Abstract
Background:
Molecular testing is increasingly used to refine the probability of cancer and assess recurrence risk in thyroid nodules with Bethesda III/IV fine needle aspiration (FNA) cytology. However, limited data exist for Bethesda V (suspicious for malignancy [SFM]) samples. This study evaluated the performance of ThyroSeq v3 (TSv3) in thyroid nodules with SFM cytology.
Methods:
In this single-institution retrospective cohort study, consecutive thyroid FNA samples diagnosed as SFM with TSv3 testing and known surgical outcome were identified. Clinical, pathology, and molecular findings were reviewed. The TSv3 Cancer Risk Classifier was used to determine molecular risk groups (MRGs). For test-negative cases diagnosed as cancer/noninvasive follicular thyroid neoplasm with papillary-like nuclear features, TSv3 was performed on the resected tumors.
Results:
Among 128 SFM samples studied, 100 (78.1%) were TSv3 positive, and 28 (21.9%) were negative. The cancer prevalence on surgery was 82.8%. Among test-positive samples, 95% were malignant and 5% benign. Among test-negative samples, 17 (60.7%) were benign and 11 (39.3%) malignant. Overall, TSv3 had a sensitivity of 89.6% (confidence interval; CI 82.4–94.1) and a specificity of 77.3% (CI 56.6–89.9). For a cancer prevalence of 50–75% expected in SFM cytology by the Bethesda system, the negative predictive value was expected to range from 71.2% to 88.1% and the positive predictive value from 79.8% to 92.2%. Among test-positive nodules, 20% were MRG-Low (mostly RAS-like alterations), 66% MRG-Intermediate (mostly BRAF-like alterations), and 14% MRG-High. Among patients with cancer, 65 (61.3%) were American Thyroid Association low risk, 25 (23.6%) intermediate risk, and 6 (5.7%) high risk. During the mean follow-up of 51.2 months (range: <1 to 470 months), 12 (13.0%) patients had disease recurrence, which was more common in MRG-High (54.6%) compared with MRG-Intermediate (9.5%) and MRG-Low (0%) cancers (p < 0.001). Upon reexamining tumors with false-negative results, half of evaluable cases had alterations likely missed due to limiting FNA sampling, and the remainder represented low-risk tumors. Potentially targetable alterations were identified in 10 samples.
Conclusions:
In this large series of SFM thyroid nodules, TSv3 further improved cancer prediction and detected RAS-like, BRAF-like, high-risk, and potentially targetable alterations, all of which may inform more optimal patient management. MRGs were associated with recurrence-free survival, offering potential preoperative cancer risk stratification.
Keywords: Bethesda V cytology, cancer recurrence, fine needle aspiration samples, molecular testing, thyroid cancer
Introduction
Molecular testing (MT) is increasingly used to refine the probability of cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and assess recurrence risk in thyroid nodules with indeterminate fine needle aspiration (FNA) cytology. These indeterminate categories include atypia of undetermined significance/follicular lesion of undetermined significance (Bethesda III), follicular neoplasm/suspicious for follicular neoplasm (Bethesda IV), and suspicious for malignancy (SFM, Bethesda V) based on The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC).1 Anywhere from 10% to 40% of thyroid FNA samples may have an indeterminate cytology diagnosis.1 Among those, SFM cytology nodules carry the highest probability of malignancy, estimated to be 50–75%, yet a significant proportion of these nodules are benign.1
Several molecular tests are currently available commercially in the United States for clinical testing of thyroid FNA samples, including Afirma Genomic Sequencing Classifier (GSC) (Veracyte, Inc.), ThyraMIR/ThyGeNEXT (Interpace Diagnostics), and ThyroSeq v3 (TSv3) (UPMC/Sonic Healthcare). However, the validation studies for these tests largely focused on Bethesda III/IV samples, with only a limited number of SFM samples included, precluding evaluation of performance characteristics for this sample type.2–4
Several studies have analyzed more SFM samples but did not contain surgical outcome data, precluding evaluation of test performance or utility.5,6 The Afirma Xpression Atlas (XA) validation included 105 SFM samples, but only analytic performance was evaluated.5 Another study reported 86 SFM or malignant cytology samples with MT (ThyGeNEXT or TSv3), but the only outcome evaluated was if optimal surgery was performed.6 As a result, it remains unknown whether currently available molecular tests can improve the management of patients with SFM cytology nodules.
In this study, we evaluated a large consecutive series of thyroid nodules with SFM cytology, TSv3 testing, and known surgical outcome to assess the ability of preoperative MT to refine the probability of cancer conferred by SFM cytology and predict tumor phenotype and risk of recurrence.
Materials and Methods
Study cohort
Under approval of the University of Pittsburgh Institutional Review Board (STUDY20120006), this retrospective cohort study identified consecutive thyroid FNA samples diagnosed as SFM with known surgical outcome and previous TSv3 testing or material available to perform testing from the UPMC laboratory information system between January 1, 2014, and May 1, 2021 (Supplementary Fig. S1). Twenty-three samples were excluded because no surgery was performed (9 test-positive, 7 test-negative, 7 without MT). Clinical data, radiology, cytology, and surgical pathology reports, and molecular data were reviewed for all cases. Surgical pathologists were not blinded to preoperative MT results. All nodules were matched with cytology and molecular results based on size and location using ultrasound and surgical pathology information. The Thyroid Imaging, Reporting and Data System (TI-RADS) score was obtained from the ultrasound report or calculated using ultrasound data, when available.7 American Thyroid Association (ATA) risk groups were determined using the 2015 criteria.8
Thyroid cancer recurrence, either structural (locoregional and/or metastatic) or biochemical, was assessed from the date of initial surgery until the most recently available follow-up.8 Biochemical recurrence was defined as detectable serum thyroglobulin in the absence of structural disease after an initial excellent response to therapy (surgery and/or radioactive iodine).8 Patients with metastasis at presentation were excluded.
Molecular analysis
For samples without previous TSv3 testing, residual material was obtained, either FNA material in a molecular preservative solution or extracted nucleic acid stored at −20°C. TSv3 was performed in the UPMC Molecular & Genomic Pathology Laboratory as previously described.9 TSv3 is a DNA- and RNA-based next-generation sequencing test that evaluates targeted regions of 112 genes for single-nucleotide variants, small insertions and deletions, copy number alterations (CNA), gene fusions, and gene expression alterations (GEA). Analysis of molecular findings using the TSv3 Genomic Classifier (GC) diagnostic algorithm reports test results as negative (low probability of cancer/NIFTP) or positive (high probability of cancer/NIFTP).4
In addition, test-positive samples were reanalyzed using the TSv3 Cancer Risk Classifier (CRC) to establish risk of cancer recurrence. Based on the CRC algorithm, samples were categorized into three molecular risk groups (MRGs): MRG-Low (RAS-like alterations and focal chromosomal-type CNA), MRG-Intermediate (BRAFV600E-like alterations and genome haploidization-type CNA), and MRG-High (TERT, TP53 mutations, typically co-occurring with other alterations), as previously described.10
Analysis of false-negative molecular test results
To investigate possible reasons for false-negative test results, histologic slides for cases with negative TSv3 on FNA cytology that were diagnosed as cancer or NIFTP on resection were reviewed by two pathologists (J.M.S. and Y.E.N.). In addition, representative slides from each tumor were blindly reviewed by another pathologist (R.R.S.) to confirm the diagnosis. TSv3 testing was then performed on unstained formalin-fixed, paraffin-embedded slides from resected tumors as previously described.9
Statistical analysis
Test sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) with Wilson confidence intervals (CIs) were calculated using surgical pathology diagnosis as the reference standard. Baseline characteristics between groups were compared using Pearson's chi-squared test. Statistical analysis was performed using R software package (version 4.0.4; R Foundation) and SPSS Statistics software (version 25.0; IBM). Bayes' theorem was used to estimate NPV and PPV for the observed sensitivity and specificity across cancer prevalences from 0% to 100%.
For the test-positive cohort evaluated for recurrence-free survival (RFS), continuous variables were compared using analysis of variance or Kruskal–Wallis tests when comparing means and medians, respectively. Categorical variables were compared with the chi-squared test for association or Fisher's exact test, where appropriate. RFS was analyzed with the Kaplan–Meier method and MRGs compared using the log-rank test. There were no recurrences in the MRG-Low, precluding any multivariable analyses. Analysis was performed using SAS OnDemand for Academics: Studio (SAS Institute). Tests were two-sided and statistical significance was set at the 0.05 level.
Results
Study cohort
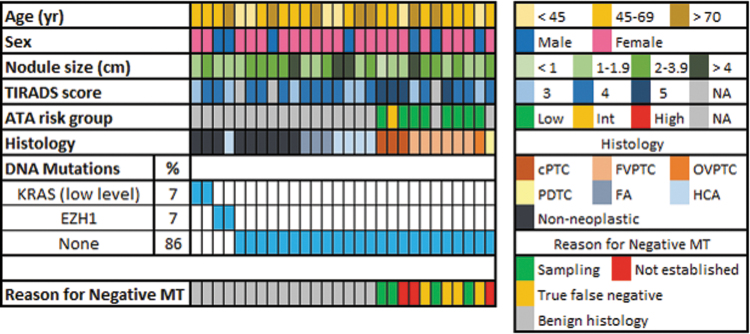
A total of 128 FNA samples with SFM (Bethesda V) cytology and known surgical outcome were identified. Among these, 89 (69.5%) had preoperative MT and 39 (30.5%) had MT performed postoperatively. Overall, 62.5% were female and median age was 56.6 years (range: 18–83 years) and median nodule size was 2.0 cm (range: 0.6–11.1 cm). On ultrasound, 27 (21.1%) nodules had a TI-RADS score of 3, 57 (44.5%) a score of 4, 36 (28.1%) a score of 5, and for 8 (6.3%), the score could not be calculated due to insufficient ultrasound data. Overall, 100 (78.1%) samples demonstrated one or more molecular alterations associated with thyroid cancer and were test-positive (Fig. 1). Twenty-eight (21.9%) samples had no cancer-related alterations or had low-level alterations and were test-negative (Fig. 2).
FIG. 1.
Oncoplot showing genetic alterations and clinicopathologic features of TSv3-positive samples with SFM cytology. *Incidental papillary microcarcinoma adjacent to sampled hyperplastic nodule. ATA, American Thyroid Association; ATC, anaplastic thyroid carcinoma; CNA, copy number alterations; PTC, papillary thyroid carcinoma; cPTC, classic type PTC; FTC, follicular thyroid carcinoma; FVPTC, follicular variant PTC; GEA, gene expression alterations; HCA, Hürthle cell adenoma; HCC, Hürthle cell carcinoma; OVPTC, oncocytic variant PTC; PDTC, poorly differentiated thyroid carcinoma; RCC, renal cell carcinoma; TSv3, ThyroSeq v3; TVPTC, tall cell variant PTC; WVPTC, Warthin-like variant PTC.
FIG. 2.
Oncoplot showing findings and clinicopathologic features of TSv3-negative sample with SFM cytology. FA, follicular adenoma; MT, molecular testing.
No differences were found between test-positive and test-negative samples with respect to patient age and sex, nodule size, and TI-RADS score (Supplementary Table S1). On resection, 106 (82.8%) nodules were cancer and 22 (17.2%) were benign. None were diagnosed as NIFTP. Overall, 87 (68.0%) patients were treated with initial total thyroidectomy and 41 (32.0%) with lobectomy. Of those with cancer, 65 (61.3%) were ATA low risk, 25 (23.6%) intermediate risk, and 6 (5.7%) high risk, while 2 (1.9%) could not be determined due to insufficient data, and for 8 (7.5%), ATA risk stratification was not applicable.8 There was no difference in cancer prevalence between samples with preoperative and postoperative MT (79.8% vs. 89.7%, respectively; p = 0.169).
Test performance
Among test-positive samples, 95 (95%) were cancer and 5 (5%) were benign on resection. Among test-negative samples, 17 (60.7%) were benign and 11 (39.3%) were cancer. Overall, in this cohort with a cancer rate of 82.8%, TSv3 had a sensitivity of 89.6% (CI 82.4–94.1), a specificity of 77.3% (CI 56.6–89.9), an NPV of 60.7% (CI 42.4–76.4), and a PPV of 95.0% (CI 88.8–97.8) (Table 1). While sensitivity and specificity are intrinsic characteristics of each test, the NPV and PPV depend on the disease prevalence in the tested population. In this cohort, the pre-test cancer/NIFTP rate was higher than the 50–75% range expected for SFM cytology by TBSRTC.1 In this expected prevalence range with the above test sensitivity and specificity, the NPV of TSv3 in SFM nodules was calculated to range from 71.2% to 88.1% and PPV from 79.8% to 92.2% (Fig. 3).
Table 1.
Performance Characteristics of ThyroSeq v3 in Thyroid Nodules with Suspicious for Malignancy Cytology in This Study
| Surgical diagnosis |
Total | ||
|---|---|---|---|
| Cancer/NIFTP | Benign | ||
| Test positive | 95 | 5 | 100 |
| Test negative | 11 | 17 | 28 |
| Total | 106 | 22 | 128 |
| Sensitivity (CI) | 89.6 (82.4–94.1) | ||
| Specificity (CI) | 77.3 (56.6–89.9) | ||
| NPV (CI) | 60.7 (42.4–76.4) | ||
| PPV (CI) | 95.0 (88.8–97.8) | ||
CI, confidence interval; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; NPV, negative predictive value; PPV, positive predictive value.
FIG. 3.
Graphical representation of NPV and PPV over a spectrum of cancer prevalences as calculated using Bayes' theorem. NPV and PPV in the expected range of cancer/NIFTP prevalence based on the Bethesda system (50–75%) are shown. NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; NPV, negative predictive value; PPV, positive predictive value.
Spectrum of molecular alterations and histologic outcomes
Among test-positive samples, 57 (57%) had BRAF mutation, including 55 V600E and 2 in-frame insertion/deletion mutations. Of these, 48 (84.2%) were diagnosed as classic papillary thyroid carcinoma (PTC), 2 (3.5%) as tall cell variant PTC, 2 (3.5%) as Warthin-like variant PTC, 4 (7.0%) as follicular variant PTC (FVPTC), and 1 (1.8%) as anaplastic thyroid carcinoma (ATC). The ATC nodule revealed co-occurring BRAFV600E and TERT mutations.
Twenty (20%) samples had RAS mutation (14 NRAS, 3 HRAS, 3 KRAS). Of these, 11 (55%) were diagnosed as FVPTC, 1 (5%) as follicular thyroid carcinoma, 2 (10%) as oncocytic variant PTC, 1 (5%) as Hürthle cell carcinoma, 2 (10%) as Hürthle cell adenoma (HCA), 2 (10%) as poorly differentiated thyroid carcinoma (PDTC), and 1 (5%) as ATC. The ATC nodule had concurrent KRAS and NRAS mutations along with EIF1AX, TERT, and TP53 mutations.
Eleven (11%) samples had a gene fusion (3 RET, 5 NTRK3, 1 ALK, 2 PPARG). Of these, 6 (54.5%) were diagnosed as classic PTC (3 RET, 2 NTRK3, 1 ALK), 4 (36.4%) as FVPTC (2 NTRK3, 2 PPARG), and 1 (9.1%) as PDTC (NTRK3).
Two (2%) samples had VHL mutations and no expression of thyroid cell markers, with test results suspicious for metastatic renal cell carcinoma (RCC), which was confirmed on resection.
Overall, 18% of test-positive samples had RAS-like alterations, 60% had BRAFV600E-like alterations, 8% had CNA of genome haploidization-type (n = 6) or focal chromosomal-type (n = 2), and 14% had high-risk alterations (TERT, TP53 mutations).
Potentially targetable alterations were identified in 10 samples. These included NTRK3, RET, and ALK fusions, which are targetable by FDA-approved inhibitors for advanced thyroid cancer or other cancer types, and BRAFV600E mutation in ATC.
MRGs and outcomes
Among test-positive samples, TSv3 CRC categorized 20% as MRG-Low, 66% as MRG-Intermediate, and 14% as MRG-High (Table 2). Of 28 patients with initial thyroid lobectomy, 6 (21.4%) had MRG-High results. MRG-Low nodules included mostly RAS mutations, and the most common histologic diagnosis was FVPTC found in 13 (65%) cases. MRG-Intermediate nodules included mostly BRAF mutations, with the most common diagnosis being classic PTC in 51 (77.3%) cases. Among MRG-High nodules, TERT promoter and TP53 mutations were identified in 11 and 2 samples, respectively, all in combination with another driver alteration. The MRG-High included nodules diagnosed as tall cell variant PTC, PDTC, or ATC. In addition, two samples with VHL mutations, consistent with metastatic RCC, were also considered MRG-High.
Table 2.
Molecular Risk Groups Based on Fine Needle Aspiration ThyroSeq v3 Testing and Histopathologic Outcomes
| MRG | No. of cases, n (%) | Histopathologic outcomes |
|---|---|---|
| Low | 20 (20) | FVPTC (65%), OVPTC (15%), FTC (5%), HCC (5%), HCA (10%) |
| Intermediate | 66 (66) | cPTC (77.3%), WVPTC (3%), FVPTC (10.6%), OVPTC (1.5%), HCC (3%), HCA (3%), HN with adjacent microcarcinoma (1.5%) |
| High | 14 (14) | PDTC (21.4%), ATC (14.3%), cPTC (14.3%), TVPTC (14.3%), FVPTC (14.3%), metastatic RCC (14.3%), HCC (7.1%) |
ATC, anaplastic thyroid carcinoma; PTC, papillary thyroid carcinoma; cPTC, classic PTC; FTC, follicular thyroid carcinoma; FVPTC, follicular variant PTC; HCA, Hürthle cell adenoma; HCC, Hürthle cell carcinoma; HN, hyperplastic nodule; MRG, molecular risk group; OVPTC, oncocytic variant PTC; RCC, renal cell carcinoma; PDTC, poorly differentiated thyroid carcinoma; TVPTC, tall cell variant PTC; WVPTC, Warthin-like variant PTC.
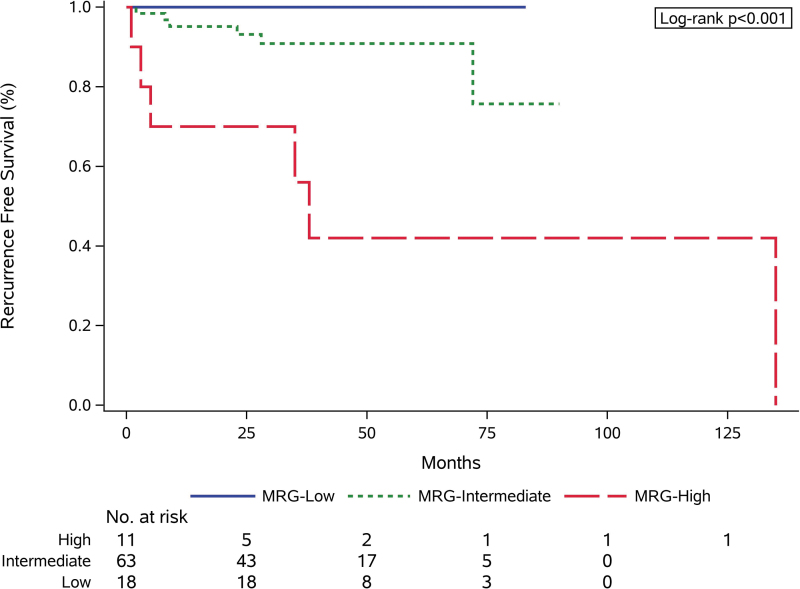
Of the 100 patients with test-positive nodules, 92 were evaluated for tumor recurrence. Five were excluded due to benign diagnosis on resection (2 MRG-Low, 3 MRG-Intermediate), and 3 due to metastatic disease at presentation (1 ATC, 2 RCC; all MRG-High). During the follow-up period (mean 51.2 months, range <1 to 470 months), 12 (13.0%) patients had disease recurrence (11 structural, 1 biochemical). Among these, 6 were MRG-High (54.6% of all MRG-High nodules) and 6 MRG-Intermediate (9.5% of all MRG-Intermediate nodules), with no recurrences found in MRG-Low nodules (Table 3). Recurrence was significantly more common in MRG-High cancers compared with MRG-Intermediate (54.6% vs. 9.5%, p < 0.001) and MRG-Low (54.6% vs. 0%, p < 0.001) cancers. Estimated 36-month RFS for MRG-High, MRG-Intermediate, and MRG-Low cancers was 56.0%, 90.9%, and 100%, respectively (Fig. 4).
Table 3.
Patient Characteristics and Outcomes of the Recurrence-Free Survival Cohort and by Molecular Risk Group
| Total (n = 92) | MRG |
p | |||
|---|---|---|---|---|---|
| Low (n = 18) | Intermediate (n = 63) | High (n = 11) | |||
| Mean age (SD), year | 52.3 (17.7) | 52.5 (19.1) | 49.3 (16.9) | 69.0 (9.3) | 0.002 |
| Female | 56 (60.9%) | 11 (61.1%) | 38 (60.3%) | 7 (63.6%) | 0.98 |
| Median nodule size (range), cm | 2.0 (0.6–11.1) | 2.5 (1.4–6.1) | 1.7 (0.6–6.7) | 4.1 (1.2–11.1) | <0.001 |
| TI-RADS score | 0.002 | ||||
| 3 | 16 (17.4%) | 7 (38.9%) | 7 (11.1%) | 2 (18.2%) | |
| 4 | 40 (43.5%) | 9 (50.0%) | 31 (49.2%) | 0 (0.0%) | |
| 5 | 32 (34.8%) | 2 (11.1%) | 22 (34.9%) | 8 (72.7%) | |
| NA | 4 (4.4%) | 0 (0.0%) | 3 (4.8%) | 1 (9.1%) | |
| Total thyroidectomy | 67 (72.8%) | 10 (55.6%) | 50 (79.4%) | 7 (62.6%) | 0.10 |
| Central node dissection | |||||
| Prophylactic | 19 (20.7%) | 4 (22.2%) | 13 (20.6%) | 2 (18.2%) | 1.00 |
| Therapeutic | 12 (13.0%) | 1 (5.6%) | 8 (12.7%) | 3 (27.3%) | 0.27 |
| Central nodal disease | 18 (19.5%) | 1 (5.6%) | 14 (22.2%) | 3 (27.3%) | 0.20 |
| Lateral nodal disease | 4 (4.4%) | 0 (0.0%) | 3 (4.8%) | 1 (9.1%) | 0.53 |
| Extrathyroidal extension | |||||
| Microscopic | 19 (20.7%) | 1 (5.6%) | 13 (20.6%) | 5 (45.5%) | 0.04 |
| Gross | 2 (2.2%) | 0 (0.0%) | 0 (0.0%) | 2 (18.2%) | 0.01 |
| ATA risk category | < 0.001 | ||||
| Low | 57 (62.0%) | 16 (88.9%) | 40 (63.5%) | 1 (9.1%) | |
| Intermediate | 24 (26.1%) | 2 (11.1%) | 19 (30.2%) | 3 (27.3%) | |
| High | 6 (6.5%) | 0 (0.0%) | 3 (4.8%) | 3 (27.3%) | |
| NA | 5 (5.4%) | 0 (0.0%) | 1 (1.6%) | 4 (36.4%) | |
| RAI | 31 (33.7%) | 4 (22.2%) | 19 (30.2%) | 8 (72.7%) | 0.01 |
| Recurrence | 12 (13.0%) | 0 (0.0%) | 6 (9.5%) | 6 (54.6%) | < 0.001 |
| Death | |||||
| All-cause | 4 (4.4%) | 1 (5.6%) | 1 (1.6%) | 2 (18.2%) | 0.05 |
| Disease specific | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 0.12 |
| Mean follow-up (range), months | 51.2 (<1–470) | 51.3 (30–83) | 46.3 (4–315) | 78.6 (<1–470) | 0.29 |
ATA, American Thyroid Association; NA, not applicable; RAI, radioactive iodine; SD, standard deviation; TI-RADS, Thyroid Imaging, Reporting and Data System.
FIG. 4.
RFS by MRG. MRG, molecular risk group; RFS, recurrence-free survival.
False-negative and false-positive molecular test results
Of the 11 false-negative samples, all of which met the adequacy criteria for MT, histologic slides were available and reviewed for 9 of the resected tumors, and all were confirmed to be cancer/NIFTP (Table 4). MT was successful in 8 of these tumors, of which 4 (50%) were test-positive and 4 (50%) were test-negative, with no association with nodule size (Table 4).
Table 4.
Test-Negative Samples with Cancer/Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features on Resection and Their Molecular Test Results on Cytology and Surgical Samples
| Case no. | Age (year) | Sex (M/F) | Nodule size (cm) | Initial pathology | Reviewed pathology | TSv3 result on FNA samples | TSv3 result on surgical tissue retesting | Reason for negative MT |
|---|---|---|---|---|---|---|---|---|
| 1 | 52 | F | 2.0 | EFVPTC with minimal capsular invasion (2.1 cm) | EFVPTC with minimal capsular invasion | Negative | Negative | False negative |
| 2 | 60 | F | 0.8 | cPTC (0.8 cm); adjacent HCA (2.0 cm) | cPTC with oncocytic features | Negative | BRAFV600E (VAF 23%), TP53 (VAF 3%) mutations | Tumor sampling |
| 3 | 74 | M | 2.2 | EFVPTC with minimal capsular invasion (2.2 cm) | FVPTC, well demarcated with focal infiltration | Negative | TERT promoter mutation (VAF 5%)/GEA | Tumor sampling |
| 4 | 27 | M | 1.5 | PTC arising in hyperplastic nodule (1.3 cm) | OVPTC arising in FA | Negative | Negative | False negative |
| 5 | 64 | F | 1.5 | cPTC with oncocytic features (2.4 cm); adjacent nodular GT | cPTC with oncocytic features | Negative | BRAFV600E mutation (VAF 28%)/GEA | Tumor sampling |
| 6 | 52 | F | 1.5 | EFVPTC with minimal capsular invasion (2.0 cm) | EFVPTC with minimal capsular invasion, possibly arising in FA | Negative | Negative | False negative |
| 7 | 60 | F | 2.0 | EFVPTC with minimal capsular invasion (1.6 cm) | EFVPTC with oncocytic features and minimal capsular invasion | Negative | Negative | False negative |
| 8 | 55 | F | 1.9 | FVPTC (1.5 cm) | TVPTC | Negative | BRAFV600E mutation (VAF 20%)/GEA | Tumor sampling |
| 9 | 76 | M | 4.9 | FVPTC (4.2 cm) | NIFTP | Negative | Noninformative/failed | NA |
| 10 | 55 | F | 2.0 | PDTC arising in PTC (1.5 cm) | Not available for review | Negative | Not available for retesting | NA |
| 11 | 20 | F | 1.9 | cPTC (1.7 cm) | Not available for review | Negative | Not available for retesting | NA |
EFVPTC, encapsulated follicular variant PTC; FA, follicular adenoma; FNA, fine needle aspiration; GEA, gene expression alterations; MT, molecular testing; TSv3, ThyroSeq v3; VAF, variant allele frequency.
The four confirmed test-negative tumors included three encapsulated FVPTC, all with minimal capsular invasion and no vascular invasion, and one well-demarcated noninvasive oncocytic PTC, possibly arising in a hyperplastic nodule or follicular adenoma with hyperplastic features. All tumors were low stage by AJCC and low risk by ATA criteria.8,11
The other four tumors with negative MT on FNA showed one or more molecular alterations upon analysis of resected tumor tissue. These included a 0.8 cm classic PTC with BRAFV600E and TP53 mutations with an adjacent 2.0 cm HCA, a 1.5 cm classic PTC with BRAFV600E mutation and GEA with adjacent nodular granulomatous thyroiditis, a tall cell variant PTC with BRAFV600E mutation and GEA, and an FVPTC with TERT mutation and GEA (Table 4). The presence of clearly identifiable genetic alterations in the tumor but not in the tested FNA samples strongly suggests that limited sampling of cancer collected for MT was the reason for the false-negative result. All tumors were low stage by AJCC with 2 low risk and 1 intermediate risk by ATA criteria (1 unable to be determined).8,11
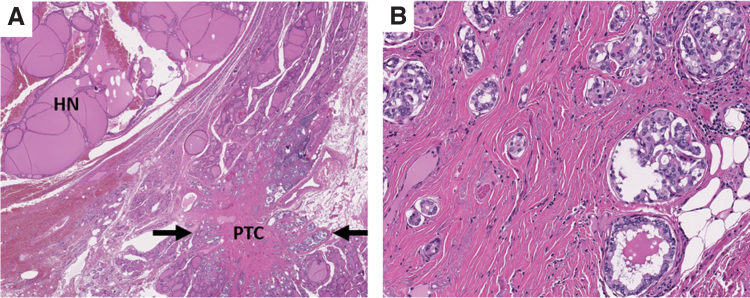
Among the five test-positive samples with benign outcomes, four were diagnosed as HCA, ranging in size from 1.1 to 6.8 cm, of which two had RAS mutation and two had genome haploidization-type CNA. In the remaining sample, a 3.5 cm complex nodule was targeted by FNA and demonstrated BRAFV600E and GNAS mutations along with GEA. On resection, this site demonstrated a 2.5 cm nodule with histologic findings of a dominant hyperplastic nodule and adjacent papillary microcarcinoma measuring 3 mm (Fig. 5). Based on these findings, the nodule of interest was considered to be benign, although it remains unclear if microcarcinoma cells were also sampled during FNA.
FIG. 5.
Representative histologic images of false-positive sample with BRAFV600E and GNAS mutations demonstrating incidental papillary thyroid microcarcinoma adjacent to a dominant hyperplastic nodule; (A) hematoxylin and eosin, 40 × magnification; (B) hematoxylin and eosin, 200 × magnification.
Discussion
In this study, we report the largest series to date of thyroid nodules with SFM (Bethesda V) cytology analyzed by TSv3 and demonstrate the ability of MT to refine the probability of cancer conferred by SFM cytology and provide preoperative cancer risk stratification.
The results of this study showed that the sensitivity and specificity of the test in SFM nodules were overall comparable with those previously observed in Bethesda III/IV samples—90% versus 94% and 77% versus 82%, respectively.4 The PPV of the test in this study was very high (95%), as expected in a cohort of patients with a high pre-test probability of cancer/NIFTP.12 In the range of cancer/NIFTP prevalence expected based on the Bethesda system for SFM cytology (50–75%), the PPV was calculated to range between 80% and 92%.1 Therefore, a positive test result is expected to further increase the probability of cancer/NIFTP in nodules with SFM cytology.
A small number of false-positive results were identified, with the majority diagnosed as HCA on resection. While most HCAs would be expected to yield Bethesda IV cytology, these cases likely had more significant nuclear atypia, prompting the SFM diagnosis. The molecular alterations commonly identified in these tumors, including RAS mutations or CNA, are characteristic of follicular-patterned neoplasms ranging from benign adenomas to NIFTP and invasive cancer. The probability of invasive cancer in nodules with genome haploidization-type CNA has a strong direct correlation with nodule size, suggesting that some of these benign tumors may be precursors for cancer.13
In test-negative nodules, the residual cancer probability was calculated to range between 12% and 29% with a pre-test cancer prevalence of 50–75% expected for SFM cytology. A subgroup of these cases were available for additional analysis, and retesting of resected cancers showed that about half of the false-negative results were caused by nonrepresentative sampling in the FNA material submitted for MT. This suggests that repeat MT on cytology smears with diagnostic SFM material or a new FNA sample could potentially mitigate this issue in some cases, although the residual cancer probability would likely remain high enough to require surgical management. However, it is reassuring that the majority of missed cancers were low risk, which could support more limited surgery (i.e., diagnostic lobectomy) in test-negative nodules.
In addition, MT can provide valuable information for preoperative cancer risk stratification, which may help to guide the extent of surgery. Current management guidelines recommend lobectomy for low-risk intrathyroidal cancers 1–4 cm in size, although preoperative risk assessment of such cancers is challenging.8 In this study, we show that even on a relatively short follow-up, MRGs correlated with disease recurrence in nodules with SFM cytology. Finding TERT and/or TP53 mutations, associated with MRG-High disease, was associated with 56% estimated 36-month RFS, which may warrant consideration for more extensive surgery. Conversely, identification of MRG-Low disease would predict low-risk follicular-patterned tumors, which could support more limited surgery. For nodules with BRAF-like alterations, which are MRG-Intermediate, the scope of surgical intervention may be further informed by nodule size and imaging studies.
Overall, this study provides further evidence for MRGs as a predictor of disease recurrence, initially demonstrated using resected cancer samples, in preoperative FNA samples with SFM cytology.10
Additional clinical use for MT in SFM samples may lie in the identification of potential therapeutic targets, which could inform therapy in patients with advanced disease. Several inhibitors are FDA approved for thyroid cancer harboring specific molecular alterations (NTRK and RET fusions, BRAFV600E mutation in ATC) or for other cancer types (ALK fusions), which can be detected preoperatively and were found in 10 samples in this cohort.14–23 Two patients with NTRK3 fusions developed recurrent disease, both with small-volume lung metastases, although based on the 2015 ATA guidelines they did not meet the criteria for TKI therapy at the time of last follow-up but could be eligible in the future.
This study has several limitations. First, it was a retrospective study, although 70% of samples had preoperative MT. Second, the length of follow-up was relatively short, and therefore, long-term associations between MRGs and recurrence characteristics remain to be established. Finally, the probability of cancer/NIFTP in this study cohort was higher than expected for SFM cytology based on the Bethesda system, and while the test performance in populations with the expected prevalence range was predicted using Bayes' theorem, the findings should be confirmed in other studies.
In summary, the results of this study showed that MT is expected to improve the diagnostic performance of cytology even in SFM samples with high pre-test cancer probability. Moreover, TSv3 can predict the histologic outcome and risk of recurrence in these nodules, offering preoperative data that may inform optimal patient management.
Supplementary Material
Acknowledgment
We thank Jessica Tebbets for administrative support and help in preparation of the article.
Authors' Contributions
J.M.S.: conceptualization, investigation, methodology, visualization, and writing (original draft, review and editing). C.T.: investigation and writing (review and editing). J.B.L.: investigation, formal analysis, and writing (review and editing). A.I.W.: investigation, resources, and writing (review and editing). A.V.N.: formal analysis, visualization, and writing (review and editing). S.I.C: writing (review and editing). R.R.S.: investigation and writing (review and editing). N.P.O.: writing (review and editing). E.K.-F.: writing (review and editing). SE.C.: writing (review and editing). M.N.N.: conceptualization, resources, and writing (review and editing). L.Y.: writing (review and editing). Y.E.N.: conceptualization, methodology, supervision, and writing (original draft, review and editing).
Author Disclosure Statement
J.M.S., C.T., J.B.L., A.I.W., A.V.N., S.I.C., R.R.S., N.P.O., E.K.-F., and L.Y. have no financial interests to disclose. S.E.C.: Supported by a generous gift from the William and Susan Johnson Fund for Endocrine Surgery Research. M.N.N., Y.E.N.: Owns intellectual property and receives royalties related to ThyroSeq from the University of Pittsburgh; also serves as a consultant for Sonic Healthcare USA.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Ali SZ, Cibas ES, eds.: The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes. 2nd ed. Springer International Publishing AG, Switzerland; 2018. [Google Scholar]
- 2. Patel KN, Angell TE, Babiarz J, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg 2018;153(9):817–824; doi: 10.1001/jamasurg.2018.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lupo MA, Walts AE, Sistrunk JW, et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagn Cytopathol 2020;48(12):1254–1264; doi: 10.1002/dc.24564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steward DL, Carty SE, Sippel RS, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: A prospective blinded multicenter study. JAMA Oncol 2019;5(2):204–212; doi: 10.1001/jamaoncol.2018.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angell TE, Wirth LJ, Cabanillas ME, et al. Analytical and clinical validation of expressed variants and fusions from the whole transcriptome of thyroid FNA samples. Front Endocrinol (Lausanne) 2019;10:612; doi: 10.3389/fendo.2019.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hier J, Avior G, Pusztaszeri M, et al. Molecular testing for cytologically suspicious and malignant (Bethesda V and VI) thyroid nodules to optimize the extent of surgical intervention: A retrospective chart review. J Otolaryngol Head Neck Surg 2021;50(1):29; doi: 10.1186/s40463-021-00500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14(5):587–595; doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 8. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26(1):1–133; doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018;124(8):1682–1690; doi: 10.1002/cncr.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yip L, Gooding WE, Nikitski A, et al. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: A matched case-control study. Cancer 2021;127(11):1779–1787; doi: 10.1002/cncr.33421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amin MB, Edge SB, Greene FL, et al. , (eds). AJCC Cancer Staging Manual. 8th ed. Springer: New York; 2017. [Google Scholar]
- 12. McIver B, Castro MR, Morris JC, et al. An independent study of a gene expression classifier (Afirma) in the evaluation of cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab 2014;99(11):4069–4077; doi: 10.1210/jc.2013-3584. [DOI] [PubMed] [Google Scholar]
- 13. Doerfler WR, Nikitski AV, Morariu EM, et al. Molecular alterations in Hurthle cell nodules and preoperative cancer risk. Endocr Relat Cancer 2021;28(5):301–309; doi: 10.1530/ERC-20-0435. [DOI] [PubMed] [Google Scholar]
- 14. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018;378(8):731–739; doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol 2020;21(2):271–282; doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov 2018;8(7):836–849; doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 17. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020;383(9):825–835; doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 2018;36(1):7–13; doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368(25):2385–2394; doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 20. Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017;389(10072):917–929; doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 21. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017;377(9):829–838; doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 22. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: Second interim analysis of the Phase III ALTA-1L Trial. J Clin Oncol 2020;38(31):3592–3603; doi: 10.1200/JCO.20.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 2020;383(21):2018–2029; doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.