Abstract
The genetic structure and evolution of a novel exchangeable meningococcal genomic island was defined for the important human pathogen Neisseria meningitidis. In 125 meningococcal strains tested, one of three unrelated nucleotide sequences, designated exl (exchangeable locus), was found between a gene required for heme utilization, hemO, and col, encoding a putative Escherichia coli collagenase homologue. The 5′ boundary of each exl cassette was the stop codon of hemO, whereas the 3′ boundary was delineated by a 33-bp repeat containing neisserial uptake sequences located downstream of col. One of the three alternative exl cassettes contained the meningococcal hemoglobin receptor gene, hmbR (exl3). In other meningococcal strains, hmbR was absent from the genome and was replaced by either a nucleotide sequence containing a novel open reading frame, exl2, or a cassette containing exl3. The proteins encoded by exl2 and exl3 had no significant amino acid homology to HmbR but contained six motifs that are also present in the lipoprotein components of the lactoferrin (LbpB), transferrin (TbpB), and hemoglobin-haptoglobin (HpuA) uptake systems. To determine the evolutionary relationships among meningococci carrying hmbR, exl2, or exl3, isolates representing 92 electrophoretic types were examined. hmbR was found throughout the population structure of N. meningitidis (genetic distance, >0.425), whereas exl2 and exl3 were found in clonal groups at genetic distances of <0.2. The commensal neisserial species were identified as reservoirs for all of the exl cassettes found in meningococci. The structure of these cassettes and their correlation with clonal groups emphasize the extensive gene pool and frequent horizontal DNA transfer events that contribute to the evolution and virulence of N. meningitidis.
The distinct syndrome of epidemic meningitis and meningococcemia caused by Neisseria meningitidis was first reported in 1805 (6). An interesting aspect of this naturally transformable human pathogen is the rapid evolution of the meningococcal genome through high-frequency recombination events and uptake of DNA (10). Because of this high rate of microevolution, the emergence of new clonal groups responsible for outbreaks of meningococcal disease can be readily detected through genetic typing (2). These approaches have identified the emergence of new clonal groups since the 1960s, the most recent being lineage III, originally found in The Netherlands in 1980 but now detected worldwide (2).
Conceptually, transformation and recombination of foreign DNA into the neisserial genome could also facilitate the incorporation of a new gene(s) into the meningococcal chromosome. DNA fragments containing uptake sequences are preferentially incorporated into the genomes of naturally transformable species such as Haemophilus influenzae (5), N. meningitidis, and N. gonorrhoeae (16). Uptake sequence-mediated DNA uptake has been proposed to be the mechanism by which sodC from Haemophilus spp. has been incorporated into the meningococcal genome (13). Transformation and recombination are also proposed to be the mechanisms through which the DNA island containing the capsule biosynthesis genes was originally inserted between the conserved genes tex and galE in the genome of N. meningitidis (22; D. S. Stephens et al., unpublished data). Further evidence suggests that this process facilitates the exchange of different capsule biosynthesis loci, resulting in capsule switching (35). Apart from the capsule loci, other meningococcus-specific genetic cassettes found so far include those encoding glutathione peroxidase (19), rotamase (30), and the RTX (repeats in toxin) toxin-like Frp proteins (37). Subtractive hybridization of meningococcal and gonococcal genomes has identified at least eight genetic islands in meningococci (11). However, the extent of the gene pool, the rate of dissemination, the mechanisms of acquisition of new genes, and the association of new genes with virulence in N. meningitidis remain poorly understood.
A comparison of the meningococcal genomes of serogroup A strain Z2491 and serogroup B strain MC58 (21, 36) has revealed that other genetic islands exist within N. meningitidis. The genes encoding the hemoglobin (Hb)-haptoglobin (Hp) receptor, hpuAB, are located on a genetic island that is present in serogroup A strain Z2491 but absent in serogroup B strain MC58. A second phase-variable meningococcal Hb receptor, encoded by hmbR, however, is present in both strains, suggesting that this receptor is necessary for iron uptake from Hb in strain MC58. Conversely, Richardson and Stojiljkovic (28) have found that many clinical meningococcal isolates have lost hmbR but have retained hpuAB. In this report, we have investigated the genetic mechanism that results in the complete loss of the hmbR locus from clinical meningococcal isolates. We have identified an exchangeable DNA island (exl) consisting of multiple cassettes that are capable of being exchanged for hmbR in the meningococcal genome. The commensal neisserial species appear to be the reservoirs for these exl cassettes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Meningococcal strains were cultured under aerobic conditions with 3.5% CO2 at 37°C on GC agar (Difco) supplemented with 0.4% glucose, 0.01% glutamine, 0.2 mg of cocarboxylase/liter, and 5 mg of Fe(NO3)3/liter. The meningococcal strains used in this study were obtained from several geographic locations over a period of years and span the genetic diversity of N. meningitidis. Invasive isolates collected as part of a prospective population-based surveillance for meningococcal disease in metropolitan Atlanta, Ga., from 1988 to the present (25, 31), serogroup A meningococcal isolates representing each clonal group (I to VIII) (kindly provided by Mark Achtman), and isolates obtained from the collection of the Centers for Disease Control and Prevention, Atlanta, Ga., were examined in this study. Nasopharyngeal isolates were provided from a study of asymptomatic carriers in the metropolitan Atlanta area (Stephens et al., unpublished). Commensal Neisseria spp. have been described previously (18).
Amplification of the exl cassettes and flanking regions.
The region surrounding the exl cassettes was PCR amplified from chromosomal preparations of 10 meningococcal isolates: NMB (32), 44/76 (38), 6940 (41), M981 (41), FAM18 (18), M1205 (35), M1843 (35), F8229 (23), GA0929 (Atlanta Surveillance Group, VA Medical Center, Atlanta, Ga.), and 6083 (18). Gonococcal strain FA1090 was used as a positive control. Region I (Fig. 1) was PCR amplified from these strains using the primer pair pqi3A (5′-CTCGATATACGTCATACCGTAAGC-3′) and pqi11 (5′-GCGAACACGACTTTGTAGAATGC-3′).
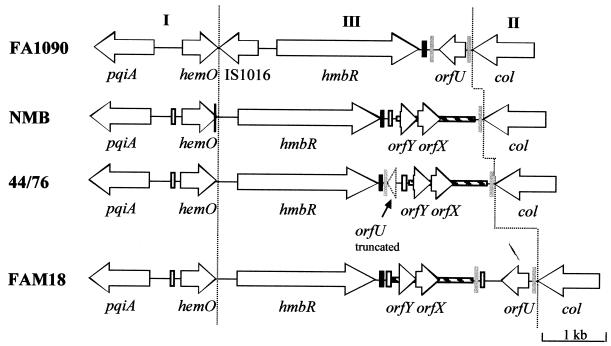
FIG. 1.
Genetic arrangement of the hmbR locus in N. gonorrhoeae strain FA1090 (accession number AF319529) and N. meningitidis strains NMB (accession numbers AF319530 and AF319531), 44/76 (accession number AF319527), and FAM18 (accession number AF319528). Each ORF is represented by an arrow that indicates the direction of transcription. The thin black vertical line indicates the position of a 23-bp sequence which is duplicated in hemO of strain NMB. The thick grey vertical lines indicate the positions of the 33-bp repeats of orfU. Black bars represent a 112-bp conserved nucleotide sequence immediately bordering the 3′ end of hmbR. White bars indicate the locations of neisserial repeat sequences (4). Hatched bars indicate the positions of the 1,280-bp sequence containing orfYX.
Southern blot analysis.
Chromosomal DNA from meningococcal strains was prepared using the method of Nath (20). Double-stranded DNA probes were labeled with digoxigenin using a Genius 2 labeling kit (Boehringer Mannheim Biochemicals) according to the manufacturer's instructions. The internal hmbR probe from N. meningitidis strain NMB was amplified with hmbR2 (5′-GAAGCTGCAACCGAAACCACACC-3′) and hmbR3 (5′-GTGGTGCTGTAGCTATACAAACCG-3′), the exl2 probe from N. meningitidis strain GA0929 was amplified with tbp1 (5′-GTCTAGCTCTTTCAGCTTGTAGTTCG-3′) and col12 (5′-GCTTTCGTCAAATCAAGGATTCAAACG-3′), and the exl3 probe from N. meningitidis strain 6083 was amplified with Acol23 (5′-GAACATACTGAGCCTGCGATGAATGC-3′) and Acol28 (5′-CATCCCCATACCTGAAGGATCTTTGG-3′). Chromosomal DNA was digested with either Bsp106 or StyI overnight and electrophoresed in a 0.7% agarose gel. Southern transfer onto a nylon membrane was performed as described by Maniatis et al. (15). Hybridization and development of the Southern blots were carried out according to the instructions for the Genius system (Boehringer).
DNA sequencing.
Automated sequencing was performed at the Emory DNA Sequencing Core Facility (VA Medical Center, Atlanta, Ga.). The sequence was analyzed using the GAP sequence comparison program of the Genetics Computer Group software package (version 7.3.1 for UNIX) and the Lasergene suite of programs (DNASTAR). All the sequences described in this manuscript have been submitted to GenBank (see below).
Multilocus enzyme electrophoresis.
Multilocus enzyme electrophoresis was carried out by established methods (39). Briefly, constitutive cytosolic enzymes were extracted and run in 11% starch gels. The band position for each of 24 cellular enzymes was established by specific staining for each enzyme. Relative enzyme mobilities (alleles) were numbered in order of increasing anodal migration, and each unique set of alleles was defined as an electrophoretic type (ET). Strains representing ET0717, ET0715, ET0712, ET700, and ET708 were included as markers for serogroup A subgroups I, II, III, IV-1, V, VI, VII, and VIII (courtesy of Tanya Popovic, Centers for Disease Control and Prevention) but were not available for exl cassette typing.
Carriage of exl cassettes in the meningococcal population.
Colony PCR was performed on each isolate using an anchor primer, pqi10 (5′-GCATTCTACAAAGTCGTGTTCGC-3′), Fpqi10 (5′-GTGAAACCTTCGGTTTGCCGGAAGG-3), or Ypqi10 (5′-GCATTTTACAAAGTCGTGTTGCG-3′), paired with a primer specific for each open reading frame (ORF) located in the exl cassette. ORF-specific primers were hmbR2 and hmbR3 for hmbR; col14 (5′-GTCAAGTTGCGCGAACGGAAATGC-3′) for exl2; Acol26 (5′-CCTGTTTTAGGTTGGTTATCGGCAGC-3′) for exl3; and A1col2 (5′-CATATCACGGTTGTTTTCAGGCTGC-3′) for exl3A. PCR amplification of an internal section of the conserved flanking gene, col, using primers col1 (5′-CGATTCGCTCGAAATCATCCACC-3′) and col2 (5′-GGTGGATGATTTCGAGCGAATCG-3′) was included as a positive control for template quality in every PCR run.
Nucleotide sequence accession numbers.
The nucleotide sequences submitted to GenBank (accession numbers in parentheses) are as follows: region I from N. gonorrhoeae strain FA1090 (AF319529), region I from N. meningitidis strain NMB (AF319530), region II and III in N. meningitidis strain FAM18 (AF319528), region II and III from N. meningitidis strain NMB (AF319531), region II and III from N. meningitidis strain 44/76 (AF319527); exl2 from N. meningitidis strain GA0929 (AF319532), exl3 from N. meningitidis strain 6083 (AF319533), exl2C from N. cinerea (AF319536), exl3L from N. lactamica AF319537), exl3A from N. meningitidis strain Z5005 (AF319534), and exl3 from N. meningitidis strain Z5035 (AF319535).
RESULTS
Arrangement of the hmbR (exl1) locus in N. meningitidis
We first studied the organization of the hmbR locus because of the reported linkage of hmbR to the lipopolysaccharide heptosyltransferase encoded by rfaC (33). Using chromosome walking, direct PCR, and sequencing, we found that rfaC was not linked to hmbR in N. meningitidis or N. gonorrhoeae (Fig. 1). DNA sequencing of the locus located upstream of hmbR an ORF which was subsequently termed hemO, as it encodes the heme oxygenase necessary for the release of iron from imported heme (40). Upstream of hemO lies a homologue of Escherichia coli pqiA which encodes a paraquat-inducible stress protein (12). The ORF downstream of hmbR was termed col and was predicted to encode a protein with 63% identity and 75% similarity to a putative collagenase from E. coli.
Region I contains pqiA and hemO and was shown by PCR to be identical in size in eight meningococcal isolates (see Materials and Methods). Region I in gonococcal strain FA1090 produced a larger PCR product which, upon sequencing, was shown to contain insertion element IS1016 and an associated 55-bp deletion located downstream of hemO (accession number AF319529). Nevertheless, a comparison of the hemO genes from gonococcal strain FA1090 and serogroup B meningococcal strain NMB (accession number AF319530) showed 88.5% nucleotide sequence identity between these two strains, indicating a high degree of conservation. Similarly, region II, which contains col (Fig. 1), was highly conserved at the nucleotide level in meningococcal strains FAM18 (accession number AF319528) and NMB (97% identity over 146 bp).
Region III was polymorphic (Fig. 1) and contained four elements that were found in different combinations in the eight meningococcal isolates and the one gonocococcal strain examined. Downstream of col in strain FA1090, a 524-bp novel ORF, orfU, flanked by two 33-bp inverted repeats (AATGC CGTCTGAAAACGGTTTCAGACGGCATT) containing neisserial uptake sequences (shown in bold) was found (B. A. Roe, S. Clifton, and D. W. Dyer, Gonococcal Genome Sequencing Project [http://www.genome.ou.edu]). The organization of orfU resembles that of many insertion sequence elements. However, in meningococcal serogroup B strain NMB, orfU was replaced with a 1,280-bp sequence that contained a 418-bp ORF, orfX, and a 248-bp ORF, orfY (accession number AF319531). OrfX shares 50% identity at the amino acid level with a hypothetical protein encoded by an ORF found in multiple copies in the genomes of Synechocystis spp., whereas OrfY shares 34% amino acid identity with a conserved hypothetical protein from Streptomyces coelicolor (26). Region III of serogroup B meningococcal strain 44/76 (accession number AF319527) and serogroup C strain FAM18 (accession number AF319528) possessed hybrid sequences containing orfYX bounded by a neisserial repeat element and the 33-bp inverted repeat and either a partial or a complete orfU gene (Fig. 1). Southern analysis of these strains indicated that both orfYX and orfU were exclusively associated with this genomic location (data not shown). The various combinations and arrangements of the elements in region III suggest that it is a site for recombination. Invariably, region III was delineated by the stop codon of hemO and the 33-bp repeat found downstream of col. Region III will be subsequently referred to as the exl (exchangeable locus) cassette.
Detection and organization of exl2 and exl3 loci in N. meningitidis
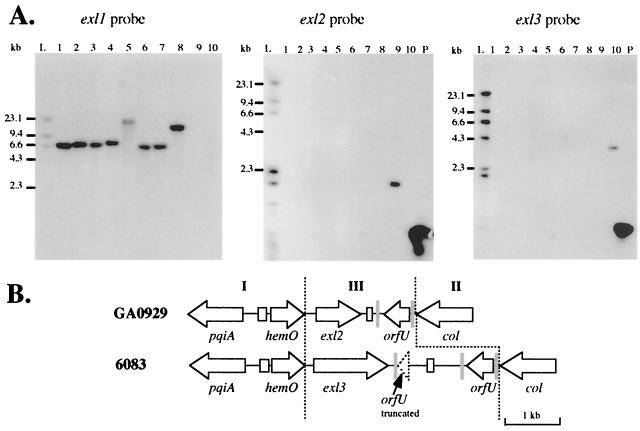
Southern analysis of a panel of 10 meningococcal isolates using an hmbR probe revealed that serogroup Y strain GA0929 and serogroup W-135 strain 6083 did not contain hmbR (Fig. 2A). The region between hemO and col from both isolates was amplified and then sequenced. Region I from strains GA0929 (accession number AF319532) and 6083 (accession number AF319533) were both 93% identical to the corresponding region in strain NMB (data not shown). Likewise, col from region II (146 bp) was 100% identical in GA0929 and 6083 and 94% identical to the same region in strain NMB. The 1,904-bp exl cassette in strain GA0929, however, diverged from the hmbR loci immediately after the stop codon of hemO (Fig. 3) and contained an 809-bp ORF designated exl2. Southern analysis of the panel of 10 meningococcal isolates using a specific probe containing an internal section of exl2 showed that none of the hmbR-containing strains carried this ORF elsewhere in their chromosomes (Fig. 2).
FIG. 2.
(A) Carriage of exl loci in meningococcal genomes is mutually exclusive. Southern blots of 10 meningococcal strains hybridized with hmbR, exl2, and exl3 probes are shown. Lanes 1 through 10 contain genomic DNAs from strains NMB, 44/76, 6940, M981, FAM18, M1205, M1843, F8229, GA0929, and 6083, respectively. Lane L contains labeled size markers, and lane P contains unlabeled probe DNA as a positive control. (B) Genetic arrangement of the exl cassettes in N. meningitidis strainsGA0929 (accession number AF319532) and 6083 (accession number AF319533). See the legend to Fig. 1 for details.
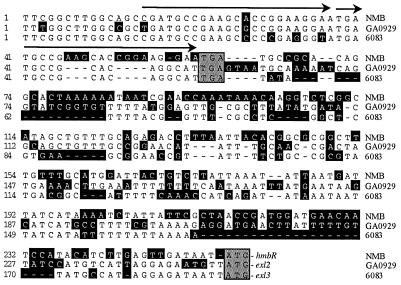
FIG. 3.
The 5′ boundary of the exl cassettes corresponds to the stop codon of hemO. Shown is the alignment of the exl cassettes from the 3′ end of hemO to the start codon of hmbR from N. meningitidis strain NMB, exl2 from N. meningitidis strain GA0929, and exl3 from N. meningitidis strain 6083. The stop codon of each hemO sequence and the start codon of each exl ORF are marked by grey boxes. Nucleotide residues that do not match the consensus sequence are enclosed in black boxes. Gaps are represented by dashes. The position of the 23-bp sequence in hemO (nucleotides 15 to 37) that is duplicated in strain NMB is indicated by arrows.
In strain 6083, region III consisted of a second, novel 3,426-bp sequence containing a 1,368-bp ORF, exl3 (Fig. 2B). The exl3 ORF contained a homopolymeric cytosine tract located in the 5′ end of the gene and therefore is potentially phase variable. Meningococcal isolates containing hmbR or exl2 did not contain exl3 elsewhere in their genomes (Fig. 2A). Although exl2 and exl3 did not have significant nucleotide sequence identity, the translated amino acid sequences from these ORFs were 55% identical (data not shown). Both predicted Exl2 and Exl3 proteins had conserved signal peptides containing a signal peptidase II cleavage site, suggesting that both could be membrane-bound lipoproteins.
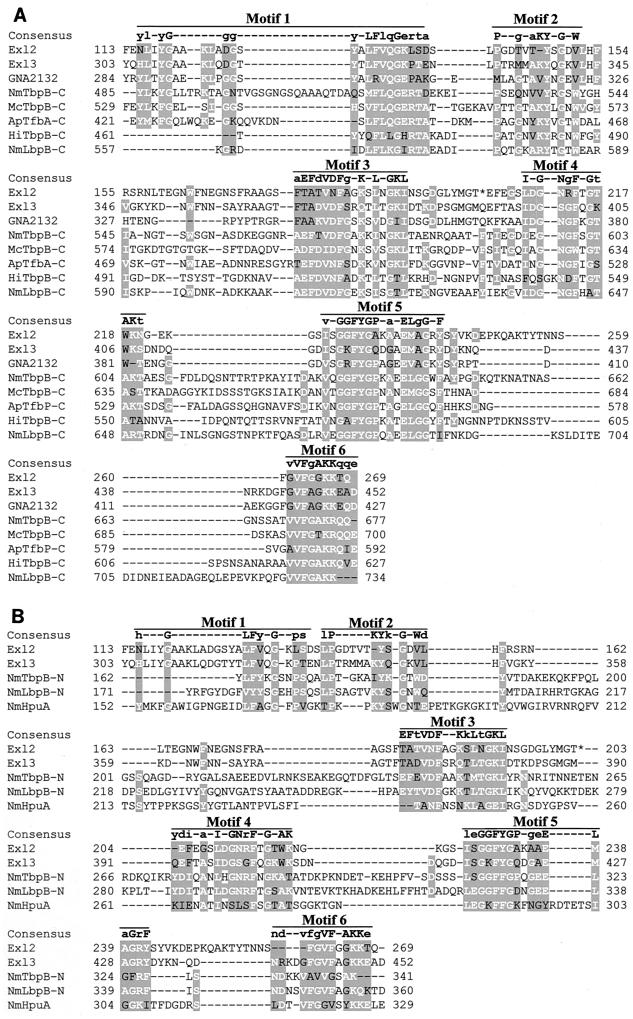
A search for amino acid sequence similarity using PSI-BLAST (1) showed that both Exl2 and Exl3 had 47% identity and 61% similarity to a putative lipoprotein (GNA2132) encoded by the ORF NMB2132 in the meningococcal genome of strain MC58 (36). GNA2132, Exl2, and Exl3 all have low sequence identity to the lipoprotein components of the meningococcal transferrin (TbpB), lactoferrin (LbpB), and Hb-Hp (HpuA) receptors (Fig. 4). Mazarin et al. (17) have previously shown that the TbpB proteins, which are generally about 700 amino acids long, consist of two domains of approximately 350 amino acid residues each. Both the carboxy- and amino-terminal domains contain the same patterns of conserved motifs, which may be involved in binding the bilobed transferrin substrate (27). Alignments of the carboxy- and amino-terminal sequences of TbpB proteins from various species with the Exl2 and Exl3 sequences revealed that six identifiable motifs, corresponding to those described by Mazarin et al. (17), were found in Exl2 and Exl3 (Fig. 4). Our alignment also reveals that, like TbpB, LbpB (741 amino acids) contains two sets of the six motifs (Fig. 4). Because they are much smaller proteins, Exl2 (269 amino acids), Exl3 (455 amino acids), and GNA2132 (427 amino acids) contain only one set of the six motifs. In this respect, these proteins are similar to HpuA (341 amino acids), a protein that is involved in Hb-Hp utilization.
FIG. 4.
Alignment of Exl2 and Exl3 with iron binding lipoproteins. The carboxy termini of Exl2, Exl3, and GNA2132 are 45% identical. These three proteins have low identity (below 25%) with the transferrin binding proteins (TbpB) of N. meningitidis (NmTbpB), Moraxella catarrhalis (McTbpB), Actinobacillus pleuropneumoniae (ApTfbB), and Haemophilus influenzae (HiTbpB); the meningococcal lactoferrin receptor (NmLbpB); and the Hb-Hp receptor (NmHpuA). Six motifs were identified in the carboxy termini (A) and amino termini (B) of the NmTbpB and NmLbpB proteins. Exl2, Exl3, GNA2132, and NmHpuA contained only one copy of the six motifs. The consensus sequence for each motif is shown above the alignments. Uppercase letters indicate residues that are conserved in the carboxy- and amino-terminal motifs, whereas lowercase letters indicate residues specific for the motif in each domain. The consensus sequence for the amino-terminal motifs (B) was determined using all nine proteins (data not shown).
Commensal Neisseria species contain hmbR, exl2, and exl3
Stojiljkovic et al. (34) previously showed that the commensal Neisseria spp., N. polysaccharea and N. perflava, contain hmbR. To determine whether exl2 and exl3 were also present in commensal Neisseria spp., Southern hybridization using exl probes was performed on a representative sample of commensal strains. Representative isolates of N. polysaccharea, N. mucosa, and N. macaca contained a locus which hybridized to the hmbR probe, whereas the exl3 probe hybridized to N. lactamica and N. flavescens. N. cinerea hybridized to the exl2 probe. Three isolates representing the commensal species N. subflava, N. elongata, and N. sicca did not bind to any of the probes. As with meningococci, hmbR, exl2, and exl3 were mutually exclusive, with only one copy of each ORF present in each genome. The linkage of pqiA to col in N. lactamica, N. flavescens, N. cinerea, and N. polysaccharea was confirmed by PCR. The organization of this region in the N. subflava and N. sicca isolates that we examined could not be confirmed, even though pqiA and col were present in their genomes (data not shown).
A comparison of the nucleotide sequence of exl2 from N. meningitidis strain GA0929 with that of its homologue in N. cinerea (accession number AF319536) indicated that meningococcal exl2 had been shortened by an internal deletion that removed 331 codons (or one-third of the ORF). The translational frame of meningococcal exl2 was maintained but contained a stop codon at position 203. These features suggest that meningococcal exl2 was a deletion version of the ORF designated exl2C in N. cinerea (data not shown).
The nucleotide sequences of the exl3-like ORF from N. lactamica (exl3L; accession number AF319537) and exl3 from N. meningitidis strain 6083 were 63% identical (data not shown). Complete analysis of an exl cassette from the serogroup A meningococcal strain Z5005, which hybridized weakly to the exl3 probe, revealed the presence of a third variant of exl3, termed exl3A (accession number AF319534). In contrast to the size variations observed in exl2 variants, all of the exl3 variants were of approximately similar sizes. For clarity, we assigned new gene names where the nucleotide sequence of an ORF was less than 90% identical to the closest homologue.
Distributions of hmbR, exl2, and exl3 in the N. meningitidis population.
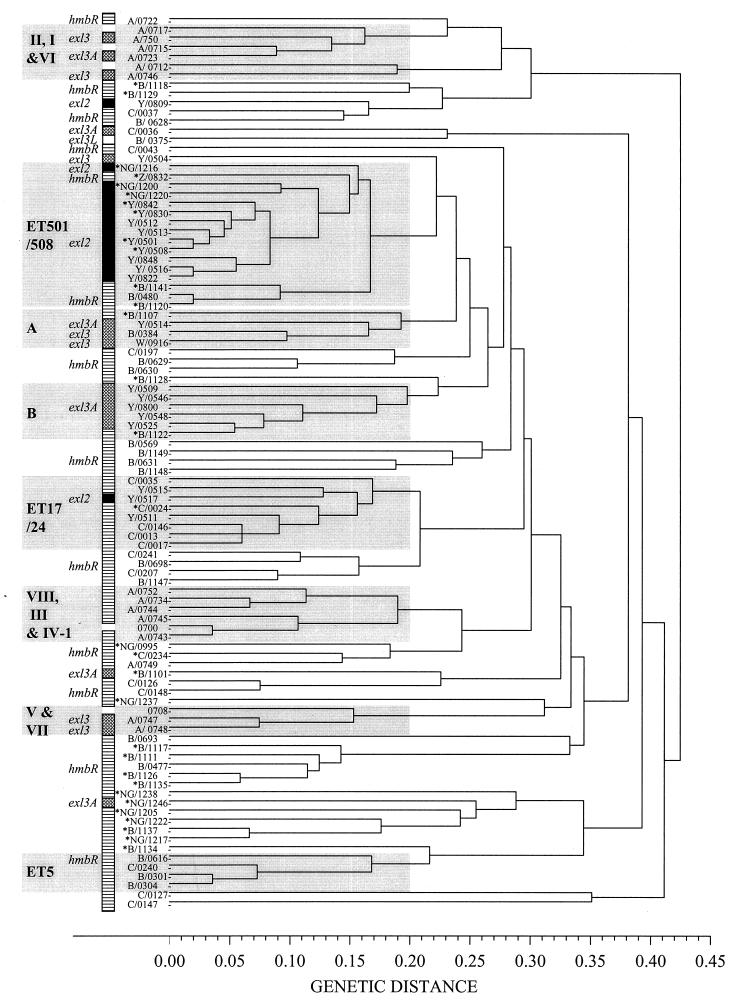
A survey of 125 meningococcal isolates representing 92 ETs (genetic distance, >0.425) was conducted to determine the distributions of hmbR, exl2, and exl3 or exl3A in the meningococcal population. Multiple isolates with the same ET contained the same exl cassette (data not shown). When categorized by serogroup, isolates of serogroups B, C, and A contained similar proportions of hmbR and exl3 or exl3A (approximately 80 and 20%, respectively). In contrast, 55% of serogroup Y strains (11 of 20) examined carried exl2, 35% (7 of 20) carried exl3 or exl3A, and 10% (2 of 20) contained hmbR. Nongroupable isolates collected from asymptomatic carriers contained hmbR, exl2, and exl3A in a ratio of 6:3:1. From this survey, no correlation of exl type with site of isolation could be found (e.g., blood, cerebrospinal fluid, or nasopharynx) (Fig. 5).
FIG. 5.
ET dendrogam showing the distribution of different exl cassettes in the meningococcal population represented by 97 ETs. The carriage of hmbR (stippled boxes), exl2 (black boxes), and exl3 or exl3A (hatched boxes) in 92 ET isolates is shown to the left of the dendrogram. The ET designations appear on the y axis in the following manner: serogroup/ET type, where serogroup is represented by the single-letter code (A, B, C, Y, W [W-135], Z, and NG [nongroupable]) and ET type is represented by the four-digit numerical code. All of the representative strains from each ET type are clinical isolates, except for those labeled with an asterisk, indicating isolation from the nasopharynx of an asymptomatic carrier. ET complexes or clonal groups are boxed in grey and are labeled accordingly. The ET clonal groups ET5 and ET17-ET24 and subgroups I, II, III, IV-1, V, VI, VII, and VIII have been described elsewhere (2). The clonal complexes ET501-ET508, A, and B are described in this study.
Analysis of the population by ET clonal group revealed that most exl2-containing serogroup Y and nongroupable isolates were members of the ET501-ET508 clonal complex (Fig. 5). Only two ETs (ET809 and ET517) containing exl2 were outside the ET501-ET508 complex. Conversely, ET501-ET508 clonal group isolates that did not express the serogroup Y capsule did not contain exl2. These data suggest a genetic linkage between serogroup Y capsule expression and carriage of exl2 in isolates from the ET501-ET508 clonal cluster.
The exl3 and exl3A ORFs were widely distributed (genetic distance, >0.4) across the meningococcal population (Fig. 5). In many instances, isolates containing exl3 and exl3A, however, were found in clonal clusters (genetic distance, <0.2). For example, representative isolates of serogroup A subgroups I, VI, V, and VII contained exl3 and exl3A, and a previously undefined complex, termed B, consisting of five ETs representing serogroup Y isolates, contained exl3A. Complex A consisted of three ETs (ET514, ET384, and ET916) and contained isolates carrying exl3 and exl3A and expressing serogroup Y, B, and W-135 capsules. Thus, the distribution of exl3 and exl3A in the meningococcal population, unlike the distribution of exl2, was not linked to a single capsular serogroup.
Acquisition, dissemination, and microevolution of exl2 and exl3.
To understand the dissemination of the exl cassettes, we analyzed the nucleotide sequences of 10 meningococcal isolates (genetic distance, >0.4) that contained exl3 alleles (allele defined as >90% identity at the nucleotide sequence level). A 290-bp region spanning the last 56 bp of hemO, the intergenic space, and the first 124 bp of the exl3 allele was amplified and sequenced. We hypothesized that if the insertion of the exl3-containing cassettes were the result of a specific integration into this region, then polymorphisms within the hemO nucleotide sequence would be randomly distributed and not linked to polymorphisms in the exl3 sequences. However, in the 10 meningococcal isolates and an N. lactamica isolate, the sequence polymorphisms in hemO and the intergenic space were linked to the sequence polymorphisms in the exl3, exl3A, and exl3L alleles (Fig. 6A). These results indicated that homologous recombination within the conserved flanking gene, hemO, is the mechanism by which the exl cassettes have spread in meningococci.
FIG. 6.
(A) Polymorphisms in the 3′ end of hemO and the intergenic space between this position and the exl3 start codon are characteristic of the exl3 alleles in N. meningitidis. Alignment of the nucleotide sequences from position 663 of hemO (position 1 in this figure) to position 288 of exl3 in 10 meningococcal isolates and N. lactamica is shown. The numbering of the polymorphic sites within the sequences is shown in vertical format above the sequence for N. lactamica exl3L (accession number AF319537). In this scheme, numbers 29 to 31 correspond to the stop codon of hemO and positions 164 to 166 correspond to the exl3 start codon. Nucleotide positions that are identical to those in N. lactamica exl3L are shown as dots, and dashes indicate spaces. Meningococcal isolates (ETs): B431 (375), 6083 (916), B472 (384), M0022 (504), M1978 (525), M1762 (509), Z5005 (723), M2436 (546), M2505 (548), and M2743 (800). (B) Polymorphisms in the first 650 bp of exl3L from N. meningitidis strain B431 (ET375) and N. lactamica exl3L. Identical nucleotides (dots) and spaces (dashes) are marked according to the reference sequence of exl3 from N. meningitidis strain 6083 (ET916). The polymorphic sites are numbered from positions 1 to 3 corresponding to the ATG start codon of exl3. Asterisks mark polymorphisms not shared between the exl3L cassettes in N. meningitidis strain B431 and N. lactamica. (C) Alignment of the complete exl3 genes from N. meningitidis strain 6083 (ET916) and N. meningitidis strain Z5035 (ET747). This alignment revealed polymorphisms in the nucleotide sequence that corresponds to the C-terminal domain of the translated protein. In this numbering scheme, positions 1 to 3 correspond to the ATG start codon of exl3 (see above for details).
To assess microevolution within the exl cassettes, we examined both exl2 and exl3 ORFs for polymorphisms. The exl2 ORF nucleotide sequences of seven isolates, five from the ET501-ET508 clonal complex and one isolate each from unrelated ET809 and ET517 (Fig. 5), were identical. Thus, the acquisition and spread of exl2 in meningococci appears to be recent, since different alleles have not yet been produced through point mutation or homologous recombination. In contrast, the nucleotide sequences of the exl3 and exl3A ORFs showed evidence of microevolution (data not shown). The first 650 bp of exl3 in strains representing ET916, ET504, ET747, and ET384 were identical, except for a single nucleotide at position 221 in ET384 (data not shown). ET916 and ET384 are closely related (complex A; genetic distance, 0.1) (Fig. 5) and thus would be expected to contain similar exl3 alleles. ET504 and ET747 are not closely related to ET916 or ET384 (genetic distances, 0.25 and 0.34, respectively) and are likely to have acquired the nearly identical exl3 alleles through horizontal exchange. Examination of the exl3 ORFs from strains representing ET747 (accession number AF319535) and ET916 (accession number AF319533) revealed that there were 69 base-pair changes, including transitions, transversions, and small deletions, over a 342-bp section of the C-terminal domain (Fig. 6C). Therefore, the meningococcal population contains multiple alleles of exl3, indicating that this region has diverged by microevolution from an ancestral homologue. Similar conclusions were made regarding exl3A from isolates representing ET548, ET800, ET525, ET546, ET509, and ET723 (data not shown).
exl3L in N. meningitidis strain B431 (ET375) contained many of the polymorphisms characteristic of exl3L in N. lactamica (Fig. 6B), in both the conserved regions (positions 1 to 297 and 450 to 650) and the central region (positions 297 to 450) of the gene. In all, 61 base-pair changes (out of 650 bp) distinguished exl3L from N. meningitidis strain B431 from that of N. lactamica. This result suggests that acquisition of the exl cassettes by meningococci has occurred multiple times.
DISCUSSION
The ability of N. meningitidis and N. gonorrhoeae to establish infections is in part due to their ability to scavenge essential nutrients, such as iron, from human tissues. Indeed, it has been recently shown that the ability to utilize transferrin is essential for gonococci to establish an infection in the human challenge model (3). Unlike many other pathogens that use siderophores to shuttle iron, meningococci and gonococci express at least four outer membrane protein receptors that are specific for transferrin (tbpAB), lactoferrin (lbpAB), Hb (hmbR), and Hb-Hp complexes (hpuAB) (7). Once heme has been transported into the cytoplasm, iron is released from the heme by a heme oxygenase-like enzyme, encoded by hemO, which is found upstream of hmbR (40).
However, during the investigation of the genetic region containing hemO, we found that many meningococcal isolates did not contain hmbR. In these isolates, hmbR was absent from the chromosome and had been replaced by two distinct DNA islands, which contain ORFs exl2 and exl3. The predicted Exl2 and Exl3 proteins had significant sequence identity with GNA2132, a highly antigenic TbpB-like protein that was recently identified as a potential vaccine candidate (24). In a broader context, Exl2, Exl3, and GNA2132 contain six motifs that are also present in TbpB, LbpB, and HpuA (Fig. 4). TbpB contains two sets of these motifs, one each in the N-terminal and C-terminal domains, which are hypothesized to be involved in binding to the bilobed substrate human transferrin (27). The function of these motifs, however, in LbpB and HpuA, which differ from TbpB in their substrate specificities, is currently unknown. The family of proteins consisting of Exl2, Exl3, and GNA2132 may represent iron binding proteins with an as-yet-undefined substrate specificity.
A comparison of the organization of the exl cassettes in meningococci and commensal Neisseria spp. suggests that each cassette arose from a specific insertion event. This concept is supported by the finding that each exl cassette is delineated by clearly defined 5′ (hemO stop codon) and 3′ (uptake sequence downstream of col) boundaries. In this respect, each cassette resembles an integron, but no other features, such as an integration site (att) or a gene encoding an integrase, could be identified (9). Since orfYX and orfU have some of the features of insertion sequence elements and are located at the 3′ boundary of each exl cassette, they may have been involved in the initial acquisition of the exl cassettes (8). Correlation of the nucleotide polymorphisms outside the 5′ boundary of the exl cassette with base-pair changes found in the cassette, however, indicates that once the cassette was inserted, it was disseminated by homologous recombination within the conserved flanking neisserial genes.
The prevalence of hmbR within the meningococcal population and in N. gonorrhoeae suggests that hmbR may have been present in a common ancestor. Although hmbR has a high degree of primary sequence conservation, alleles of this gene have been previously identified (34), indicating that hmbR has undergone microevolution within meningococci. Examination of the exl cassettes containing exl3 and exl3A within the meningococcal population also revealed that these genes have undergone microevolution, and evidence of transfer of identical exl3 and exl3A alleles to distantly related strains indicates that horizontal transfer has occurred in the meningococcal population. The finding that exl3 and exl3A are mostly confined to a few clonal groups suggests, however, that these alleles have only recently been acquired by meningococci and are slowly being dispersed by homologous recombination outside these organisms.
The detection of only one allele of the exl2-containing cassette suggests that it has only recently been acquired by meningococci. Further, the exl2-containing DNA cassette is largely restricted to strains within the ET501-ET508 clonal complex. Interestingly, the ET501-ET508 clonal complex has a relatively short history, as it has emerged over the last decade as a major causative agent of serogroup Y disease in the United States (29). The presence of the exl2-containing DNA cassette in meningococci offers the unique opportunity to track the rates of microevolution and dissemination of a novel gene within the meningococcal population.
In this report, we have defined an exchangeable DNA island that has been inserted into a single site in the meningococcal chromosome and that consists of at least three distinct cassettes. One of these cassettes encodes the Hb receptor, HmbR, while the other two cassettes encode unique proteins, Exl3 or Exl3A and Exl2. It is currently unclear why so many different strategies have been used by N. meningitidis to change the patterns of expression of the Hb receptors HpuAB and HmbR. Apart from phase-variable expression, the genes of both receptors can be completely replaced or removed from the genome. It is possible that complete removal of HmbR has been developed as a way of replacing a relatively antigenically stable outer membrane protein (34) in the meningococcal population. For many other outer membrane proteins, such as TbpB (14), a high level of antigenic variation is necessary for maintenance in the meningococcal population.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI-33517–08 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
We thank John K. Davies and Harry Sakellaris for critical reading of the manuscript. We are also grateful to Lane Pucko for assistance in preparing the manuscript.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caugant D A. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS. 1998;106:505–525. [PubMed] [Google Scholar]
- 3.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 4.Correia F F, Inouye S, Inouye M. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J Biol Chem. 1988;263:12194–12198. [PubMed] [Google Scholar]
- 5.Deich R A, Smith H O. Mechanism of homospecific DNA uptake in Haemophilus influenzae transformation. Mol Gen Genet. 1980;177:369–374. doi: 10.1007/BF00271475. [DOI] [PubMed] [Google Scholar]
- 6.DeVoe I W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982;46:162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genco C A, Desai P J. Iron acquisition in the pathogenic Neisseria. Trends Microbiol. 1996;4:179–184. doi: 10.1016/0966-842x(96)10029-9. [DOI] [PubMed] [Google Scholar]
- 8.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 9.Hall R M, Collis C M, Kim M J, Partridge S R, Recchia G D, Stokes H W. Mobile gene cassettes and integrons in evolution. Ann N Y Acad Sci. 1999;870:68–80. doi: 10.1111/j.1749-6632.1999.tb08866.x. [DOI] [PubMed] [Google Scholar]
- 10.Holmes E C, Urwin R, Maiden M C J. The influence of recombination on the population structure and evolution of the human pathogen Neisseria meningitidis. Mol Biol Evol. 1999;16:741–749. doi: 10.1093/oxfordjournals.molbev.a026159. [DOI] [PubMed] [Google Scholar]
- 11.Klee S R, Nassif X, Kusecek B, Merker P, Beretti J-L, Achtman M, Tinsley C R. Molecular and biological analysis of eight genetic islands that distinguish Neisseria meningitidis from the closely related pathogen Neisseria gonorrhoeae. Infect Immun. 2000;68:2082–2095. doi: 10.1128/iai.68.4.2082-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh Y-S, Roe J-H. Isolation of a novel paraquat-inducible (pqi) gene regulated by the soxRS locus in Escherichia coli. J Bacteriol. 1995;177:2673–2678. doi: 10.1128/jb.177.10.2673-2678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroll J S, Wilks K E, Farrant J L, Langford P R. Natural genetic exchange between Haemophilus and Neisseria: intergeneric transfer of chromosomal genes between major human pathogens. Proc Natl Acad Sci USA. 1998;95:12381–12385. doi: 10.1073/pnas.95.21.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linz B, Schenker M, Zhu P, Achtman M. Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol Microbiol. 2000;36:1049–1058. doi: 10.1046/j.1365-2958.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 16.Mathis L S, Scocca J J. Haemophilus influenzae and Neisseria gonorrhoeae recognize different specificity determinants in the DNA uptake step of genetic transformation. J Gen Microbiol. 1982;128:1159–1161. doi: 10.1099/00221287-128-5-1159. [DOI] [PubMed] [Google Scholar]
- 17.Mazarin V, Rokbi B, Quentin-Millet M J. Diversity of the transferrin-binding protein Tbp2 of Neisseria meningitidis. Gene. 1995;158:145–146. doi: 10.1016/0378-1119(95)00151-u. [DOI] [PubMed] [Google Scholar]
- 18.McAllister C F, Stephens D S. Analysis in Neisseria meningitidis and other Neisseria species of genes homologous to the FKBP immunophilin family. Mol Microbiol. 1993;10:13–23. doi: 10.1111/j.1365-2958.1993.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore T D E, Sparling P F. Isolation and identification of a glutathione peroxidase homolog gene, gpxA, present in Neisseria meningitidis but absent in Neisseria gonorrhoeae. Infect Immun. 1995;63:1603–1607. doi: 10.1128/iai.63.4.1603-1607.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath K. A rapid DNA isolation procedure from petri dish grown clinical bacterial isolates. Nucleic Acids Res. 1990;18:6462. doi: 10.1093/nar/18.21.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhill J, Achtman M, James K D, Bentley S D, Churcher C, Klee S R, Morelli G, Basham D, Brown D, Chillingworth T, Davies R M, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail M A, Rajandream M-A, Rutherford K M, Simmonds M, Skelton J, Whitehead S, Spratt B G, Barrell B G. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 22.Petering H, Hammerschmidt S, Frosch M, van Putten J P M, Ison C A, Robertson B D. Genes associated with the meningococcal capsule complex are also found in Neisseria gonorrhoeae. J Bacteriol. 1996;178:3342–3345. doi: 10.1128/jb.178.11.3342-3345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pine L, Quinn F, Ewing E, Jr, Birkness K, White E, Stephens D, Ribot E. Evaluation of the chick embryo for the determination of relative virulence of Neisseria meningitidis. FEMS Microbiol Lett. 1995;130:37–44. doi: 10.1016/0378-1097(95)00181-4. [DOI] [PubMed] [Google Scholar]
- 24.Pizza M, Scarlato V, Masignani V, Giuliani M M, Arico B, Comanducci M, Jennings G T, Baldi L, Bartolini E, Capecchi B, Galeotti C L, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood D W, Jeffries A C, Saunders N J, Granoff D M, Venter J C, Moxon E R, Grandi G, Rappuoli R. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 25.Raymond N J, Reeves M, Ajello G, Baughman W, Gheesling L L, Carlone G M, Wenger J D, Stephens D S. Molecular epidemiology of sporadic (endemic) serogroup C meningococcal disease. J Infect Dis. 1997;176:1277–1284. doi: 10.1086/514123. [DOI] [PubMed] [Google Scholar]
- 26.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 27.Retzer D M, Yu R H, Schryvers A B. Identification of sequences in human transferrin that bind to the bacterial receptor protein, transferrin-binding protein B. Mol Microbiol. 1999;32:111–121. doi: 10.1046/j.1365-2958.1999.01331.x. [DOI] [PubMed] [Google Scholar]
- 28.Richardson A R, Stojiljkovic I. HmbR, a hemoglobin-binding outer membrane protein of Neisseria meningitidis, undergoes phase variation. J Bacteriol. 1999;181:2067–2074. doi: 10.1128/jb.181.7.2067-2074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstein N E, Perkins B A, Stephens D S, Lefkowitz L, Cartter M L, Danila R, Cieslak P, Shutt K A, Popovic T, Schuchat A, Harrison L H, Reingold A L. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180:1894–1901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 30.Sampson B A, Gotschlich E C. Neisseria meningitidis encodes an FK506-inhibitable rotamase. Proc Natl Acad Sci USA. 1992;89:1164–1168. doi: 10.1073/pnas.89.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens D S, Hajjeh R A, Baughman W S, Harvey R C, Wenger J D, Farley M M. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann Intern Med. 1995;123:937–940. doi: 10.7326/0003-4819-123-12-199512150-00007. [DOI] [PubMed] [Google Scholar]
- 32.Stephens D S, Swartley J S, Kathariou S, Morse S A. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect Immun. 1991;59:4097–4102. doi: 10.1128/iai.59.11.4097-4102.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stojiljkovic I, Hwa V, Larson J, Lin L, So M, Nassif X. Cloning and characterization of the Neisseria meningitidis rfaC gene encoding α 1,5 heptosyltransferase I. FEMS Microbiol Lett. 1997;151:41–49. doi: 10.1111/j.1574-6968.1997.tb10392.x. [DOI] [PubMed] [Google Scholar]
- 34.Stojiljkovic I, Larson J, Hwa V, Anic S, So M. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartley J S, Marfin A A, Edupuganti S, Liu L-J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tettelin H, Saunders N J, Heidelberg J, Jeffries A C, Nelson K E, Eisen J A, Ketchum K A, Hood D W, Peden J F, Dodson R J, Nelson W C, Gwinn M L, DeBoy R, Peterson J D, Hickey E K, Haft D H, Salzberg S L, White O, Fleischmann R D, Dougherty B A, Mason T, Cieko A, Parksey D S, Blair E, Cittone H, Clark E B, Cotton M D, Utterback T R, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith H O, Fraser C M, Moxon E R, Rappuoli R, Venter J C. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 37.Thompson S A, Wang L L, Sparling P F. Cloning and nucleotide sequence of frpC, a second gene from Neisseria meningitidis encoding a protein similar to RTX cytotoxins. Mol Microbiol. 1993;9:85–96. doi: 10.1111/j.1365-2958.1993.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 38.Wakarchuk W, Martin A, Jennings M, Moxon E R, Richards J C. Functional relationships of the genetic locus encoding the glycosyltransferase enzymes involved in expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. J Biol Chem. 1996;271:19166–19173. doi: 10.1074/jbc.271.32.19166. [DOI] [PubMed] [Google Scholar]
- 39.Woods T C, Helsel L O, Swaminathan B, Bibb W F, Pinner R W, Gellin B G, Collin S F, Waterman S H, Reeves M W, Brenner D J, Broome C V. Characterization of Neisseria meningitidis serogroup C by multilocus enzyme electrophoresis and ribosomal DNA restriction profiles (ribotyping) J Clin Microbiol. 1992;30:132–137. doi: 10.1128/jcm.30.1.132-137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Hunt D J, Richardson A R, Stojiljkovic I. Use of heme compounds as iron sources by pathogenic neisseriae requires the product of the hemO gene. J Bacteriol. 2000;182:439–447. doi: 10.1128/jb.182.2.439-447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zollinger W D, Mandrell R E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977;18:424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]