalli S, Mehta JL, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022 doi: 10.1186/S12933-022-01455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. doi: 10.1136/BMJ.N71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 doi: 10.1136/BMJ.L4898. [DOI] [PubMed] [Google Scholar]
- 22.Charaya K, Shchekochikhin D, Andreev D, Dyachuk I, Tarasenko S, Poltavskaya M, et al. Impact of dapagliflozin treatment on renal function and diuretics use in acute heart failure: a pilot study. Open Heart. 2022;9:e001936. doi: 10.1136/OPENHRT-2021-001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, et al. Dapagliflozin in patients recently hospitalized with heart failure and mildly reduced or preserved ejection fraction. J Am Coll Cardiol. 2022 doi: 10.1016/J.JACC.2022.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF) Eur J Heart Fail. 2020;22:713–722. doi: 10.1002/EJHF.1713. [DOI] [PubMed] [Google Scholar]
- 25.Boorsma EM, Beusekamp JC, ter Maaten JM, Figarska SM, Danser AHJ, van Veldhuisen DJ, et al. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. 2021;23:68–78. doi: 10.1002/EJHF.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S, et al. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF) Circulation. 2022 doi: 10.1161/CIRCULATIONAHA.122.059038. [DOI] [PubMed] [Google Scholar]
- 27.Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/S41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim A, Ghaleb R, Mansour H, Hanafy A, Mahmoud NM, Elsharef MA, et al. Safety and efficacy of adding dapagliflozin to furosemide in type 2 diabetic patients with decompensated heart failure and reduced ejection fraction. Front Cardiovasc Med. 2020 doi: 10.3389/FCVM.2020.602251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMOA2030183/SUPPL_FILE/NEJMOA2030183_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki S, Yamada T, Watanabe T, Morita T, Furukawa Y, Kawasaki M, et al. Effect of empagliflozin as an add-on therapy on decongestion and renal function in patients with diabetes hospitalized for acute decompensated heart failure: a prospective randomized controlled study. Circ Heart Fail. 2021;14:327–338. doi: 10.1161/CIRCHEARTFAILURE.120.007048. [DOI] [PubMed] [Google Scholar]
- 31.Thiele K, Rau M, Hartmann NK, Möller M, Möllmann J, Jankowski J, et al. Empagliflozin reduces markers of acute kidney injury in patients with acute decompensated heart failure. ESC Heart Fail. 2022 doi: 10.1002/EHF2.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullens W, Verbrugge FH, Nijst P, Tang WHW. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J. 2017;38:1872–1882. doi: 10.1093/EURHEARTJ/EHX035. [DOI] [PubMed] [Google Scholar]
- 33.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. 2021;83:503–528. doi: 10.1146/ANNUREV-PHYSIOL-031620-095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/DOM.13126. [DOI] [PubMed] [Google Scholar]
- 35.Honda S, Nagai T, Nishimura K, Nakai M, Honda Y, Nakano H, et al. Long-term prognostic significance of urinary sodium concentration in patients with acute heart failure. Int J Cardiol. 2018;254:189–194. doi: 10.1016/J.IJCARD.2017.08.053. [DOI] [PubMed] [Google Scholar]
- 36.Tersalvi G, Dauw J, Gasperetti A, Winterton D, Cioffi GM, Scopigni F, et al. The value of urinary sodium assessment in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2021;10:216–223. doi: 10.1093/EHJACC/ZUAA006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodson DZ, Griffin M, Mahoney D, Raghavendra P, Ahmad T, Turner J, et al. Natriuretic response is highly variable and associated with 6-month survival: insights from the ROSE-AHF trial. JACC Heart Fail. 2019;7:383–391. doi: 10.1016/J.JCHF.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins SP, Jenkins CA, Baughman A, Miller KF, Storrow AB, Han JH, et al. Early urine electrolyte patterns in patients with acute heart failure. ESC Heart Fail. 2019;6:80–88. doi: 10.1002/EHF2.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagaito M, Imamura T, Joho S, Ushijima R, Nakamura M, Kinugawa K. Renoprotective effects of sodium glucose cotransporter 2 inhibitors in type 2 diabetes patients with decompensated heart failure. BMC Cardiovasc Disord. 2021 doi: 10.1186/S12872-021-02163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagaito M, Imamura T, Joho S, Ushijima R, Nakamura M, Kinugawa K. Efficacy of continuing SGLT2 inhibitors on outcomes in patients with acute decompensated heart failure. Int Heart J. 2021;62:885–890. doi: 10.1536/IHJ.21-022. [DOI] [PubMed] [Google Scholar]
- 41.Mullens W, Martens P. Empagliflozin and renal sodium handling: an intriguing smart osmotic diuretic. Eur J Heart Fail. 2021;23:79–82. doi: 10.1002/EJHF.2086. [DOI] [PubMed] [Google Scholar]
- 42.Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WHW, et al. The kidney in congestive heart failure: “are natriuresis, sodium, and diuretics really the good, the bad and the ugly?”. Eur J Heart Fail. 2014;16:133–142. doi: 10.1002/EJHF.35. [DOI] [PubMed] [Google Scholar]
- 43.McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, et al. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail. 2020;8:537–547. doi: 10.1016/J.JCHF.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martín E, López-Aguilera J, González-Manzanares R, Anguita M, Gutiérrez G, Luque A, et al. Impact of canagliflozin in patients with type 2 diabetes after hospitalization for acute heart failure: a cohort study. J Clin Med. 2021;10:1–11. doi: 10.3390/JCM10030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.