Abstract
Background & objectives:
The pandemic caused by the SARS-CoV-2 has been a threat to humankind due to the rapid spread of infection and appearance of multiple new variants. In the present study, we report the dynamics and persistence of immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in asymptomatic and symptomatic COVID-19 patients by chemiluminescent assay.
Methods:
A total of 463 serum samples from 218 SARS-CoV-2 PCR-positive patients were collected over a period of 124 days post-onset of disease (POD). Antibody levels were measured by chemiluminescence bioanalyzer. Neutralizing antibody titres were assessed by plaque reduction neutralization test (PRNT) for SARS-CoV-2.
Results:
Both IgM and IgG started appearing from day five post-infection in symptomatic and asymptomatic patients. IgM antibody response peaked around day 35 POD and rapidly diminished thereafter, with the last IgM-positive sample observed at 90 days POD. IgG antibody response peaked around 45 days POD and persisted till 124 days. The chemiluminescence immunoassay (CLIA) results showed a moderate correlation (R=0.5846, P<0.001) compared with PRNT. Additional analysis indicated a neutralizing titre of 250 corresponded to 12.948 AU/ml of YHLO iFlash SARS-CoV-2 IgG units.
Interpretation & conclusions:
Both symptomatic and asymptomatic COVID-19 patients seem to initiate production of antibody responses from day five of onset of disease. Although the CLIA gives high sensitivity and specificity and also its binding IgG antibody titres may correlate moderately with protective immunity, our results indicate that the values of binding antibody alone may not be a perfect guide to represent virus neutralization titre during donor selection for plasma therapy. However, IgM and IgG antibody detection may help in monitoring the status of disease progression and burden in the community.
Key words: Antibody response, chemiluminescence assay, neutralizing antibody, SARS-CoV-2, quantitative detection, YHLO
The pandemic caused by the SARS-CoV-2 is a threat to humankind due to the rapid spread of infection and appearance of new variants1-5. Viral RNA detection is the assay of choice for the confirmation of COVID-19 infection. The high numbers of asymptomatic infections have created difficulty in the estimation of the true disease burden6. Such information is crucial to evaluate herd immunity through the assessment of antibody response to this virus.
The studies reported worldwide indicate differential antibody responses and kinetics among COVID-19 patients7. Bauer8 noticed variability in SARS-CoV-2 immunoglobulin M (IgM) responses, while the immunoglobulin G (IgG) antibodies remain stable and appear in high titres. The highly variable IgM antibody responses and the early appearance of IgG antibodies and its importance in recovery from the infection have led to exhaustive analyses of IgG antibody responses.
Validated serological assays are required for studying the sero-epidemiology of COVID-19 and persistence of antibodies. Among the available commercial serological assays, the chemiluminescence immunoassay (CLIA) platform was reported to show high sensitivity and specificity9,10. In the present study, we report the dynamics and sero-persistence of IgG and IgM antibodies in asymptomatic and symptomatic COVID-19 patients. The antibody units were compared with the antibody titres using a virus neutralization assay and to assess the utility of CLIA in plasma therapy11.
Material & Methods
The study was conducted between June and November 2020 at the ICMR-National Institute of Virology (NIV), Pune, after obtaining approval from the Institutional Human Ethics Committee. A total of 463 serum samples were collected from 218 SAR-CoV-2 PCR-positive patients over a period of 124 days post-onset of disease (POD). Of these 218 patients, 109 patients provided samples a single time and the remaining 109 patients provided samples multiple times (39 patients provided sample at two time points, 16 provided sample at three time points, 42 provided sample at four time points and 12 patients provided sample at five time points). The median age of the patients was 37 yr with interquartile range (IQR) of 27-51 years.
A total of 138 patients (63.30%) were less than 50 yr of age and the remaining 80 (36.70%) were above 50 yr. Of these 218 patients, 131 were males. Among them, 45 were asymptomatic (20.64%) and 173 (79.36%) were symptomatic. For the asymptomatic patients, the duration for IgM and IgG antibody time kinetics was recorded as days post-first PCR positivity and for symptomatic patients as days POD. Of the 463 clinical samples tested for antibody kinetics, 373 (80.56%) samples were from symptomatic patients while 90 (19.44%) samples were from asymptomatic patients. The median number of samples collected was three among patients who were tested at more than one time point. The last sample was collected on day 124 POD. One hundred serum samples collected before the SARS-CoV-2 outbreak were used as negative control samples in CLIA.
IgA and IgM antibodies were measured by utilizing IgG (Shenzhen YHLO Biotech Co., Ltd., China, C86095G) and IgM (Shenzhen YHLO Biotech Co., Ltd., China, C86095m) detection kits using YHLO iFlash 1800 Chemiluminescence bioanalyzer (Shenzhen YHLO Biotech Co., Ltd., China)12,13.
YHLO iFlash 1800 bioanalyzer is a paramagnetic particle-based CLIA based on detection of antibodies against nucleoprotein (N) and spike (S) proteins of SARS-CoV-2. The sample testing was performed strictly according to the manufacturers’ instructions. Chemiluminescent reaction was measured as relative light units. The detection threshold for both IgM and IgG tests was10 AU/ml, wherein all samples above this value were considered as positive. Neutralizing antibody titres were assessed by plaque reduction neutralization test (PRNT) for SARS-CoV-214. Of the total 463 samples, a subset of 83 samples was tested by PRNT.
Statistical analysis: Descriptive statistics (mean, median and IQR) were calculated for continuous variables and counts and percentages for categorical variables. Mann-Whitney U test was performed to compare the differences between the two groups. Pearson’s correlation was drawn to evaluate correlation between the two methods. The analysis was performed on GraphPad Prism 9 (San Diego, CA, USA).
Results & Discussion
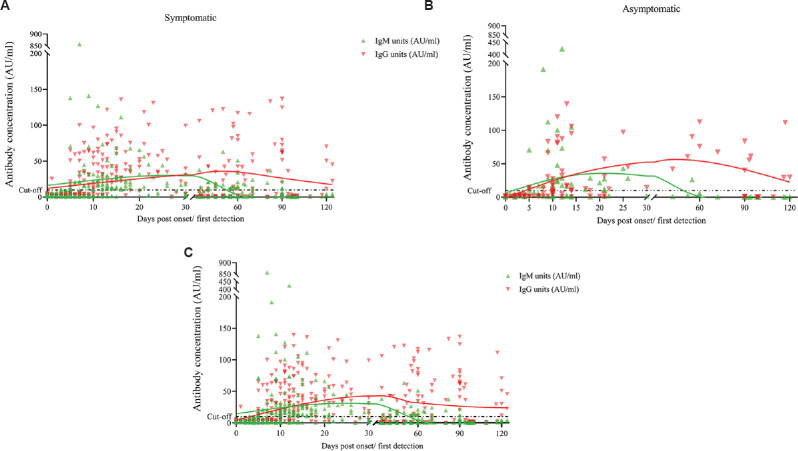
None of the blood samples collected before the COVID-19 pandemic tested positive for SARS-CoV-2-specific antibodies by the selected kits, which indicated high specificity of the tests. The detailed antibody kinetics is shown in Figure 1. IgM and IgG were detected from day five post-infection in both symptomatic and asymptomatic patients. However, only one sample from the symptomatic category showed appearance of IgM antibodies as early as day three. The IgM response of symptomatic and asymptomatic patients showed a similar trend with a single peak at around 35 days POD (Fig. 1A and B). A significant difference was noted in IgM antibody levels (P<0.01) between patients aged <50 yr (average IgM titres: 13.68 AU/ml) vs. patients >50 yr (average IgM titres: 42.41 AU/ml) (Fig. 2), which was in contradiction to the reports from Jiang et al6, while IgG levels did not differ significantly. A total of 17 (7.83%) patients were negative by YHLO and later confirmed the same by PRNT. Further studies are required to assign whether these samples are true non-responders or may have given a false-negative result due to a delayed immune response, timeframe of sample collection or sensitivity of the assay.
Fig. 1.
Kinetics of IgM and IgG antibodies in COVID-19 patients. Scatter plot for SARS-CoV-2. IgM (green) and IgG (red) antibodies units plotted against time with trend lines obtained from the cumulative values from 0 to 120 days POD. (A) Distribution of IgM and IgG antibody positivity in 373 serum collected from 173 symptomatic patients; and (B) ninety serum collected from 45 asymptomatic patients. (C) Profile of IgM and IgG antibody positivity in all 463 samples collected from 218 patients. The assay cut-off (dotted line) was10 AU/ml for both IgM and IgG detection kits. POD, post-onset of disease.
Fig. 2.
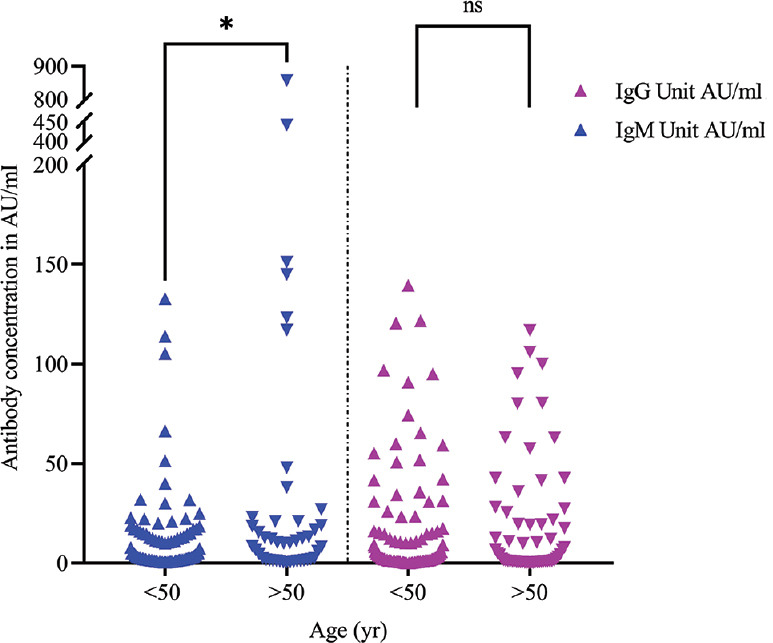
Age-specific distribution of IgM and IgG antibodies in SARS-CoV-2 samples. Distribution of IgM (blue) and IgG (violet) antibody positivity (AU/ml) among COVID-19 patients of age >50 (n=138) and <50 yr (n=80): *P<0.01 in IgM titres (GMT<50 yr -18.41 AU/ml and GMT >50 yr - 52.60 AU/ml). POD, post-onset of disease; ns, non-significant
For understanding the kinetics of IgM and IgG antibodies, about 90 per cent (196/218) of the patients developed IgG by 20 days post-infection, while the IgM detection was reduced to 68.8 per cent (150/218) by 40 days POD or from the day of PCR positivity.
During the acute phase (0-21 days POD) of infection, IgM antibodies showed relatively high titres with higher per cent positivity as compared to IgG titres (P<0.01) (Table). IgM titres exhibited an increase in titres and reached a plateau around 35 days POD, following which we observed a distinct decline up to 55 days POD (Fig. 1). The cumulative average of IgM titres was negative (>10 AU/ml) around day 55 POD, although the last IgM-positive sample was observed on day 90 POD. The cumulative average of IgG antibody titres remained negative (>10 AU/ml) during the first week post-infection and showed a considerable increase till 45 days POD followed by decline in IgG antibody titres by 124 days POD. The cumulative average of IgG antibody titres remained positive during the study period, except during the first week. This showed a timeframe for waning of IgM antibody response and also indicated about the longitivity of IgG-based response (Fig. 1C). Our study displayed a little contradictory finding with Wang et al15 and Vogelzang et al16, who reported the decline of IgM antibody in 12th wk but persistence of IgG antibodies at high levels up to three months and a subsequent decline.
Table .
Profiling of SARS-CoV- 2 immunoglobulin M (IgM) and immunoglobulin G (IgG) antibody detection in COVID-19 patients
| Time points of sample collection (POD in days) | IgM+/IgG− n (%) | IgM+/IgG+ n (%) | IgM−/IgG+ n (%) | IgM−/IgG− n (%) | P |
|---|---|---|---|---|---|
| 0-10 (n=197) | 38 (19.29) | 63 (31.98) | 25 (12.69) | 71 (36.04) | <0.01 |
| 11-20 (n=87) | 15 (17.24) | 50 (57.47) | 16 (18.39) | 6 (6.90) | |
| 21-30 (n=35) | 5 (14.39) | 20 (57.14) | 10 (28.57) | 0 | |
| 31-40 (n=21) | 1 (4.76) | 5 (23.81) | 14 (66.67) | 1 (4.46) | |
| 41-50 (n=22) | 0 | 9 (40.91) | 12 (54.55) | 1 (4.55) | |
| 51-60 (n=27) | 0 | 11 (40.74) | 13 (48.15) | 3 (11.11) | |
| 61-70 (n=12) | 0 | 4 (33.33) | 7 (58.33) | 1 (8.33) | |
| 71-80 (n=4) | 0 | 1 (25) | 2 (50) | 1 (25) | |
| 81-90 (n=26) | 0 | 1 (3.85) | 21 (80.77) | 4 (15.38) | |
| 91-100 (n=20) | 0 | 0 | 18 (90) | 2 (10) | |
| 111-124 (n=12) | 0 | 0 | 91.67 (88.88) | 2 (8.33) |
IgM and/IgG antibody positivity was compared at different time points till 124 days POD or first positive PCR detection (n=463). The percentage positivity of SARS CoV-2 IgM and/IgG at different time points POD or first positive PCR detection revealed significant difference in antibody detection between PODs with a Mann-Whitney U test for unpaired comparison P<0.01. IgM+, IgM antibody positive against SARS-CoV-2; IgG+, IgG antibody positive against SARS-CoV-2; IgM−, IgM antibody negative against SARS-CoV-2; IgG−, IgG antibody negative against SARS-CoV-2; n, number of samples collected in each category; POD, post-onset of disease
Of the 463 samples tested with YHLO CLIA, a subset of 83 samples was tested with live virus neutralization assay (POD day 4-92).The comparison indicated the moderate Pearson’s correlation (R=0.5846, P<0.001). The samples for PRNT were selected randomly, keeping the distribution of symptomatic and asymptomatic samples. In addition, another 83 PRNT-negative samples were also tested by CLIA. Using PRNT as gold standard, the sensitivity and specificity of YHLO were assessed and found to be 92.77 and 98.79 per cent, respectively.
The Ministry of Health and Family Welfare (MoHFW), Government of India, recommended 640 neutralization antibody titres for the selection of convalescent plasma therapy17. The US-FDA recommends Ortho VITROS IgG S/C level of 12 for the selection of convalescent plasma for therapy18. In our study, YHLO iFlash SARS-CoV-2 IgG concentration of 12.948 AU/ml compared to 12 units of Ortho VITROS IgG S/C which was corresponding to 250 neutralizing titres. The 640 titres recommended by MoHFW were corresponding to 87 AU/ml of YHLO iFlash SARS-CoV-2 IgG concentration17.
Although various studies reported on antibody appearance and profile of IgM and IgG among the COVID-19 patients, the observations remained variable. The sensitivity and specificity of the assay methods used will have an effect on the variability of results.
The results indicated that the values of binding antibody assays alone would not be a perfect guide for donor selection for plasma therapy. These can be used as tools for screening the large number of donor samples to confirm with neutralization assays. The small sample size and the need of long-term follow up were the limitations of this study.
Our study observations have led to the finding that symptomatic status of an individual does not impact the mounted immune response. The anti-SARS-CoV-2 IgG antibodies response peaked at 45 days POD but showed overall response till 124 days. The binding IgG antibody concentration correlated with protective immunity. The decline in IgM antibody after 35 days POD and the rare appearance up to 90 POD reemphasizes that detection of IgM antibody may not be reliable in active case management. However, both IgM and IgG antibody detection may be a useful tool in identifying the exposure to SARS-CoV-2 and in monitoring the status of disease progression in the community. Further, due to the reported variants of SARS-CoV-2 worldwide, better understanding of humoral immune response will help in recognizing epidemiological linkage, development of effective therapeutics, preventive modalities and improved diagnostic methods.
Footnotes
Financial support & sponsorship: The study was funded by the Department of Health Research, Ministry of Health and Family Welfare, Government of India, New Delhi.
Conflicts of Interest: None.
References
- 1.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti P, Caraffa A, Gallenga CE, Kritas SK, Frydas I, Younes A, et al. The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J Biol Regul Homeost Agents. 2021;35:1–4. [PubMed] [Google Scholar]
- 3.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–5. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 4.Burki T. Understanding variants of SARS-CoV-2. Lancet. 2021;397:462. doi: 10.1016/S0140-6736(21)00298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava S, Banu S, Singh P, Sowpati DT, Mishra RK. SARS-CoV-2 genomics:An Indian perspective on sequencing viral variants. J Biosci. 2021;46:22. doi: 10.1007/s12038-021-00145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang C, Wang Y, Hu M, Wen L, Wen C, Wang Y, et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Transl Immunology. 2020;9:e1182. doi: 10.1002/cti2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonelli F, Sarasini A, Zierold C, Calleri M, Bonetti A, Vismara C, et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58:e01224–20. doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer G. The variability of the serological response to SARS-corona virus-2:Potential resolution of ambiguity through determination of avidity (functional affinity) J Med Virol. 2021;93:311–22. doi: 10.1002/jmv.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parai D, Dash GC, Choudhary HR, Peter A, Rout UK, Nanda RR, et al. Diagnostic accuracy comparison of three fully automated chemiluminescent immunoassay platforms for the detection of SARS-CoV-2 antibodies. J Virol Methods. 2021;292:114121. doi: 10.1016/j.jviromet.2021.114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontou PI, Braliou GG, Dimou NL, Nikolopoulos G, Bagos PG. Antibody tests in detecting SARS-CoV-2 infection:A meta-analysis. Diagnostics (Basel) 2020;10:319. doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U. S. Food and Drug Administration. COVID-19 convalescent plasma. [accessed on August 24, 2020]. Available from: https://www.fda.gov/media/141480/download .
- 12.US Food &Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. [accessed on December 17, 2020]. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-applications-inds-cber-regulated-products/recommendations-investigational-covid-19-convale scent-plasma .
- 13.Infantino M, Grossi V, Lari B, Bambi R, Perri A, Manneschi M, et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies:An Italian experience. J Med Virol. 2020;92:1671–5. doi: 10.1002/jmv.25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshpande GR, Sapkal GN, Tilekar BN, Yadav PD, Gurav Y, Gaikwad S, et al. Neutralizing antibody responses to SARS-CoV-2 in COVID-19 patients. Indian J Med Res. 2020;152:82–7. doi: 10.4103/ijmr.IJMR_2382_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Li J, Li H, Lei P, Shen G, Yang C. Persistence of SARS-CoV-2-specific antibodies in COVID-19 patients. Int Immunopharmacol. 2021;90:107271. doi: 10.1016/j.intimp.2020.107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelzang EH, Loeff FC, Derksen NIL, Kruithof S, Ooijevaar-de Heer P, vanMierlo G, et al. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J Immunol. 2020;205:3491–9. doi: 10.4049/jimmunol.2000767. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health and Family Welfare, Directorate General of Health Services (EMR Division), Government of India. Clinical Management Protocol:COVID-19. [accessed on December 17, 2020]. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf .
- 18.US Food and Drug Administration. FDA issues emergency use authorization for convalescent plasma as potential promising COVID–19 treatment, another achievement in administration's fight against pandemic. [accessed on December 17, 2020]. Available from: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment .