Abstract
Introduction
Focal epilepsies are diseases of neuronal excitability affecting macroscopic networks of cortical and subcortical neural structures. These networks (“epileptogenic networks”) can generate pathological electrophysiological activities during seizures, and also between seizures (interictal period). Many works attempt to describe these networks by using quantification methods, particularly based on the estimation of statistical relationships between signals produced by brain regions, namely functional connectivity (FC).
Results
FC has been shown to be greatly altered during seizures and in the immediate peri-ictal period. An increasing number of studies have shown that FC is also altered during the interictal period depending on the degree of epileptogenicity of the structures. Furthermore, connectivity values could be correlated with other clinical variables including surgical outcome.
Significance
This leads to a conceptual change and to consider epileptic areas as both hyperexcitable and abnormally connected. These data open the door to the use of interictal FC as a marker of epileptogenicity and as a complementary tool for predicting the effect of surgery.
Aim
In this article, we review the available data concerning interictal FC estimated from intracranial electroencephalograhy (EEG) in focal epilepsies and discuss it in the light of data obtained from other modalities (EEG imaging) and modeling studies.
Impact statement
In this article, we review the concept of the epileptogenic network and explained the basic notions of functional connectivity (FC) and the potential biases when studying it using intracranial EEG (iEEG). We report the current body of published data using iEEG. These data demonstrate that even at temporal distance from epileptic seizures there are differential changes in FC between areas epileptic or not. It appears that the connectivity of epileptic zone remains relatively preserved and higher than the connectivity of the nonepileptic zone (decreased). These data could help in locating epileptic areas and predicting the surgical outcome.
Keywords: drug-resistant epilepsy, EEG, epilepsy surgery, functional connectivity, partial epilepsy, SEEG
Introduction
Focal refractory epilepsy and the concept of epileptogenic network
Epilepsy is a serious and highly prevalent neurological disease, affecting more than 1% of the population worldwide (Fiest et al, 2016), and associated with a significant overmortality rate and frequent comorbidities (Thurman et al, 2017). Approximately 60% of patients with epilepsies have focal seizures (Hauser et al, 1991). Pharmacoresistance to antiseizure medications remains a major issue for up to one-third of patients (Kwan et al, 2000). In these cases, epilepsy resective surgery, when possible, is the best option (Dwivedi et al, 2017; Ryvlin et al, 2014; Wiebe et al, 2001). For these patients with drug-resistant focal epilepsies, the main prognostic factor of surgery is to achieve complete resection of the epileptogenic zone (EZ), the latter being defined on the basis of multimodal data (clinical, electroencephalograhy [EEG], magnetic resonance imaging [MRI], positron emission tomography [PET]).
In some cases, noninvasive data are not sufficient to accurately define the EZ and its relationship with eloquent cortices. In these cases, intracranial EEG (iEEG) recordings are mandatory (Isnard et al, 2018; Jayakar et al, 2016). Such iEEG recordings have led to the observation that focal epilepsy is often organized as a network with regard to the spatial organization of EZ, pattern of seizure propagation, and connectivity alteration induced by recurrent seizures (for a review, see Bartolomei et al, 2017). In the last 15 years, the notion of “epileptogenic networks” has become more and more popular in epileptology (Bartolomei et al, 2017) since its first description in the early 2000s (Bartolomei et al, 2001; Spencer, 2002).
In this context, the important breaking point is to replace the concept of epileptic focus (Rosenow and Luders, 2001) by that of epileptogenic networks. This model involves spatiotemporal dynamics in the genesis of ictal and interictal activities between a more or less extended set of distant brain regions. In this model, there is a hierarchy of brain regions ranked according to their epileptogenicity: (1) EZ network, (2) propagation zone (PZ) network, and (3) noninvolved zone (NIZ) network (Fig. 1). Indeed, the analysis of iEEG recordings of seizures often reveals an involvement of multiple cerebral structures, sometimes with different types of discharges (more or less rapid) and with variable delays of involvement. It is thus not always easy to define the limits of the area to be resected, especially in the case of short involvement times and extensive rapid discharge at seizure onset.
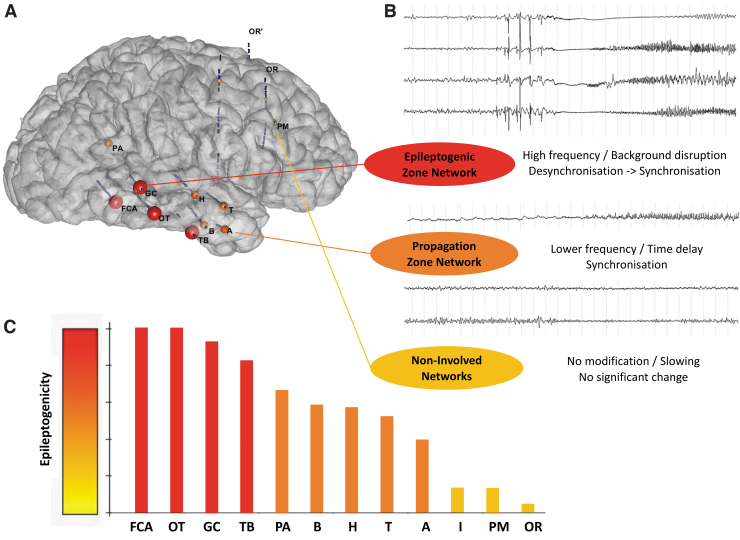
FIG. 1.
Represents a seizure recorded in SEEG where we observe a simultaneous coimplication of several distinct/distant cerebral areas at the time of the initiation and the propagation of the seizure. The frequent observation of this simultaneous involvement of a “network” of brain areas during seizures, as well as the connectivity changes observed at the time of the seizures (not shown here), led us to propose the concept of the three “networks”: EZN, PZN, and NIN. (A) A 3D representation of the epileptogenicity of the sampled area in a patient with SEEG recordings. EZN are the nodes with a big red sphere, PZN are the nodes with a medium orange sphere, and NIN are the nodes with a small yellow sphere. (B) Example of SEEG traces during a seizure within the EZN, PZN, and NIN; and summary of the most frequent observed changes within each zone in terms of signal properties. (C) Bar plot of the level of epileptogenicity for the nodes sampled in this example, showing a gradual decrease of epileptogenicity (A, right middle temporal gyrus anterior part; B, right middle temporal gyrus anterior part; FCA, right middle temporal gyrus posterior part; GC, right superior temporal gyrus posterior part; H, right planum temporale; I, right middle frontal gyrus posterior part; OR, right superior frontal gyrus posterior part; OT, right middle temporal gyrus posterior part; PA, right angular gyrus; PM, right middle frontal gyrus anterior part; SEEG, stereo-EEG; T, right planum polare; TB, right inferior temporal gyrus anterior part). 3D, three dimensional; EZN, epileptogenic zone network; NIN, noninvolved zone network; PZN, propagation zone network.
Quantified analyses of seizure onsets actually show a gradient of epileptogenicity between the different structures involved (Bartolomei et al, 2017; Bartolomei et al, 2008). The EZ network concerns the regions involved earlier in the seizure with the most rapid discharges, whereas the PZ network concerns regions involved later or through less rapid discharges. Interestingly, studies with other modalities (PET and MRI) also show such a gradient in the observed alterations (structural connectivity and metabolic, respectively) (Besson et al, 2017; Lagarde et al, 2020).
The MRI-visible lesion and the areas generating interictal paroxysms could, according to the patient, belong to one or more of the above ictal-defined (epileptogenic, propagation, and noninvolved) networks. While numerous studies have shown modified brain synchrony during seizures (preictal, ictal, and postictal states) (see review in Bartolomei et al, 2017), cerebral connectivity is also notably altered during the interictal period. However, preictal, ictal, and interictal data reflect distinct pathophysiological processes and should not be mixed up when interpreting the existing literature. We focus the present review on the functional connectivity (FC) alterations observed remotely from seizures, that is, in the interictal period.
Functional connectivity
FC refers to the statistical link that can exist between activities recorded from distinct brain structures, reflecting more or less synchronized functioning of underlying neuronal populations. The first methods of FC analysis from EEG signals were developed in the 1950s (Barlow and Brazier, 1954), and the first application to ictal signals in the 1980s (Gotman, 1983). The methods have developed in the following years, particularly with the rise of computers and digital EEG systems. Today, the range of methods that can be used to estimate FC is wide. These methods have been evaluated in studies using simulated signals (Ansari-Asl et al, 2006; Wang et al, 2014). The conclusion of these studies is that no method is universal (i.e., most efficient in all the situations tested). They show different performances depending on the type of model and the data used (electrophysiology or functional MRI [fMRI]).
Another observation is a strong influence of the choice of the frequency band of interest (Courtens et al, 2016). Still, methods belonging to the family of linear and nonlinear correlations proved to be a good compromise in various tested models (Wang et al, 2014; Wendling et al, 2009).
FC at the macroscopic scale can be measured by EEG (scalp or intracranial), magnetoencephalography (MEG), and fMRI. fMRI is an indirect marker of neural activity through the hemodynamic response. It should be noted that this hemodynamic response is delayed after the variation in neural activity (e.g., about 5 sec after the start of a stimulus) (Logothetis, 2008). Thus, if the fMRI allows whole-brain exploration, derived measures of connectivity can only be estimated over relatively slow timescales (seconds). The data concerning MRI connectivity in focal refractory epilepsies have been reviewed in previous works (Bernhardt et al, 2013; Tavakol et al, 2019) and are not the topic of this review.
Both MEG and EEG offer a high temporal resolution and allow for analysis of neural activity at the millisecond scale. Despite whole brain covering, with some limitations for deep structures, their spatial resolution is limited to the study of regions with a volume of 1 cm3. Moreover, it is necessary to solve the inverse problem to go from the sensor to the brain source level. In this context, volume conduction effect and source leakage may influence the results of connectivity. However, several methods have been developed, notably source imaging and also specific connectivity analyses (e.g., imaginary part of coherence), to limit this problem (He et al, 2019). It should be noted that this problem of volume conduction is not absent in iEEG either. A review concerning interictal FC data in EEG and MEG can be found in van Mierlo et al (2019).
iEEG recordings bring complementary information to EEG/MEG data by a higher spatial resolution (with sampling at the mm3 level) and excellent temporal resolution (msec, equivalent to scalp EEG and MEG), yet incomplete brain sampling. Two main techniques of iEEG recording are being used in routine: electrocorticography (ECoG) and stereo-EEG (SEEG). Several studies have now confirmed that SEEG has a lower morbidity rate than subdural recordings (Jehi et al, 2021; Katz and Abel, 2019; Mullin et al, 2016; Tandon et al, 2019). SEEG has currently gained worldwide popularity due to its favorable morbidity profile, superior coverage of subcortical structures, and the ability to perform multilobar or bilateral explorations without the need for craniotomy. It is noteworthy that the assessment of FC and brain networks is not superimposable when using SEEG or ECoG data (Bernabei et al, 2021).
SEEG allows to record the activity of multiple and distant brain regions, which are more likely to reveal large-scale network activities, which probably explains why the development of the concept of epileptogenic networks began with the pioneering work in the field (Bancaud and Talairach, 1992; Bartolomei et al, 1999; Chauvel et al, 1987). However, the number of SEEG electrodes being obviously limited, the spatial sampling remains incomplete, which requires certain precautions to be taken when analyzing and interpreting FC and network metrics from graph theory (see Graph measures sub-section).
An SEEG electrode records local field potentials (LFP) corresponding to the electrical activity of cooperative activity in neuronal populations. Generally speaking, LFP depend on the geometry of dendrites and on the features of the dipole constituted by sinks and sources at the dendrites and soma of pyramidal cells. It is well admitted that LFP reflect several underlying processes such as synaptic potentials, afterpotentials of somatodendritic spikes, and voltage-gated membrane oscillations (Wendling and Lopes da Silva, 2018). Regarding space, they reflect the mean activity of a neuronal population between 1 mm3 and 1 cm3 depending on the geometrical features of the extracellular electrode (micro- to mesoscale) and on the level of synchrony (Logothetis, 2003). In the specific case of SEEG, the exact dimension of the recorded neuronal population is not accurately known.
Still, in the last years, several SEEG studies have brought important knowledge on the alterations in interictal FC that are observed in focal refractory epilepsy. This review aims at providing a detailed description of these data, highlighting methodological issues that should be considered for data interpretation and discussing the remaining questions to be addressed.
Overview of Methods for Measuring FC
FC estimation
It has been hypothesized that the synchronization of neuronal oscillations between cerebral areas may allow transfer of information in the brain. Therefore, several quantitative methods have been developed to assess the statistical relationship between signals, namely the FC. There is now a plethora of available methods, each with its own advantages and disadvantages. These can be distinguished according to some of their characteristics, which we briefly detail below. Interested readers can find a more detailed review on the subject in Bastos and Schoffelen (2016) and He et al (2019).
A first distinction can be made between the methods, model-based (e.g., linearity assumed for correlation or granger causality) and model-free (e.g., mutual information, transfer entropy, and nonlinear correlation being sensitive to both linear and nonlinear interactions). The simplest measure for estimating linear interactions is the Pearson correlation coefficient, which measures the linear relationship between two variables. Other forms of nonlinear coupling exist such as cross-frequency coupling (where the phase or amplitude of a certain frequency interacts with the phase or amplitude of another frequency), and then, other metrics sensitive to this nonlinear coupling have been developed.
For example, as the relationship between signals in epilepsy may be more complex than a simple shift, Pijn and da Silva (1993) have thus proposed to use a nonlinear model for the transformation, which consists in a nonparametric analysis aiming at quantifying the correlation of a signal Y on a signal X, independently of the type of the relationship between the two signals. This is a more flexible method, while keeping the number of parameters reasonably low (too many parameters would lead to “overfit” that is, good description of any relationship including noise). In reference to the r2, this nonlinear measure has been named h2 (nonlinear correlation coefficient).
In practice, in a sliding window, a piecewise linear regression is performed between each pair of signals. The h2 is the coefficient of determination, which measures the goodness of fit of the regression (equivalent to the r2 used in linear regression). The h2 is bounded between 0 (no correlation) and 1 (maximal correlation) and is asymmetric. This method has been shown to be sensitive to the following: nonlinear relationships between signals (Lopes da Silva et al, 1989), phase-to-phase coupling (Wendling et al, 2009), amplitude-to-amplitude coupling (Wendling et al, 2009), signals generated by nonlinear systems and coupled linearly or nonlinearly (Wang et al, 2014; Wendling et al, 2009), asymmetric relationships between signals (Lopes da Silva et al, 1989), and signals containing epileptic discharges generated by neural mass models (Wendling et al, 2009).
Regarding the cross-frequency coupling, the h2 is usually calculated in broadband and is independent of the frequency, which does not allow to evaluate this aspect. However, it is theoretically possible to calculate it on sub-band filtered signals to evaluate the cross-frequency coupling, and in this case, the results are close to those obtained with a linear correlation. Finally, h2 is not a particularly designed method for evaluating phase–amplitude coupling. Other methods that do not assume a linear relationship have been developed such as mutual information or transfer entropy (Bastos and Schoffelen, 2016) and have shown a good sensitivity to nonlinear relationships (Wang et al, 2014).
Another important aspect is the distinction between methods that are computed from the time (e.g., correlation, cross-correlation, mutual information, transfer entropy) or frequency domain representation of the signals (e.g., coherence, phase locking value, phase slope index). For the latter, the equivalent of correlation in the frequency domain is coherence, introduced in the context of epilepsy by Brazier (Brazier and Casby, 1952). This measure allows measuring the strength of linear coupling at different frequencies. The coherence measure (ranging between 0 and 1) is based on the Fourier transform that decomposes each signal as a set of sine waves at different frequencies, each having an amplitude and a phase (temporal shift at a given frequency).
The profile of amplitudes across frequencies is the spectrum. The coherence is simply the correlation of the Fourier coefficients across several time windows. Importantly, this requires averaging across several time windows—coherence between one time window and another one would be by construction 1. The coherence method assumes that signals are linked both in amplitude and frequency. This can be separated in a measure of amplitude correlations only and a measure of phase locking only (i.e., constant delays between signals at a given frequency across time windows, independently of the amplitude). To be noted, interictal signals containing transient patterns (e.g., interictal epileptiform discharges [IEDs]) may not be described well by stable sine waves of the Fourier transform. Thus, wavelet transform has been proposed, which is based on the nonstationary wavelet transform, and correlation can be applied to the wavelet coefficients (Amini et al, 2011).
One may be also interested in the direction of the flow of information in connectivity analyses, and several methods have been developed to assess the directionality (e.g., cross-correlation, granger causality, transfer entropy), whereas some others cannot estimate this feature (e.g., correlation, mutual information, coherence, phase locking value). One of the easiest ways for this estimation is to look to propagation delays and then compute the correlation for shifted versions of one signal with respect to the other. The shift with the highest correlation is retained, together with the respective correlation value. This is cross-correlation, which is adapted when one signal is simply a delayed version of the other with no transformation. Using nonlinear correlation (h2), directionality could be estimated from the delay of the shift maximizing the h2 value and/or the asymmetry of the values (Wendling et al, 2001).
Other methods have attempted to quantify the causal relationship between time series such as granger causality and transfer entropy. The principle underlying Granger causality can be described as follows: X “granger causes” Y if Y is better modeled using both the past of X and the past of Y than only using the past of Y. This is a stronger statement that simple correlation. Indeed, if signals happen to be oscillations at a constant frequency, then granger causality will find that the relationship is low—one oscillation can be well predicted by its own past alone (this is also possible to detect in a repetition of the events where one would search for constant phase relationship, i.e., phase locking).
Another important methodological point in the estimation of FC is how to deal with the common input problem. Indeed, the functional interaction between a pair of signals could be caused by a common input from a third source (that may have not been considered/sampled). To limit this problem, methods using multivariate analysis have been developed. Their principle is that information from all channels is taken into account when estimating the FC between any pair of channels (e.g., directed transfer function [DTF], partial directed coherence [PDC], but also partial version of cross-correlation linear or not) (Astolfi et al, 2008; Astolfi et al, 2007; Astolfi et al, 2005; Florin et al, 2010).
Methodological considerations
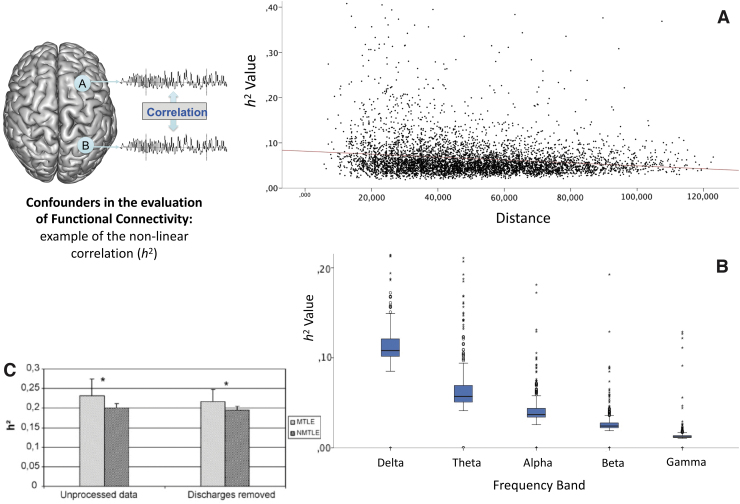
In iEEG FC analyses, several parameters may influence the results algorithms and parameters used (Ansari-Asl et al, 2006; Wang et al, 2014), montage and reference used (Bartolomei et al, 1999; Bastos and Schoffelen, 2016), duration of the period analyzed (Chu et al, 2012; Goodale et al, 2020; Kramer et al, 2011; Wang et al, 2014), power spectrum of the signals (Müller et al, 2008), distance between recorded channels (Goodale et al, 2020; Lagarde et al, 2018; Warren et al, 2010), effect of the partial spatial sampling (Conrad et al, 2020), signal-to-noise ratio (Bastos and Schoffelen, 2016), and IEDs (Bartolomei et al, 2013; Bettus et al, 2008)] and may lead to detection of spurious connectivity. It should be kept in mind during the interpretation of results (Fig. 2). For more details about methodological considerations, see Supplementary Data and the reviews by Bastos and Schoffelen (2016) and He et al (2019).
FIG. 2.
Illustrates the confounders influencing the estimation of functional connectivity through the example of the nonlinear correlation coefficient (h2). (A) The connectivity value decreases with the increase of intercontact distance (possible solutions: normalization by the distance (using noninvolved zone data)/multivariate analysis using the distance-effect). (B) The connectivity values are lower for higher frequencies (possible solution: sub-band analysis/comparison of the frequency content before analysis). (C) The IEDs (unprocessed data) increase slightly the connectivity value without changing the global trend of difference between zones (higher connectivity when mesiotemporal structures are epileptogenic MTLE vs. nonepileptogenic NMTLE). *Statistically significant. IEDs, interictal epileptiform discharges; MTLE, mesio-temporal lobe epilepsy; NMTLE, non mesio-temporal lobe epilepsy.
Graph measures
Connectivity measures across all channels of interest (that can in addition evolve with time) lead to a high amount of data that can be potentially very complex to analyze. It is thus interesting to summarize these data using a mathematical tool such as graph theory. In this framework, channels (single contacts in monopolar or pairs of contact in bipolar montage) can be seen as nodes of a graph, and the value of connectivity between these channels as the link (edge) between these nodes. The advantage of this representation is to summarize the specific properties of the studied network and help to analyze its topology. Topology analysis aims at quantifying the organization of the network, nodes with dense connectivity, organization of the connections between nodes, and so on (for further details, see Fornito et al, 2016).
Schematically, two types of measures have been proposed. The first type measures the way a node (or a set of nodes = zone) is integrated in the whole network (local topology). The second type measures the overall organization of the network (global topology). Local topology includes several measures of “centrality,” that is, the importance of a node in terms of its connectivity to the rest of the nodes (highly or weakly connected = high or low centrality value). Several metrics could be used (e.g., degree, node strength, betweenness centrality [BC], eigenvector centrality), each with its own advantage and inconvenience that we will not detail here (interested readers may look at specific reviews: Fornito et al, 2016; Fornito et al, 2013; Gleichgerrcht et al, 2015; Vecchio et al, 2017). In the case of directed FC measures, one can estimate the ingoing and outgoing centrality of each node.
Global topology gives the relationship between short-range and long-range connections and then the efficiency of the whole network. For example, the small-world topology is characterized by an efficient balance with several short-range and some long-range connections. This is frequently seen in real-life examples in many fields, for example, transportation networks (the important nodes or “hubs” are the large cities that connected with each other, while smaller cities only need to be connected to the nearest hub), including brain organization.
Graph theory allows simplifying the analysis of complex networks such as those of the human brain (Stam, 2004) and helps the comparison between patients. However, an important point concerning the use of graph theory metrics in iEEG studies is the bias related to incomplete spatial sampling. Thus, when we refer to centrality or topology in these studies, we are not speaking in terms of absolute values (at the scale of the entire brain as can be explored by fMRI, EEG, or MEG), but in terms of relative values within the subnetwork of explored structures. These metrics nevertheless make it possible to rank the structures according to their degree of connectivity, and to appreciate the modifications of topology within a subnetwork related to its epileptic character or not.
Another important problem is the risk of biasing the estimates of these metrics since some structures can be oversampled in iEEG which, if we consider all possible connections, risks overestimating the centrality of these structures. It is necessary to control this bias by limiting to one the value of connectivity between regions of interest before performing the graph theory analysis. The centrality metrics must be normalized (e.g., by the theoretical maximum of centrality) to take into account the difference in the number of sampled regions between patients. Moreover, not all centrality metrics are equally robust to the problem of incomplete sampling. It appears that the simplest ones (node strength) have good performance and some methods have been suggested to estimate the confidence interval of these metrics (Conrad et al, 2020).
FC in the Interictal State
Links between FC and epileptogenicity
Table 1 synthesizes data from studies using ECoG and/or SEEG recordings, as described above.
Table 1.
Summary of Studies on Interictal Functional Connectivity Using Intracranial EEG
| Authors | Population | Recording modality | Methods | Main results |
|---|---|---|---|---|
| ECoG ± depth electrodes | ||||
| Towle et al (1998) | Twenty-five patients Tumors in the sensory-motor region or temporal lobectomy |
ECoG During surgery (five with also presurgical recording) |
Coherence | Local zone of increased FC nearby/within EZ |
| Arnhold et al (1999) | One patient with mesial TLE One patient with neocortical epilepsy |
ECoG+depth electrodes (during presurgical monitoring) | “Interdependence” | Greater FC within the EZ than within the NIZ |
| Schevon et al (2007) | Nine patients with neocortical epilepsy | ECoG (during presurgical monitoring) | Mean phase coherence | Area of local hypersynchrony (stable in time) overlapping with the EZ |
| Ortega et al (2008) | Twenty-nine patients with TLE | ECoG (during surgery) | Linear correlation, mutual information, and phase synchronization | Cluster of local hypersynchrony in the epileptic temporal lobe (no good correlation with the EZ) |
| Dauwels et al (2009) | Six patients with neocortical epilepsy | ECoG+depth electrodes (during presurgical monitoring) | Cross-correlation, Granger causality (directed transfer function), phase synchrony, magnitude coherence | Area of hypersynchrony correlates with the EZ |
| Warren et al (2010) | Comparison of patients with chronic pain (n = 2) and with epilepsy (n = 4) | ECoG (during presurgical monitoring) | Linear correlation, mean phase coherence | Disconnection of EZ from the NIZ and decrease of connectivity within the NIZ |
| Wilke et al (2011) | Twenty-five patients with neocortical epilepsy | ECoG (during presurgical monitoring) | Directed transfer function (gamma band) | Correlation between “active node” and EZ (lower than interictal spikes) |
| Park and Madsen (2018) | Twenty-five patients (10 TLE) | ECoG+depth electrodes | Granger causality | FC predicts better than chance the EZ/RZ |
| Shah et al (2019b) | Twenty-seven patients (18 TLE) | ECoG+depth electrodes | Linear correlation | Gradual decrease of FC: within-RZ > RZ-OUT > within-OUT |
| SEEG | ||||
| Mormann et al (2000) | Seventeen patients with TLE | SEEG (bilateral temporal mesial sampling) | Mean phase coherence | Increased FC in the epileptogenic side |
| Bettus et al (2008) | Twenty-one patients with mesial TLE +14 with nonmesial TLE | SEEG | Nonlinear correlation (h2) | FC within mesiotemporal structures is higher when these structures belong to EZ |
| Bettus et al (2011) | Five patients with TLE | SEEG | Nonlinear correlation (h2) | Higher FC within the EZ and the irritative zone than within the NIZ (in beta only) Leading role of the EZ |
| Varotto et al (2012) | Ten patients with FCD II (neocortical epilepsy) | SEEG | Partial directed coherence | Increase in outgoing connections (30–80 Hz) in FCD compared with other structures of the EZ Increase in the betweenness centrality within the FCD |
| Van Diessen et al (2013) | Twelve patients with TLE | SEEG (bilateral temporal mesial sampling) | Phase Lag Index | Disconnection of the epileptogenic mesiotemporal structures from their contralateral nonepileptogenic homologous |
| Bartolomei et al (2013) | Eleven patients with mesial TLE + eight with nonmesial TLE | SEEG | Synchronization likelihood | FC within temporal structures is higher when these structures belong to EZ |
| Lagarde et al (2018) | Fifty-nine patients with FCD or NDT (20 TLE) | SEEG | Nonlinear correlation (h2) | Gradual decrease of FC: EZ > PZ > NIZ FC between EZ-PZ > PZ-NIZ Leading role of the EZ |
| Goodale et al (2020) | Fifteen patients (12 TLE) | SEEG | Imaginary coherence | FC in EZ and EZ-NIZ higher than NIZ Higher clustering coefficient, betweenness centrality within EZ Predictive accuracy = 80.4% |
| Narasimhan et al (2020) | Twenty-five patients (18 TLE) | SEEG | Mutual information, imaginary coherence, partial directed coherence, directed transfer entropy | Gradual decrease of FC: EZ > PZ > Irritative zone > NIZ EZ with higher inward FC Predictive accuracy = 88%, better if combination of connectivity methods |
| Paulo et al (2022) | Thirty-two patients (18 TLE) | SEEG | Imaginary coherence, partial directed coherence (in alpha band) | Stability across time (days) Higher FC in EZ than in NIZ This difference decreased with the antiseizure medication withdrawal when using imaginary coherence |
| Jiang et al (2022) | Twenty-seven patients (23 TLE) | SEEG | Directed transfer function, cross-frequency directionality | Higher inward strength in EZ than in NIZ Information flow from NIZ high-frequency activity to EZ low-frequency activity |
Studies are presented in chronological order. If the definition of the epileptic areas (epileptogenic, seizure-onset zone, etc. …) is variable from one study to another, for simplicity, we summarized the data with the terms EZ and NIZ. For studies using ECoG recordings, we precise if the recordings were performed during a surgery procedure (with anesthesia) or long-term monitoring.
ECoG, electrocorticography; EZ, epileptogenic zone; FC, functional connectivity; FCD, focal cortical dysplasia; h2, nonlinear correlation coefficient; irritative zone, area with interictal spikes but no ictal discharge; NDT, neurodevelopmental tumor; NIZ, noninvolved zone; PZ, propagation zone; RZ, resection zone; SEEG, stereo-EEG; TLE, temporal lobe epilepsy.
ECoG studies
Several studies have investigated iEEG functional interictal connectivity in epilepsy using ECoG data. First, Towle et al (1998) demonstrated areas of locally increased coherence on ECoG within/nearby the epileptic zone, mostly in patients with temporal lobe epilepsy (TLE). Following studies using a mix of ECoG and depth-electrodes within hippocampi confirmed a greater interdependence between structures belonging to the EZ compared with structures in the NIZ (Arnhold et al, 1999; Dauwels et al, 2009). Schevon et al (2007) found areas of increased synchrony using several distinct FC measures (correlation, phase synchrony, coherence magnitude, granger causality) with stability over time, in a group of nine patients. In this study, the authors found a strong overlap between the EZ and areas of hypersynchrony for both ECoG and depth electrodes and, using mean phase coherence, found areas of local hypersynchrony (between 2 and 5 cm) with temporal stability across time.
This suggested that an increase in local synchrony could be a marker of epileptogenicity. Nevertheless, the overlap was not always perfect and the hypersynchrony clusters were sometimes located at the edge of, and not within, the EZ. Moreover, complete resection of areas of local hypersynchrony was associated with a favorable surgical outcome. Ortega et al (2008) described a cluster of local hypersynchrony within the temporal neocortex of patients with TLE explored using intraoperative ECoG. In this study, the authors failed to find a correlation between complete resection of the clusters of local hypersynchrony and surgical outcome. This could be explained by the fact that most of their patients benefited from anterior temporal lobectomy and that ECoG is mostly sensitive to the activity of lateral temporal neocortex. Wilke et al (2011) also showed a change in BC obtained with DTF in gamma band within the EZ.
Finally, only one iEEG study by Warren et al (2010) was able to compare data from patients with epilepsy with patients without epilepsy (implanted for refractory facial pain), using ECoG, linear correlation, and mean phase coherence. This study demonstrated that FC (1) between the EZ and other nonepileptogenic areas and (2) between the nonepileptogenic areas was lower than the corresponding connections in controls. These results suggested a disconnection of epileptogenic structures from nonepileptogenic structures and a decrease of connectivity within the nonepileptogenic cortices.
SEEG studies
The ECoG recording technique has several limitations (limited spatial sampling, exclusion of mesial cortical and subcortical structures, relatively indirect recording of cortical activity, mostly unilateral sampling, and, for some reports, recordings performed under anesthesia for intraoperative ECoG). SEEG studies may overcome some of these limitations. A first set of SEEG studies used bilateral recordings with two depth electrodes implanted along the posterior–anterior axis of both hippocampi in patients with mesiotemporal epilepsy for whom the question of bilateral epileptogenicity was raised. This type of implantation allows for comparison of epileptogenic versus nonepileptogenic mesiotemporal structures (hippocampus and amygdala mostly).
Mormann et al (2000) showed a local increase in synchrony (mean phase coherence) between the structures of the EZ, in a group of 17 patients. Following studies using the same approach confirmed these findings (Arnhold et al, 1999; Dauwels et al, 2009) and showed a disconnection (lower strength and eigenvector centrality) of the EZ from the contralateral analogous (Van Diessen et al, 2013).
Other studies have then used a more complete SEEG sampling typically including neocortical temporal and extratemporal cortices. Three studies compared connectivity across the mesiotemporal structures according to their epileptogenicity (patients with mesiotemporal epilepsy compared with patients with extratemporal epilepsy), using a nonlinear analysis through the h2 method (Bettus et al, 2011; Bettus et al, 2008) or the synchronization likelihood (Bartolomei et al, 2013). The authors have found a higher connectivity between these structures when they belong to the EZ (Bartolomei et al, 2013; Bettus et al, 2011; Bettus et al, 2008). A methodological question was to know the role of interictal spikes and actually they could increase the connectivity values (Bartolomei et al, 2013; Bettus et al, 2008). However, the suppression of sections comprising interictal spikes did not change the results of both FC (Bettus et al, 2008; Jiang et al, 2022) and graph theory metrics (Bartolomei et al, 2013).
Varotto et al (2012) specifically addressing FC in patients with focal cortical dysplasia (FCD) type II estimated the role of the epileptogenic lesion itself. They showed an increase in outgoing connections between 30 and 80 Hz in FCD compared with the other structures of the EZ. Using measures of centrality from graph theory, this study also showed higher BC values within the FCD.
We recently reported a study of interictal FC (nonlinear correlation, h2) in a large series of 59 patients with various forms of focal epilepsies not limited to temporal epilepsies and with a broad SEEG sampling (Lagarde et al, 2018). In this study, the different epileptogenic networks were first defined as precisely as possible by quantifying the ictal activity recorded in SEEG, thanks to the epileptogenicity index (Bartolomei et al, 2008). Thus, the epileptogenic networks were defined as EZ, PZ, and NIZ networks. Independently of the intercontact distance, there was a gradual decrease of FC from the EZ (disclosing the highest connectivity) to the PZ (characterized by an intermediate level of connectivity), and finally, to NIZ (with the lowest connectivity). Moreover, the areas belonging to the EZ were preferentially connected with the areas belonging to the PZ. The EZ was also more interconnected than connected to the NIZ.
This result confirmed the findings of previous studies showing a trend for a “disconnection” of the epileptogenic structures with the nonepileptic brain areas (Van Diessen et al, 2013; Warren et al, 2010). Our findings were consistent in broadband, but also in frequency subbands. The directionality of connectivity (estimated from time delays) did not allow for identification of significant leaders in broadband analysis, but the EZ was found to be the leading zone in alpha and beta frequency bands. Goodale et al (2020) confirmed our findings and found a higher clustering coefficient, nodal BC, and edge BC for the epileptogenic areas. They also fitted a model to predict the epileptogenicity of structures based on the connectivity measured and obtained an accuracy of 80.4% (sensitivity 82.5% and specificity of 60.4%).
Narasimhan et al (2020) extended these data using several methods for connectivity estimation: imaginary coherence, mutual information, PDC, and directed transfer entropy. The definition of the EZ was not quantified in the study and not precisely defined, but authors found a gradual decrease of connectivity values from epileptogenic to propagation to irritative (defined as noninvolved during seizure) and to NIZ. Looking at the predictive value of interictal connectivity to predict the epileptogenicity of the structures, the authors found the best predictive value (area under the curve [AUC]) for the three following methods (by decreasing performance): undirected mutual information, DTF, and undirected imaginary coherence. Furthermore, the combination of connectivity measures improved the predictive value moderately (+4% on AUC). Notably, the model performed equally well in the subset of patients who were seizure-free after surgery (higher confidence in the exact definition of the EZ).
Recently, Paulo et al (2022) investigated the impact of time in epilepsy monitoring unit, changes in antiseizure medication doses, seizure burden, and differences between eyes-closed formal resting states and eyes-open pseudoresting states on interictal SEEG FC (imaginary coherence and PDC computed in the alpha band). They confirmed that nondirected and inward connectivity measures are higher in the EZ compared with the NIZ, but most importantly demonstrated that: (1) FC measures are stable over time; (2) FC measures are not influenced by the seizure burden; (3) antiseizure medication dose may influence some FC measures (imaginary coherence with a smaller difference between EZ and NIZ in case of smaller dose of antiseizure medication) but not some others (PDC); and (4) the type of resting state (formal eyes-closed or not) may influence some FC measures (PDC with a higher difference between EZ and NIZ during formal eyes-closed resting state), but not some others (imaginary coherence).
Finally, Jiang et al (2022) looked specifically at the asymmetry of connectivity between EZ and NIZ. They observed that resting-state information flows from NIZ to EZ across all frequencies and that FC measures remain stable across several periods of recording. Moreover, using cross-frequency coupling analyses, they showed that information from NIZ high-frequency activities lead EZ low-frequency activity. Based on these features, the authors found that a random forest classifier had an accuracy of 88% to predict the EZ.
These studies discussed above used a definition of the EZ based on iEEG biomarkers (visual or quantified) and have of course an inherent limitation as these markers do not have a perfect performance. It is possible that in some cases, especially in nonseizure free patients, the EZ has been misjudged. This is possibly suggested by some studies showing differences in connectivity according to the surgical outcome (see below). Thus, future studies focusing only on seizure-free patients after complete surgery of their EZ, thus with an a priori correct definition, would be useful to control this potential bias. To control this potential bias, some authors have also focused their analyses on an operational definition of the EZ, namely the resection zone (RZ).
Although this definition is pragmatic and close to clinical practice of epilepsy surgery, it has several disadvantages, including the following: exclusion of nonoperated patients (often more complex cases with a wider EZ and counting up to 50% of SEEG-explored patients), inclusion of nonepileptogenic regions within this zone (e.g., anterior temporal lateral neocortex in standard anterior temporal lobectomy/lateral structures on the surgical access to mesial EZ), and exclusion of some EZs (e.g., located in eloquent cortices). Following such methodology, Park and Madsen (2018) demonstrated that interictal connectivity (estimated from Granger causality on a mix of depth-electrodes and ECoG recordings in 25 patients) predicts significantly better than the chance location of EZ/RZ. Shah et al (2019b) have recently replicated this finding in a study on 27 patients recorded using a mix of ECoG and depth electrodes (with a majority of ECoG).
An interesting method in this study is the normalization by a spatially constrained null model. In line with previous findings, these authors showed a gradual decrease of connectivity values (edge weight) from the following: (1) connections within the RZ (highest values), (2) connections linking RZ and non-RZs, and (3) connections within non-RZ (lowest values).
Taken as a whole, data from SEEG studies demonstrated a higher connectivity of the EZ than NIZ and that EZ is relatively disconnected from the NIZ. These findings seem robust across time and methods used. Moreover, the information from connectivity could be useful to locate the EZ.
Cortico-cortical-evoked potential studies
Directed FC can also be estimated from cortico-cortical-evoked potentials (CCEPs) generated by direct cortical electrical stimulation using intracranial electrodes (Boulogne et al, 2016; Trebaul et al, 2018). The foundations of this approach were laid more than 50 years ago when the Saint-Anne's team in Paris proposed to study the functional connections between the hippocampus and the amygdala from SEEG recordings (Buser et al, 1969). CCEPs consist in delivering bipolar single-pulse electrical stimulations in a given region and probing other regions for significant evoked potentials that reflect actual anatomical connections between the stimulated and the responding regions (either direct or passing through synapses that delay the evoked response). CCEPs correspond to early potentials beginning in the first 100 msec after the stimulation. From the presence or the absence of CCEP responses, it is possible to infer the directed (and causal) connectivity between two areas and then to obtain a connectivity matrix.
Table 2 synthesizes data from studies using CCEPs to infer anatomo-functional/effective connectivity in iEEG recordings. Lacruz et al (2007) failed to observe a difference in the hemispheric CCEPs according to their epileptogenicity. However, following studies using a more refined methodology achieved to observe connectivity differences according to the epileptogenic nature of the structures. For example, looking at a centrality metric derived from CCEP, Keller et al (2014) found higher in-degree in the seizure-onset zone than outside (without difference in out-degrees). Interestingly, Parker et al (2018) reported results that are very close to SEEG studies demonstrating the following: (1) a higher effective connectivity for the connections within the EZ, in comparison with the connections within zones outside the EZ; and a (2) higher outdegree and normalized outdegree for contacts belonging to the EZ.
Table 2.
Summary of Studies on Connectivity Using Cortico-Cortical-Evoked Potential
| Authors | Population | Recording's modality | Main results |
|---|---|---|---|
| Lacruz et al (2007) | Fifty-one patients | ECoG + depth electrodes (frontal and temporal recordings) | No difference in ipsilateral and contralateral hemispheric CCEP according to the side of the EZ |
| Keller et al (2014) | Fifteen patients (10 TLE) | ECoG + depth electrodes | Higher in-degree in EZ (no difference in out-degree) |
| Parker et al (2018) | Seven patients with neocortical epilepsy | SEEG | Higher FC within-EZ than within-NIZ Higher out-degree of EZ |
| Zhao et al (2019) | Eight patients (three TLE) | ECoG (during presurgical monitoring) | Higher FC of EZ > NIZ Higher degree centrality and shorter path length of EZ |
| Guo et al (2020) | Twenty-five patients (10 TLE) | SEEG | Gradual decrease of FC: within-EZ > between EZ-PZ > within-PZ > between EZ-NIZ and PZ-NIZ > within-NIZ No difference in directionality |
Studies are presented in chronological order.
CCEP, cortico-cortical evoked potential.
Zhao et al (2019) also confirmed a higher connectivity within the EZ in comparison with the non-EZs, with a significantly higher degree centrality and nodal shortest path length (not significant for BC, clustering coefficient, and local efficiency). It should be noted that, in these studies, the majority of other nodal graph theoretical parameters (in-degree, clustering coefficient, centrality) showed no significant difference between epileptogenic and nonepileptogenic contacts (Parker et al, 2018; Zhao et al, 2019), pointing out the importance of the choice of the centrality measure (Coito et al, 2019; Geier and Lehnertz, 2017).
Following these studies, Guo et al (2020) demonstrated in 25 patients, recorded using SEEG electrodes, that directed connectivity differs significantly between EZ and non-EZ after controlling for the intercontact distance. They found a decrease of connectivity from intra-EZ connections (with the highest value) to “between epileptogenic and propagation zones”; to “intrapropagation zone connections”; to connections “between noninvolved zone and propagation or epileptogenic zone”; to “intranoninvolved zone connections” (with the lowest value). The authors did not find significant directionality between zones (e.g., no difference between connectivity values from EZ to PZ, and connectivity values from PZ to EZ).
Overall, CCEP studies confirmed findings from resting-state SEEG studies and demonstrated a higher connectivity within EZ and PZ, than within NIZ.
Changes in network topology
Varotto et al (2020) found a higher BC (i.e., ratio between the number of shortest paths passing through a specific node and the total number of shortest paths in the network) within FCD type II than outside the lesion area. Goodale et al (2020) found also higher centrality metrics within the EZ (BC and clustering coefficient, the latter reflecting the degree to which nodes tend to cluster together). Using CCEPs, Zhao et al (2019) found a higher degree centrality and a shorter path length within the EZ. Therefore, studies suggest higher centrality of the EZ.
Bartolomei et al (2013), looking at the SEEG data of 11 patients with mesial TLE in comparison with 8 patients with nonmesial TLE, found an increase in the clustering coefficient and path length within the epileptogenic temporal lobe. This result suggested a more regular organization of the FC between temporal structures when they belong to the EZ. These studies suggest that some measures of centrality may be correlated with the degree of epileptogenicity of structures. However, the choice of the metric used seems crucial and future studies are needed.
Directionality of FC
Several studies investigated the directionality of connectivity to identify the leading zone. This directionality could be estimated from the causality method (Granger causality and its extensions such as PDC and DTF) or using delays and/or asymmetry of nonlinear correlation values. The literature in the domain is not unanimous with some studies suggesting a leading role (higher out-connectivity) of the EZ (Bettus et al, 2011; Lagarde et al, 2018; Varotto et al, 2012) and some others suggesting a higher inward connectivity toward the EZ (Jiang et al, 2022; Narasimhan et al, 2020; Paulo et al, 2022; Vlachos et al, 2017). Moreover, using CCEPs, Parker et al (2018) highlighted the higher outward connectivity of the EZ, whereas Guo et al (2020) did not find a significant difference between the inward and outward connectivity of the EZ. These discrepancies could be due to the variable methods used for connectivity estimation.
To be noted, some studies using the same method, namely the PDC, have opposite results (Narasimhan et al, 2020; Paulo et al, 2022; Varotto et al, 2012). Another explanation could be the impact of interictal spikes on connectivity directionality, because some authors included spikes in their analyses (Bettus et al, 2011; Lagarde et al, 2018; Varotto et al, 2012) and others did not (Narasimhan et al, 2020; Paulo et al, 2022). Therefore, despite the relatively low effect of epileptic spikes on the overall connectivity value (Bartolomei et al, 2013; Bettus et al, 2008; Jiang et al, 2022; Park and Madsen, 2018), it is possible that spikes affect the directionality of connectivity (Karunakaran et al, 2018). Further studies looking at the effect of spikes and methods used for estimating the directionality of the connectivity of the EZ are still needed.
Association with other variables
Van Dellen et al (2009) investigated the effect of epilepsy duration on FC (phase lag index) in the temporal neocortex. They showed a decrease in connectivity value with an increase in epilepsy duration, and a more random configuration of the network (decrease of the clustering coefficient and of the small-world index) in case of a longer epilepsy duration. The authors did not find a significant association for age at epilepsy onset and seizure frequency. Unfortunately, the information about the type of epilepsy (mesiotemporal or laterotemporal) and the type of surgery performed was not available in this study. It is therefore difficult to speculate on the precise epileptogenicity of the studied structures. Bartolomei et al (2013) suggested that the topology of interictal networks evolves with time. They observed an increase of the “small-worldness” (S index) with the increase in epilepsy duration (being essentially due to a decrease of the path length over time).
While the pattern of connectivity changes during a peri-ictal state (pre-, per-, and postictal) is increasingly known (Bartolomei et al, 2004; Courtens et al, 2016; review in Bartolomei et al, 2017), few studies have focused on the temporal variation/dynamics of interictal FC measured from iEEG recordings. Geier and Lehnertz (2017) have shown fluctuation of the degree of centrality of brain regions across long-term intracranial recordings on timescales of hours to days, with strong contributions of daily rhythms. This study found differences in the variation across time of centrality metric between the EZ and noninvolved regions, with a slightly higher variation for EZ. The amplitude of variation was also higher using BC rather than strength centrality.
However, Kramer et al (2011) demonstrated the emergence of a stable network pattern across long-term recordings. Similarly, others studies have reported stability of the FC results across days of iEEG recordings in patients with epilepsy (Dauwels et al, 2009; Jiang et al, 2022; Mormann et al, 2000; Paulo et al, 2022; Schevon et al, 2007). This result strengthens the confidence in the use of connectivity data for EZ delineation purpose in practice. However, it remains unclear what could be the added value of the estimation of interictal FC dynamics into the delineation of the EZ, and further studies on the topic are needed.
Association with surgical outcome
Table 3 summarizes the data concerning the association between FC data and surgical outcome. Schevon et al (2007) have first suggested a link between the level of interictal FC impairment and postsurgical prognosis. Antony et al (2013) analyzed the FC (linear correlation) between mesiotemporal and neocortical structures in 23 patients operated for drug refractory TLE. They found that the smaller the mean and the variation of the connectivity values, the better the surgical outcome. The performance of connectivity data was very good (accuracy = 87%) for distinguishing patients categorized with a favorable and unfavorable postsurgical outcome, but the association with the SEEG-defined EZ was not formerly studied. In contrast, Goodale et al (2020) suggest that patients with higher connectivity within the RZ could have a more favorable surgical outcome.
Table 3.
Links Between Functional Connectivity Data and Postsurgical Seizure Outcome
| Authors | Population | Recording modality | Methods | Main results |
|---|---|---|---|---|
| Schevon et al (2007) | Nine patients with neocortical epilepsy | ECoG (during presurgical monitoring) | Mean phase coherence | Complete resection of area of local hypersynchrony associated with good outcome |
| Ortega et al (2008) | Twenty-nine patients with TLE | ECoG (during surgery) | Linear correlation, mutual information, and phase synchronization | No correlation between complete resection of cluster of local hypersynchrony and postsurgical outcome |
| Antony et al (2013) | Twenty-three patients with TLE | SEEG | Linear correlation | Better outcome if weaker overall FC and more homogenous overall FC (less outlier with high FC) |
| Lagarde et al (2018) | Fifty-nine patients with FCD or NDT (20 TLE) | SEEG | Nonlinear correlation (h2) | Worse outcome is higher FC within NIZ |
| Grobelny et al (2018) | Thirty-six patients | ECoG (during presurgical monitoring) | Granger causality | Worse outcome if higher overall betweenness centrality and more outlier with high values |
| Goodale et al (2020) | Fifteen patients (12 TLE) | SEEG | Imaginary coherence | Better outcome if higher FC within-RZ |
| Shah et al (2019b) | Twenty-seven patients (18 TLE) | ECoG + depth electrodes | Linear correlation | Better outcome if higher FC within RZ Better outcome with higher overlap between RZ and nodes with the highest FC |
| Guo et al (2020) | Twenty-five patients (10 TLE) | SEEG | CCEP | Better outcome if higher FC within EZ |
| Paulo et al (2022) | Thirty-two patients (18 TLE) | SEEG | Imaginary coherence, partial directed coherence (in alpha band) | No difference in EZ FC between patients being Engel I or not |
| Jiang et al (2022) | Twenty-seven patients (23 TLE) | SEEG | Directed transfer function, cross-frequency directionality | Larger within-frequency information flow asymmetry between EZ and NIZ is associated with favorable outcome |
Studies are presented in chronological order. For studies using ECoG recordings, we precise if the recordings were performed during a surgery procedure (with anesthesia) or long-term monitoring.
Recently, and in the same vein, Shah et al (2019b) found that patients with favorable outcomes had higher within-RZ connectivity than patients with an unfavorable outcome. Moreover, the higher the overlap between the RZ and the nodes with the highest strength, the better the postsurgical seizure outcome. In addition, the distinction between patients with a good or bad outcome was better using beta-band connectivity. Nevertheless, the great variability of values across patients seems to preclude easy application at the individual level because of the difficulty to set a common threshold. A study using CCEPs to estimate directed FC showed a positive correlation between the values of FC within the EZ and the surgical outcome (Guo et al, 2020).
These results are in line with a study using MEG showing that higher connectivity within the RZ was associated with a favorable outcome (Englot et al, 2015). Looking at the directionality of the FC, Jiang et al (2022) demonstrated that a larger resting-state, within-frequency information flow asymmetry between EZ and NIZ was associated with a favorable seizure outcome. Interestingly, using this information, a random forest classifier had an accuracy of 86% to predict the surgical outcome.
In our abovementioned study, we have suggested that a larger disturbance of cerebral connectivity (higher connectivity outside EZ and PZ, i.e., within-NIZ and between PZ-NIZ) is associated with worse prognosis (Lagarde et al, 2018). Similar findings were recently reported using ECoG and BC: patients not seizure free after surgery had a higher value of BC in the interictal and postictal period, and a greater proportion of extreme-valued BC nodes (Grobelny et al, 2018). However, the individual predictive value of these connectivity measures on the postsurgical prognosis is not yet known. Future studies may investigate how these connectivity data can be used in clinical practice to predict the chance of surgical success.
Overall, studies showed that the higher the connectivity within the EZ and the larger the difference with the connectivity of the NIZ, the better the surgical outcome. The potential predictive value for surgical outcome obtained from presurgical connectivity has been also reported in several works using tractography MRI data. It was suggested that pathologically increased limbic and extralimbic structural connectivity can explain worse seizure outcome after epilepsy surgery (Bonilha et al, 2015; Bonilha et al, 2013), including a role of thalamocortical connectivity (He et al, 2017; Keller et al, 2015). Noteworthy, these studies reported a better predictive value of connectivity than the sole usual clinical variables for postsurgical outcome (He et al, 2017; Keller et al, 2015; Morgan et al, 2019).
Connectivity changes induced by neurostimulation techniques
Another important and growing field in epilepsy surgery is neurostimulation: vagal nerve stimulation (VNS), deep-brain stimulation, and responsive neurostimulation (Fisher and Velasco, 2014; Fisher et al, 2010; Geller et al, 2017; Jobst et al, 2017; Ryvlin et al, 2021; Ryvlin et al, 2014). Studies have evaluated the impact of these techniques on iEEG FC. Bartolomei et al (2016) focused on VNS-induced connectivity changes (comparison of ON and OFF periods) in five patients explored by SEEG. They found that nonresponder patients exhibited an increase in overall connectivity, while the responder patients exhibited a decrease. These findings are in line with scalp EEG studies showing an association between the ability of VNS to decrease FC during ON periods and its efficacy (Bodin et al, 2015; Sangare et al, 2020). Moreover, VNS was able to induce some changes of connectivity within the EZ (increase in one nonresponder patient and decrease in the responder one) (Bartolomei et al, 2016). Yu et al (2018) focused on the effect of anterior nucleus of the thalamus (ANT) deep brain stimulation (DBS) in nine patients explored by SEEG. In this study, authors found that high-frequency stimulation of the ANT decreased the connectivity of the brain networks during ON periods. In line with these results, a recent study has shown that pulvinar stimulation can decrease the duration of temporal seizures and the associated alteration of consciousness (Filipescu et al, 2019), and that in responders, stimulation leads to a decrease in synchrony between the extratemporal brain regions (Deutschová et al, 2021).
These preliminary studies suggested that neuromodulation techniques may act also by modulating FC. Further studies investigating systematically the effect of different parameters of neuromodulation on large-scale SEEG connectivity under various and neurostimulation protocols would be interesting, to help choosing optimal parameters to use for VNS or DBS. This is especially important as different frequencies seem to induce different changes on connectivity (Bartolomei et al, 2016; Yu et al, 2018).
Comparison with Other Modalities
Magnetic resonance imaging
Only a few studies have compared iEEG and MRI FC. Bettus et al (2011) compared FC as extracted from SEEG recordings with that obtained from fMRI. This latter study reported some multimodal agreement: (1) in the directionality of connectivity, the EZ influenced the NIZ; and (2) in the decrease of FC between the EZ and the NIZ (delta band SEEG) and within the NIZ in fMRI. Nevertheless, there were some discrepancies with an increase in SEEG FC (in the beta band) in the EZ and secondary irritative zone (IZ2), while the blood-oxygen-level dependent FC was decreased in IZ2. These results can be explained by the fact that the two modalities do not study the same processes, especially at different timescales.
Ridley et al (2017) looked at the comparison between FC recorded simultaneously in fMRI and SEEG. They showed that there was a good intermodality correlation in nonepileptic areas, but an alteration of this correlation in epileptic regions.
Besson et al (2017), focusing on the link between the structural connectivity alterations according to the brain network involved during seizure as defined by SEEG in patients with temporal lobe epilepsies, revealed that structural connectivity was significantly preserved within epileptic zones (EZ and PZ) and decreased in nonepileptic structures. Taken together with other structural connectivity MRI studies, these findings suggest that: (1) areas involved in seizure generation and propagation (e.g., thalamus in TLE) have a relatively preserved (higher) structural connectivity (Besson et al, 2017; Bonilha et al, 2012; Dinkelacker et al, 2015); and (2) other remote areas have a widely decreased structural connectivity (Besson et al, 2014). This pattern of local “hyperconnectivity” within epileptic structures combined with widespread “hypoconnectivity” outside these areas is concordant with iEEG findings and other studies on FC extracted from noninvasive electrophysiological recording (see below).
This similarity confirms both the tight relationships between structural and FC known to exist in healthy conditions (Goni et al, 2014; Honey et al, 2007), and the increased structural–functional correlation in epilepsy (Wirsich et al, 2016).
MEG/EEG
MEG studies have demonstrated an increase in FC between regions within the EZ network (Englot et al, 2015; Juárez-Martinez et al, 2018; Nissen et al, 2016; Wu et al, 2014), associated with a decrease in FC between these regions and other noninvolved brain regions (Englot et al, 2015; Nissen et al, 2016). Interestingly, Englot (source-space MEG connectivity) found that the degree of global reductions in FC was related to epilepsy duration and frequency of consciousness-impairing seizures, and thus may reflect the deleterious effects of seizures on brain networks over time (Englot et al, 2015). Moreover, in this study, the increased regional connectivity appears to be a marker of favorable seizure outcome after surgery. Changes in network topology have also been identified in MEG (Chavez et al, 2010; Horstmann et al, 2010), in particular an increase in the BC in the network of epileptogenic regions (Nissen et al, 2018; Nissen et al, 2017).
Nevertheless, the correlation between MEG and iEEG connectivity seems limited, as well as the ability of connectivity results to distinguish between resection areas and nonresection areas (Juárez-Martinez et al, 2018; Nissen et al, 2018). These discrepancies could be due to the metrics used to analyze connectivity (Coito et al, 2019) and further studies are needed.
Some scalp EEG studies have shown a decrease in the influence (in terms of out-connections) of the default mode network, and an increase of in-connections into the epileptic hippocampus in patients with TLE (Coito et al, 2016). Coito et al used high-density EEG and source-space FC in IED-free epochs (60 sec). They demonstrated that patients had a significantly reduced connectivity within regions belonging to the default-mode network. Moreover, the strongest connections arose from the posterior cingulate cortex in controls and from the epileptic hippocampus in patients. Noteworthy, the connectivity results differ according to the disease duration, the cognitive (learning deficit) and psychiatric status (depression or not).
Using a similar methodology in epochs without IEDs, Verhoeven et al (2018) applied two-class random forest classifiers on FC and were able to differentiate healthy control from patients, and left from right TLE, at the individual level. The classification achieved a high accuracy, sensitivity, and specificity (between 85% and 95%). As in the previous study by Coito et al (2015), the most important features for diagnosis were the outflows from the left and right medial temporal lobe. However, it was important to consider the whole connectome (i.e., including connectivity values of several brain areas in the predictive model) to achieve correct classification.
Overall, FC data from noninvasive recording techniques (EEG and MEG) confirmed the higher connectivity within the EZ and highlighted the widespread hypoconnectivity outside the EZ.
Summary
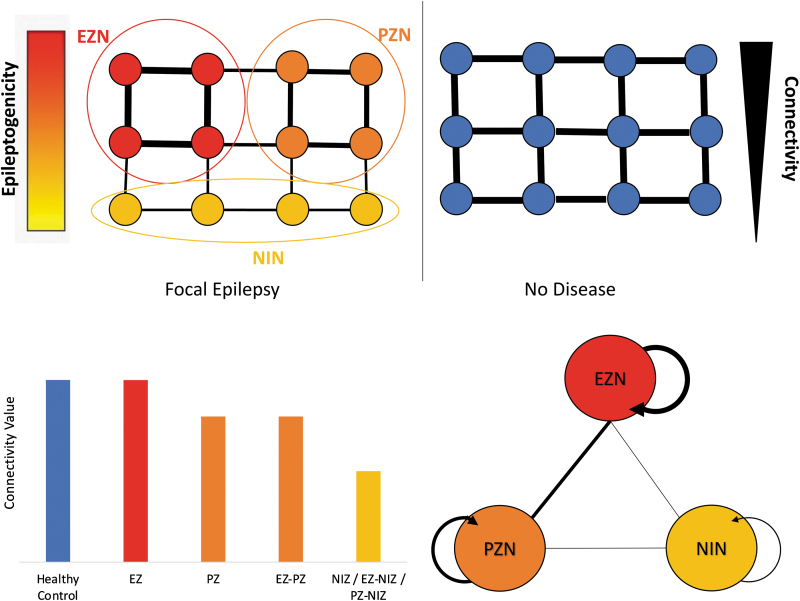
Taken as a whole, the data about connectivity from iEEG studies and from whole-brain noninvasive modalities [especially MEG (Englot et al, 2015) and structural MRI data (Besson et al, 2017)] suggested that focal refractory epilepsies are associated with a global profile of large-scale network alteration, including the following: (1) a wide decrease of connectivity outside the EZ; (2) an EZ exhibiting, rather than an increase, a relative preservation of its intrinsic connectivity (Fig. 3); (3) an EZ with a preferential connection to PZ and with a relative disconnection from NIZ.
FIG. 3.
Synthesizes the main data on interictal functional connectivity. In comparison with healthy control, the EZN shows a relatively preserved connectivity, while the NIN has a decreased connectivity. PZN shows an intermediate level of connectivity. EZN is preferentially connected to the PZN and relatively disconnected of the NIN. For the clarity of the figure, all nodes are not interconnected, and the connectivity is considered as equal between all nodes in healthy condition (it is not the case in human brain). The directionality is not represented in this graph as the existing data are contradictory on the role of the epileptogenic zone: sender or receiver?
From Excitability to Connectivity: Conceptual Comments
While most historical works have focused on the importance of the hyperexcitability of epileptic areas, it is increasingly apparent that the underlying connectivity is also essential for understanding the organization of interictal events and seizures. This hypothesis was suggested early on by Wendling et al (2005, 2001) using a computational model. The authors showed that the interictal-ictal transition was not explained solely by the excitation/inhibition balance, but rather by the interactions between pyramidal cells and interneuron populations. Noteworthy, this concept has also been highlighted in experimental models (in vivo and in vitro); some authors have demonstrated how specific changes in the topology or synaptic strength can impact brain epileptogenicity (Morgan and Soltesz, 2008; Netoff et al, 2004). It has also been shown that a minor change in the topology of a network can explain the emergence of explosive changes in synchrony as observed during the generation and propagation of epileptic seizures (Wang et al, 2017).
The phenomenological model has shown that the network's ability to generate seizures is highly dependent on its topology. Hebbink et al (2017) have demonstrated that: (1) even in the presence of a hyperexcitable node, some networks do not generate seizures; (2) the existence of a driving node greatly increases the number of seizures; and (3) the presence of reciprocal connections between two nodes could act as a stabilizer reducing the number of seizures. Proix et al (2014), using a different phenomenological model (Jirsa et al, 2014), systematically explored the impact of excitability and coupling values on the behavior of two oscillators mimicking the activity of two brain regions.
Variations in these two parameters were sufficient to induce different behaviors: (1) systematic seizure propagation, (2) partial seizure propagation, (3) change in the seizure-generating area across time, (4) lack of seizure propagation, and (5) lack of seizure genesis. This study has clearly demonstrated that both excitability and coupling of brain structures determine the behavior of epileptic network. These concepts were recently confirmed in studies demonstrating that the dynamics of focal seizures is related to the underlying structural connectivity and that seizure spread is tightly controlled by structural connections (Proix et al, 2017; Shah et al, 2019a).
This principle is used in large-scale seizure modeling called “virtual epileptic patient” that is a whole-brain model (based on the architecture of the “virtual brain”) using the structural connectivity of the patient coupled with a neural mass model, able to reproduce epileptic seizure dynamics (Jirsa et al, 2017; Jirsa et al, 2014) and to simulate the pattern of seizure spread. Interestingly this model was able to reproduce in silico the spatiotemporal dynamics of seizure evidenced in SEEG at the patient level (Jirsa et al, 2017; Makhalova et al, 2022; Proix et al, 2018; Proix et al, 2017).
Conclusions and Perspectives
The different studies reviewed in this article were performed mainly with the aim of discriminating the EZ from less epileptogenic regions from invasive EEG data. Our results as well as data from the literature suggest a potential interest to distinguish the EZ from other areas (Narasimhan et al, 2020). In fact, an important point from our data (Lagarde et al, 2018) is the small difference in connectivity strengths between EZ and PZ. This observation makes it difficult for connectivity methods to distinguish between EZ and PZ, a classic goal of the presurgical workup. However, it suggests that there is probably more a gradient of epileptogenicity than a true “clear cut” difference between these regions. Furthermore, because EZ has high intrinsic connectivity but low connectivity to nonepileptic areas, centrality measures (obtained by averaging the connectivity values of these two types of connections) may be insufficient to accurately determine EZ.
Thus, the use of other markers may be relevant (e.g., directionality). Indeed, the literature suggests that only the EZ behaves as a “module” (higher connectivity within the EZ than between the EZ and other areas) (Lagarde et al, 2018), and then, some specific graph theory metrics could be markers to be tested in further studies. In addition, the added value of markers from connectivity analyses compared with classical markers of epileptogenicity such as ictal (e.g., epileptogenicity index) (Bartolomei et al, 2008) and interictal (e.g., spikes, high frequency oscillations) (Roehri and Bartolomei, 2019) neuromarkers could also be investigated.
Moving forward, several studies have recently examined the value of simulation/modeling methods in surgical decision-making. Most studies used ictal/peri-ictal ECoG data (An et al, 2019; Goodfellow et al, 2016; Junges et al, 2019; Khambhati et al, 2016; Kini et al, 2019; Lopes et al, 2018; Lopes et al, 2017; Müller et al, 2018; Olmi et al, 2019; Steimer et al, 2017) and only one used interictal ECoG data (Sinha et al, 2017). Further studies examining the added value of this simulation/modeling technique in surgical decision-making based on interictal SEEG FC data could be useful.
Further study could also consider performing connectivity analysis over IED-centered time windows and especially looking at directionality (Bou Assi et al, 2020). Such an analysis could add information about the differences between spikes inside and outside the EZ and help to delineate the EZ more effectively from the interictal period. The goal would be to find markers of epileptogenicity from spike propagation. These methods could be compared with results obtained from co-occurrences (Bourien et al, 2005; Lambert et al, 2018; Malinowska et al, 2014), which have proven to identify interictal spike networks.
Ethical Publication Statement
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supplementary Material
Authors' Contributions
S.L. performed the literature review, drafted the article, and created the tables and figures. C.-G.B., F.W., and F.B. contributed to the writing of the article, including its critical review. All authors approved the final form of the article.
Author Disclosure Statement
None of the authors has any conflict of interest to disclose.
Funding Information
Some of the discussed studies, from our team, and this review have been carried within the following grants: FHU EPINEXT and DHU-Imaging, A*MIDEX project (ANR-11-IDEX-0001-02) funded by the “Investissements d'Avenir” French Government program managed by the French National Research Agency (Agence Nationale de la Recherche [ANR]); “VIBRATIONS” ANR-13-PRTS-0011-01, funded by ANR and Direction Générale de l'Offre de Santé (DGOS); European Union's Horizon 2020 research and innovation programme (grant agreement No 855109; ERC-SyG 2019) funded by the European Research Council (ERC).
Supplementary Material
References
- Amini L, Jutten C, Achard S, et al. Directed differential connectivity graph of interictal epileptiform discharges. IEEE Trans Biomed Eng 2011;58(4):884; doi: 10.1109/TBME.2010.2099227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Bartolomei F, Guye M, et al. Optimization of surgical intervention outside the epileptogenic zone in the virtual epileptic patient (VEP). PLoS Comput Biol 2019;15(6):1–25; doi: 10.1371/journal.pcbi.1007051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari-Asl K, Senhadji L, Bellanger J-J, et al. Quantitative evaluation of linear and nonlinear methods characterizing interdependencies between brain signals. Phys Rev E 2006;74(3):031916; doi: 10.1103/PhysRevE.74.031916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony AR, Alexopoulos AV, González-Martínez JA, et al. Functional connectivity estimated from intracranial EEG predicts surgical outcome in intractable temporal lobe epilepsy. PLoS One 2013;8(10):e77916; doi: 10.1371/journal.pone.0077916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold J, Grassberger P, Lehnertz K, et al. A robust method for detecting interdependences: Application to intracranially recorded EEG. Phys D 1999;134(4):419–430; doi: 10.1016/S0167-2789(99)00140-2 [DOI] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, et al. Assessing cortical functional connectivity by linear inverse estimation and directed transfer function: Simulations and application to real data. Clin Neurophysiol 2005;116(4):920–932; doi: 10.1016/j.clinph.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, et al. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum Brain Mapp 2007;28(2):143–157; doi: 10.1002/hbm.20263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, et al. Tracking the time-varying cortical connectivity patterns by adaptive multivariate estimators. IEEE Trans Biomed Eng 2008;55(3):902–913; doi: 10.1109/TBME.2007.905419 [DOI] [PubMed] [Google Scholar]
- Bancaud J, Talairach J.. Clinical Semiology of Frontal Lobe Seizures. Raven Press: New York, NY, 1992; pp. 3–58. [PubMed] [Google Scholar]
- Barlow JS, Brazier MAB. A note on a correlator for electroencephalographic work. Electroencephalogr Clin Neurophysiol 1954;6:321–325; doi: 10.1016/0013-4694(54)90036-x [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Bettus G, Stam CJ, et al. Interictal network properties in mesial temporal lobe epilepsy: A graph theoretical study from intracerebral recordings. Clin Neurophysiol 2013;124(12):2345–2353; doi: 10.1016/j.clinph.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Chauvel P, Wendling F.. Epileptogenicity of brain structures in human temporal lobe epilepsy: A quantified study from intracerebral EEG. Brain 2008;131(7):1818–1830; doi: 10.1093/brain/awn111 [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Lagarde S, Wendling F, et al. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia 2017;58(7):1131–1147; doi: 10.1111/epi.13791 [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Trébuchon A, Bonini F, et al. What is the concordance between the seizure onset zone and the irritative zone? A seeg quantified study. Clin Neurophysiol 2016;127(2):1157–1162; doi: 10.1016/j.clinph.2015.10.029 [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Wendling F, Bellanger JJ, et al. Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clin Neurophysiol 2001;112(9):1746–1760; doi: 10.1016/S1388-2457(01)00591-0 [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Wendling F, Régis J, et al. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Res 2004;61(1–3):89–104; doi: 10.1016/j.eplepsyres.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Wendling F, Vignal JP, et al. Seizures of temporal lobe epilepsy: identification of subtypes by coherence analysis using stereo-electro-encephalography. Clin Neurophysiol 1999;110(10):1741–1754; doi: 10.1016/S1388-2457(99)00107-8 [DOI] [PubMed] [Google Scholar]
- Bastos AM, Schoffelen J-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Syst Neurosci 2016;9:1–23; doi: 10.3389/fnsys.2015.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabei JM, Campbell Arnold T, Shah P, et al. Electrocorticography and stereo EEG provide distinct measures of brain connectivity: Implications for network models short, running title: ECoG, SEEG, and networks in epilepsy. Brain Commun 2021;11(3):fcab156; doi: 10.1093/braincomms/fcab156/6319016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Hong S, Bernasconi A, et al. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 2013;7:1–14; doi: 10.3389/fnhum.2013.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson P, Bandt SKK, Proix T, et al. Anatomic consistencies across epilepsies: A stereotactic-EEG informed high-resolution structural connectivity study. Brain 2017;140(10);2639–2652; doi: 10.1093/brain/awx181 [DOI] [PubMed] [Google Scholar]
- Besson P, Dinkelacker V, Valabregue R, et al. Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage 2014;100:135–144; doi: 10.1016/j.neuroimage.2014.04.071 [DOI] [PubMed] [Google Scholar]
- Bettus G, Ranjeva JP, Wendling F, et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One 2011;6(5):e20071; doi: 10.1371/journal.pone.0020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res 2008;81(1):58–68; doi: 10.1016/j.eplepsyres.2008.04.020 [DOI] [PubMed] [Google Scholar]
- Bodin C, Aubert S, Daquin G, et al. Responders to vagus nerve stimulation (VNS) in refractory epilepsy have reduced interictal cortical synchronicity on scalp EEG. Epilepsy Res 2015;113:98–103; doi: 10.1016/j.eplepsyres.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Bonilha L, Helpern JA, Sainju R, et al. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 2013;81(19):1704–1710; doi: 10.1212/01.wnl.0000435306.95271.5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Jensen JH, Baker N, et al. The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology 2015;84(18):1846–1853; doi: 10.1212/WNL.0000000000001548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Martz GU, et al. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 2012;83(9):903–909; doi: 10.1136/jnnp-2012-302476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Assi E, Zerouali Y, Robert M, et al. Large-scale desynchronization during interictal epileptic discharges recorded with intracranial EEG. Front Neurol 2020;11:1–12; doi: 10.3389/fneur.2020.529460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulogne S, Ryvlin P, Rheims S.. Single and paired-pulse electrical stimulation during invasive EEG recordings. Rev Neurol (Paris) 2016;172(3):174–181; doi: 10.1016/j.neurol.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Bourien J, Bartolomei F, Bellanger JJ, et al. A method to identify reproducible subsets of co-activated structures during interictal spikes. Application to intracerebral EEG in temporal lobe epilepsy. Clin Neurophysiol 2005;116(2):443–455; doi: 10.1016/j.clinph.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Brazier MAB, Casby JU. Crosscorrelation and autocorrelation studies of electroencephalographic potentials. EEG Clin Neurophysiol 1952;4(2):201–211; doi: 10.1016/0013-4694(52)90010-2 [DOI] [PubMed] [Google Scholar]
- Buser P, Bancaud J, Talairach J, et al. Amygdalo-hippocampal interconnections in man. Physiological study during stereotaxic explorations. Electroencephalogr Clin Neurophysiol 1969;26(6):637. [PubMed] [Google Scholar]
- Chauvel P, Buser P, Badier JM, et al. The “epileptogenic zone” in humans: Representation of intercritical events by spatio-temporal maps. Rev Neurol (Paris) 1987;143(5):443–450. [PubMed] [Google Scholar]
- Chavez M, Valencia M, Navarro V, et al. Functional modularity of background activities in normal and epileptic brain networks. Phys Rev Lett 2010;104(11):1–4; doi: 10.1103/PhysRevLett.104.118701 [DOI] [PubMed] [Google Scholar]
- Chu CJ, Kramer MA, Pathmanathan J, et al. Emergence of stable functional networks in long-term human electroencephalography. J Neurosci 2012;32(8):2703–2713; doi: 10.1523/JNEUROSCI.5669-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coito A, Biethahn S, Tepperberg J, et al. Interictal epileptogenic zone localization in patients with focal epilepsy using electric source imaging and directed functional connectivity from low-density EEG. Epilepsia Open 2019;4(2):281–292; doi: 10.1002/epi4.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coito A, Genetti M, Pittau F, et al. Altered directed functional connectivity in temporal lobe epilepsy in the absence of interictal spikes: A high density EEG study. Epilepsia 2016;57(3):402–411; doi: 10.1111/epi.13308 [DOI] [PubMed] [Google Scholar]
- Coito A, Plomp G, Genetti M, et al. Dynamic directed interictal connectivity in left and right temporal lobe epilepsy. Epilepsia 2015;56(2):207–217; doi: 10.1111/epi.12904 [DOI] [PubMed] [Google Scholar]
- Conrad EC, Bernabei JM, Kini LG, et al. The sensitivity of network statistics to incomplete electrode sampling on intracranial EEG. Netw Neurosci 2020;4(2):484–506; doi: 10.1162/netn_a_00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtens S, Colombet B, Trébuchon A, et al. Graph measures of node strength for characterizing preictal synchrony in partial epilepsy. Brain Connect 2016;6(7):530–539; doi: 10.1089/brain.2015.0397 [DOI] [PubMed] [Google Scholar]
- Dauwels J, Eskandar E, Cash S.. Localization of seizure onset area from intracranial non-seizure EEG by exploiting locally enhanced synchrony. In: Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC 2009 2009; pp. 2180–2183. [DOI] [PubMed] [Google Scholar]
- van Dellen E, Douw L, Baayen JC, et al. Long-term effects of temporal lobe epilepsy on local neural networks: A graph theoretical analysis of corticography recordings. PLoS One 2009;4(11):e8081; doi: 10.1371/journal.pone.0008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschová B, Pizzo F, Giusiano B, et al. Ictal connectivity changes induced by pulvinar stimulation correlate with improvement of awareness. Brain Stimul 2021;14(2):344–346; doi: 10.1016/j.brs.2021.01.021 [DOI] [PubMed] [Google Scholar]
- Van Diessen E, Hanemaaijer JI, Otte WM, et al. Are high frequency oscillations associated with altered network topology in partial epilepsy? Neuroimage 2013;82:564–573; doi: 10.1016/j.neuroimage.2013.06.031 [DOI] [PubMed] [Google Scholar]
- Dinkelacker V, Valabregue R, Thivard L, et al. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: Enhanced connectivity per hippocampal voxel. Epilepsia 2015;56(8):1217–1226; doi: 10.1111/epi.13051 [DOI] [PubMed] [Google Scholar]
- Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med 2017;377(17):1639–1647; doi: 10.1056/NEJMoa1615335 [DOI] [PubMed] [Google Scholar]
- Englot DJ, Hinkley LB, Kort NS, et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain 2015;138(8):2249–2262; doi: 10.1093/brain/awv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiest KM, Jetté N, Roberts JI, et al. The prevalence and incidence of epilepsy: A systematic review and meta-analysis. Neurology 2016;43(S1):S3–S50; doi: 10.1212/WNL.0000000000003509 [DOI] [PubMed] [Google Scholar]
- Filipescu C, Lagarde S, Bartolomei F.. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia 2019;60(4):e25–e30; doi: 10.1111/epi.14677 [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51(5):899–908; doi: 10.1111/j.1528-1167.2010.02536.x [DOI] [PubMed] [Google Scholar]
- Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol 2014;10(5):261–270; doi: 10.1038/nrneurol.2014.59 [DOI] [PubMed] [Google Scholar]
- Florin E, Gross J, Pfeifer J, et al. The effect of filtering on granger causality based multivariate causality measures. Neuroimage 2010;50(2):577–588; doi: 10.1016/j.neuroimage.2009.12.050 [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M.. Graph analysis of the human connectome: Promise, progress, and pitfalls. Neuroimage 2013;80:426–444; doi: 10.1016/j.neuroimage.2013.04.087 [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Bullmore ET. Fundamentals of Brain Network Analysis. Academic Press; 2016. [Google Scholar]
- Geier C, Lehnertz K.. Long-term variability of importance of brain regions in evolving epileptic brain networks. Chaos An Interdiscip J Nonlinear Sci 2017;27(4):043112; doi: 10.1063/1.4979796 [DOI] [PubMed] [Google Scholar]
- Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 2017;58(6):994–1004; doi: 10.1111/epi.13740 [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Kocher M, Bonilha L.. Connectomics and graph theory analyses: Novel insights into network abnormalities in epilepsy. Epilepsia 2015;56(11):1660–1668; doi: 10.1111/epi.13133 [DOI] [PubMed] [Google Scholar]
- Goni J, van den Heuvel MP, Avena-Koenigsberger A, et al. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci U S A 2014;111(2):833–838; doi: 10.1073/pnas.1315529111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale SE, González HFJ, Johnson GW, et al. Resting-state SEEG may help localize epileptogenic brain regions. Neurosurgery 2020;86(6):792–801; doi: 10.1093/neuros/nyz351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow M, Rummel C, Abela E, et al. Estimation of brain network ictogenicity predicts outcome from epilepsy surgery. Sci Rep 2016;6(1):29215; doi: 10.1038/srep29215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J. Measurement of small time differences between EEG channels: Method and application to epileptic seizure propagation. Electroencephalogr Clin Neurophysiol 1983;56(5):501–514; doi: 10.1016/0013-4694(83)90235-3 [DOI] [PubMed] [Google Scholar]
- Grobelny BT, London D, Hill TC, et al. Betweenness centrality of intracranial electroencephalography networks and surgical epilepsy outcome. Clin Neurophysiol 2018;129(9):1804–1812; doi: 10.1016/j.clinph.2018.02.135 [DOI] [PubMed] [Google Scholar]
- Guo Z-H, Zhao B-T, Toprani S, et al. Epileptogenic network of focal epilepsies mapped with cortico-cortical evoked potentials. Clin Neurophysiol 2020;131(11):2657–2666; doi: 10.1016/j.clinph.2020.08.012 [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940. –1980. Epilepsia 1991;32(4):429–445; doi: 10.1111/j.1528-1157.1991.tb04675.x [DOI] [PubMed] [Google Scholar]
- He B, Astolfi L, Valdes-Sosa PA, et al. Electrophysiological brain connectivity: theory and implementation. IEEE Trans Biomed Eng 2019;66(7):2115–2137; doi: 10.1109/TBME.2019.2913928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Doucet GE, Pustina D, et al. Presurgical thalamic hubness predicts surgical outcome in temporal lobe epilepsy. Neurology 2017;88(24):2285–2293; doi: 10.1212/WNL.0000000000004035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbink J, Meijer H, Huiskamp G, et al. Phenomenological network models: Lessons for epilepsy surgery. Epilepsia 2017;58(10):e147–e151; doi: 10.1111/epi.13861 [DOI] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, et al. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A 2007;104(24):10240–10245; doi: 10.1073/pnas.0701519104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann M, Bialonski S, Noennig N, et al. Clinical neurophysiology state dependent properties of epileptic brain networks: Comparative graph—Theoretical analyses of simultaneously recorded EEG and MEG. Clin Neurophysiol 2010;121(2):172–185; doi: 10.1016/j.clinph.2009.10.013 [DOI] [PubMed] [Google Scholar]
- Isnard J, Taussig D, Bartolomei F, et al. French guidelines on stereoelectroencephalography (SEEG). Neurophysiol Clin 2018;48(1):5–13; doi: 10.1016/j.neucli.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Jayakar P, Gotman J, Harvey AS, et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia 2016;57(11):1735–1747; doi: 10.1111/epi.13515 [DOI] [PubMed] [Google Scholar]
- Jehi L, Morita-Sherman M, Love TE, et al. Comparative effectiveness of stereo-EEG versus subdural grids in epilepsy surgery. Ann Neurol 2021;90(6):927–939; doi: 10.1002/ana.26238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Kokkinos V, Ye S, et al. Interictal SEEG resting-state connectivity localizes the seizure onset zone and predicts seizure outcome. Adv Sci 2022;9(18):e2200887; doi: 10.1002/advs.202200887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsa VK, Proix T, Perdikis D, et al. The virtual epileptic patient: Individualized whole-brain models of epilepsy spread. Neuroimage 2017;145(Pt B):377–388; doi: 10.1016/j.neuroimage.2016.04.049 [DOI] [PubMed] [Google Scholar]
- Jirsa VK, Stacey WC, Quilichini PP, et al. On the nature of seizure dynamics. Brain 2014;137(8):2210–2230; doi: 10.1093/brain/awu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst BC, Kapur R, Barkley GL, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 2017;58(6):1005–1014; doi: 10.1111/epi.13739 [DOI] [PubMed] [Google Scholar]
- Juárez-Martinez EL, Nissen IA, Idema S, et al. Virtual localization of the seizure onset zone: Using non-invasive MEG virtual electrodes at stereo-EEG electrode locations in refractory epilepsy patients. Neuroimage Clin 2018;19:758–766; doi: 10.1016/j.nicl.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junges L, Lopes MA, Terry JR, et al. The role that choice of model plays in predictions for epilepsy surgery. Sci Rep 2019;9(1):1–12; doi: 10.1038/s41598-019-43871-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Rollo MJ, Kim K, et al. The interictal mesial temporal lobe epilepsy network. Epilepsia 2018;59(1):244–258; doi: 10.1111/epi.13959 [DOI] [PubMed] [Google Scholar]
- Katz JS, Abel TJ. Stereoelectroencephalography versus subdural electrodes for localization of the epileptogenic zone: What is the evidence? Neurotherapeutics 2019;16(1):59–66; doi: 10.1007/s13311-018-00703-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Honey CJ, Entz L, et al. Corticocortical evoked potentials reveal projectors and integrators in human brain networks. J Neurosci 2014;34(27):9152–9163; doi: 10.1523/JNEUROSCI.4289-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Richardson MP, Schoene-Bake JC, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol 2015;77(5):760–774; doi: 10.1002/ana.24376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambhati AN, Davis KA, Lucas TH, et al. Virtual cortical resection reveals push-pull network control preceding seizure evolution. Neuron 2016;91(5):1170–1182; doi: 10.1016/j.neuron.2016.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini LG, Bernabei JM, Mikhail F, et al. Virtual resection predicts surgical outcome for drug resistant epilepsy. Brain 2019;142(12):3892–3905; doi: 10.1093/brain/awz303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Lepage KQ, et al. Emergence of persistent networks in long-term intracranial EEG recordings. J Neurosci 2011;31(44):15757–15767; doi: 10.1523/JNEUROSCI.2287-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ, Wan PAK. Early identification of refractory epilepsy. N Engl J Med 2000;342(5):314–319; doi: 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- Lacruz ME, García Seoane JJ, Valentin A, et al. Frontal and temporal functional connections of the living human brain. Eur J Neurosci 2007;26(5):1357–1370; doi: 10.1111/j.1460-9568.2007.05730.x [DOI] [PubMed] [Google Scholar]
- Lagarde S, Boucekine M, McGonigal A, et al. Relationship between PET metabolism and SEEG epileptogenicity in focal lesional epilepsy. Eur J Nucl Med Mol Imaging 2020;47(13):3130–3142; doi: 10.1007/s00259-020-04791-1 [DOI] [PubMed] [Google Scholar]
- Lagarde S, Roehri N, Lambert I, et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain 2018;141(10):2966–2980; doi: 10.1093/brain/awy214 [DOI] [PubMed] [Google Scholar]
- Lambert I, Roehri N, Giusiano B, et al. Brain regions and epileptogenicity influence epileptic interictal spike production and propagation during NREM sleep in comparison with wakefulness. Epilepsia 2018;59(1):235–243; doi: 10.1111/epi.13958 [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 2003;23(10):3963–3971; doi: 10.1523/JNEUROSCI.23-10-03963.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature 2008;453(7197):869–878; doi: 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Lopes MA, Richardson MP, Abela E, et al. An optimal strategy for epilepsy surgery: Disruption of the rich-club? PLoS Comput Biol 2017;13(8):e1005637; doi: 10.1371/journal.pcbi.1005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MA, Richardson MP, Abela E, et al. Elevated ictal brain network ictogenicity enables prediction of optimal seizure control. Front Neurol 2018;9:1–7; doi: 10.3389/fneur.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva F, Pijn JP, Boeijinga P.. Interdependence of EEG signals: Linear vs. nonlinear associations and the significance of time delays and phase shifts. Brain Topogr 1989;2(1–2):9–18; doi: 10.1007/BF01128839 [DOI] [PubMed] [Google Scholar]
- Makhalova J, Medina Villalon S, Wang H, et al. Virtual epileptic patient brain modeling: Relationships with seizure onset and surgical outcome. Epilepsia 2022;63(8):1942–1955; doi: 10.1111/epi.17310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska U, Badier JM, Gavaret M, et al. Interictal networks in magnetoencephalography. Hum Brain Mapp 2014;35(6):2789–2805; doi: 10.1002/hbm.22367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mierlo P, Höller Y, Focke NK, et al. Network perspectives on epilepsy using EEG/MEG source connectivity. Front Neurol 2019;10:1–13; doi: 10.3389/fneur.2019.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RJ, Soltesz I.. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci U S A 2008;105(16):6179–6184; doi: 10.1073/pnas.0801372105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Rogers BP, Anderson AW, et al. Divergent network properties that predict early surgical failure versus late recurrence in temporal lobe epilepsy. J Neurosurg 2019;132(5):1324–1333; doi: 10.3171/2019.1.jns182875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P, et al. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys D Nonlinear Phenom 2000;144(3):358–369; doi: 10.1016/S0167-2789(00)00087-7 [DOI] [Google Scholar]
- Müller M, Rummel C, Schindler K, et al. Virtual resection for predicting the outcome of epilepsy surgery. Epileptologie 2018;35:162–170. [Google Scholar]
- Müller M, Schindler K, Goodfellow M, et al. Evaluating resective surgery targets in epilepsy patients: A comparison of quantitative EEG methods. J Neurosci Methods 2018;305:54–66; doi: 10.1016/j.jneumeth.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JP, Shriver M, Alomar S, et al. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia 2016;57(3):386–401; doi: 10.1111/epi.13298 [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Kundassery KB, Gupta K, et al. Seizure-onset regions demonstrate high inward directed connectivity during resting-state: An SEEG study in focal epilepsy. Epilepsia 2020;61(11):2534–2544; doi: 10.1111/epi.16686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, Clewley R, Arno S, et al. Epilepsy in small-world networks. J Neurosci 2004;24(37):8075–8083; doi: 10.1523/JNEUROSCI.1509-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen IA, van Klink NEC, Zijlmans M, et al. Brain areas with epileptic high frequency oscillations are functionally isolated in MEG virtual electrode networks. Clin Neurophysiol 2016;127(7):2581–2591; doi: 10.1016/j.clinph.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Nissen IA, Stam CJ, Reijneveld JC, et al. Identifying the epileptogenic zone in interictal resting-state MEG source-space networks. Epilepsia 2017;58(1):137–148; doi: 10.1111/epi.13622 [DOI] [PubMed] [Google Scholar]
- Nissen IA, Stam CJ, van Straaten ECW, et al. Localization of the epileptogenic zone using interictal MEG and machine learning in a large cohort of drug-resistant epilepsy patients. Front Neurol 2018;9:1–11; doi: 10.3389/fneur.2018.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmi S, Petkoski S, Guye M, et al. Controlling seizure propagation in large-scale brain networks. PLoS Comput Biol 2019;15(2):1–23; doi: 10.1371/journal.pcbi.1006805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega GJ, Menendez De La Prida L, Sola RG, et al. Synchronization clusters of interictal activity in the lateral temporal cortex of epileptic patients: Intraoperative electrocorticographic analysis. Epilepsia 2008;49(2):269–280; doi: 10.1111/j.1528-1167.2007.01266.x [DOI] [PubMed] [Google Scholar]
- Park EH, Madsen JR. Granger causality analysis of interictal IEEG predicts seizure focus and ultimate resection. Neurosurgery 2018;82(1):99–109; doi: 10.1093/neuros/nyx195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CS, Clayden JD, Cardoso MJ, et al. Structural and effective connectivity in focal epilepsy. Neuroimage Clin 2018;17:943–952; doi: 10.1016/j.nicl.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo DL, Wills KE, Johnson GW, et al. SEEG functional connectivity measures to identify epileptogenic zones: Stability, medication influence, and recording condition. Neurology 2022; 98(20):e2060–e2072; doi: 10.1212/WNL.0000000000200386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijn JP, da Silva F.. Propagation of electrical activity: Nonlinear associations and time delays between EEG signals. In: Basic Mechanisms of the EEG. (Zschocke S, Speckmann E-J. eds.) Birkhäuser Boston: Boston, MA; 1993; pp. 41–61; doi: 10.1007/978-1-4612-0341-4_4 [DOI] [Google Scholar]
- Proix T, Bartolomei F, Chauvel P, et al. Permittivity coupling across brain regions determines seizure recruitment in partial epilepsy. J Neurosci 2014;34(45):15009–15021; doi: 10.1523/JNEUROSCI.1570-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proix T, Bartolomei F, Guye M, et al. Individual brain structure and modelling predict seizure propagation. Brain 2017;140(3):641–654; doi: 10.1093/brain/awx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proix T, Jirsa VK, Bartolomei F, et al. Predicting the spatiotemporal diversity of seizure propagation and termination in human focal epilepsy. Nat Commun 2018;9(1):1088; doi: 10.1038/s41467-018-02973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley B, Wirsich J, Bettus G, et al. Simultaneous intracranial EEG-fMRI shows inter-modality correlation in time-resolved connectivity within normal areas but not within epileptic regions. Brain Topogr 2017;30(5):639–655; doi: 10.1007/s10548-017-0551-5 [DOI] [PubMed] [Google Scholar]
- Roehri N, Bartolomei F. Are high-frequency oscillations better biomarkers of the epileptogenic zone than spikes? Curr Opin Neurol 2019;32(2):213–219; doi: 10.1097/wco.0000000000000663 [DOI] [PubMed] [Google Scholar]
- Rosenow F, Luders HO. Presurgical evaluation of epilepsy patients. Brain 2001;124(Pt 9):1683–1700; doi: 10.3390/medicina44080076 [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Cross JH, Rheims S.. Epilepsy surgery in children and adults. Lancet Neurol 2014;13(11):1114–1126; doi: 10.1016/S1474-4422(14)70156-5 [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Rheims S, Hirsch LJ, et al. Neuromodulation in epilepsy: State-of-the-art approved therapies. Lancet Neurol 2021;20(12):1038–1047; doi: 10.1016/S1474-4422(21)00300-8 [DOI] [PubMed] [Google Scholar]
- Sangare A, Marchi A, Pruvost-Robieux E, et al. The effectiveness of vagus nerve stimulation in drug-resistant epilepsy correlates with vagus nerve stimulation-induced electroencephalography desynchronization. Brain Connect 2020;10(10):566–577; doi: 10.1089/brain.2020.0798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Cappell J, Emerson R, et al. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage 2007;35(1):140–148; doi: 10.1016/j.neuroimage.2006.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Ashourvan A, Mikhail F, et al. Characterizing the role of the structural connectome in seizure dynamics. Brain 2019a;142(7):1955–1972; doi: 10.1093/brain/awz125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Bernabei JM, Kini LG, et al. High interictal connectivity within the resection zone is associated with favorable post-surgical outcomes in focal epilepsy patients. Neuroimage Clin 2019b;23:101908; doi: 10.1016/j.nicl.2019.101908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Dauwels J, Kaiser M, et al. Predicting neurosurgical outcomes in focal epilepsy patients using computational modelling. Brain 2017;140(2):319–332; doi: 10.1093/brain/aww299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia 2002;43(3):219–227; doi: 10.1046/j.1528-1157.2002.26901.x [DOI] [PubMed] [Google Scholar]
- Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci 2014;15:683–695. [DOI] [PubMed] [Google Scholar]
- Steimer A, Müller M, Schindler K, et al. Predictive modeling of EEG time series for evaluating surgery targets in epilepsy patients. Hum Brain Mapp 2017;38(5):2509–2531; doi: 10.1002/hbm.23537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N, Tong BA, Friedman ER, et al. Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol 2019;76(6):672–681; doi: 10.1001/jamaneurol.2019.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakol S, Royer J, Lowe AJ, et al. Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: from focal lesions to macroscale networks. Epilepsia 2019;60(4):1–12; doi: 10.1111/epi.14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Logroscino G, Beghi E, et al. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia 2017;58(1):17–26; doi: 10.1111/epi.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Syed I, Berger C, et al. Identification of the sensory/motor area and pathologic regions using ECoG coherence. Electroencephalogr Clin Neurophysiol 1998;106(1):30–39; doi: 10.1016/S0013-4694(97)00082-5 [DOI] [PubMed] [Google Scholar]
- Trebaul L, Deman P, Tuyisenge V, et al. Probabilistic functional tractography of the human cortex revisited. Neuroimage 2018;181:414–429; doi: 10.1016/j.neuroimage.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varotto G, Tassi L, Franceschetti S, et al. Epileptogenic networks of type II focal cortical dysplasia: A stereo-EEG study. Neuroimage 2012;61(3):591–598; doi: 10.1016/j.neuroimage.2012.03.090 [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Maria Rossini P.. Connectome: Graph theory application in functional brain network architecture. Clin Neurophysiol Pract 2017;2:206–213; doi: 10.1016/j.cnp.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven T, Coito A, Plomp G, et al. Automated diagnosis of temporal lobe epilepsy in the absence of interictal spikes. Neuroimage Clin 2018;17:10–15; doi: 10.1016/j.nicl.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos I, Krishnan B, Treiman D, et al. The concept of effective inflow: Application to interictal localization of the epileptogenic focus from IEEG. IEEE Trans Biomed Eng 2017;64(9):2241–2252; doi: 10.1109/TBME.2016.2633200 [DOI] [PubMed] [Google Scholar]
- Wang HE, Bénar CG, Quilichini PP, et al. A systematic framework for functional connectivity measures. Front Neurosci 2014;8:1–22; doi: 10.3389/fnins.2014.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tian C, Dhamala M, et al. A small change in neuronal network topology can induce explosive synchronization transition and activity propagation in the entire network. Sci Rep 2017;7(1):561; doi: 10.1038/s41598-017-00697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CP, Hu S, Stead M, et al. Synchrony in normal and focal epileptic brain: The seizure onset zone is functionally disconnected. J Neurophysiol 2010;104:3530–3539; doi: 10.1152/jn.00368.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Ansari-Asl K, Bartolomei F, et al. From EEG signals to brain connectivity: A model-based evaluation of interdependence measures. J Neurosci Methods 2009;183(1):9–18; doi: 10.1016/j.jneumeth.2009.04.021 [DOI] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Bellanger JJ, et al. Interpretation of interdependencies in epileptic signals using a macroscopic physiological model of the EEG. Clin Neurophysiol 2001;112(7):1201–1218; doi: 10.1016/S1388-2457(01)00547-8 [DOI] [PubMed] [Google Scholar]
- Wendling F, Hernandez A, Bellanger J-J, et al. Interictal to ictal transition in human temporal lobe epilepsy: insights from a computational model of intracerebral EEG. J Clin Neurophysiol 2005;22(5):343–356; doi: 0000. 4691-200510000-00006 [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Lopes da Silva F.. Dynamics of EEGs as signals of neuronal populations: Models and theoretical considerations. In: Electroencephalography, Basic Principles, Clinical Applications, and Related Fields. (Schomer DL, Lopes da Silva FH. eds.) Oxford Academic: New York, NY; 2017. [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345(5):311–318; doi: 10.1056/NEJM200108023450501 [DOI] [PubMed] [Google Scholar]
- Wilke C, Worrell G, He B.. Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia 2011;52(1):84–93; doi: 10.1111/j.1528-1167.2010.02785.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsich J, Perry A, Ridley B, et al. Whole-brain analytic measures of network communication reveal increased structure-function correlation in right temporal lobe epilepsy. Neuroimage Clin 2016;11:707–718; doi: 10.1016/j.nicl.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ge S, Zhang R, et al. Neuromagnetic coherence of epileptic activity: An MEG study. Seizure 2014;23(6):417–423; doi: 10.1016/j.seizure.2014.01.022 [DOI] [PubMed] [Google Scholar]
- Yu T, Wang X, Li Y, et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain 2018;141(9):2631–2643; doi: 10.1093/brain/awy187 [DOI] [PubMed] [Google Scholar]
- Zhao C, Liang Y, Li C, et al. Localization of epileptogenic zone based on cortico-cortical evoked potential (CCEP): A feature extraction and graph theory approach. Front Neuroinform 2019;13:1–9; doi: 10.3389/fninf.2019.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.