Abstract
Apyrase from potato (Solanum tuberosum) is a divalent metal ion-dependent enzyme that catalyzes the hydrolysis of nucleoside di- and tri-phosphates with broad substrate specificity. The enzyme is widely used to manipulate nucleotide levels such as in the G protein-coupled receptor (GPCR) field where it is used to deplete guanine nucleotides to stabilize nucleotide-free ternary agonist-GPCR-G protein complexes. Potato apyrase is available commercially as the native enzyme purified from potatoes or as a recombinant protein, but these are prohibitively expensive for some research applications. Here, we report a relatively simple method for the bacterial production of soluble, active potato apyrase. Apyrase has several disulfide bonds, so we co-expressed the enzyme bearing a C-terminal (His)6 tag with the E. coli disulfide isomerase DsbC at low temperature (18 °C) in the oxidizing cytoplasm of E. coli Origami B (DE3). This allowed low level production of soluble apyrase. A two-step purification procedure involving Ni-affinity followed by Cibacron Blue-affinity chromatography yielded highly purified apyrase at a level of ~ 0.5 mg per L of bacterial culture. The purified enzyme was functional for ATP hydrolysis in an ATPase assay and for GTP/GDP hydrolysis in a GPCR-G protein coupling assay. This methodology enables the time- and cost-efficient production of recombinant apyrase for various research applications.
Keywords: Nucleotide Phosphohydrolase, Recombinant protein expression, E. coli trxB gor, Cibacron blue dye affinity chromatography, GPCR-G protein coupling BRET assay
Introduction
Apyrase enzymes are diphosphate phosphohydrolases that catalyze the hydrolysis of extracellular nucleoside di- and tri-phosphates to the mono-phosphate forms with the release of inorganic phosphate [1]. These divalent metal ion-dependent (Ca2+ or Mg2+) enzymes have broad nucleotide substrate specificity and are present in various organisms where they have functions including regulation of nucleotide levels in blood vessels, inhibition of platelet aggregation, and regulation of purinergic receptor signaling [1]. Many apyrase enzymes are membrane bound, but soluble apyrase isoforms have been purified from the potato Solanum tuberosum and these are the most extensively characterized apyrase enzymes [2-4]. One of these was cloned by Handa and Guidotti and given the gene name RROP1 [5]. This encodes a protein of molecular mass ~50 kDa with eight cysteine residues that could form up to four disulfide bonds.
Apyrase enzymes are valuable for research applications including degradation of nucleotides in DNA pyrosequencing [6] and manipulation of extracellular ATP/ADP levels [7]. In the G protein-coupled receptor (GPCR) field apyrase is widely used to deplete GTP/GDP to stabilize the nucleotide-free ternary agonist-GPCR-G protein complex for purification and structural studies [8, 9]. It is also used in cell-based bioluminescence resonance energy transfer (BRET) assays that measure GPCR-G protein coupling [10-12]. Commercial sources of apyrase include the enzyme purified from potatoes (Sigma) and a recombinant form of potato apyrase produced in E. coli (New England Biolabs), but these are expensive and can suffer from contaminants that interfere with assays [13]. Recombinant potato apyrase has been produced in the eukaryotic Pichia pastoris expression system as a secreted protein to a level of 1 mg/L culture, purified by hydrophobic interaction and ion exchange chromatography, and demonstrated to be functional in pyrosequencing [14]. Recombinant apyrase enzymes from mosquito or potato have also been produced in the simpler prokaryotic E. coli expression system, but these involved refolding insoluble protein from inclusion bodies [15, 16]. There is a report of expression of an active potato apyrase isoform in E. coli BL21 (DE3) when co-expressed with heat shock chaperones, but the expression level was unclear and the enzyme was not purified [17].
Here we show that the potato apyrase of Handa and Guidotti [5] can be easily and cheaply produced in a soluble, properly folded form by co-expression with the disulfide isomerase DsbC in the oxidizing cytoplasm of the mutant E. coli trxB gor strain Origami B (DE3). The expression level was low, but a relatively simple two step purification involving Ni-affinity and Cibacron blue affinity chromatography yielded highly purified, functional apyrase to a level of ~0.5 mg/L culture. The methodology reported here enables time- and cost-effective production of recombinant apyrase for various research applications.
Materials and Methods
Reagents
General chemicals and the phosphate assay kit (MAK308) were from Sigma-Aldrich. Restriction enzymes and commercial apyrase were from New England Biolabs. Chelating Sepharose fast flow resin and the pre-packed HiTrapBlue HP column were from GE Healthcare or Cytiva. Ni-NTA agarose resin was from Qiagen. Coelenterazine H BRET substrate was from Nanolight. Dialysis membrane was from Spectrum Labs.
Plasmid construction
A synthetic gene encoding Solanum tuberosum apyrase (UniProt P80595; gene RROP1) was ordered as a GeneArt string (ThermoFisher) with codon optimization for E. coli. The region encoding residues 35-454 was PCR amplified with primers that appended NcoI (5’-GTAGCTCCATGGGCCGTCGTCATCTGCTGAGC-3’) and Xhol (5’-GTAGCTCTCGAGGCTGCTTGCAACACGAATTTTG-3’) restriction endonuclease sites at the 5’ and 3’ ends, respectively. The digested PCR product was ligated into the NcoI and XhoI sites of the IPTG-inducible, T7 promoter-driven pSumoT7amp vector (LifeSensors, Malvern PA). This resulted in the removal of the Sumo-coding sequence to yield the expression vector pAP507 that expresses apyrase residues 35-454 with an additional Met-Gly at the N-terminus and Leu-Glu-(His)6 at the C-terminus. The coding sequence of the plasmid was verified by automated DNA sequencing at the OUHSC Laboratory of Molecular Biology and Cytometry Research core facility. The plasmid pAP339, which expresses the E. coli disulfide isomerase DsbC without its signal sequence from the 2nd multiple cloning site of pACYCDuet1 (Novagen), was previously described [18].
Protein expression
E. coli BL21 (DE3) competent cells were transformed with the pAP507 apyrase-H6 expression vector with selection on LB agar plates containing 50 μg/ml ampicillin. E. coli Origami B (DE3) competent cells were co-transformed with the pAP507 apyrase-H6 and pAP339 DsbC expression vectors with selection on LB agar plates containing 50 μg/ml ampicillin and 25 μg/ml chloramphenicol. After colony purification the expression strains were stored as glycerol stocks at −80 °C. For small-scale expression tests, 2 ml LB + 50 μg/ml ampicillin (pAP507 alone) or LB + 50 μg/ml ampicillin, 25 μg/ml chloramphenicol (pAP507 + pAP339) liquid cultures were inoculated and grown at 37 °C, 225-250 rpm overnight. The next morning 50 ml of tryptone-phosphate (TP) media [19] + 0.2% glucose and the appropriate antibiotic(s) in a 250 ml Erlenmeyer flask was inoculated with 100 μl of the overnight culture and grown at 37 °C, 225 rpm to an optical density ~0.6 at 600 nm. The cultures were moved to an incubator at 18 °C with shaking for 15 min to cool down and then induced with IPTG as indicated and shaking was continued overnight. The cells were harvested by centrifugation and the cell pellets were stored at −80 °C for later processing.
For large-scale expression, a 50 ml LB + 50 μg/ml ampicillin, 25 μg/ml chloramphenicol starter culture was inoculated from the glycerol stock of Origami B (DE3) with pAP507 and pAP339 and grown overnight at 37 °C, 225-250 rpm. The next morning four 4 L baffled shake flasks each containing 1.5 L of TP media + 0.2% glucose, 50 μg/ml ampicillin, 25 μg/ml chloramphenicol were each inoculated with 7.5 ml of the overnight culture and grown at 37 °C, 165 rpm to an optical density ~0.5 to 0.6 at 600 nm. The temperature was reduced to 18 °C with shaking for 30 min for cool down and then IPTG was added to 50 μM final concentration and shaking was continued overnight. The cells were harvested by centrifugation and two separate 3L pellets were each resuspended in ~150 ml of 50 mM Tris-HCl, pH 7.5, 10% glycerol, 150 mM NaCl, 40 mM Imidazole and stored at −80 °C for later use.
Protein purification
For small-scale tests of expression, solubility, and Ni-NTA agarose binding the frozen cell pellets from the 50 ml cultures were thawed and resuspended at 33 ml/gram cell pellet in a buffer of 50 mM Tris-HCl, pH 7.5, 10% glycerol, 150 mM NaCl, 40 mM Imidazole, 0.25 mM β-mercaptoethanol, and 0.05% Tween-20 (Ni Buffer A). 5 ml of the resuspended cells were sonicated using the microtip of a Branson Sonifier with cooling on ice. 4.5 ml of the lysate was centrifuged at maximum speed at 4 °C in a benchtop microcentrifuge (Eppendorf) for 10 min and the supernatant was added to 100 μl Ni-NTA resin (Qiagen) equilibrated in Ni Buffer A. After a 30 min incubation on ice with occasional mixing, the resin was pelleted by gentle low speed centrifugation, the supernatant was discarded, and the resin was washed with 1 ml of buffer A three times. The resin was then eluted by addition of 200 μl of Ni Buffer A supplemented with imidazole to 300 mM. 15 μl of the total lysate, soluble fraction after the first centrifugation, and the Ni-NTA elution fractions were analyzed by non-reducing SDS-PAGE with Coomassie blue-staining.
For large scale purification, we used an AKTA Purifier or AKTA PURE FPLC system for the column wash and gradient elution steps. All steps were performed at 4 °C or on ice. The courses of the purifications were followed by SDS-PAGE with Coomassie blue staining. One 3L pellet was thawed and final concentrations of 0.25 mM β-mercaptoethanol (βME), 0.05% Tween-20, and 0.1 mg/mL hen egg white lysozyme were added. The resuspended cells were sonicated using the large tip of a Branson Sonifier with cooling in a rosette flask packed in ice water. The lysate was centrifuged at 25,000 xg for 45 min at 4°C in a JA25.50 rotor using a highspeed centrifuge (Beckman). The supernatant was loaded using a peristaltic pump onto a 50 mL Ni-chelating sepharose column equilibrated in Ni Buffer A. The column was washed with four column volumes of 3% Ni Buffer B – the same as Ni Buffer A except with 500 mM imidazole – and eluted with a linear gradient from 3% Buffer B to 100% over 200 mL. 10 mL fractions were collected. The selected apyrase fractions were pooled and dialyzed (MWCO 6-8 kDa membrane) at 4°C overnight against 1L of buffer containing 25 mM Tris-HCl, pH 7.5, 10% glycerol, 50 mM NaCl, 0.25 mM DTT, and 0.05% Tween-20.
The next morning 1 mM CaCl2 was added to the dialyzed sample and it was slowly loaded using a peristaltic pump onto a 5 mL HiTrap Blue HP column equilibrated in a buffer of 25 mM Tris-HCl, pH 7.5, 10% glycerol, 50 mM NaCl, 1 mM CaCl2, 0.25 mM DTT, and 0.033% Tween-20 (Blue Buffer A). The column was washed with eight column volumes of Blue Buffer A and eluted with a linear gradient over 75 mL of 0-100% Blue Buffer B – 25 mM Tris-HCl, pH 7.5, 10% glycerol, 2.5 M NaCl, 20-25 mM EDTA, 0.25 mM DTT, 0.033% Tween-20 and urea at 0, 0.5 or 2 M. 3 mL fractions were collected. The apyrase-containing fractions were pooled and dialyzed overnight against the same 1L of dialysis buffer used above except with the addition of 0.1 mM CaCl2. The following morning the dialysis bag was transferred to 1L of fresh storage buffer containing 25 mM Na HEPES, pH 7.5, 50% glycerol, 50 mM NaCl, 0.1 mM CaCl2, 0.25 mM DTT, and 0.05% Tween-20 for another overnight dialysis. The final sample was then aliquoted for storage at −80°C. Final protein concentration and yield were determined using a Bradford assay (Bio-Rad) with a BSA standard curve.
LC-MS
In-gel tryptic digest followed by LC-MS was used to confirm the identity of the purified protein as apyrase. This experiment was performed by the OUHS mass spec core facility. The putative apyrase-H6 SDS-PAGE gel band was excised and destained with 200 μl 25 mM ammonium biocarbonate in 1:1 acetonitrile/water. Destain solution was removed and 30 μl of 50 mM TCEP was added with incubation at 60 °C for 10 min to reduce disulfide bonds. The reducing buffer was replaced with 30 μl alkylation buffer 100 mM iodoacetamide for 1 hr at room temperature in the dark. The gel slice was washed with destain solution and trypsin was added to 1:50 (w:w) enzyme:protein with incubation overnight at 37 °C. The digest was desalted on a Pierce C18 spin column before being subjected to LC-MS using a Thermo Scientific Dionex Ultimate 3000 RSLC nano liquid chromatography system coupled to a Thermo Scientific Orbitrap Fusion Lumos mass spectrometer.
Colorimetric ATPase assay
A time course was employed to measure ATP hydrolysis at 1 min, 5 min, 10 min, 15 min, and 30 min of enzymatic activity using the Sigma phosphate assay kit. Final reaction conditions were 50 μM ATP and 0.5 nM apyrase in 50 μL volumes. A 96-well assay plate was prepared with 40 μL of 1X Buffer – 20 mM MES, pH 6.5, 50 mM NaCl, 5 mM CaCl2, 1 mM DTT, and 0.05% Tween-20 (prepared from 10X Buffer – NEB) – with or without 1.25X ATP (62.5 μM). 10 μL volumes containing 5X concentrations (2.5 nM) of apyrase were added to wells of the assay plate at room temperature. A buffer-only control was included in this addition step. Enzymatic activity was halted with the addition of 100 μL Malachite Green Reagent. The plate was incubated for 30 min at room temperature for color development. Once finished, samples were measured for absorbance at 620 nm using a PolarStar Omega plate reader (BMG Labtech). Results were analyzed against a phosphate standard curve and plotted using GraphPad Prism.
Permeabilized cell BRET assay for GPCR-G protein coupling
HEK 293 cells (ATCC) were propagated on 6-well plates according to the supplier’s protocol. Cells were transfected with plasmid DNA encoding β2AR-Rluc8 (0.8 μg), Gαs-long (0.6 μg), Venus-1-155-Gγ2 (0.3 μg), Venus-155-239-Gβ1 (0.3 μg) using linear polyethyleneimine MAX (PEI MAX; MW 40,000) and were used for experiments 24 hours later [10-12]. Cells were washed twice with permeabilization buffer (KPS) containing 140 mM KCl, 10 mM NaCl, 1 mM MgCl2, 0.1 mM potassium EGTA, 20 mM NaHEPES (pH 7.2), harvested by trituration, permeabilized in KPS containing 10 μg ml−1 high-purity digitonin, and transferred to opaque white 96-well plates. After addition of 10 μM isoproterenol, 48 nM apyrase and 5 μM coelenterazine H, BRET was measured every 0.5 second using a BMG PolarStar plate reader. GDP and GDPβS (100 μM final concentrations) were injected at 15 and 75 second time points.
Results and Discussion
Plasmid construction and small-scale tests of expression and Ni-NTA resin binding
A T7 promoter-driven, IPTG-inducible bacterial expression plasmid was constructed to express potato apyrase lacking its signal sequence and with a C-terminal (His)6 tag for purification. Apyrase contains eight Cys residues with the potential for four disulfide bonds. We used Colabfold [20] to predict the structure of apyrase with AlphaFold2 [21], which revealed that six of the cysteine residues were likely to form three disulfide bonds, while the remaining two were in the reduced form (Supplementary Fig. 1). Given the need to form three disulfide bonds we tested expression of apyrase in the oxidizing cytoplasm of the trxB gor mutant E. coli strain Origami B (DE3). To further improve the environment for disulfide formation we co-transformed with a plasmid that expressed the E. coli disulfide isomerase DsbC, which has been shown to improve production of complex disulfide-containing proteins [22, 23].
Small-scale (50 ml) expression, solubility, and batch Ni-NTA resin binding experiments were conducted using rich tryptone-phosphate (TP) growth media to allow for more biomass because we expected that a low apyrase expression level might be required for expression of soluble, properly folded protein. Low-level production of a soluble protein with molecular mass ~50 kDa consistent with apyrase-H6 was observed with addition of IPTG inducer at either a low (25 μM) or high (400 μM) concentration and low temperature (18 °C) overnight expression, and the protein was enriched by binding/elution from Ni-NTA resin (Fig. 1A). The higher IPTG concentration appeared to yield slightly more apyrase, however, this concentration yielded only ½ the cell biomass as compared to the lower IPTG concentration. Similar experiments performed using LB media yielded a lower level of apyrase (data not shown). For comparison, we also tested expression of apyrase-H6 in the “wild-type” E. coli strain BL21 (DE3) without co-expression of DsbC. High-level expression of apyrase was observed with both low and high IPTG concentrations, but the protein was present only in the total lysate fraction indicating that it was insoluble and likely misfolded (Fig. 1B).
Figure 1.
Small-scale expression, solubility test, and Ni-NTA resin binding for apyrase produced in two E. coli expression strains. Shown are non-reducing SDS-PAGE 12% gels analyzing the samples for apyrase-H6 co-expressed with DsbC in Origami B (DE3) (A) or apyrase-H6 expressed in BL21(DE3) (B). T=total cell lysate; S=soluble fraction; E=elution from Ni-NTA resin. The gels were Coomassie-blue stained. The asterisk designates the apyrase band.
Large scale expression and purification of potato apyrase
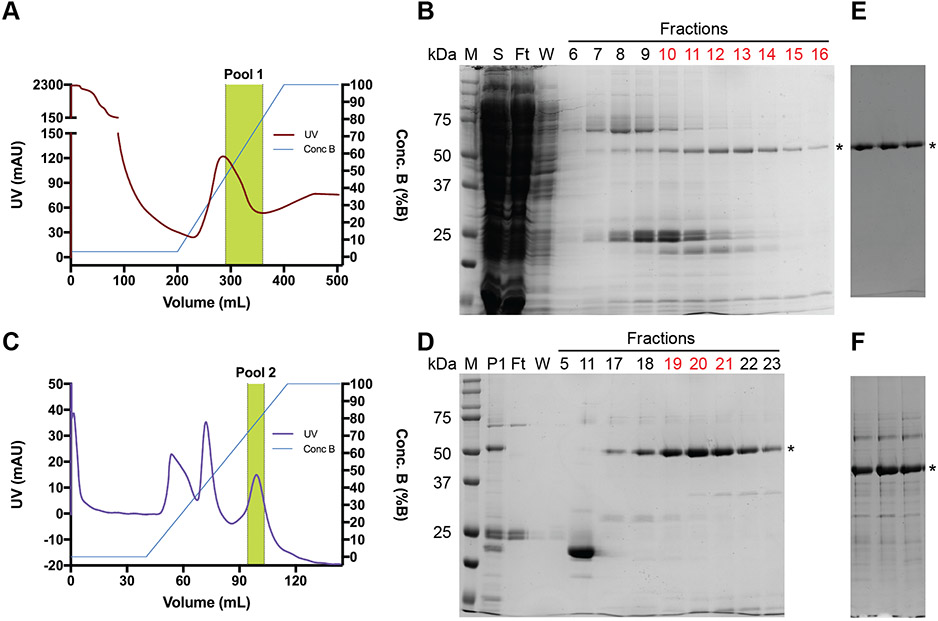
Large-scale expression was performed with a 6 L culture volume using the Origami B (DE3) strain and 50 μM IPTG for induction overnight at 18 °C. The cultures were harvested by centrifugation as two separate 3L pellets in ~150 mL of resuspension buffer because of the large biomass. A single preparation started with a resuspended 3L pellet. The cells were lysed by sonication followed by centrifugation and loading of the supernatant on a Ni-chelating Sepharose column. After washing, the column was eluted with a linear gradient of increasing imidazole concentration (Fig. 2A). We found the gradient elution to work better than a step elution because of the low-level expression of apyrase (not shown). The gradient elution allowed better separation from E. coli contaminant proteins that stick to the Ni resin, but the apyrase was still not sufficiently pure after the single Ni column step (Fig. 2B). Fractions 10-16 were pooled as Pool 1, which yielded ~6.4 mg of protein.
Figure 2.
Large-scale purification of apyrase-H6. (A) Ni Sepharose chromatogram. (B) Non-reducing SDS-PAGE 12% gel analyzing the Ni column fractions. (C) HiTrap Blue HP chromatogram for the purification that included 0.5 M urea in Blue Buffer B. (D) Non-reducing SDS-PAGE 12% gel analyzing the Blue column fractions for the purification that included 0.5 M urea in Blue Buffer B. (E) Non-reducing SDS-PAGE 12% gel for the Blue column apyrase-H6 fractions that were pooled for the purification that lacked urea in Blue Buffer B. (F) Non-reducing SDS-PAGE 12% gel for the Blue column apyrase-H6 fractions that were pooled for the purification that included 2 M urea in Blue Buffer B. S=supernatant; FT=flow-through; W=wash; P1=Pool 1. The gels were Coomassie-blue stained. The asterisk designates the apyrase band. Fractions labeled in red text were pooled.
We next turned to the use of Cibacron blue affinity chromatography because it can be a powerful step for nucleotide binding proteins [24] and it was previously used in the purification of native potato apyrase from potatoes [2, 5]. We were surprised to find that in our initial attempts the bacterially produced apyrase did not stick to Cibacron blue resin. In our hands, addition of calcium to the sample was necessary for apyrase to bind the resin. The calcium level in the E. coli cytoplasm may be too low for the overexpressed apyrase to be calcium-bound, or alternatively calcium may have been stripped from apyrase during the Ni column chromatography. Pool 1 from the Ni column was dialyzed overnight to lower the imidazole and NaCl concentrations, and then 1 mM CaCl2 was added with gentle mixing. The sample was loaded on a Cibacron blue column, followed by washing and a gradient elution with increasing NaCl and EDTA +/− urea at varying concentration. In the presence of calcium the apyrase sticks tightly to Cibacron blue and is difficult to fully elute even with 2.5 M NaCl and 20 to 25 mM EDTA. We found that inclusion of urea at 0.5 to 2 M in Buffer B improved the elution yield, albeit with the caveat that as the urea concentration was raised more contaminants co-eluted with apyrase. Ultimately, we settled on 0.5 M urea as a reasonable compromise between elution yield and purity. Fig. 2C shows a gradient elution using 0.5 M urea in buffer B. Apyrase eluted last and fractions 19-21, which were of good purity (Fig. 2D), were pooled as Pool 2. Fig. 2E, and 2F show the apyrase fractions that were pooled from preps that used no urea or 2 M urea in Buffer B, respectively. Pool 2 was dialyzed overnight to lower the urea and EDTA concentrations and then a final overnight dialysis to storage buffer was performed prior to storage as aliquots at −80 °C. The final yields obtained from 3L starting cultures using 0, 0.5, or 2M urea in Cibacron blue buffer B were 0.97, 1.2, and 2.3 mg total protein, respectively. Regardless of the elution method, it is a good idea to thoroughly wash the Cibacron blue column with buffer containing 2 M urea before storage and future usage.
Bacterially produced potato apyrase is functional in ATPase and GPCR-G protein coupling assays
We compared our recombinant enzyme to commercial potato apyrase from New England Biolabs, which is also recombinant from E. coli, although their method of expression and purification is not provided. Our two samples that were eluted from the Cibacron blue column with 0 or 0.5 M urea exhibited good purity on SDS-PAGE as compared to the NEB apyrase (Fig. 3A), although our protein was a bit bigger (~50 kDa) than the NEB enzyme (~47 kDa). Apparently, NEB uses a different construct or isoform than we used. Nonetheless, since our protein ran slightly bigger than its calculated molecular mass of ~47.5 kDa, we confirmed its identity by excising the SDS-PAGE band and conducting trypsinization followed by LC-MS. This validated our recombinant protein as potato apyrase (Supplementary Excel File). We note that in the original publication reporting the cloning of the potato apyrase isoform used here, the enzyme purified from potatoes was reported to lack glycosylation and run as a ~50 kDa band on SDS-PAGE [5]. In a time-course colorimetric ATPase assay that detects the inorganic phosphate released as ATP is hydrolyzed by the enzyme, our apyrase samples were comparable in activity to NEB apyrase (Fig. 3B). Apyrase purified using 2 M urea in Cibacron Blue Buffer B was also active in this assay (Supplementary Fig. 2). These data indicated that our bacterially produced apyrase-H6 was properly folded and functional for hydrolysis of ATP.
Figure 3.
Colorimetric in vitro ATPase assay for apyrase-H6 activity. (A) Non-reducing SDS-PAGE 12% gel comparing recombinant apyrase from NEB with our samples purified using 0 (JK270) or 0.5 M urea (JK271) in Blue Buffer B. The gel was Coomassie-blue stained. (B) Time course ATPase assay with the indicated apyrase at 0.5 nM and ATP at 50 μM. The plot shows representative results from one of three independent replicate assays performed on different days.
Last, we tested the activity of our bacterially produced apyrase in the bioluminescence resonance energy transfer (BRET) assay for GPCR-G protein coupling that was the original impetus for developing a cheap and efficient method for apyrase production because commercial apyrase is a significant expense for this assay. The assay has been used for many different GPCRs [10-12]. HEK293 cells are co-transfected with plasmids expressing the GPCR of interest C-terminally tagged with the BRET donor Rluc8, untagged Gα subunit, and Gβγ tagged with the BRET acceptor mVenus. The cells are permeabilized by gentle digitonin treatment to allow exogenous apyrase addition to deplete cytosolic GDP and GTP to stabilize the nucleotide-free ternary agonist-GPCR-G protein complex that would otherwise exist only transiently in the cell (Fig. 4A). In this assay using the human β2-adrenergic receptor and Gs heterotrimer our recombinant apyrase allowed formation of a stable ternary complex (Fig. 4B). Notably, both GDP and GTP have been shown to destabilize this complex [8, 9, 11]. Therefore, to demonstrate the action of our recombinant apyrase in real-time, GDP or the non-hydrolyzable analog GDPβS was injected after establishing the baseline BRET signal. Injection of GDP destabilized the ternary complex as indicated by a rapid decrease in the BRET signal, but this effect was quickly reversed by the enzymatic activity of apyrase (Fig. 4B). In contrast, GDPβS injection irreversibly destabilized the complex. Although it may appear that the BRET signal failed to fully recover to baseline in the GDP-treated sample, this may simply be due to a slow “rundown” in the assay. For example, some of the G protein may be lost to the supernatant or solubilized when released from the receptor due to the cell permeabilization. Regardless of the cause, these data indicated that our bacterially produced apyrase was functional for hydrolysis of GTP and GDP.
Figure 4.
Cell-based BRET GPCR-G protein coupling assay dependent on apyrase depletion of guanine nucleotides. (A) Format of the assay in which apyrase depletes GDP and GTP to stabilize the nucleotide-free ternary isoproterenol-β2AR-Gs complex. (B) Real-time BRET assay with permeabilized HEK293 cells expressing β2AR-Rluc8, untagged Gαs, and mVenus-Gβγ. Apyrase-H6 (48 nM) purified using 0.5 M urea in Blue Buffer B and isoproterenol agonist (10 μM) were used to stabilize the ternary complex and establish a baseline BRET signal followed by injection of guanine nucleotides (100 μM) as indicated by the arrows. The plot shows mean ± SD of four independent experiments performed in quadruplicate.
Conclusions
Here we showed that potato apyrase can be easily and cheaply produced in bacteria in a soluble form and purified by a relatively simple two step chromatography procedure obviating the need for complex refolding procedures starting from insoluble inclusion bodies. The expression level was relatively low, but it is probably difficult to express high levels of soluble, active enzyme in the cytoplasm of E. coli due to toxicity. Our expression level was about half that reported for secretion from the yeast Pichia pastoris [14], but production using E. coli is quicker, simpler, and cheaper. Importantly, the yields reported here are adequate to provide the recombinant enzyme in quantities that can support numerous assays. This methodology should be of value to researchers in fields where apyrase is used to manipulate nucleotide levels.
Supplementary Material
Supplementary Figure 1. A predicted apyrase structure using AlphaFold2. Six cysteine residues (yellow) displaying three disulfide bonds and two free residues.
Supplementary Figure 2. Colorimetric in vitro ATPase time course assay for apyrase-H6 activity with the indicated apyrase concentrations. The plot shows a single result from samples purified using 2 M urea (AP595) in Blue Buffer B.
Supplementary File 3. Excel file with LC-MS results for the excised band in Figure 3 from JK270.
Highlights.
Potato apyrase expressed in soluble form in E. coli Origami B (DE3)
Simple two-step Ni- and Cibacron Blue-affinity chromatography purification
Highly purified apyrase at a yield of ~0.5 mg per L of culture
Bacterially produced potato apyrase is functional for nucleotide hydrolysis
Acknowledgements
We thank Huaiwen Wang and the OUHSC mass spectrometry core facility for the in-gel trypsinization and LC-MS experiment to validate the 50 kDa SDS-PAGE band as apyrase.
Funding
This work was supported by the National Institutes of Health [grant numbers R01GM104251 (AAP), R01GM130142 (NAL) and R35GM145284 (NAL)].
Footnotes
CRediT author statement for PEP-D-22-00283
Jordan A. Karim: Methodology, Investigation, Visualization, Writing-Original Draft, Writing-Review & Editing
Nevin A. Lambert: Methodology, Investigation, Visualization, Writing-Original Draft, Writing-Review & Editing, Funding acquisition
Augen A. Pioszak: Conceptualization, Methodology, Investigation, Visualization, Writing-Original Draft, Writing-Review & Editing, Supervision, Funding acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Plesner L, Ecto-ATPases: identities and functions. International review of cytology 158 (1995) 141–214. [DOI] [PubMed] [Google Scholar]
- [2].Kettlun AM, Uribe L, Calvo V, Silva S, Rivera J, Mancilla M, Valenzuela MA, Traverso-Cori A, Properties of two apyrases from solanum tuberosum. Phytochemistry 21 (1982) 551–558. [Google Scholar]
- [3].Traverso-Cori A, Traverso S, Reyes H, Different molecular forms of potato apyrase. Archives of biochemistry and biophysics 137 (1970) 133–142. [DOI] [PubMed] [Google Scholar]
- [4].Valenzuela MA, Del Campo G, Marin E, Traverso-Cori A, Effects of protein-modifying reagents on an isoenzyme of potato apyrase. The Biochemical journal 133 (1973) 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Handa M, Guidotti G, Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum). Biochemical and biophysical research communications 218 (1996) 916–923. [DOI] [PubMed] [Google Scholar]
- [6].Ronaghi M, Uhlen M, Nyren P, A sequencing method based on real-time pyrophosphate. Science 281 (1998) 363, 365. [DOI] [PubMed] [Google Scholar]
- [7].Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB, ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience 8 (2005) 752–758. [DOI] [PubMed] [Google Scholar]
- [8].Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK, Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 477 (2011) 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yao XJ, Velez Ruiz G, Whorton MR, Rasmussen SG, DeVree BT, Deupi X, Sunahara RK, Kobilka B, The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proceedings of the National Academy of Sciences of the United States of America 106 (2009) 9501–9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jang W, Adams CE, Liu H, Zhang C, Levy FO, Andressen KW, Lambert NA, An inactive receptor-G protein complex maintains the dynamic range of agonist-induced signaling. Proceedings of the National Academy of Sciences of the United States of America 117 (2020) 30755–30762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Okashah N, Wan Q, Ghosh S, Sandhu M, Inoue A, Vaidehi N, Lambert NA, Variable G protein determinants of GPCR coupling selectivity. Proceedings of the National Academy of Sciences of the United States of America 116 (2019) 12054–12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Okashah N, Wright SC, Kawakami K, Mathiasen S, Zhou J, Lu S, Javitch JA, Inoue A, Bouvier M, Lambert NA, Agonist-induced formation of unproductive receptor-G12 complexes. Proceedings of the National Academy of Sciences of the United States of America 117 (2020) 21723–21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Madry C, Arancibia-Carcamo IL, Kyrargyri V, Chan VTT, Hamilton NB, Attwell D, Effects of the ecto-ATPase apyrase on microglial ramification and surveillance reflect cell depolarization, not ATP depletion. Proceedings of the National Academy of Sciences of the United States of America 115 (2018) E1608–E1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nourizad N, Ehn M, Gharizadeh B, Hober S, Nyren P, Methylotrophic yeast Pichia pastoris as a host for production of ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum). Protein expression and purification 27 (2003) 229–237. [DOI] [PubMed] [Google Scholar]
- [15].Dong F, Fu Y, Li X, Jiang J, Sun J, Cheng X, Cloning, expression, and characterization of salivary apyrase from Aedes albopictus. Parasitology research 110 (2012) 931–937. [DOI] [PubMed] [Google Scholar]
- [16].Wujak M, Banach M, Porowinska D, Piskulak K, Komoszynski M, Isolation and bioinformatic analysis of seven genes encoding potato apyrase. Bacterial overexpresssion, refolding and initial kinetic studies on some recombinant potato apyrases. Phytochemistry 93 (2013) 8–17. [DOI] [PubMed] [Google Scholar]
- [17].Porowinska D, Czarnecka J, Komoszynski M, Chaperones are necessary for the expression of catalytically active potato apyrases in prokaryotic cells. Applied biochemistry and biotechnology 173 (2014) 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Warner ML, Bell T, Pioszak AA, Engineering high-potency R-spondin adult stem cell growth factors. Molecular pharmacology 87 (2015) 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moore JT, Uppal A, Maley F, Maley GF, Overcoming inclusion body formation in a high-level expression system. Protein expression and purification 4 (1993) 160–163. [DOI] [PubMed] [Google Scholar]
- [20].Mirdita M, Schutze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M, ColabFold: making protein folding accessible to all. Nature methods 19 (2022) 679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D, Highly accurate protein structure prediction with AlphaFold. Nature 596 (2021) 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bessette PH, Aslund F, Beckwith J, Georgiou G, Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proceedings of the National Academy of Sciences of the United States of America 96 (1999) 13703–13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moad HE, Pioszak AA, Reconstitution of R-spondin:LGR4:ZNRF3 adult stem cell growth factor signaling complexes with recombinant proteins produced in Escherichia coli. Biochemistry 52 (2013) 7295–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Subramanian S, Dye-ligand affinity chromatography: the interaction of Cibacron Blue F3GA with proteins and enzymes. CRC critical reviews in biochemistry 16 (1984) 169–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. A predicted apyrase structure using AlphaFold2. Six cysteine residues (yellow) displaying three disulfide bonds and two free residues.
Supplementary Figure 2. Colorimetric in vitro ATPase time course assay for apyrase-H6 activity with the indicated apyrase concentrations. The plot shows a single result from samples purified using 2 M urea (AP595) in Blue Buffer B.
Supplementary File 3. Excel file with LC-MS results for the excised band in Figure 3 from JK270.