Abstract
Introduction
Bacillus Calmette–Guérin (BCG) has been shown to have protective effects against respiratory viruses. We conducted a scoping review of the literature to clarify the available evidence regarding the effect of BCG therapy in preventing respiratory complications of coronavirus disease 2019 (COVID-19).
Methods
We searched PubMed, Embase, CENTRAL, Scopus, and Web of Science for related studies up to October 2022.
Results
In total, 35 publications and trials were included. One animal study, two observational studies, and six finalized trials measured the effect of BCG administration on respiratory complications of COVID-19. The remaining publications included eight unfinished trials, 12 ecological studies, and six observational studies that did not directly measure respiratory complications but assessed overall mortality of the disease and were included as an adjunct to our study. All trials involved vaccinating adults to protect them against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, and measured respiratory symptoms or the need for intensive respiratory support as the primary or secondary aim of the study. One trial that exclusively included at-risk adults between 18 and 60 years old showed a decreased chance of respiratory complications as the secondary outcome of the study. Another trial that exclusively evaluated this effect on the elderly (60 years and older) as the primary aim of the study reported no protective effect against respiratory complications. The remaining literature provided mostly inconclusive evidence.
Conclusion
The majority of the literature on the protective effect of BCG against respiratory complications of COVID-19 is inconclusive.
Keywords: COVID-19, SARS-CoV-2, pulmonary complication, respiratory system, immunotherapy, BCG, scoping review
Summary
Respiratory complications contribute to the majority of deaths from coronavirus disease 2019(COVID-19). Bacillus Calmette–Guérin (BCG) vaccine is known to provide heterologous immunity against respiratory viral infections and is already available on the market. In this scoping review, we performed a thorough literature search, procuring evidence that supports or rejects the protective effects of BCG on respiratory complications of COVID-19. We found one clinical trial showing that at-risk adults between the ages of 18 and 60 who received BCG vaccine were less likely to develop severe COVID-19, or to require oxygen therapy or hospitalization. Another trial showed no protective effect of BCG on respiratory complications in the elderly (60 years or older). The remaining literature was mainly inconclusive.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by a single-stranded positive-sense RNA virus with a genomic length of around 30,000 nucleotides, belonging to the family Coronaviridae and the genus Betacoronavirus. It was first named 2019-nCoV, which was then changed to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1,2 Since December 2019, when the Chinese Center for Disease Control and Prevention reported a respiratory disease with unknown cause in Wuhan town, this disease has spread quickly around the world, and in March 2020, the World Health Organization (WHO) declared a global pandemic for the virus. According to the WHO, as of November 16th 2022, there were more than 632.95 million people infected and almost 6.59 million deaths worldwide (https://covid19.who.int).3 Infected individuals with this virus may show mild to severe symptoms such as fever, dry cough, sore throat, tiredness, loss of taste or smell, pains, nasal congestion, dyspnea, headache, diarrhea, nausea, vomiting, and, rarely, cutaneous lesions. This infection can develop and lead to severe illness and death; elderly individuals with multiple comorbidities are at highest risk of complications.1,4 The WHO reports a worldwide mortality rate of 1.1% for COVID-19;3 roughly 63% of these deaths can be attributed to respiratory complications, based on a report by von Stillfried et al.5,6
Since the start of the pandemic, BCG vaccine has been suggested as a potential protector against COVID-19, as it is known to provide heterologous immunity, ie immunity against pathogens other than itself, especially against respiratory viral infections. Moreover, BCG is already available on the market.4,7,8 This vaccine was originally developed by Dr Albert Calmette and Camille Guérin in 1921, from Mycobacterium bovis, as a vaccine against tuberculosis and its complications.9,10 This vaccine has been the most widely used vaccine worldwide for a century11,12 and is well known for giving a non-specific long-term immune boost against a wide range of pathogens, such as Leishmania species, malaria, Candida albicans, and influenza virus, through reprogramming of the innate immune cells. The immune activation properties of BCG are used by urologists for adjuvant immunotherapy of early forms of bladder cancer.11,13,14 However, it should be kept in mind that despite its benefits and applications, BCG therapy, like many other treatments, can have side effects and risks. Although pulmonary involvement is rare (0.3– 0.7%) and systemic BCG infection is reported in less than 1% of cases, when tuberculosis/COVID-19 coinfection occurs, there may be a synergism between the two pathogens and increased susceptibility to COVID-19, with a consequent rapid respiratory worsening and, eventually, death.15,16
Given this evidence, we hypothesize that BCG may affect the respiratory complications of COVID-19. The aim of this review was to find available evidence on the role of BCG vaccination in respiratory complications of COVID-19.
Materials and Methods
The study was a scoping review designed according to the 2005 guideline by Arksey and O’Malley,17 which was later refined by Colquhoun et al18 and adopted by the Joanna Briggs Institute.19 The purpose was “exploration by including unlimited study designs, settings and outcomes”.17 The organization of information was based on the PRISMA model20 and the recommendations of PRISMA-ScR.21
Search Strategy
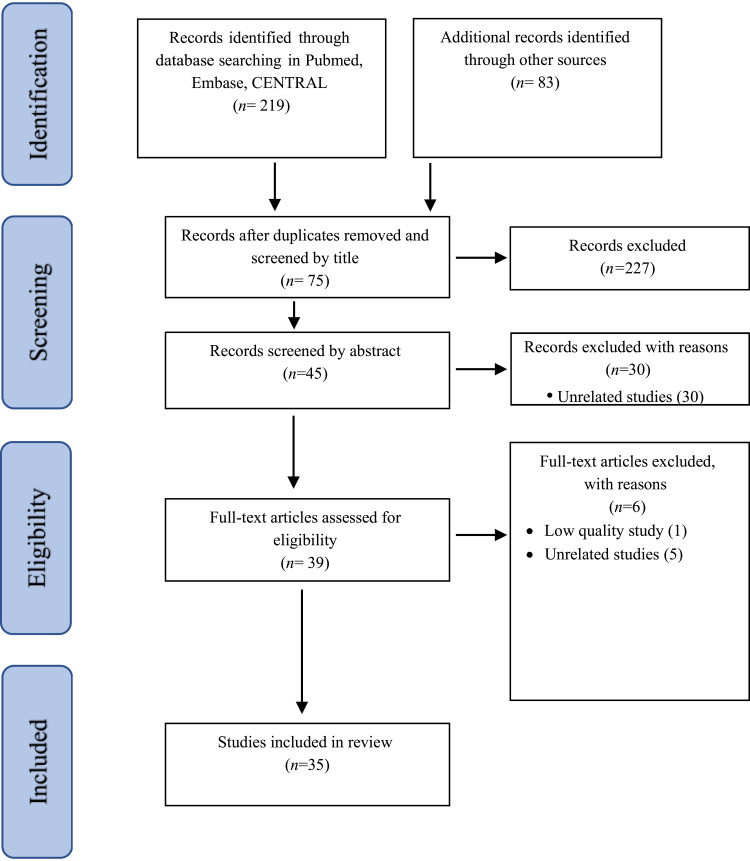
Our focus was “evaluating the effect of the BCG immunotherapy on respiratory complications caused by COVID-19”. We used the Rayyan platform22 for our search; details of the search strategy are available in Appendix 1. In short, we searched all published articles up to October 2022 using Medical Subject Headings (MeSH), Emtree language, and text words in the following databases: PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), supplemented with a manual search (ie not using Rayyan) in Scopus and Web of Science. Studies could be included in the review if 1) they were carried out in vivo, on the relationship between BCG immunotherapy and respiratory complications caused by COVID-19; and 2) no limitation to language was applied to the software. The Rayyan software automatically removed duplicates. The remaining exclusions were performed manually by three investigators. The following study types were excluded: letters, replies, reviews, editorials, communications, and conference abstracts. Unrelated articles, ie articles that were not pertinent to our focus, were excluded after reading the title, abstract, and the main manuscript, as described in Figure 1, along with those without full text access, preprints, or unpublished articles; however, in case of unfinished trials, the trial number and a short summary are provided in Table 1. When there were numerous publications on the same cohort, all articles were reported, with special focus on the differences in their analysis leading to different conclusions.
Figure 1.
Process of identification and inclusion of studies – PRISMA flow diagram.
Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Creative Commons.
Table 1.
Characteristics of Clinical Trials
| Clinical Trial | Start and End Dates | Phase | Samples | Follow-Up | Inclusion Criteria | Main Outcome | Status | Results | Respiratory Complications | Funder | Country | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBR-4kjqtg | 20.08.2020–31.08.2021 | II | 68 controls (no placebo) vs 70 BCG Moscow | 180 days via telemedicine | Healthcare workers (HCWs) exposed to SARS-CoV-2; male or female; 18 years or older; positive history of previous BCG vaccination and negative history of COVID-19 | Incidence of SARS-CoV-2 infection | Finalized | Groups were not significantly different in their possibility of acquiring the infection. Adverse events: only mild local lesion | Possibly no worsening of respiratory complications by BCG. Protection could not be proven | CNPq/MCTI | Brazil | [23] |
| NCT04373291 | 18.05.2020–01.10.2021 | III | 1293 | 180 days | Hospital personnel in participating hospital for more than 22 hours per week; male or female; 18 years or older | Absenteeism from work | Recruitment completed | No study results reported | Not measured directly – hospital admissions as secondary outcome indirect measures | Bandim Health Project | Denmark | [24] |
| NCT04327206 | 30.03.2020–27.05.2022 | III | 6828 controls vs BCG Danish | Every 3 months until 12 months, in person | HCWs; male or female; 18 years or older | Incidence of symptomatic or severe COVID- 19 at 6 months | Finalized | Only serology results available: BCG vaccination reduced cytokines associated with severe disease | Incidence and severity of febrile respiratory illness – no results available | Murdoch Childrens Research Institute | Australia, Netherlands, Spain, UK, and Brazil | [25,26] The BRACE trial |
|
NCT04328441 NCT03987919 |
24.03.2020–31.03.2021 | III | 758 placebo vs 753 BCG Danish | 12 months by weekly questionnaire via app | HCWs with exposure to SARS-CoV-2; 18 years or older; male or female | Reduce HCW absenteeism due to illness during the COVID-19 pandemic | Finalized | No significant difference in absenteeism due to any cause or incidence of documented SARS-CoV-2. Adverse events: only mild | No difference in incidence of respiratory symptoms | UMC Utrecht | Netherlands | [27] |
| NCT0441733 | 16.04.2020–01.05.2021 | IV | 1006 placebo vs 1008 BCG Danish or Denmark | 12 months by weekly or monthly questionnaire via app or phone | Male or female; elderly ≥60 years | Cumulative incidence of respiratory tract infection (RTI) requiring medical intervention | Finalized | No significant difference in incidence of RTI or documented SARS-CoV-2 infection. Adverse events: only mild | No difference in incidence of RTI or dyspnea | Radboud University Medical Center | Netherlands | [28] |
| NCT04414267 | 26.05.2020–19.04.2021 | III | 153 placebo vs 148 BCG Moscow or India | 6 months | Male or female; age ≥50 years plus history of at least one of the following: coronary heart disease; chronic obstructive pulmonary disease; Charlson’s comorbidity index (CCI) >3. Negative serum testing for immunoglobulin G and M against SARS-CoV-2; skin tuberculin test diameter less than 10 mm | Incidence of COVID-19 and presence of anti-SARS-CoV-2 antibody | Finalized | BCG revaccination resulted in 68% relative risk reduction of infection at 6 months. No difference in infection incidence at 3 months. Adverse event: only mild local lesion | Respiratory symptoms and their severity were asked about in questionnaires. No significant difference between groups | Hellenic Institute for the Study of Sepsis | Greece | [29] The ACTIVATE-2 trial |
| Clinical Trials Registry – India (CTRI number CTRI/2020/07/026668) | 10.2020–12.2021 | III | 249 placebo vs 246 BCG India | 1, 3, 6, and 9 months | Male or female with underlying conditions (poorly controlled diabetes, chronic kidney or lung disease, etc); 18–60 years old | Risk of SARS-CoV-2 infection | Finalized | BCG arm had 8.4% reduction in the incidence of probable infection | BCG arm had significantly lower incidence of severe COVID-19, oxygen requirement, and hospitalization (p=0.03) | Three hospitals in different areas of India | India | [30] The BRIC trial |
| NCT04632537 | 07.12.2020–23.03.2021 | III | – | 6 months | HCWs exposed to SARS-CoV-2; male or female; 18–64 years old | Risk of SARS-CoV-2 infection and disease severity | Withdrawn (funding issues) | – | Oxygen/intensive care/mechanical ventilation requirement; incidence of self-reported respiratory symptoms – no results available | Henry M. Jackson Foundation for the Advancement of Military Medicine | United States | – |
| NCT04348370 | 20.04.2020–05.2022 | IV | 1800 | 6 months | HCWs directly exposed to SARS-CoV-2; male or female; 18–75 years old | Risk of SARS-CoV-2 infection and disease severity | Active, not recruiting | No study results reported | Oxygen/intensive care/mechanical ventilation requirement – no results available | Texas A&M University | United States | The BADAS trial |
| NCT04362124 | 08.2020–11.2021 | III | – | 360 days | HCWs directly exposed to SARS-CoV-2; male or female; 18–65 years old; negative COVID-19 test and asymptomatic | Incidence of SARS-CoV-2 infection | Withdrawn (funding issues) | – | Severe COVID-9 – no results available | Universidad de Antioquia | Colombia | – |
| NCT04659941 | 01.10.2020–01.10.2022 | II | 752 | 6 months | HCWs never infected with SARS-CoV-2; male or female; 18 years or older | Incidence of SARS-CoV-2 infection | Active, not recruiting | No study results reported | Severe disease – no results available | Universidade Federal do Rio de Janeiro | Brazil | – |
| NCT04369794 | 01.10.2020–30.08.2023 | III | 186 placebo vs 175 BCG Brazil or India | Weekly for 4–6 weeks, in person | Infected with SARS-CoV-2 within the past 14 days; male or female; 18 years or older | Safety of BCG revaccination in COVID-19 convalescent patients | Active, not recruiting | Adverse events: mostly mild local lesions. Possibility of increased dyspnea in the second week post-BCG | Higher proportion of dyspnea in the BCG recipients in the second week, which may have been due to failed randomization | University of Campinas | Brazil | [10,31] The BATTLE trial |
| NCT04461379 | 21.07.2020–01.01.2021 | III | 908 | 6 months | HCWs directly exposed to SARS-CoV-2; male or female; 18 years or older; | Incidence of SARS-CoV-2 infection | Active, not recruiting | No study results reported | Oxygen/intensive care/mechanical ventilation requirement; mortality associated with respiratory disease – no results available | Hospital Universitario Dr. Jose E. Gonzalez | Mexico | – |
| NCT04350931 | 20.04.2020–01.12.2020 | III | 900 | Daily | HCWs exposed to SARS-CoV-2; male or female; 18 years or older | Incidence of SARS-CoV-2 infection | Unknown | No study results reported | Symptom of dyspnea | Ain Shams University | Egypt | – |
Data Extraction
The first three authors separately screened the titles and abstracts and double-screened a portion of the articles for inclusion and exclusion criteria. The following information was extracted from each article: study reference or identification code of clinical trials, start and end dates of the clinical trials and cohorts, phase of the clinical trials, sponsor, inclusion criteria, sample size, follow-up details, primary aim, clinical trial status, results, and how the respiratory complications were assessed. Abstract data were extracted by the first and third authors and a detailed assessment was added by the second author.
Results
The primary search detected 302 citations (219 from electronic databases and 83 from others), 94 of which were duplicates. The titles of the remaining 208 articles were screened by the first three authors, leaving 75 abstracts that were screened independently, thus leaving 35 full-text articles for further screening. All the remaining 35 studies met inclusion and exclusion criteria of this study and were included (Figure 1 – PRISMA flow diagram). Disagreements regarding the inclusion of articles were resolved by discussion. Overall, 14 trial reports were identified, with the results of only six being available. Only one trial assessed respiratory complications as the main outcome and the remaining studies reported them as secondary outcomes. Out of the remaining 21 studies, only three directly assessed respiratory complications. The remaining studies indirectly assessed these complications using mortality or hospitalization rates, and are mentioned at the end of this section as an adjunct to the review. Population-based studies (ecological studies), explained in the adjunct section, are summarized in Supplementary Table Appendix 2.
Discussion
Animal Studies
The study by Kaufmann et al32 is the only animal study in this scoping review. They vaccinated mice and hamsters with BCG. After 4 weeks or 6 months, they infected them with various strains of SARS-CoV-2 and the influenza A virus through different routes (intranasally or intratracheally). They observed no difference between vaccinated and non-vaccinated mice in terms of weight loss, pulmonary viral load, or morbidity from SARS-CoV-2. In contrast, they observed an protective effect of BCG against influenza A virus. They concluded that BCG’s heterologous protection against respiratory viruses depends on the virus. Their study is limited by the very small sample size of each group (as small as three in some groups).
Observational Studies
Su et al33 retrospectively analyzed a cohort of the Taiwanese population with an available record of BCG vaccination. They aimed to assess the severity of COVID-19, especially respiratory syndromes, based on BCG vaccination status. Since data were only available for individuals born after 1985, their study subjects were a maximum of 33 years old. Data on COVID-19 cases were extracted between January 21st and March 19th 2021. A total of 328 individuals were included, 112 of whom were unvaccinated. They allocated included subjects into two age groups: 4–24 and 25–33 years old. In the 4–24-year-old age group, only dix individuals were unvaccinated. There was no substantial difference in COVID-19 severity between groups. Their study is limited by the sample size being far too small to detect an extremely rare event (respiratory complications of COVID-19 in individuals younger than 33 years).
Aksu et al34 performed a cross-sectional report of COVID-19 patients with pneumonia referred to a state hospital in Istanbul, Turkey, between March 11th and June 10th 2020. They looked for a correlation between childhood BCG vaccination and the severity of respiratory complications in their patients. BCG vaccination was confirmed by visualizing the BCG scar together with the subject’s self-declaration of vaccination. Their final sample size was 123. Patients either had severe COVID-19 pneumonia and were hospitalized (tachypnea with respiratory rate >30 breaths/min, room oxygen saturation <90% plus bilateral diffuse pulmonary infiltrate, N=89 [72.4%]) or had a mild condition suitable for follow-up on an outpatient basis (N=34 [27.6%]). They found that BCG vaccination was less common in the severe cases; however, the difference was not statistically significant.
Our comment: The country of Turkey has had a BCG program for 70 years and the mean age of patients in this study was 49.7±13.3 years; however, only 68.5% of the severe cases were BCG vaccinated (68.5% of severe cases vs 88.2% of mild cases). The severe cases had a mean age of 58.2±13.2 years. This means that, if the distribution of age in severe cases was normal and BCG truly had no effect on the probability of severe cases, then, based on the z score, 81% of them should have been younger than 70 years and therefore BCG vaccinated. The lower than expected percentage of BCG vaccination speaks in favor of BCG’s protective effect in decreasing the chance of severe COVID-19. However, the distribution of age was probably NOT normal, and we are talking about a program that supposedly started 70 years ago, so we cannot expect 100% of individuals born after the initiation of the program to have been vaccinated.
Severe cases in this study also had lower income and were more likely to be diabetic. Therefore, the authors performed multivariate analysis on their sample size of 123 to assess seven variables in their patients (age, gender, income, smoking, diabetes, and hypertension, as well as BCG vaccination status). The multivariate analysis only showed income and age as independent predictors, and BCG vaccination was not shown to be protective against severe pneumonia in COVID-19 patients. However, their sample size was too small for this analysis.
Trials
Our search strategy identified 14 reports of trials on the effect of BCG vaccination intervention on COVID-19 and respiratory complications. Details of each trial and their results are available in Table 1. Only six trials are currently finalized with reported results. All trials were designed for adults. Two of them included only older subjects (≥50 and ≥60 years old). Four trials had a maximum inclusion age (18–60 years, 18–65 years in two studies, and 18–75 years). Only one trial excluded individuals with a positive skin tuberculin test. Most of the trials (10/14) solely included healthcare workers (HCWs). Five of the trials on HCWs included only those with some level of exposure to COVID-19 patients.
Two of the trials on HCWs required participants to have never been infected by SARS-CoV-2 at the time of intervention, as proven by negative serological testing. In contrast, one trial specifically included recently infected individuals and did not limit their inclusion criteria to HCWs. All studies excluded patients with the possibility of immunodeficiency, including HIV infection, chronic steroid use, taking chemotherapy agents, etc.
The results of only six trials are currently available. In terms of BCG adverse events, all trials reported the possible safety of this vaccine in adults at risk of COVID-19 or those recovering from COVID-19. A small lesion at the injection site was reported in the majority of BCG recipients. Dionato et al31 described the lesions in detail. Only one study found evidence of BCG’s protective effect against respiratory complications: the BRIC trial by Sinha et al30 assessed the possibility of respiratory failure as the secondary aim of their study by following up their participants for 1, 3, 6, and 9 months, observing for the incidence of COVID-19 complicated with high respiratory rate (>30 breaths/min), low oxygen saturation (<90% on room air), severe respiratory distress, or a diagnosis of acute respiratory distress syndrome (ARDS).
The BRIC trial exclusively included subjects between 18 and 60 years old at risk of severe COVID-19: those with poorly controlled diabetes, or chronic kidney, lung, or cardiovascular disease. The results of their trial showed reduced incidences of severe COVID-19, oxygen requirement, and hospitalization (p=0.03 for all) in the BCG arm.
The BATTLE trial is the only trial to have been performed on COVID-19 convalescent patients. The primary aim was to assess potential synergistic effects of BCG on COVID-19 symptoms and concerns regarding possible worsening of the disease. The inclusion criterion in this trial was recently infected adults (<14 days since the initiation of symptoms). All participants in this trial had been BCG vaccinated at birth through Brazil’s national program. The results of the BATTLE trial are published in two separate articles because each article used a different strategy for analyzing the results (different handling of missing data and different symptom progression analysis). The article by Jalalizadeh et al10 concluded that BCG does not worsen COVID-19 symptoms, including respiratory symptoms. The results of Dionato et al,31 however, show possible worsening of dyspnea during the second week in the BCG arm. The findings of the second article are further modified by sensitivity analysis, showing that the higher prevalence of dyspnea could have been due to a higher prevalence of chronic pulmonary disease in the BCG arm (failed randomization). The results of both studies are limited by the fact that all the included patients had mild disease and were relatively healthy and young (average age 41 years). In short, the BATTLE trial showed that BCG-revaccinating adults with mild COVID-19 is unlikely to worsen respiratory complications of the disease. The trial, however, could not prove benefits from BCG revaccination in terms of respiratory complications.
Moorlag and colleagues performed two trials, one in elderly people, ≥60 years old,28 and another on HCWs.27 The primary end point of the first trial was initially the cumulative incidence of hospital admission due to COVID-19 in the elderly; however, it was later changed to the cumulative incidence of aggravated respiratory symptoms due to rare incidence of hospitalization. This trial was the only trial to assess BCG protection against respiratory complications as the main outcome. The second trial was conducted on HCWs, with the primary end point of reduced absenteeism from work, and evaluated respiratory symptoms as the secondary outcome. Both trials followed up patients on a weekly or monthly basis after BCG injection, and continued to follow up their participants for one year. Neither trial reported a significant difference in the incidence of respiratory tract infections. Dos Anjos et al23 carried out a trial on HCWs exposed to SARS-CoV-2, with the main purpose of reducing the incidence of SARS-CoV-2 infection. Their results also showed no significant difference in this incidence. As the secondary aim of their study, they observed no difference in their participants in terms of respiratory complications.
As the secondary aim of the study, Moorlag et al35 measured the humoral response to COVID-19 and influenza infection in the BCG recipients, comparing them to placebo recipients. This study can be juxtaposed with the animal study by Kaufmann et al32 mentioned at the beginning of this section, with similar findings: both studies found an enhanced response to influenza but not to SARS-CoV-2. Kaufmann et al32 concluded that BCG’s protection against respiratory viruses depends on the type of the virus.
There are two important differences between the BATTLE and BRIC trials and the trial conducted by Moorlag et al.28 First, the age of participants is significantly different. Moorlag et al28 injected BCG into elderly patients, >60 years of age, expecting a boost in their immune system. The immune system in the elderly is limited, as shown by the replacement of thymus by fat tissue and the vulnerability of the elderly to a newly mutated cold virus. Following BCG administration, Moorlag et al28 found an enhanced response to influenza infection, which has been around for decades, meaning that their elderly subjects had been previously exposed to this virus when their immune system was younger and more adaptable. It can be postulated that the immune system loses its ability to adapt to novel infections over time. Moorlag et al28 also showed that the elderly subjects failed to enhance their immune response to SARS-CoV-2, which is a novel virus. Vaccinating this population is therefore less likely to be effective, as BCG is expected to enhance trained immunity, which has the components of the “rigid” innate immunity but is also expected to “adapt” to some extent.
The other important difference between the trials is the participants’ previous exposure to BCG. The BATTLE trial was held in Brazil, which has a national program for BCG vaccination of all newborns, and an old BCG scar was evident in the majority of their participants, indicating that all of them had possibly been exposed to this vaccine at birth. The BRIC trial was held in India, with similar BCG status (vaccination of all newborns after 1948).36 Participants in the Moorlag et al28 trial were heterologous; around one-fourth of them had previously been BCG vaccinated (median 49 years before) and around one-fifth of them were uncertain of their previous vaccination status. The trial managers proportionately distributed the participants with different previous exposure between control and intervention groups through randomization, and therefore this confounder probably did not interfere with the conclusion of their main end point (cumulative incidence of respiratory tract infection). Their secondary end point, on the other hand, showed a significant difference: the humoral response against COVID-19 was more robust if the subject had previously been vaccinated.
The humoral analysis in the BATTLE trial partially explains this phenomenon. BCG vaccination in this study caused a less specific humoral response to SARS-CoV-2, meaning that the antibody response against a new virus after BCG vaccination becomes heterologous. The authors postulated that this prepares the immune system for future mutations of the virus. The previously BCG vaccinated subjects in the Moorlag et al study28 have therefore acquired the old unmutated coronavirus and produced immunity against its possible mutation forms as well through heterologous antibody production.
One important limitation of all of the included trials is the lack of information on the COVID-19 vaccination status of participants. All trials started before the COVID-19 vaccine became widely available, but most of the finalized studies finished after the COVID-19 vaccine had become partially available. The study participants may have been partially vaccinated; however, this information is only mentioned in one of the trials, which is not yet finalized (NCT04659941).
Importantly, the analysis of the lesion in participants in the BATTLE trial shows that those who received placebo could occasionally develop lesions after the injection of placebo (normal saline). The injection site for this vaccine is typically not cleaned with alcohol, in order to avoid killing the BCG. It can therefore be assumed that during this type of injection, some normal flora of the skin is occasionally introduced to the intracutaneous area. The heterologous immune boost after BCG vaccination can therefore be slightly attributed to the occasional intracutaneous introduction of the skin flora. This could result in smaller differences between placebo and intervention groups in trials and lead to the wrong conclusion that BCG vaccination is not effective; however, as seen in the lesion analysis of the BATTLE trial, the effect is probably small.
One reason that these trials have different results could be due to the different strains of BCG used by each trial (Table 1). Differences in the immunogenicity of strains have been shown in previous studies.37 The BATTLE trial used two different strains of BCG and performed a separate analysis to compare the COVID-19 symptoms and lesions of participants based on the BCG strain. No differences were observed from this comparison.37
Adjunct
Studies That Did Not Directly Assess Respiratory Complications
Many of the identified studies did not directly measure respiratory complications. They indirectly measured them using substitute indices: CMR, CFR, and rate of hospitalization. CMR is case mortality rate, defined as the number of deaths from COVID-19 in all individuals (infected or not infected), usually shown as the number of deaths per million individuals. CFR is case fatality rate and is measured as the number of deaths in those infected with SARS-CoV-2, indicating how deadly the infection was for those who tested positive. Some studies calculated this as death per case per day (DPC/day). There are important considerations for replacing respiratory complications with CFR and CMR (explained in the following paragraph) and there is potential for multiple types of bias. However, we did not exclude these studies, in order to increase the amount of evidence in our hands. In the following paragraphs, we first explain how to approximate respiratory complications based on the available measures; we then explain the studies in detail so that the reader can cautiously assess them with discretion and identify any potential bias.
Respiratory complications are the main cause of death in COVID-19.5 As von Stillfried et al5 showed, roughly 63% of all COVID-19 deaths were due to respiratory complications: 52% due to diffuse alveolar damage (DAD) and the remainder due to superinfection, pulmonary embolism, and others. We can therefore attribute roughly 63% of any observed effect on mortality to changes in respiratory complications. However, respiratory complications may be disproportionately affected by our intervention; for example, BCG may have a more significant effect on reducing superinfections by generating heterologous immunity. Furthermore, not all respiratory complications lead to death.
The long-awaited report from von Stillfried et al5 also showed that only 86% of deaths that occurred in the setting of a positive SARS-CoV-2 test were in fact due to the virus and the remaining 14% occurred as a coincidence. This was statistically expected as the virus became widespread, ie when an event becomes very common, coincidences also become common. For example, an elderly man with multiple comorbidities who is about to succumb to his illnesses is more likely than the average person to spend time in places that put him at high risk of acquiring SARS-CoV-2 infection (hospitals, hospice care, elderly care centers).
CFR and CMR: While CFR is a measurement of how deadly the infection is in those who acquire it, CMR counts the number of related deaths in a population regardless of the number of infections. CFR is therefore dependent on the testing rate in the community, meaning that if the community rarely provides testing, asymptomatic individuals or those with mild disease will not be diagnosed and therefore CFR will be measured as higher than reality. CMR is less affected by this bias but, as we know, COVID-19 is highly political and both CMR and CFR could be inaccurately reported for financial and political reasons. Pharmaceutical lobbyists may inflate the numbers to gain more public funding and the politicians may lower the numbers to gain public approval.
Ecological Studies
Our search strategy identified 12 ecological studies.38–49 Ecological studies are epidemiological studies that find differences based on the geographical location of their subjects (populations). The results of ecological studies that analyzed countries’ performance during the COVID-19 pandemic based on their national BCG immunization status are summarized in Supplementary Table Appendix 2. This table provides comparison of the variables used in each study, the timing of their study during the COVID-19 pandemic, and their results. None of the ecological studies directly measured respiratory complications of COVID-19. We therefore briefly explore them in this article. In short, the studies varied greatly in their methodology and their conclusions varied greatly as well. Three studies found no evidence of BCG protection against COVID-19. Nine studies, conversely, found different types of national BCG vaccination program to improve the country’s CMR.
In the Supplementary Table, we describe the potential confounders that each study included in its analysis and whether they were shown to be a possible source of bias. All studies were performed very early in the pandemic. All studies identified age as the main confounder, ie COVID-19 mortality was dependent on the age of the population. Some studies added many variables to their analysis to account for confounders. This was considered both a strength and a limitation of the studies: although adding confounders is always beneficial in studies, the sample size should be large and the source of the added information must be accurate. Hassan et al50 performed an ecological study limited to the country of Nigeria (not included in the table), comparing different states of Nigeria based on their BCG coverage and other factors. Their analysis showed lower CMRs in states with higher BCG coverage. However, their analyses also showed multiple possible confounders. Importantly, some argue that the BCG vaccination effect is confounded by the fact that people in countries with a BCG vaccination policy have a higher level of exposure to Mycobacterium spp.51–53
Other Studies
Bates et al54 extracted health data of USA military veterans on March 17th 2021, and compared COVID-19 cases and fatality in those who were born in countries with a national BCG vaccination program to those who were born in other countries and the USA. BCG vaccination was based on the BCGAtlas.org 2020 update. The study found lower CFRs in those who were likely to have received BCG in infancy than in those who were not (0.8% vs 3.2%).
Strengths:
The authors performed two types of analysis based on two study designs: a case–control analysis and a retrospective cohort analysis, leading to slightly different conclusions.
They considered the possibility of the BCG effect waning in time and performed an analysis to assess this, which was possible owing to their large sample size. No evidence of better BCG protection in younger ages was found from this analysis. This analysis is important given that some studies have shown the effect of BCG effect to wane over time, especially if there is no environmental exposure to Mycobacterium spp.52,55
Limitations:
There was no direct analysis of respiratory complications. CFR was used as a substitute but the cause of death was only assumed to be due to COVID-19.
Large exclusions for multiple reasons excluded 32% of the initial sample. They excluded many individuals for many reasons. Those older than 75 years of age were excluded because they were unlikely to be vaccinated. Individuals younger than 20 were excluded because they were not veterans but children of veterans. They excluded those younger than 30 years from mortality analysis because death was unlikely at their age, even though the sample size was large enough for rare case analysis. They also excluded employees of Veterans Affairs from the data, as well as individuals who died before January 1st 2020, because they wanted to make sure that the cause of death was COVID-19 (the first death from COVID-19 in the USA was recorded on February 28th 2020). Therefore, their data were not very clear on the cause of death; all deaths within cases of positive COVID-19 were considered as COVID-19 mortality. In the statistical analysis section of the manuscript, they mention that they excluded anyone who had not had a primary care visit in the past 2 years, justifying that this excludes those who typically receive healthcare from outside Veterans Affairs. Despite these wide exclusions, they did not perform any analysis of the missing data. They only performed a sensitivity analysis for exclusion of those who had not had any primary care visits in the past 2 years, which changed the results on the impact of ethnicity on CFR.
A delay in recording was noticed, and their data were not up to date at the time of extraction (March 17th 2021).
The control group in this study was a random sample of 500,000 veterans who had “never tested” for SARS-CoV-2. To compensate for the enormous bias this creates, the authors manually added a “proportionate” sample of those who had tested negative. In the final analysis, some countries are disproportionately represented, eg Mexico has more infected cases than controls, while most countries are represented by a 3 to 2 control to case ratio.
The BCG policy of some countries is unclear. Some countries administered booster BCG shots at ages 7 and 14. This information was ignored in the final analysis as it was incompletely available. Some countries started the vaccination at age 13, and those individuals were considered unvaccinated in the study.
The male sex was the predominant gender in their data. The data showed infected males to be considerably older (mean age 61 vs 50 years) and with higher CFR (3.4% mortality in males compared to 0.8% in females, not adjusted for age). Widows had a surprisingly higher CFR than average (6.5%, not adjusted for age). Widows in this study had a higher CFR than those who were divorced or separated (3.3%). The reason for this observation could be due to an increased chance of a subject being near the age of death if their spouse has already died, because spouses tend to have a similar diet and lifestyle. It could also point to the health benefits of living with a partner.
The study classified the BCG status of subjects based on country of birth, even though their analysis also showed “ethnicity” to be a major confounder in COVID-19 deaths.
de Chaisemartin and de Chaisemartin56 focused on Sweden’s BCG program, and specifically the sudden discontinuation of national BCG vaccination in April 1975. They hypothesized that if BCG had any protective effect, it would manifest as a sudden change in COVID-19 infection, hospitalization, or death rates of individuals born after this date compared to those born immediately before. Their regression discontinuity analysis showed no abrupt change in infection rate or mortality. They concluded that BCG has no COVID-19 protective effect.
Strength:
The groups were very similar, except for the age difference, which was considered in their analysis by looking at “abrupt change in trend” rather than looking at absolute difference.
Limitations:
The data were extracted on May 17th 2020, very early in the pandemic, when the number of infected cases was very small and limited to certain groups of the population. The small case number is evident in their graph, showing almost zero cases of infection per 1000 individuals born in 2001.
They assessed three aspects of COVID-19 epidemiology: number of cases per 1000 inhabitants, number of COVID-19 hospitalizations per 1000 inhabitants, and number of COVID-19 deaths per 1000 inhabitants. They did not assess respiratory complications or risk of death from infection (CFR); CFR was the main outcome in the study by Bates et al,54 which showed a difference between BCG vaccinated and unvaccinated individuals.
Even though the country abruptly stopped the BCG vaccination of newborns in 1975, it continued vaccinating other populations for a few years (15-year-olds, military recruits). However, the study authors argue that those vaccination policies did not interfere with their 1975 cohort.
This study can be compared to a similar study from Israel, by Hamiel et al.57 BCG vaccination of newborns was stopped in Israel in 1982; Hamiel et al57 acquired infection and mortality rates in Israelis who were tested for SARS-CoV-2 between March 1st and April 5th 2020. They grouped them based on their year of birth into two groups: those born before the policy halt (1979–1981) and those born a few years afterward (1983–1985). Unlike the Swedish study, they did not assess “abrupt change in trend”; rather, they compared the two groups using two-sided Fisher’s exact test. They found no difference in positive SARS-CoV-2 test results between the BCG vaccinated and unvaccinated groups. Only one person in each group was hospitalized, so they were unable to compare mortality. Importantly, the BCG vaccinated group in their study was on average 5 years older (40 years vs 35 years), and it is important to keep in mind that early in the pandemic, SARS-CoV-2 was more likely to be discovered in older individuals as older people were more likely to be tested (the graphs in de Chaisemartin and de Chaisemartin’s56 study clearly show this trend). Therefore, the equal infection rate in the two groups speaks in favor of BCG vaccinated individuals, because by default, the older group should have had a higher infection rate.
Hupert et al58 modeled the impact of various “heterologous” vaccines on the progress of the pandemic in the USA during the fall and winter of 2020. They did not separate the BCG variable in their model and analyzed many non-SARS-CoV-2 vaccines together: polio, MMR, influenza, BCG, and other vaccines that are poorly described in their manuscript and Supplemental Material. There are some serious issues regarding the source of their data; it is unclear where they obtained the information for vaccination coverage and how they labeled an individual as “BCG vaccinated”.
Moorlag et al35 retrospectively followed a cohort of recently BCG-vaccinated adults during the first months of the pandemic in the Netherlands by collecting information between February 27th and April 30th 2020 via a digital survey. Their main purpose was assessing the safety of recent BCG vaccination. They compared 266 volunteers who had been vaccinated in the past 5 years to 164 volunteer controls who had never been vaccinated. The two groups were not randomized and were different in age distribution and exposure to SARS-CoV-2; vaccinated individuals were younger and more likely to be HCWs. They concluded that BCG vaccination does not increase the chance of hospitalization, as none of the vaccinated individuals were hospitalized. The rate of infection did not differ between the groups, even though the BCG vaccinated participants were more exposed and more likely to be HCWs. Furthermore, during the period of the study, mostly HCWs were tested for the virus in the Netherlands, meaning that the rate of confirmed infection in this study could be biased. The study also evaluated differences in self-reported COVID-19 symptoms, and found reduced frequency of most symptoms, and especially fatigue, in the BCG group.
Conclusion
The strongest evidence in favor of protective effect of BCG on respiratory complications of COVID-19 is currently provided by the BRIC trial by Sinha et al;30 as the secondary outcome of their study, Sinha et al assessed the incidence of respiratory complications from SARS-CoV-2 infection in 495 at-risk individuals between the ages of 18 and 60. Their results show that BCG can reduce these complications in subjects with chronic diseases, such as poorly controlled diabetes or chronic kidney, lung, or cardiovascular disease. The trial by Moorlag et al28 failed to show protective effects of BCG in elderly people (60 years or older). The BATTLE trial showed that the possibility of worsening of COVID-19 symptoms with the BCG injection is low. All trials reported a mild local lesion as the only adverse effect of BCG injection, and rare serious side effects (pulmonary or disseminated BCG infection) were not reported.
The remaining evidence regarding this topic is, unfortunately, inconclusive and does not clearly either support or reject the theory: respiratory symptoms were not the main outcome of the remaining trials; therefore, their power may have been insufficient to detect a significant difference. The animal study suggests that BCG can protect mice against respiratory complications of influenza and not SARS-CoV-2, suggesting that this vaccine’s heterologous immunity coverage depends on the virus. The ecological studies (population-based studies) and the observational studies that measured mortality indices from COVID-19 show a possible protective effect of BCG vaccination in large populations; however, their results are mainly inconclusive and their analyses had a high risk of bias.
Funding Statement
This review did not receive any funding.
Disclosure
Cristiane G da Costa and Mehrsa Jalalizadeh are co-first authors for this study. The authors declare that they have no competing interests in this work.
References
- 1.Kumari P, Gupta UD, Bhagyawant SS. Bacillus Calmette-Guerin as a quick and temporary solution to coronavirus disease-2019. Int J Mycobacteriol. 2021;10(2):105. doi: 10.4103/ijmy.ijmy_86_21 [DOI] [PubMed] [Google Scholar]
- 2.Khongthaw, Dulta K, Chauhan PK, Kumar V, Ighalo JO. Lycopene: a therapeutic strategy against coronavirus disease 19 (COVID- 19). Inflammopharmacology. 2022;30(6):1955–1976. doi: 10.1007/s10787-022-01061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus Disease (COVID-19). World Health Organization; 2022. [Google Scholar]
- 4.Maheshwari N, Jain A. Is there a rationale for using bacillus Calmette–Guerin vaccine in coronavirus infection? Viral Immunol. 2021;34(5):300–306. doi: 10.1089/vim.2020.0079 [DOI] [PubMed] [Google Scholar]
- 5.von Stillfried S, Bülow RD, Röhrig R, et al. First report from the German COVID-19 autopsy registry. Lancet Public Health. 2022;15:100330. doi: 10.1016/j.lanepe.2022.100330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108 [DOI] [PubMed] [Google Scholar]
- 7.Ramesh S. 100-year-old TB vaccine now being tested for Covid-19, India may conduct a trial too. Print. 2020.
- 8.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315–323.e9. doi: 10.1016/j.cell.2020.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charoenlap S, Piromsopa K, Charoenlap C. Potential role of Bacillus Calmette-Guérin (BCG) vaccination in COVID-19 pandemic mortality: epidemiological and immunological aspects. Asian Pac J Allergy Immunol. 2020;38(3):150–161. doi: 10.12932/AP-310520-0863 [DOI] [PubMed] [Google Scholar]
- 10.Jalalizadeh M, Buosi K, Dionato FAV, et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID‐19: clinical evolution, adverse events, and humoral immune response. J Intern Med. 2022;292(4):654–666. doi: 10.1111/joim.13523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo N, Brooks NA, Zlotta AR, et al. 100 years of Bacillus Calmette–guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18(10):611–622. doi: 10.1038/s41585-021-00481-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–1180. doi: 10.1016/S0140-6736(06)68507-3 [DOI] [PubMed] [Google Scholar]
- 13.Hegarty PK, Sfakianos JP, Giannarini G, DiNardo AR, Kamat AM. COVID-19 and Bacillus Calmette-guérin: what is the link? Eur Urol Oncol. 2020;3(3):259–261. doi: 10.1016/j.euo.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandi N, Bartalena L, Mosconi C, Golfieri R. A unique case of miliary pulmonary tuberculosis induced by bacillus Calmette-Guérin intravesical instillation with COVID-19 superinfection. S Afr J Radiol. 2021;25(1):1. doi: 10.4102/sajr.v25i1.2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mousquer GT, Peres A, Fiegenbaum M. Pathology of TB/COVID-19 Co-infection: the phantom menace. Tuberculosis. 2021;126:102020. doi: 10.1016/j.tube.2020.102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 18.Colquhoun HL, Levac D, O’Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67(12):1291–1294. doi: 10.1016/j.jclinepi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 19.Peters MD, Godfrey CM, McInerney P, Soares CB, Khalil H, Parker D. The Joanna Briggs Institute reviewers’ manual 2015: methodology for JBI scoping reviews. 2015.
- 20.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 21.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dos Anjos LRB, da Costa AC, Cardoso ADRO, et al. Efficacy and safety of BCG revaccination with M. bovis BCG Moscow to prevent COVID-19 infection in health care workers: a randomized phase II clinical trial. Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.841868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen AMR, Schaltz-Buchholzer F, Benfield T, et al. Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic: a structured summary of a study protocol for a randomised controlled trial in Denmark. Trials. 2020;21(1):799. doi: 10.1186/s13063-020-04714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittet LF, Messina NL, Gardiner K, et al. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: protocol for a randomised controlled trial (BRACE trial). BMJ Open. 2021;11(10):e052101. doi: 10.1136/bmjopen-2021-052101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messina NL, Germano S, McElroy R, et al. Off‐target effects of bacillus Calmette–Guérin vaccination on immune responses to SARS‐CoV‐2: implications for protection against severe COVID‐19. Clin Transl Immunol. 2022;11(4). doi: 10.1002/cti2.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ten Doesschate T, van der Vaart TW, Debisarun PA, et al. Bacillus Calmette-Guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin Microbiol Infect. 2022;28(9):1278–1285. doi: 10.1016/j.cmi.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorlag SJCFM, Taks E, ten Doesschate T, et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis. 2022;75(1):e938–e946. doi: 10.1093/cid/ciac182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsilika M, Taks E, Dolianitis K, et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.873067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Ajayababu A, Thukral H, et al. Efficacy of Bacillus Calmette–Guérin (BCG) vaccination in reducing the incidence and severity of COVID-19 in High-Risk Population (BRIC): a phase III, multi-centre, quadruple-blind randomised control trial. Infect Dis Ther. 2022;11(6):2205–2217. doi: 10.1007/s40121-022-00703-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dionato FAV, Jalalizadeh M, Buosi K, et al. BCG vaccine safety in COVID-19 convalescent adults: BATTLE a randomized controlled trial. Vaccine. 2022;40(32):4603–4608. doi: 10.1016/j.vaccine.2022.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann E, Khan N, Tran KA, et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38(10):110502. doi: 10.1016/j.celrep.2022.110502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su WJ, Chang CH, Wang JL, Chen SF, Yang CH. COVID-19 severity and neonatal BCG vaccination among young population in Taiwan. Int J Environ Res Public Health. 2021;18(8):4303. doi: 10.3390/ijerph18084303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aksu K, Naziroğlu T, Özkan P. Factors determining COVID-19 pneumonia severity in a country with routine BCG vaccination. Clin Exp Immunol. 2020;202(2):220–225. doi: 10.1111/cei.13507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moorlag SJCFM, van Deuren RC, van Werkhoven CH, et al. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: a retrospective cohort study. Cell Rep Med. 2020;1(5):100073. doi: 10.1016/j.xcrm.2020.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahariya C. A brief history of vaccines & vaccination in India. Indian J Med Res. 2014;139(4):491–511. [PMC free article] [PubMed] [Google Scholar]
- 37.Jalalizadeh M, Giacomelli CF, Patricia AF, et al. Comparing Bacillus Calmette-Guérin (BCG) strains in convalescent COVID-19 patients. Immunotherapy. 2023;2023:1. [DOI] [PubMed] [Google Scholar]
- 38.Hidvegi M, Nichelatti M. BCG vaccination policy and salmiak consumption are inversely associated with COVID-19 death rates in Europe. SSRN Electron J. 2020. doi: 10.2139/ssrn.3596914 [DOI] [PubMed] [Google Scholar]
- 39.Hensel J, McAndrews KM, McGrail DJ, Dowlatshahi DP, LeBleu VS, Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep. 2020;10(1):18377. doi: 10.1038/s41598-020-75491-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chimoyi L, Velen K, Churchyard GJ, Wallis R, Lewis JJ, Charalambous S. An ecological study to evaluate the association of Bacillus Calmette-Guerin (BCG) vaccination on cases of SARS-CoV2 infection and mortality from COVID-19. PLoS One. 2020;15(12):e0243707. doi: 10.1371/journal.pone.0243707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guerin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11(1):774. doi: 10.1038/s41598-020-80787-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma A, Kumar sharma S, Shi Y, et al. BCG vaccination policy and preventive chloroquine usage: do they have an impact on COVID-19 pandemic? Cell Death Dis. 2020;11(7):516. doi: 10.1038/s41419-020-2720-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urashima M, Otani K, Hasegawa Y, Akutsu T. BCG vaccination and mortality of COVID-19 across 173 Countries: an Ecological Study. Int J Environ Res Public Health. 2020;17(15):5589. doi: 10.3390/ijerph17155589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak S, Jolly MK, Nandi D. Countries with high deaths due to flu and tuberculosis demonstrate lower COVID-19 mortality: roles of vaccinations. Hum Vaccin Immunother. 2021;17(9):2851–2862. doi: 10.1080/21645515.2021.1908058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wassenaar TM, Buzard GS, Newman DJ. BCG vaccination early in life does not improve COVID‐19 outcome of elderly populations, based on nationally reported data. Lett Appl Microbiol. 2020;71(5):498–505. doi: 10.1111/lam.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li WX. Worldwide inverse correlation between Bacille Calmette–Guérin (BCG) immunization and COVID-19 mortality. Infection. 2021;49(3):463–473. doi: 10.1007/s15010-020-01566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogimi C, Qu P, Boeckh M, Bender Ignacio RA, Zangeneh SZ. Association between live childhood vaccines and COVID-19 outcomes: a national-level analysis. Epidemiol Infect. 2021;149:e75. doi: 10.1017/S0950268821000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szigeti R, Kellermayer D, Trakimas G, Kellermayer R, Roques P. BCG epidemiology supports its protection against COVID-19? A word of caution. PLoS One. 2020;15(10):e0240203. doi: 10.1371/journal.pone.0240203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassan Z, Hashim MJ, Khan G. Population risk factors for COVID-19 deaths in Nigeria at sub-national level. Pan Afr Med J. 2020;35. doi: 10.11604/pamj.supp.2020.35.2.25258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh S, Kishore D, Singh RK, Pathak C, Ranjan K. Higher BCG‐induced trained immunity prevalence predicts protection from COVID‐19: implications for ongoing BCG trials. Clin Transl Discov. 2022;2(2). doi: 10.1002/ctd2.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh S, Maurya RP, Singh RK, Morrison TE. “Trained immunity” from Mycobacterium spp. exposure or BCG vaccination and COVID-19 outcomes. PLoS Pathog. 2020;16(10):e1008969. doi: 10.1371/journal.ppat.1008969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arslan Gulen T, Bayraktar M, Yaksi N, Kayabas U. Is the course of COVID‐19 associated with tuberculin skin test diameter? A retrospective study. J Med Virol. 2022;94(3):1020–1026. doi: 10.1002/jmv.27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates MN, Herron TJ, Lwi SJ, Baldo JV. BCG vaccination at birth and COVID-19: a case-control study among U.S. military veterans. Hum Vaccin Immunother. 2022;18(1). doi: 10.1080/21645515.2021.1981084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menzies D. Interpretation of repeated tuberculin tests. Am J Respir Crit Care Med. 1999;159(1):15–21. doi: 10.1164/ajrccm.159.1.9801120 [DOI] [PubMed] [Google Scholar]
- 56.de Chaisemartin C, de Chaisemartin L. Bacille Calmette-Guérin vaccination in infancy does not protect against Coronavirus Disease 2019 (COVID-19): evidence from a natural experiment in Sweden. Clin Infect Dis. 2021;72(10):e501–e505. doi: 10.1093/cid/ciaa1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323(22):2340. doi: 10.1001/jama.2020.8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hupert N, Marín-Hernández D, Gao B, Águas R, Nixon DF. Heterologous vaccination interventions to reduce pandemic morbidity and mortality: modeling the US winter 2020 COVID-19 wave. Proc Natl Acad Sci U S A. 2022;119(3). doi: 10.1073/pnas.2025448119 [DOI] [PMC free article] [PubMed] [Google Scholar]