Abstract
Aims/Introduction
This study aimed to investigate the clinical significance and antigen specificity of autoantibodies to insulinoma‐associated antigen‐2 (IA‐2A) by radioimmunoassay (RIA; IA‐2A‐RIA) and enzyme‐linked immunosorbent assay (ELISA; IA‐2A‐ELISA) in Japanese patients with type 1 diabetes.
Materials and Methods
A total of 338 type 1 diabetic patients were enrolled, including 38 fulminant type 1 diabetes, 168 acute‐onset type 1 diabetes and 137 slowly‐progressive type 1 diabetes (SPIDDM). The concordance, correlation of autoantibody titer, and the relationship between IA‐2A and progression to the insulin‐deficient state were examined. Also, competitive assay was used to examine the antigen specificity.
Results
The prevalence of IA‐2A‐ELISA was 4–5% lower than that of IA‐2A‐RIA in both the acute‐onset type 1 diabetes and SPIDDM, but the diagnostic sensitivities of both subtypes, when measured in combination with glutamic acid decarboxylase autoantibody, were comparable. The diagnosis of type 1 diabetes using either the RIA or ELISA methods showed substantial agreement with the exponential correlation of autoantibody titers detected by RIA and ELISA. Among the SPIDDM patients, the fasting C‐peptide for IA‐2A‐positive cases by ELISA, but not the RIA method, was significantly lower than in the negative cases (P < 0.05). Furthermore, IA‐2A‐ELISA proved superior to the RIA method in predicting the progression to insulin deficiency in SPIDDM. Competitive analysis showed that even sera with discrepant results by RIA and ELISA have IA‐2‐specific autoantibodies.
Conclusion
These results suggest that IA‐2A‐ELISA is a reliable marker not only for the diagnosis of type 1 diabetes, but also for the prediction of future insulin dependency; that is, detection of IA‐2A‐ELISA helps identify a subtype of SPIDDM patients who would likely progress onto insulin‐deficient state.
Keywords: Autoantibodies, Autoantigen, Insulinoma‐associated antigen‐2, Type 1 diabetes
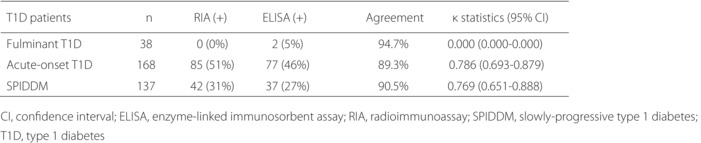
In the present study, we showed that the agreement between radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA) for insulinoma‐associated antigen‐2 autoantibodies (IA‐2A) by the RSR Ltd. (Cardiff, UK) was 94.7% (κ statistic = 0.000, 95% confidence interval 0.000–0.000) for fulminant type 1 diabetes, 89.3% (κ statistic = 0.786, 95% confidence interval 0.693–0.879) for acute‐onset type 1 diabetes and 90.5% for slowly‐progressive type 1 diabetes (κ statistic = 0.769, 95% confidence interval 0.651–0.888), and the diagnostic sensitivities of autoimmune‐mediated type 1 diabetes when measured in combination with glutamic acid decarboxylase autoantibody were comparable between the RIA and ELISA methods. Furthermore, we reported that RSR IA‐2A‐ELISA is more useful than RSR IA‐2A‐RIA in identifying the subtype of slowly‐progressive type 1 diabetic patients who would likely progress onto an insulin‐deficient state.
INTRODUCTION
Autoantibodies to insulinoma‐associated antigen‐2 (IA‐2A) are important serological markers for predicting and diagnosing type 1 diabetes. The Diabetes Autoantibody Standardization Program or Islet Autoantibody Standardization Program have been formed to standardize and improve anti‐islet autoantibody assay performance among laboratories 1 , 2 . Among the participating laboratories in these standardization programs, radioligand binding assays showed a remarkable degree of concordance in detecting IA‐2A 2 , whereas at the same time, various methodological improvements have been also developed. These methods include radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA) by RSR Ltd. (Cardiff, UK), both well‐established tests for the analysis of IA‐2A, and widely distributed throughout the world as commercial kits. These kits have proven to perform well, achieving high sensitivity and specificity in the Diabetes Autoantibody Standardization Program IA‐2A workshops 1 . Recently, IA‐2A measurement has shifted from RSR‐RIA to RSR‐ELISA in Japan, making it necessary to change the interpretation and correspondence, including the positive/negative judgment of the RIA method. We, therefore, carried out the present study as a research project under the committee of type 1 diabetes of the Japan Diabetes Society to investigate the clinical characteristics of Japanese patients with type 1 diabetes who showed discrepant IA‐2A measurements using RSR‐RIA and RSR‐ELISA. Furthermore, we carried out a competitive assay to evaluate whether or not the discrepancy of IA‐2A results between RSR‐RIA and RSR‐ELISA is due to non‐specific binding.
MATERIALS AND METHODS
Participants
A total of 343 serum samples from type 1 diabetic patients were collected from 10 contributing institutes for the present study through the committee of the Japan Diabetes Society and the Japanese Type 1 Diabetes Database (TIDE‐J) study. The inclusion criteria of the TIDE‐J study were described previously 3 . This study comprised 38 patients with fulminant type 1 diabetes, 168 with acute‐onset type 1 diabetes and 137 with slowly‐progressive type 1 diabetes (SPIDDM), and a diagnosis of type 1 diabetes was made based on the criteria set by the committee of the Japan Diabetes Society 4 , 5 , 6 . Details of the patients' clinical characteristics are shown in Table 1. A diagnosis of SPIDDM was made if patients showed positive for glutamic acid decarboxylase autoantibody (GADA) and/or islet cell antibodies at any time during the disease course irrespective of anti‐islet autoantibody status at the time of the study. Diabetic ketoacidosis, including soft‐drink ketosis (ketoacidosis), was seen at diagnosis in 25 patients with SPIDDM. The study protocols were approved by the ethics committee of the Japan Diabetes Society and each institute participating in this project, and informed consent was obtained from all participants. Serum samples were stored at −20°C until use.
Table 1.
Clinical characteristics
| Fulminant type 1 diabetes | Acute‐onset type 1 diabetes | SPIDDM | |
|---|---|---|---|
| n | 38 | 168 | 137 |
| Female | 18 (47%) | 101 (60%) | 70 (51%) |
| Onset age (years) | 44.9 ± 15.4 | 41.0 ± 17.7 | 50.6 ± 15.0 |
| Duration (years) | 2.3 ± 3.1 | 3.7 ± 5.7 | 7.5 ± 9.6 |
| BMI (kg/m2) | 21.6 ± 2.9 | 20.9 ± 3.2 | 22.8 ± 4.0 |
| DKA at onset (+/−) | 34/4 | 118/35 | 25/102 |
| Insulin therapy (+/−) | 38/0 | 168/0 | 99/38 |
| GADA (+) | 2 (5%) | 129 (77%) | 85 (62%) |
| HLA‐DRB1*04:05 (+) | 19/64 (30%) | 61/252 (24%) | 61/194 (31%) |
| HLA‐DRB1*09:01 (+) | 16/64 (25%) | 72/252 (29%) | 74/194 (38%) |
BMI, body mass index; DKA, diabetic ketoacidosis; GADA, glutamic acid decarboxylase autoantibody; F‐CPR, fasting C‐peptide; HLA, human leukocyte antigen; RIA, radioimmunoassay; SPIDDM, slowly‐progressive type 1 diabetes.
Anti‐islet autoantibody measurement
RSR‐RIA IA‐2A (IA‐2A‐RIA) were determined by liquid‐phase RIA using 125I‐labeled recombinant human IA‐2 as a tracer reagent, as previously described 7 . Results were read from a calibration curve constructed in the same run with the calibrators and expressed in U/mL. The cut‐off value for the IA‐2A‐RIA was 0.4 U/mL. RSR‐ELISA IA‐2A (IA‐2A‐ELISA) and GADA were determined using bivalent ELISA using biotinylated IA‐2 and GAD65, respectively, as previously described 8 . The results were read from a calibration curve constructed in the same run with the calibrators and expressed in U/mL. The cut‐off value for the IA‐2A‐ELISA and GADA was 0.6 U/mL and 5.0 U/mL, respectively. The intra‐ and interassay coefficients of variation for the IA‐2A‐RIA were 2.5–2.8% and 3.8–5.3%, respectively, whereas those for the IA‐2A‐ELISA were 2.0–3.3% and 4.0–6.5%, respectively 9 . In the Islet Autoantibody Standardization Program 2018 workshop (Lab ID: 1801), the assay sensitivities and specificities achieved were 72% and 98% for IA‐2A, and 90% and 98% for GADA, respectively.
Competition assay
Antigen specificity was examined by competitive binding experiments with unlabeled recombinant human IA‐2. The assay format was identical to the RSR‐RIA and RSR‐ELISA. Initially, to identify the optimal amount of recombinant IA‐2 protein, five sera with high levels of IA‐2A were incubated for 1 h at room temperature with three different concentrations of unlabeled IA‐2 varying from 2.5 μg to 250 μg/mL, before taking IA‐2A measurements (Figure S1). Based on this preliminary experiment, further competition assays were carried out using 250 μg/mL of unlabeled IA‐2 protein. The percentage of serum samples that showed inhibition after adding recombinant IA‐2 protein was calculated by [(U/mL without competitor − U/mL with competitor)/U/mL without competitor] × 100.
Statistical analysis
All results are expressed as the mean ± standard deviation or median (range), and the categorical variables were compared using the χ2‐test and Fisher's exact test where appropriate. Differences in non‐parametric data were tested using the Mann–Whitney U‐test or Kruskal–Wallis test, and the correlation between autoantibody titer was analyzed using the Spearman's rank correlation test. Curve fitting analysis was carried out using various regression models, including linear, quadratic, exponential and reciprocal models to determine which best fit the correlation between IA‐2A‐RIA level and IA‐2A‐ELISA level, as well as the correlation between duration of SPIDDM and autoantibody levels. Patients were divided into four groups according to their RIA/ELISA results for IA‐2A, as follows: group 1 (RIA‐positive/ELISA‐positive); group 2 (RIA‐positive/ELISA‐negative); group 3 (RIA‐negative/ELISA‐positive); and group 4 (RIA‐negative/ELISA‐negative). Agreement between the IA‐2A‐RIA and IA‐2A‐ELISA was assessed with the κ statistic, which is a measure of the strength of agreement on a scale from 0 to 1 10 . Multiple logistic regression analyses evaluating the association between the IA‐2A positivity and clinical and immunogenetic parameters were also carried out. A P‐value of <0.05 was considered statistically significant. Statistical analysis for the present study was carried out using StatView statistical software (version 5.0; SAS Institute, Cary, NC, USA) and SigmaPlot software (version 14.5; Systat Software Inc., San Jose, CA, USA).
RESULTS
Prevalence and concordance of IA‐2A‐RIA and IA‐2A‐ELISA
The prevalence of IA‐2A‐RIA and IA‐2A‐ELISA for each subtype was 0 and 5% for fulminant type 1 diabetes, 51 and 46% for acute‐onset type 1 diabetes, and 31 and 27% for SPIDDM, respectively. Thus, the prevalence of IA‐2A‐ELISA was 4–5% lower than that of IA‐2A‐RIA in both the acute‐onset type 1 diabetes and SPIDDM. However, the diagnostic sensitivities of autoimmune‐mediated type 1 diabetes when measured in combination with GADA, the first anti‐islet autoantibodies measured under Japanese health insurance, were comparable between the RIA and ELISA methods (Figure S2). Concordance between the IA‐2A‐RIA and IA‐2A‐ELISA in acute‐onset type 1 diabetes and SPIDDM is shown in Figure 1. Approximately 7% and 3% of patients in both subtypes were positive only for IA‐2A‐RIA and IA‐2A‐ELISA, respectively. Furthermore, the agreement between the IA‐2A‐RIA and IA‐2A‐ELISA was 94.7% (κ statistic = 0.000, 95% 95% confidence interval 0.000–0.000) for fulminant type 1 diabetes, 89.3% (κ statistic = 0.786, 95% 95% confidence interval 0.693–0.879) for acute‐onset type 1 diabetes and 90.5% for SPIDDM (κ statistic = 0.769, 95% confidence interval 0.651–0.888; Table 2). Curve fitting analysis using linear and non‐linear regression was carried out to determine which best fit the correlation between IA‐2A‐RIA level and IA‐2A‐ELISA level (Table S1). The exponential regression model was a better fit than other models both in the acute‐onset type 1 diabetes and SPIDDM (Figure 2a,b). We also carried out the same analyses in patients with the range IA‐2A‐RIA <20 U/mL separately, and the exponential regression curves were still the best fit for both subtypes (Figure S3).
Figure 1.
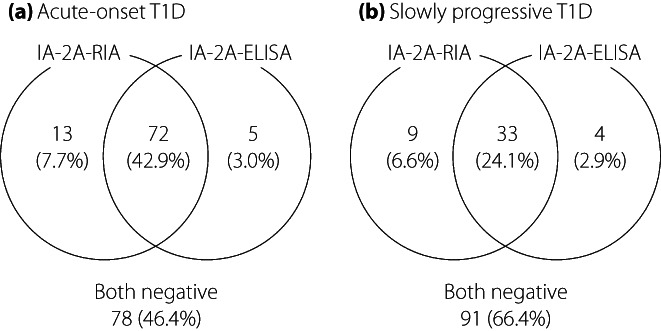
Concordance between the result of insulinoma‐associated antigen‐2 (IA‐2A) by radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA) in patients with (a) acute‐onset type 1 diabetes and (b) slowly‐progressive type 1 diabetes.
Table 2.
Agreement between the insulinoma‐associated antigen‐2 by radioimmunoassay and the insulinoma‐associated‐2 by enzyme‐linked immunosorbent assay with sera from type 1 diabetes
| Type 1 diabetic patients | n | RIA (+) | ELISA (+) | Agreement | κ statistics (95% CI) |
|---|---|---|---|---|---|
| Fulminant type 1 diabetes | 38 | 0 (0%) | 2 (5%) | 94.7% | 0.000 (0.000–0.000) |
| Acute‐onset type 1 diabetes | 168 | 85 (51%) | 77 (46%) | 89.3% | 0.786 (0.693–0.879) |
| SPIDDM | 137 | 42 (31%) | 37 (27%) | 90.5% | 0.769 (0.651–0.888) |
CI, confidence interval; ELISA, enzyme‐linked immunosorbent assay; RIA, radioimmunoassay; SPIDDM, slowly‐progressive type 1 diabetes.
Figure 2.
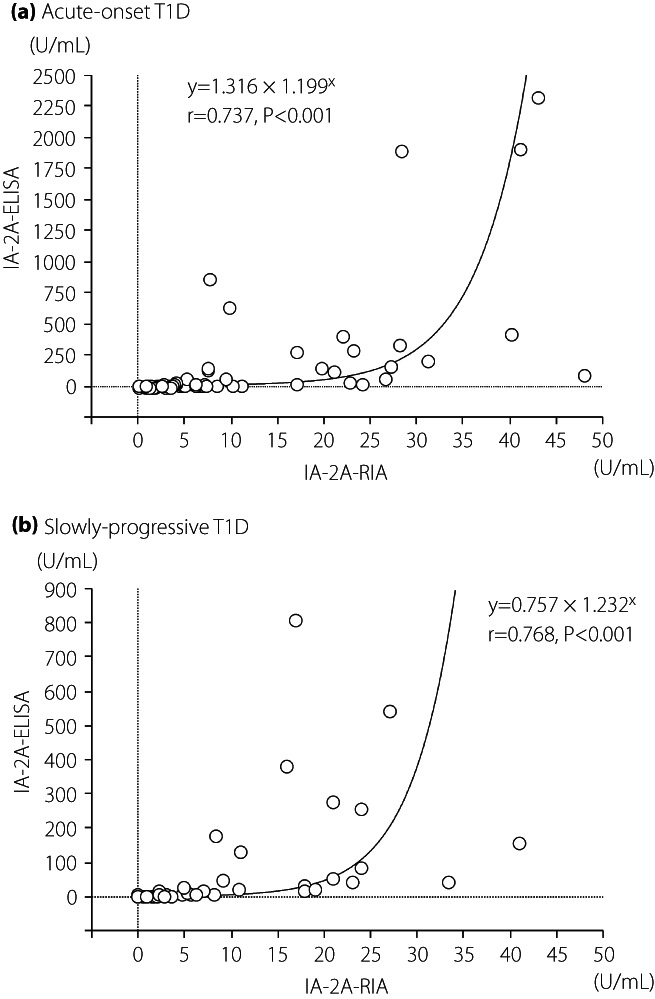
Correlation between the level of insulinoma‐associated antigen‐2 (IA‐2A) by radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA) in patients with (a) acute‐onset type 1 diabetes and (b) slowly‐progressive type 1 diabetes. Autoantibody‐positive sera by either assay were used in this analysis.
Clinical and immunogenetic characteristics of acute‐onset type 1 diabetic patients based on the positivity of IA‐2A‐RIA and IA‐2A‐ELISA
Table 3 summarizes the clinical and immunogenetic characteristics of acute‐onset type 1 diabetic patients among the four groups based on the positivity or negativity of IA‐2A‐RIA and IA‐2A‐ELISA. There were no significant differences among the four groups regarding gender, onset age, duration of diabetes, the prevalence of diabetic ketoacidosis at onset, body mass index, fasting C‐peptide (F‐CPR) level, mean GADA level and the frequencies of disease susceptible HLA‐DRB1*04:05 and ‐DRB1*09:01. However, there was a significant difference in GADA prevalence among the four groups. This significantly higher prevalence of GADA was observed in both IA‐2A‐RIA‐positive (P = 0.0047) and IA‐2A‐ELISA‐posivive patients (P = 0.0039). Furthermore, the mean IA‐2A level in group 1 (RIA+/ELISA+) was significantly higher than that in group 2 (RIA+/ELISA−) or group 3 (RIA−/ELISA+).
Table 3.
Clinical characteristics according to groups in 168 patients with acute‐onset type 1 diabetes
| Group 1 (RIA‐positive and ELISA‐positive) | Group 2 (RIA‐positive and ELISA‐negative) | Group 3 (RIA‐negative and ELISA‐positive) | Group 4 (RIA‐negative and ELISA‐negative) | P‐value | |
|---|---|---|---|---|---|
| n | 72 | 13 | 5 | 78 | |
| Female | 46 (64%) | 8 (62%) | 4 (80%) | 43 (55%) | NS |
| Onset age (years) | 39.0 ± 18.4 | 43.1 ± 19.8 | 48.4 ± 22.5 | 42.0 ± 16.5 | NS |
| Duration (years) | 3.3 ± 4.2 | 2.0 ± 2.9 | 2.0 ± 2.4 | 4.4 ± 7.2 | NS |
| DKA at onset (+/−) | 50/22 | 12/1 | 3/2 | 53/25 | NS |
| BMI (kg/m2) | 21.0 ± 3.3 | 20.1 ± 2.3 | 21.5 ± 6.0 | 20.8 ± 3.0 | NS |
| F‐CPR (ng/mL) | 0.53 ± 0.46 | 0.36 ± 0.28 | 1.03 ± 1.28 | 0.64 ± 0.81 | NS |
| GADA (+) | 64 (89%) | 9 (69%) | 3 (60%) | 53 (68%) | 0.015 |
| GADA (U/mL) | 1,000.8 ± 906.5 | 929.4 ± 1,017.0 | 748.8 ± 1,086.8 | 690.7 ± 807.2 | NS |
| IA‐2A‐RIA (U/mL) | 9.9 ± 11.4 | 1.6 ± 1.0 | NA | NA | <0.0001 |
| IA‐2A‐ELISA (U/mL) | 153.2 ± 427.4 | NA | 1.6 ± 1.0 | NA | 0.012 |
| HLA‐DRB1*04:05 (+) | 25/112 (26%) | 5/14 (36%) | 1/6 (17%) | 30/120 (25%) | NS |
| HLA‐DRB1*09:01 (+) | 36/112 (40%) | 4/14 (29%) | 2/6 (33%) | 30/120 (25%) | NS |
Autoantibody levels were determined in patients positive for corresponding autoantibody.
BMI, body mass index; DKA, diabetic ketoacidosis; ELISA, enzyme‐linked immunosorbent assay; F‐CPR, fasting C‐peptide; GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen; RIA, radioimmunoassay; SPIDDM, slowly‐progressive type 1 diabetes; N.S., not significant.
Clinical and immunogenetic characteristics of SPIDDM patients based on the positivity of IA‐2A‐RIA and IA‐2A‐ELISA
Table 4 shows the clinical and immunogenetic characteristics of SPIDDM patients among the four groups. Of note, the duration of diabetes in group 4 was significantly longer than in group 1, and the level of F‐CPR was significantly different among the four groups. F‐CPR in SPIDDM patients positive for IA‐2A‐ELISA was significantly lower than in IA‐2A‐ELISA‐negative patients (Table S2, P = 0.006). However, there was no difference in F‐CPR levels between IA‐2A‐RIA‐positive and ‐negative patients. Similarly, as with the acute‐onset type 1 diabetes results, the prevalence of GADA was significantly different among the four groups, and the mean IA‐2A level in group 1 (RIA+/ELISA+) was significantly higher than that in group 2 (RIA+/ELISA−) or group 3 (RIA−/ELISA+). Regarding class II HLA, the allele frequency of HLA‐DRB1*04:05 and DRB1*09:01 was 32% (19/60) and 62% (34/60) in IA‐2A‐ELISA‐positive patients, and 31% (42/134) and 30% (40/134) in IA‐2A‐negative patients, respectively. Thus, HLA‐DRB1*09:01, but not DRB1*04:05, was associated with only the IA‐2A‐ELISA positivity (P = 0.0004). After adjustment for the clinical and immunogenetic parameters (age at onset, sex, duration, GADA positivity and the presence of HLA‐DRB1*09:01), the association of IA‐2A‐ELISA positivity, but not IA‐2A‐RIA positivity, with HLA‐DRB1*09:01 was still significant (Table S3).
Table 4.
Clinical characteristics according to groups in 137 patients with slowly‐progressive type 1 diabetes
| Group 1 (RIA‐positive and ELISA‐positive) | Group 2 (RIA‐positive and ELISA‐negative) | Group 3 (RIA‐negative and ELISA‐positive) | Group 4 (RIA‐negative and ELISA‐negative) | P‐value | |
|---|---|---|---|---|---|
| n | 33 | 9 | 4 | 91 | |
| Female | 21 (64%) | 5 (56%) | 2 (50%) | 42 (46%) | NS |
| Onset age (years) | 47.5 ± 15.9 | 55.7 ± 18.2 | 55.3 ± 6.9 | 51.1 ± 14.6 | NS |
| Duration (years) | 4.2 ± 8.2 | 2.3 ± 2.4 | 14.3 ± 10.4 | 8.9 ± 10.1* | 0.006 |
| DKA at onset (+/−) | 8/25 | 2/7 | 1/3 | 14/77 | NS |
| BMI (kg/m2) | 21.3 ± 3.5 | 21.4 ± 3.4 | 19.8 ± 1.1 | 23.8 ± 4.1** | 0.012 |
| F‐CPR (ng/mL) | 0.91 ± 0.81 | 1.76 ± 1.88 | 0.53 ± 0.27 | 1.70 ± 1.46** | 0.042 |
| GADA (+) | 32 (97%) | 5 (56%) | 3 (75%) | 45 (49%) | <0.0001 |
| GADA (U/mL) | 1191.3 ± 950.0 | 134.4 ± 169.3 | 800.8 ± 1053.8 | 658.8 ± 847.6 | NS |
| IA‐2A‐RIA (U/mL) | 12.2 ± 10.3 | 1.0 ± 0.4 | NA | NA | <0.0001 |
| IA‐2A‐ELISA (U/mL) | 95.7 ± 178.4 | NA | 1.4 ± 0.8 | NA | 0.030 |
| HLA‐DRB1*04:05 (+) | 19/56 (34%) | 8/16 (50%) | 0/4 (0%) | 34/118 (29%) | NS |
| HLA‐DRB1*09:01 (+) | 31/56 (55%) | 2/16 (13%) | 3/4 (75%) | 38/118 (32%) | <0.0001 |
Autoantibody levels were determined in patients positive for corresponding autoantibody.
*P < 0.005 versus group 1. **P < 0.05 versus group 1.
BMI, body mass index; DKA, diabetic ketoacidosis; ELISA, enzyme‐linked immunosorbent assay; F‐CPR, fasting C‐peptide; GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen; NA, not applicable; NS, not significant; RIA, radioimmunoassay.
Although patients should have at least one autoantibody (mainly GADA) during the course of the diabetes to diagnose SPIDDM, some autoantibodies might disappear in patients with longer duration. Therefore, we investigated the relationship between disease duration and the prevalence and level of autoantibodies in SPIDDM patients. The prevalence of GADA was higher than that of IA‐2A‐RIA and IA‐2A‐ELISA, and declined slowly throughout the disease course. The declines in positivity with disease duration were similar between IA‐2A‐RIA and IA‐2A‐ELISA (Figure 3). Consequently, the diagnostic sensitivity of SPIDDM decreased to 51% in patients with disease duration of ≥5 years. There were no associations between the disease duration and the levels of autoantibodies in autoantibody‐positive patients (Figure S4).
Figure 3.
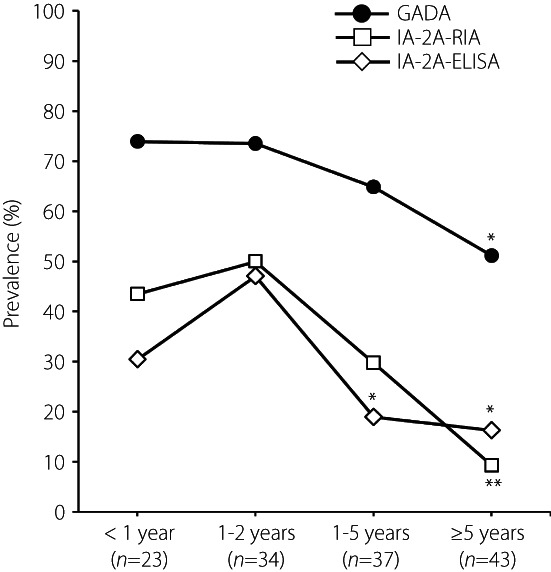
Correlation of anti‐islet autoantibody positivity with duration of diabetes in patients with slowly‐progressive type 1 diabetes. ELISA, enzyme‐linked immunosorbent assay; GADA, glutamic acid decarboxylase autoantibody; RIA, radioimmunoassay. *P < 0.05 vs group 1–2 years; **P < 0.05 vs groups <1, 1–2, and 3–5 years.
Association between progression to insulin‐deficient state and IA‐2A positivity in SPIDDM patients
As the presence of IA‐2A‐ELISA, but not IA‐2A‐RIA, was associated with lower endogenous insulin secretion in SPIDDM patients, we also analyzed the serial change of F‐CPR in 57 TIDE‐J SPIDDM patients. During the 3 years since enrollment, 32 of 57 patients (56%) progressed to an insulin‐deficient state, as defined by F‐CPR <0.6 ng/mL 5 . Table 5 shows the clinical characteristics between progressors and non‐progressors in patients with SPIDDM. Progressors were characterized by a younger age of onset, lower body mass index, higher frequency of diabetic ketoacidosis at onset, lower F‐CPR at enrollment and higher frequency of GADA than in non‐progressors. In addition, the decreased percentage of F‐CPR from the baseline (ΔF‐CPR) was significantly lower in the progressor group than in the non‐progressor group. The frequency of IA‐2A‐ELISA‐positive SPIDDM was significantly higher in the progressor group than in the non‐progressor group (53% vs 16%, P = 0.006), whereas there was no difference in frequency of IA‐2A‐RIA between the two groups. Furthermore, the mean ΔF‐CPR was significantly larger in IA‐2A‐ELISA‐positive patients than in IA‐2A‐ELISA‐negative patients (−44.0 ± 49.1% vs −10.3 ± 52.1%, P < 0.05). However, there was no significant relationship between IA‐2A‐RIA positivity and ∆F‐CPR (−35.2 ± 58.6% vs −13.7 ± 47.4%, P = 0.09).
Table 5.
Clinical characteristics between progressor and non‐progressor in patients with slowly‐progressive type 1 diabetes
| Progressor | Non‐progressor | P‐value | |
|---|---|---|---|
| n | 32 | 25 | |
| Female | 17 (53%) | 7 (28%) | NS |
| Onset age (years) | 50.3 ± 15.0 | 59.2 ± 12.2 | 0.024 |
| Duration (years) | 2.7 ± 1.9 | 2.1 ± 2.2 | NS |
| BMI (kg/m2) | 20.8 ± 2.9 | 24.3 ± 3.6 | 0.002 |
| DKA at onset (+/−) | 14/14 | 4/21 | 0.009 |
| F‐CPR at enrollment (ng/mL) | 0.77 ± 0.70 | 2.09 ± 1.41 | <0.0001 |
| ∆F‐CPR (%) | −54.1 ± 41.2 | 11.7 ± 42.7 | <0.0001 |
| IA‐2A groups | |||
| Group 1 (RIA‐positive and ELISA‐positive) | 16 (50%) | 4 (16%) | 0.008 |
| Group 2 (RIA‐positive and ELISA‐negative) | 2 (6%) | 4 (16%) | NS |
| Group 3 (RIA‐negative and ELISA‐positive) | 1 (3%) | 0 (0%) | NS |
| Group 4 (RIA‐negative and ELISA‐negative) | 13 (41%) | 17 (68%) | 0.040 |
| GADA (+) | 29 (91%) | 9 (36%) | <0.0001 |
| HLA‐DRB1*04:05 (+) | 13 (41%) | 10 (40%) | NS |
| HLA‐DRB1*09:01 (+) | 14 (44%) | 11 (44%) | NS |
Autoantibody levels were determined in patients positive for corresponding autoantibody. ∆F‐CPR indicates decreased percentage of fasting C‐peptide from baseline.
BMI, body mass index; DKA, diabetic ketoacidosis; ELISA, enzyme‐linked immunosorbent assay; F‐CPR, fasting C‐peptide; GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen; NA, not applicable; NS, not significant; RIA, radioimmunoassay.
Antigen‐specificity of IA‐2 in discrepant type 1 diabetic patients between IA‐2A‐RIA and IA‐2A‐ELISA
To elucidate whether the discrepancy in IA‐2A results between RIA and ELISA is associated with non‐specific binding to IA‐2 antigen, we carried out competitive binding experiments with unlabeled recombinant IA‐2 protein. These experiments used 31 sera from group 2 and 22 from group 1 using the RIA method, whereas 12 sera from group 3 and 19 from group 1 were employed using the ELISA method. The percentage of inhibition was 91.3 ± 10.4% for group 2 and 93.9 ± 5.7% for group 1 using the RIA method, and 97.7 ± 7.8% for group 3 and 100.0 ± 0.0% for group 1 using the ELISA method, respectively. Additionally, the levels of IA‐2A turned negative in all sera used in these experiments after adding 250 μg/mL of unlabeled IA‐2 protein.
DISCUSSION
In the present study, we showed that: (i) the prevalence of IA‐2A‐ELISA was 4–5% lower than that of IA‐2A‐RIA; (ii) the concordance rate for both IA‐2A assays was 90–95% for type 1 diabetic patients; (iii) the IA‐2A‐ELISA was a more useful marker than the RIA method for identifying SPIDDM patients who progress to the insulin‐deficient state at an early stage; and (iv) both IA‐2A assays detected antigen‐specific autoantibodies. When measured in combination with GADA, the two different commercial assay kits used to measure IA‐2A by RIA or ELISA were almost equivalent in diagnostic sensitivity. Therefore, as GADA is the first anti‐islet autoantibody in line to be measured under Japanese health insurance, the IA‐2A‐ELISA makes for a suitable alternative method to the discontinued RIA method for the diagnosis of autoimmune‐mediated type 1 diabetes in patients with acute‐onset type 1 diabetes and SPIDDM. However, it is not perfect, as we found that the correlation between IA‐2A levels by RIA and ELISA (r = 0.65–0.77) was lower than in the case of GADA‐RIA and GADA‐ELISA (r > 0.8), which was consistent with a previous report 1 . These results show that, in patients with type 1 diabetes, it might be difficult to estimate the IA‐2A‐ELISA level from the antibody level of the IA‐2A‐RIA. The discordant results between the two assays might be related to the discordant epitopes of IA‐2 peptide recognized by the RIA and the ELISA methods, although the IA‐2 molecules used in these two kits are identical.
To compare the clinical significance of IA‐2A‐RIA and IA‐2A‐ELISA, patients with type 1 diabetes were divided into four groups based on the positivity of both methods, and the clinical characteristics in patients with acute‐onset type 1 diabetes and SPIDDM were compared (Tables 3 and 4). In addition to a higher frequency of GADA in IA‐2A‐positive patients than in IA‐2A‐negative patients, the IA‐2A levels in group 1 were significantly higher than in group 2 (RIA) or group 3 (ELISA) in both type 1 diabetes subtypes. However, as both assays detected IA‐2‐spcific autoantibodies in our competitive binding experiment, we believe this outcome might be related to the different epitopes or affinities of IA‐2A between the patients positive for both RIA and ELISA and those with discrepant results.
Interestingly, patients with SPIDDM who were positive for IA‐2A‐ELISA had a higher frequency of HLA‐DRB1*09:01 than IA‐2A‐ELISA‐negative patients (Table 4). Furthermore, logistic regression analysis showed that IA‐2A‐ELISA positivity, but not IA‐2A‐RIA positivity, was independently associated with the presence of HLA‐DRB1*09:01 (Table S3). The previous studies reported that HLA‐DRB1*09:01 was susceptible HLA class II allele in SPIDDM patients, and the prevalence of IA‐2A was higher in those carrying HLA‐DRB1*09:01 11 , 12 , 13 . Thus, it is speculated that the association between SPIDDM patients and HLA‐DRB1*09:01 might be due to the presence of IA‐2A.
Regarding patients with SPIDDM, it is important to identify which predictive marker is best for identifying the progression to the insulin‐deficient state, as this could assist in maintaining good glycemic control and, therefore, avoid diabetic complications. Previous reports state that high titer, high affinity and the middle‐epitope of GADA act as predictive markers of future insulin dependency in patients with SPIDDM 14 , 15 , 16 . Furthermore, screening for other anti‐islet autoantibodies was shown to help increase the predictive power regarding GADA‐positive SPIDDM 14 , 15 . As IA‐2A‐ELISA‐positive SPIDDM patients had a lower F‐CPR than IA‐2A‐RIA‐positive individuals in our cross‐sectional dataset, we further investigated whether IA‐2A‐ELISA is superior to IA‐2A‐RIA in predicting insulin‐deficiency by using the longitudinal dataset in the TIDE‐J study. As shown in Table 5, the prevalence of IA‐2A‐ELISA, but not IA‐2A‐RIA, was significantly higher in the progressor group than in the non‐progressor group. Furthermore, there was a significant relationship between ∆F‐CPR and IA‐2A‐ELISA positivity, but not IA‐2A‐RIA positivity. These results suggest that IA‐2A‐ELISA is more useful than IA‐2A‐RIA in identifying high‐risk SPIDDM patients associated with the faster decline in β‐cell function, as well as early progression to the insulin‐dependent state. In patients with SPIDDM, it has been reported that patients with low‐affinity anti‐islet autoantibodies have prolonged preservation of residual β‐cell function 16 . Furthermore, the IA‐2A‐ELISA used in the present study utilizes the same principle, bivalent autoantibody method, as the RSR GADA‐ELISA, which has been reported to detect only high‐affinity antibodies 8 . With all these factors considered, it is speculated that the discordance between IA‐2A‐ELISA and IA‐2A‐RIA in predicting disease progression might be linked to the diversity of autoantibody affinity detected by each assay.
The present study had several limitations to report. First, the number of participants was relatively small, which might affect the concordance rate and correlation between the two assays. Therefore, further investigation using a larger cohort is required to confirm our results. Second, the duration of type 1 diabetes after onset in participants varied from short to long, so further investigations involving new‐onset patients are necessary. Third, the participants in this study did not include childhood‐onset type 1 diabetes, so further investigation involving those patients is warranted.
In summary, the present study showed that the IA‐2A‐ELISA is a reliable marker not only for the diagnosis of type 1 diabetes, but also for the prediction of SPIDDM progression when compared with the IA‐2A‐RIA. We conclude that using ELISA to detect IA‐2A might help identify a subtype of SPIDDM patients who would likely progress onto an insulin‐deficient state.
DISCLOSURE
Akihisa Imagawa received honorarium or consultation fees from Astellas Pharma Inc.; grants or research support from Astra Zeneca K. K., Taiho Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Merck Biopharma Co., Ltd., Parexel International Inc., Shionogi Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; Daisuke Chujo received honorarium for lectures from Eli Lilly and Company, Tomoyasu Fukui received research funding from Cosmic Corp. Junnosuke Miura received honorarium for lectures from Taisho Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Novartis Pharma KK, Eli Lilly Japan K.K., Sanofi K.K., Johnson & Johnson K.K., Abbott Japan LLC, Terumo Corporation, Boehringer Ingelheim Co., Ltd., Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Mylan EPD G.K., Kowa Company, Ltd., Astra Zeneca K.K. and Astellas Pharma Inc.; medical consult fees from Kanro Inc.; and manuscript fees from Novo Nordisk Pharma Ltd. The other authors declare no conflict of interest.
Approval of the research protocol: This study protocol was approved by the Ethics Committee of Japan Diabetes Society.
Informed consent: Informed consent was obtained from all participants.
Registry and the registration no. of the study/trial: Approval date of Registry 1 November 2018, and approval number 30‐008‐(2).
Animal studies: N/A.
Supporting information
Figure S1 | Insulinoma‐associated antigen‐2‐competitive binding curve for radioimmunoassay(+)/enzyme‐linked immunosorbent assay(+) sera.
Figure S2 | Prevalence of insulinoma‐associated antigen‐2 by radioimmunoassay and enzyme‐linked immunosorbent assay in combination with glutamic acid decarboxylase autoantibody.
Figure S3 | Correlation between the level of insulinoma‐associated antigen‐2 by radioimmunoassay and enzyme‐linked immunosorbent assay in patients with (a) acute‐onset type 1 diabetes and (b) slowly‐progressive type 1 diabetes with the range insulinoma‐associated antigen‐2 by radioimmunoassay <20 U/mL.
Figure S4 | Correlation between the level of (a) glutamic acid decarboxylase autoantibody, (b) insulinoma‐associated antigen‐2 by radioimmunoassay and (c) insulinoma‐associated antigen‐2 by enzyme‐linked immunosorbent assay with duration of diabetes in patients with slowly‐progressive type 1 diabetes.
Table S1 | Comparing the curve fitting data of the different models.
Table S2 | Association between fasting C‐peptide levels and insulinoma‐associated antigen‐2 positivity in patients with type 1 diabetes.
Table S3 | Logistic regression analysis for the association of insulinoma‐associated antigen‐2 positivity with clinical and genetic parameters among slowly‐progressive type 1 diabetes.
ACKNOWLEDGMENTS
This research has not received any specific grants from funding agencies in the public, commercial or not‐for‐profit sectors.
REFERENCES
- 1. Törn C, Mueller PW, Schlosser M, et al. Diabetes antibody standardization program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen‐2. Diabetologia 2008; 51: 846–852. [DOI] [PubMed] [Google Scholar]
- 2. Bingley PJ, Bonifacio E, Mueller PW. Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes 2003; 52: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 3. Chujo D, Imagawa A, Yasuda K, et al. Japanese type 1 diabetes database study (TIDE‐J): rationale and study design. Diabetol Int 2021; 13: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig 2012; 3: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawasaki E, Maruyama T, Imagawa A, et al. Diagnostic criteria for acute‐onset type 1 diabetes mellitus (2012): report of the Committee of Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus. J Diabetes Investig 2014; 5: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka S, Ohmori M, Awata T, et al. Diagnostic criteria for slowly progressive insulin‐dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the committee on slowly progressive insulin‐dependent (type 1) diabetes mellitus of the Japan diabetes society. Diabetol Int 2015; 6: 1–7. [Google Scholar]
- 7. Masuda M, Powell M, Chen S, et al. Autoantibodies to IA‐2 in insulin‐dependent diabetes mellitus. Measurements with a new immunoprecipitation assay. Clin Chim Acta 2000; 291: 53–66. [DOI] [PubMed] [Google Scholar]
- 8. Kawasaki E, Okada A, Uchida A, et al. Discrepancy of glutamic acid decarboxylase 65 autoantibody results between RSR radioimmunoassay and enzyme‐linked immunosorbent assay in patients with type 1 diabetes is related to autoantibody affinity. J Diabetes Investig 2019; 10: 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawasaki E, Miwa M, Tanaka M. Basic and clinical evaluation of ELISA assay kits (cosmic) for GADAb and IA‐2Ab. Jpn J Med Pharm Sci 2011; 66: 345–352 (in Japanese). [Google Scholar]
- 10. Pottumarthy S, Morris AJ, Harrison AC, et al. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J Clin Microbiol 1999; 37: 3229–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katahira M, Segawa S, Maeda H, et al. Effect of human leukocyte antigen class II genes on acute‐onset and slow‐onset type 1 diabetes in the Japanese population. Hum Immunol 2010; 71: 789–794. [DOI] [PubMed] [Google Scholar]
- 12. Chen W, Chen X, Zhang M, et al. The association of human leukocyte antigen class II (HLA II) haplotypes with the risk of latent autoimmune diabetes of adults (LADA): evidence based on available data. Gene 2021; 767: 145177. [DOI] [PubMed] [Google Scholar]
- 13. Takeda H, Kawasaki E, Shimizu I, et al. Clinical, autoimmune, and genetic characteristics of adult‐onset diabetic patients with GAD autoantibodies in Japan (Ehime Study). Diabetes Care 2002; 25: 995–1001. [DOI] [PubMed] [Google Scholar]
- 14. Kasuga A, Maruyama T, Nakamoto S, et al. High‐titer autoantibodies against glutamic acid decarboxylase plus autoantibodies against insulin and IA‐2 predicts insulin requirement in adult diabetic patients. J Autoimmun 1999; 12: 131–135. [DOI] [PubMed] [Google Scholar]
- 15. Kawasaki E, Nakamura K, Kuriya G, et al. Autoantibodies to insulin, insulinoma‐associated antigen‐2, and zinc transporter 8 improve the prediction of early insulin requirement in adult‐onset autoimmune diabetes. J Clin Endocrinol Metab 2010; 95: 707–713. [DOI] [PubMed] [Google Scholar]
- 16. Krause S, Landherr U, Agardh CD, et al. GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care 2014; 37: 1675–1680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Insulinoma‐associated antigen‐2‐competitive binding curve for radioimmunoassay(+)/enzyme‐linked immunosorbent assay(+) sera.
Figure S2 | Prevalence of insulinoma‐associated antigen‐2 by radioimmunoassay and enzyme‐linked immunosorbent assay in combination with glutamic acid decarboxylase autoantibody.
Figure S3 | Correlation between the level of insulinoma‐associated antigen‐2 by radioimmunoassay and enzyme‐linked immunosorbent assay in patients with (a) acute‐onset type 1 diabetes and (b) slowly‐progressive type 1 diabetes with the range insulinoma‐associated antigen‐2 by radioimmunoassay <20 U/mL.
Figure S4 | Correlation between the level of (a) glutamic acid decarboxylase autoantibody, (b) insulinoma‐associated antigen‐2 by radioimmunoassay and (c) insulinoma‐associated antigen‐2 by enzyme‐linked immunosorbent assay with duration of diabetes in patients with slowly‐progressive type 1 diabetes.
Table S1 | Comparing the curve fitting data of the different models.
Table S2 | Association between fasting C‐peptide levels and insulinoma‐associated antigen‐2 positivity in patients with type 1 diabetes.
Table S3 | Logistic regression analysis for the association of insulinoma‐associated antigen‐2 positivity with clinical and genetic parameters among slowly‐progressive type 1 diabetes.


