Abstract
Purpose
Bisphosphonates (BPs) have a powerful effect on reducing bone resorption and improving the survival of patients with breast cancer. We aimed to investigate the impact of BP treatment on the prevention of recurrence, metastasis, and death of breast cancer survivors in the perimenopausal period.
Methods
The search strategy aimed to identify both published and unpublished studies in PubMed, Web of Science, Scopus, Embase, ProQuest, and Google Scholar in March 2021. Two independent reviewers assessed quantitative papers selected for retrieval for methodological validity before being included in the review using standardized critical appraisal instruments from the Joanna Briggs Institute (JBI) Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI). Statistical meta-analysis was performed using Review Manager (RevMan) 5.4 statistical software when the data were homogenous. Meta-analysis was performed by calculating the effect size (hazard ratio; HR) and 95% confidence intervals (CIs).
Results
Twenty-one studies were eligible for this systematic review and meta-analysis. The overall The HRs for disease-free survival (DFS) and overall survival (OS) in women who received BPs were 0.89 (95% CI, 0.83–0.97; p = 0.005), and 0.75 (95% CI, 0.63–0.89; p = 0.001), respectively. The results showed that BPs had a significant effect on the prevention of locoregional (HR, 0.64; 95% CI, 0.42–0.97; p = 0.04), bone (95% CI, 0.74–0.95; p ≤ 0.001), and distant metastases (HR, 0.77; 95% CI, 0.62–0.94; p = 0.01). In the subgroup analysis based on study design, the only insignificant HR in the included randomized controlled trials (RCTs) was that of locoregional metastasis.
Conclusion
Although BPs have a promising effect on DFS, OS, and bone metastasis of perimenopausal women survivors of breast cancer, more RCTs are needed to evaluate their effect on other survivors’ outcomes.
Keywords: Breast Neoplasms, Diphosphonates, Menopause, Recurrence, Systematic Review
INTRODUCTION
Breast cancer is one of the most prevalent cancers worldwide, accounting for 685,000 global deaths by 2020. Although breast cancer is more prevalent in developed countries, more than half the deaths are reported from developing countries. Moreover, the 5-year survival rate of breast cancer is high in North America and Japan, and low in African countries [1]. Based on the results of a study in the USA, the incidence of breast cancer increased between 2009 and 2018, although the mortality rate did not increase [2]. Similar to most other cancers, breast cancer is preventable. Creating public awareness about breast cancer, reducing exposure to risk factors, and chemoprevention are the main steps for preventing breast cancer [1]. Treatment of breast cancer includes neoadjuvant chemotherapy, surgery for operable tumors, radiotherapy, adjuvant chemotherapy, and/or endocrine therapy. Surgery in metastatic breast cancer has a palliative role rather than providing survival benefit [3]. Breast cancer metastasis is responsible for nearly 12% of breast cancer diagnoses, lacks effective treatment, and is the primary cause of mortality in this cancer type. Bone metastasis is one of the most prevalent metastases in breast cancer and can lead to such morbidities. In addition to regular follow-up, there should be some method to prevent metastasis and recurrence and improve the lifestyle of patients with bone metastasis, one of which is the use of bisphosphonates (BPs).
BPs were first discovered in the 19th century [4]. Considering their structure, they have a high affinity for hydroxyapatite and are one of the most potent suppressors of bone resorption [5]. Irrespective of their type, BPs have a major role in treating osteoporosis and preventing bone loss and fractures. In addition, they help improve breast cancer outcomes, especially with long-term use (more than one year) [6,7].
A review study proved that BPs have a significant anticancer effect in preclinical settings, such as in animal models and cell cultures of different types of cancers [8].
A study demonstrated that BPs have a powerful effect in reducing bone resorption and improving the survival of breast cancer patients, especially those with low estrogen levels [9].
Recent studies have shown that doses of BPs utilized for osteoporosis could improve the survival of patients with early-stage breast cancer compared to that with denosumab, a human monoclonal antibody [10].
One of the most potent BPs is zoledronic acid, which is highly effective in the treatment of breast cancer metastasis [11]. Disseminated tumor cells (DTC) and circulating tumor cells (CTC) are accused of initiating metastasis in breast cancer. Recently, a study in 2021 evaluated the role of zoledronic acid on DTC and CTC in the serum of patients with breast cancer. Their results showed that DTC and CTC levels were meaningfully low compared to the baseline in the BPs group [12]. Although a number of studies have shown that BPs could play a role in lowering cardiovascular events and breast cancer incidence [13,14], some of them failed to demonstrate any relationship [15,16].
We conducted a systematic review to identify the effect of BPs on recurrence, death, and overall metastasis in perimenopausal women with a history of breast cancer. The quantitative objective was to estimate the effect of BPs on the prevention of recurrence of breast cancer in perimenopausal women.
METHODS
This systematic review and meta-analysis was conducted according to the Cochrane Collaboration Handbook and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [17]. The proposed systematic review was conducted following the Joanna Briggs Institute (JBI) methodology for systematic reviews [18].
Inclusion criteria
Types of participants
The quantitative component of this review included women with a history of breast cancer. Breast cancer survivors are women who have been diagnosed with breast cancer from the point of diagnosis through, and after treatment.
Types of intervention(s)
The quantitative component of the review entailed studies that evaluated the effect of BPs.
Types of comparison
The quantitative components of this review were placebo, observation controls, and other non-BP controls. Delayed BP therapy and denosumab-treatment controls were excluded from the present study.
Types of outcomes
This review included the studies that contained the following outcome measures: 1) Disease-free survival (DFS) was defined as the time from randomization to tumor recurrence or death in RCTs or from the start time of diagnosis in non-randomized studies; 2) Overall survival (OS) was defined as the time from diagnosis to death from any cause or to the last follow-up. The other secondary endpoints were locoregional, bone, and distant metastases.
Types of studies
The quantitative component of the review was interventional or observational studies, including randomized controlled trials (RCTs), cohort, and case-control studies that reported events of cancer recurrence as the primary or secondary outcomes. When multiple reports from the same study were published at different time points of follow-up, we included the data after the longest follow-up period in the analyses. RCTs that were not initially designed to study cancer recurrence were excluded.
Search strategy
The search strategy aimed to identify published and unpublished studies. This study used a three-step search strategy. An initial limited search of MEDLINE was performed, followed by an analysis of the text words, including the title and abstract. Subsequently, the second search using all identified keywords and index terms was undertaken across all included databases in March 2021. Finally, the reference lists of all the identified reports and articles were searched for additional studies. Studies published in any language and on any date were included in this review (Appendix 1).
The aforementioned databases included Cochrane Library, MEDLINE (PubMed), Web of Science, Scopus, Embase, ProQuest, Google Scholar, SID, Magiran, and IranDoc for Persian literature.
Moreover, unpublished studies were searched from seminars and congresses and included.
Study selection
Following the search, all identified citations were collated and uploaded to EndNote, and duplicate studies were eliminated. Two independent reviewers screened titles and abstracts to assess the eligibility of the studies based on the inclusion and exclusion criteria. Full texts of potentially relevant studies were retrieved, and details of citations were imported into the JBI System for the Unified Management, Assessment, and Review of Information (JBI SUMARI) (Joanna Briggs Institute, Adelaide, Australia). Two reviewers assessed the full text of the selected citations according to the eligibility criteria. Explanations for excluding studies in full-text stages that did not meet the eligibility criteria were recorded. The reviewers resolved disagreements raised at each level of the study selection process through discussion or with the opinion of a third reviewer. The search results and details of the screening process are presented in the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram [10].
Assessment of methodological quality
Two independent reviewers assessed the quantitative papers selected for retrieval for methodological validity before being included in the meta-analysis using standardized critical appraisal instruments from the Cochrane Handbook. The items under consideration were selection bias including random sequence generation and allocation concealment, performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors), attrition bias, and reporting bias. The JBI Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI) (https://jbi.global/critical-appraisal-tools) was used for other studies that were not eligible for inclusion in the meta-analysis. Probable disagreements between the reviewers were resolved through discussion or by a third reviewer (Table 1).
Table 1. Quality assessment of the included studies according to the related JBI appraisal checklists.
| Author | Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jallouk et al. [21] | 2021 | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Banys et al. [24] | 2013 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Body et al. [37] | 2003 | Unclear | Unclear | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Delmas et al. [19] | 1997 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Diel et al. [33] | 1998 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Eidtmann et al. [25] | 2010 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ishikawa et al. [27] | 2017 | Yes | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kristensen et al. [38] | 2008 | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Perrone et al. [30] | 2019 | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes |
| Rack et al. [22]* | 2010 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Saarto et al. [35] | 2003 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Q1: Was true randomization used for the assignment of participants to the treatment groups? Q2: Was allocation to the treatment groups concealed? Q3: Were the treatment groups similar at the baseline? Q4: Were the participants blinded to the treatment assignment? Q5: Were those delivering treatment blind to treatment assignment? Q6: Were outcome assessors blinded to the treatment assignment? Q7: Were the treatment groups treated identically, other than the intervention of interest? Q8: Was follow-up complete and, if not, were differences between groups in terms of their follow-up adequately described and analyzed? Q9: Were the participants analyzed in the groups to which they were randomized? Q10: Were the outcomes measured in the same way for the treatment groups? Q11: Were the outcomes measured reliably? Q12: Was appropriate statistical analysis used? Q13: Was the trial design appropriate, and were any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial?
*Q1: Is it clear in the study what is the ‘cause’ and what is the ‘effect’ (i.e., there is no confusion about which variable comes first)? Q2: Were the participants included in any comparison similar? Q3: Were the participants included in any comparison receiving similar treatment/care other than the exposure or intervention of interest? Q4: Was there any control group? Q5: Were there multiple measurements of the outcome, both pre- and post-intervention, or exposure? Q6: Was follow-up complete and, if not, were differences between groups in terms of their follow-up adequately described and analyzed? Q7: Were the outcomes of the participants included in any comparison measured in the same way? Q8: Were the outcomes measured reliably? Q9: Was appropriate statistical analysis used?
Data collection
Two independent reviewers extracted data from the included articles using the modified standardized JBI data extraction tool. Specific details were selected from the included studies, including the first author, publication year, study design, country, estrogen or progesterone receptor status, duration of BP use, and study outcomes. Any disagreement among the reviewers was resolved through discussion.
Data synthesis
Statistical meta-analysis was performed using Review Manager (RevMan) 5.4 statistical software (Cochrane Collaboration, London, UK) when the data were homogenous. Meta-analysis was performed by calculating the effect size (hazard ratio; HR) and 95% confidence intervals (CIs). The standard error of each study was calculated from the existing data. Statistical significance for all analyses was set at p < 0.05 (two-tailed). Heterogeneity was calculated using the I2 test. In the present meta-analysis, I2 > 50% and a significance level of p < 0.10 for Cochran’s Q were considered clinically important heterogeneity. Significant heterogeneity was identified in the fixed-effects model, prompting the use of the random-effects model to calculate the cumulative effect size and CI. Furthermore, because not more than ten studies were included in the meta-analysis, a funnel plot was not generated to assess publication bias. The findings were presented in a narrative form, including tables and figures, where statistical pooling was not possible, which was performed to aid data presentation, if appropriate. We only included the latest version of the published data from the same research team in this regard.
RESULTS
Study inclusion
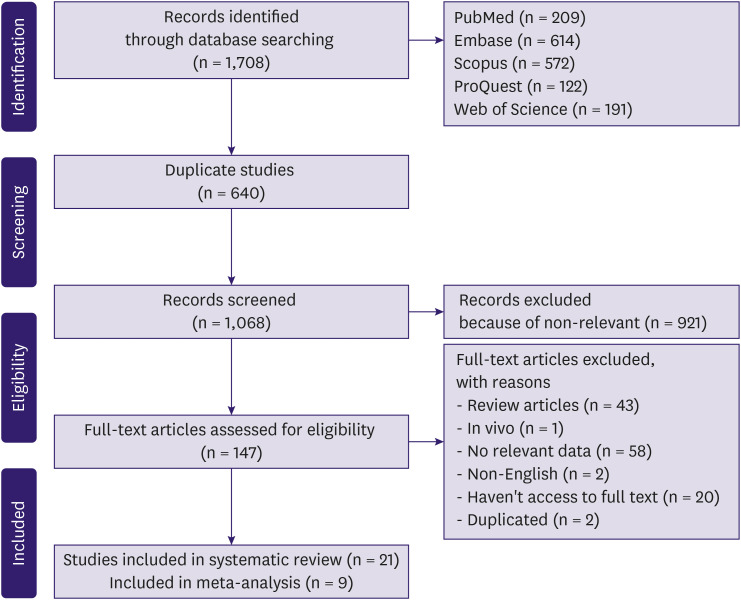
During the electronic search, manual search, and reference check, we identified 1,708 citations. After omitting duplicate citations, 640 studies remained for screening. Altogether, 147 studies were selected based on their titles and abstracts and 125 studies were excluded in the full-text selection step. Finally, 21 studies were included in the critical appraisal process and in this study. Additional information regarding the selection process is presented in the PRISMA flowchart (Figure 1).
Figure 1. Search and selection process of the systematic review.
Methodological quality
The JBI appraisal checklist critically assessed the eligible studies to detect possible biases. According to this evaluation, most studies were of appropriate quality. The results of the evaluation of eligible studies and checklists are presented in Table 1.
Characteristics of included studies and findings
The reviewed studies included three phase III randomized controlled trials, three cohort studies, and two retrospective studies, and the others were randomized or non-randomized phase II, open-label clinical trials [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. A summary of the study design and outcomes measured from these studies is shown in Tables 2 and 3.
Table 2. The included studies’ characteristics.
| # | Author | Year | Country | Study design | The regimen of control group | Type of BP | Type of breast cancer | Mean age | Menstruation | Case (n) | Control (n) | F/U (mon) | Duration of BP use (mon) | HER2 | PR | ER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Korde et al. [28] | 2017 | USA | Cohort study | Hormone therapy | Alendronate, risedronate, zoledronate | AJCC stage | 64.2 | Post: 1,527, Pre: 191 | 302 | 1,511 | 11.8 | 41.2 | - | BP: PR+: 238, PR−: 56 | BP: ER+: 257, ER−: 37 |
| BP: I: 172, IIA: 57, IIB: 15, IIIA: 23, IIIB: 6 | Post: 266 | Post: 1,261 | Control: PR+: 1,131, PR−: 345 | Control: ER+: 1,269, ER−: 209 | ||||||||||||
| Control: I: 788, IIA: 299, IIB: 154, IIIA: 114, IIIB: 38 | ||||||||||||||||
| 2 | Rack et al. [22] | 2010 | Germany | Non-randomized phase II trial | Cytostatic treatment, hormone therapy | Zoledronate | G1–G3 | 53.1–54.2 | Post: 107, Pre: 49 | 31 | 141 | 39 | 6 | - | - | - |
| Post: 89 | Post: 18 | |||||||||||||||
| 3 | Hadji et al. [20] | 2013 | Germany | Retrospective | Endocrine therapy - chemotherapy | Alendronate, clodronate, ibandronate, zoledronic acid | Stage | 59.5–64.9 | - | 937 | 630 | 12–24 | 3 ≤ | Case: 125, Control: 75 | Case: 937, Control: 630 | Case: 937, Control: 630 |
| T1: BP: 620, control: 401 | ||||||||||||||||
| Zoledronic acid 4 mg IV two to three times per year; ibandronate 50 mg/day PO, 150 mg/month PO, or 4 mg to 6 mg IV every 3 months; clodronate 1,600 mg/day PO; or alendronate 70 mg/week PO. | T2, 3, or 4: BP: 317, control: 229 | HER2−: BP: 750, Control: 517 | ||||||||||||||
| Nodal | ||||||||||||||||
| N0: BP: 537, control: 413 | ||||||||||||||||
| N+: BP: 400, control: 217 | ||||||||||||||||
| Histologic grade | ||||||||||||||||
| BP: G1,G2: 662, G3: 275 | ||||||||||||||||
| Control: G1,G2: 421, G3: 209 | ||||||||||||||||
| 4 | Gnant et al. [26] | 2014 | Italy | RCT | Goserelin, tamoxifen, anastrozole | Zoledronic acid, 4 mg given intravenously every 6 months) | T1–T3 | 45–45.5 | Post: 5, Pre: 1,798 | 900 | 903 | 94.4 | 36 | - | Case: 803, Control: 804 | Case: 835, Control: 848 |
| Post: 2 | Post: 3 | |||||||||||||||
| 5 | Coleman et al. [31] | 2018 | UK | Randomized controlled, phase 3 | Standard adjuvant systemic therapy | Zoledronic acid, 4 mg zoledronic acid given intravenously every 3–4 weeks for the first six doses, every 3 months for eight doses, and every 6 months for five doses to complete 5 years of treatment | Grade | 18 ≤ | Pre: 1,503, Post: 1,532 | 1,681 | 1,678 | 117 | 60 | BP: HER2+: 192, HER2−: 648 | BP: PR+: 725, PR−: 382 | BP: ER+: 1,318, ER−: 350 |
| BP: I: 146, II: 731, III: 765 | Post: 766 | Post: 765 | Control: HER2+: 223, HER2−: 604 | Control: PR+: 699, PR−: 424 | Control: ER+: 1,315, ER−: 356 | |||||||||||
| Control: I: 141, II: 708, III: 787 | ||||||||||||||||
| Node | ||||||||||||||||
| BP: 0: 30, 1–3: 1,042, ≥4: 604 | ||||||||||||||||
| Control: 0: 32, 1–3: 1,033, ≥4: 607 | ||||||||||||||||
| Stage | ||||||||||||||||
| BP: I: 542, II: 850, III: 228, IV: 58 | ||||||||||||||||
| Control: I: 523, II: 867, III: 228, IV: 59 | ||||||||||||||||
| 6 | Paterson et al. [29] | 2012 | USA | Multicentre, placebo-controlled, randomised trial | Endocrine therapy - chemotherapy | Clodronate, 1,600 mg daily for 3 years | Nodal | 49 ≥ to 50 ≤ | - | 1,656 | 1,655 | 90.7 | 36 | - | - | - |
| 1–3: BP: 18, control: 295 | ||||||||||||||||
| 4 or more: BP: 7, control: 114 | ||||||||||||||||
| 7 | Perrone et al. [30] | 2019 | Italy | Phase 3 randomised trial | Letrozole, tamoxifen | Zoledronic acid | G1–G3 | 44.7–45.2 | Pre: 1,065 | 355 | 710 | 64 | 60 | Case: 47, Control: 98 | Case: 346, Control: 685 | - |
| 8 | Banys et al. [24] | 2013 | Germany | Controlled randomized open-label multi-center study | Endocrine treatment chemotherapy | Zoledronic acid, intravenous zoledronic acid every 4 weeks for 24 months | Grading | - | Pre: 55, Post: 31 | 40 | 46 | 88 | 24 | BP: HER2+: 5, HER2−: 30 | BP: PR+: 24, PR−: 11 | BP: ER+: 30, ER−: 5 |
| BP: I/II: 33, III: 5 | Post: 26 | Post: 29 | Control: HER2+: 5, HER2−: 28 | Control: PR+: 23, PR−: 11 | Control: ER+: 30, ER−: 4 | |||||||||||
| Control: I/II: 35, III: 5 | ||||||||||||||||
| Nodal+: BP: 4, control: 6 | ||||||||||||||||
| Nodal−: BP: 35, control: 35 | ||||||||||||||||
| 9 | Ishikawa et al. [27] | 2017 | Japan | Randomized phase II trial | FEC100 Paclitaxel | Zoledronic acid, (4 mg) 3–4 times weekly for 7 weeks | IIA–IIIB | 20–70 | - | 93 | 95 | 36 | 36–60 | Case: 0, Control: 0 | - | - |
| (T ≥ 3.0 cm and node negative, or T ≥ 2.0 cm and cytologically or pathologically defined as node positive) Zoledronic acid (4 mg) 3–4 times weekly for 7 weeks | ||||||||||||||||
| 10 | Powles et al. [34] | 2006 | UK | Randomized double-blind, placebo-controlled trial | Chemotherapy tamoxifen | Clodronate, 1,600 mg/day during a 2 year | Stage 1–3 | 52.8 ± 10.6 | Pre: 530, Post: 539 | 530 | 539 | 67.2 | 24 | - | BP: PR+: 112, PR−: 79 | BP: ER+: 245, ER−: 136 |
| BP: TI: 137, TII: 304, TIII: 48 | 52.7 ± 10.5 | Post: 265 | Post: 274 | Control: PR+: 116, PR−: 75 | Control: ER+: 240, ER−: 136 | |||||||||||
| Control: TI: 143, TII: 305, TIII: 52 | ||||||||||||||||
| 11 | Aft et al. [32] | 2010 | USA | Open label, randomised, phase 2 trial | Epirubicin, docetaxel | Zoledronic acid, 4mg intravenous ZOL every 3 weeks for 1 year (total 17 doses) | BP: GI: 7, GII: 20, GIII: 33 | 18–56 | Pre: 64, Post: 55 | 60 | 59 | 60 | 12 | Case: 13, Control: 10 | BP: PR+: 24, PR−: | BP: ER+: 32, ER−: 28 |
| Control: GI: 2, GII: 28, GIII: 29 | Post: 29 | Post: 26 | Control: PR+: 31, PR−: | Control: ER+: 34, ER−: 28 | ||||||||||||
| 12 | Kristensen et al. [38] | 2008 | Sweden | Randomized Clinical trial | CMF, CEF | Pamidronate, 150 mg twice daily for 4 years | BP: GI: 35, GII: 181, GIII: 151 | 39 ≥ to 69 | Pre: 634, Post: 318 | 460 | 493 | 120 ≤ | 36 | - | BP: PR+: 51, PR−: 135 | BP: ER+: 62, ER−: 278 |
| Control: GI: 23, GII: 202, GIII: 168 | Post: 152 | Post: 166 | Control: PR+: 54, PR−: 136 | Control: ER+: 85, ER−: 261 | ||||||||||||
| 13 | Body et al. [37] | 2003 | USA | Double-blind, placebo-controlled, parallel-group, multicentre, phase III study | Endocrine treatment chemotherapy | Gp1: Ibandronate (2 mg) (n = 154), Gp2: Ibandronate (6 mg) (n = 154), every 3–4 weeks for up to 2 years | - | 55.3 | - | Gp1: 154 | 158 | 12.67 | 19.5 | - | - | - |
| Gp2: 154 | ||||||||||||||||
| 14 | Delmas et al. [19] | 1997 | UK | Double-Blind, Placebo-Controlled | Placebo | Risedronate, eight cycles oral risedronate 30 mg/d daily for 2 weeks followed by 10 weeks of no drug (12 weeks per cycle) | - | 36–55 | Pre: 0, Post: 53 | 27 | 26 | 36 | 36 | - | - | - |
| Post: 27 | Post: 0 | |||||||||||||||
| 15 | Eidtmann et al. [25] | 2010 | UK | Open-label, multicenter, randomized study | Letrozole | Zoledronic acid, immediate zoledronic acid (ZOL; 4 mg every 6 months) or delayed ZOL (initiated only for fracture or high risk thereof) | Stage I–IIIa | 58 | Post: 884, Pre: 176 | 524 | 536 | 36 | 11.84 | - | - | - |
| Post: 438 | Post: 448 | |||||||||||||||
| 16 | Diel et al. [33] | 1998 | Germany | Prospective, randomized, non–placebo-controlled study | Tamoxifen, CMF, CEF | Clodronate, 1,600 mg of oral clodronate per day for two years | BP: T1: 59, T2: 71, T3: 17, T4: 10 | 51 | Post: 189, Pre: 113 | 157 | 145 | 36 | 24 | - | BP: PR+: 85, PR−: 51 | BP: ER+: 104, ER−: 35 |
| Control: T1: 54, T2: 67, T3: 18, T4: 6 | Post: 101 | Post: 88 | Control: PR+: 72, PR−: 42 | Control: ER+: 84, ER−: 34 | ||||||||||||
| Nodal | ||||||||||||||||
| BP: N0: 77, N1 or N2: 80 | ||||||||||||||||
| Control: N0: 66, N1 or N2: 79 | ||||||||||||||||
| G1, or II: BP: 93, control: 92 | ||||||||||||||||
| GIII: BP: 44, control: 34 | ||||||||||||||||
| 17 | Saarto et al. [35] | 2003 | Finland | Randomized Controlled Trial | CMF | Clodronate, 1,600 mg daily for 3 years | BP: T1: 71, T2: 59, T3: 9 | 52 | Post: 134, Pre: 148 | 139 | 143 | 120 | 36 | - | BP: PR+: 70, PR−: 62 | BP: ER+: 85, ER−: 48 |
| Control: T1: 66, T2: 65, T3: 9 | Post: 72 | Post: 62 | Control: PR+: 86, PR−: 44 | Control: ER+: 97, ER−: 33 | ||||||||||||
| 18 | Ahn et al. [23] | 2014 | Korea | Retrospective | Aromatase inhibitors | Zoledronic acid, 4 mg of ZA was administered intravenously every 3 to 6 months | Histologic grade | 56–57 | Post: 235 | 77 | 158 | 68 | 45 | Case: 13, Control: 30 | Case: 64, Control: 132 | Case: HER+: 74, HER−: 3 |
| BP: I or II: 61, III: 8 | Post: 77 | Post: 158 | Control: HER+: 148, HER−: 10 | |||||||||||||
| Control: I or II: 130, III: 19 | ||||||||||||||||
| T stage | ||||||||||||||||
| BP: T1: 57, T2: 19, T3: 1 | ||||||||||||||||
| Control: T1: 101, T2: 55, T3: 2 | ||||||||||||||||
| N stage | ||||||||||||||||
| BP: N0: 50, N1: 20, N2: 7, N3: 0 | ||||||||||||||||
| Control: N0: 90, N1: 55, N2: 9, N3: 4 | ||||||||||||||||
| Stage | ||||||||||||||||
| BP: SI: 39, SII: 31, SIII: 7 | ||||||||||||||||
| Control: SI: 59, SII: 85, SIII: 14 | ||||||||||||||||
| 19 | Jallouk et al. [21] | 2021 | USA | Randomized placebo-controlled phase II clinical trial in | Chemotherapy | Zoledronic acid, 4 mg intravenous ZOL every 3 weeks for 1 year (17 total doses) | Stage II–III (≥T2 and/or ≥N1) | 47–49 | Post: 55, Pre: 64 | 60 | 59 | 172.8 | 12 | Case: 13, Control: 10 | Case: 24, Control: 31 | Case: 32, Control: 34 |
| Grade | Post: 29 | Post: 26 | ||||||||||||||
| BP: GI: 7, GII: 20, GIII: 33 | ||||||||||||||||
| Control: GI: 2, GII: 28, GIII: 29 | ||||||||||||||||
| 20 | Aft et al. [39] | 2012 | USA | Randomized clinical trial | Chemotherapy | Zoledronic acid, 4 mg intravenous ZOL every 3 weeks for 1 year (total 17 doses) | Stage II–III | 49–50 | Post: 55, Pre: 64 | 59 | 60 | 61.9 | 12 | Case: 13, Control: 10 | - | Case: 32, Control: 34 |
| Grade I: 7 in BP; 2 in control | Post: 26 | Post: 29 | ER+/HER2+: 6 in BP; 5 in control | |||||||||||||
| Grade II: 20 in BP; 28 in control | ER+/HER2−: 26 in BP; 29 in control | |||||||||||||||
| Grade III: 33 in BP; 29 in control | ER−/HER2+: 7 in BP; 5 in control | |||||||||||||||
| ER−/HER2−: 21 in BP; 19 in control | ||||||||||||||||
| 21 | von Minckwitz et al. [36] | 2013 | Germany | Open-label, randomized, controlled phase III trial | Observation | Ibandronate | Tumor stage | 40–60 | Post: 1,549, Pre: 1,431 | 1996 | 998 | 38.7 | 24 | BP: HER2+: 415, HER2−: 1,467 | - | - |
| BP: pT1: 635, pT2: 1,112, pT3: 202, pT4: 41 | Post: 1,023 | Post: 526 | ||||||||||||||
| Control: pT1: 320, pT2: 557, pT3: 103, pT4: 14 | ||||||||||||||||
| Grade | ||||||||||||||||
| BP: G1: 63, G2: 987, G3: 943 | ||||||||||||||||
| Control: G1: 33, G2: 520, G3: 442 | ||||||||||||||||
| Node | ||||||||||||||||
| BP: N1: 7,361, N2: 696, N3: 539 | ||||||||||||||||
| Control: N1: 370, N2: 362, N3: 266 |
BP = bisphosphonate; F/U = follow-up; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor; ER = estrogen receptor; AJCC = American Joint Committee on Cancer; PO = per os; RCT = randomized controlled trial; CMF = cyclophosphamide, methotrexate, fluorouracil; CEF = cyclophosphamide, epirubicin, and fluorouracil.
Table 3. The data extraction table of the effect of BPs on the study outcomes.
| Author | Recurrence | Locoregional recurrence | Recurrence distant | Metastases | Bone metastases | Distant metastases | Death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | |
| Korde et al. [28] | - | - | 6 | 88 | 15 | 224 | - | - | - | - | - | - | 20 | 288 |
| Rack et al. [22] | 3 | 31 | - | - | - | - | - | - | - | - | - | - | 2 | 15 |
| Hadji et al. [20] | 69 | 137 | - | - | - | - | - | - | - | - | - | - | 44 | 91 |
| Gnant et al. [26] | - | - | 25 | 40 | 67 | 58 | 44 | 45 | - | - | - | - | 0 | 4 |
| Coleman et al. [31] | - | - | 110 | 106 | 403 | 431 | - | - | 224 | 188 | - | - | 62 | 63 |
| Paterson et al. [29] | - | - | 53 | 53 | 90 | 113 | - | - | - | - | - | - | 34 | 30 |
| Perrone et al. [30] | - | - | 4 | 24 | - | - | - | - | - | - | 19 | 64 | 8 | 28 |
| Banys et al. [24] | - | - | 1 | 4 | - | - | - | - | - | - | 3 | 5 | 1 | 5 |
| Ishikawa et al. [27] | - | - | 2 | 5 | 15 | 14 | - | - | - | - | - | - | 6 | 4 |
| Powles et al. [34] | - | - | - | - | - | - | 130 | 167 | 51 | 72 | - | - | 98 | 129 |
| Aft et al. [32] | - | - | - | - | - | - | 14 | 14 | - | - | - | - | - | - |
| Kristensen et al. [38] | - | - | - | - | - | - | - | - | 93 | 88 | - | - | - | - |
| Body et al. [37] | - | - | - | - | - | - | - | - | Gp1: 101 | 105 | Gp1: 59 | 53 | Gp1: 16 | 25 |
| Gp2: 106 | Gp2: 35 | Gp2: 23 | ||||||||||||
| Delmas et al. [19] | 1 | 1 | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| Eidtmann et al. [25] | - | - | 2 | 10 | 20 | 30 | - | - | - | - | - | - | 11 | 18 |
| Diel et al. [33] | - | - | - | - | - | - | 46 | 94 | 12 | 25 | 21 | 42 | 6 | 22 |
| Saarto et al. [35] | - | - | - | - | - | - | 76 | 60 | 44 | 42 | 27 | 15 | 60 | 47 |
| Ahn et al. [23] | - | - | 0 | 4 | - | - | - | - | - | - | 1 | 19 | - | - |
| Jallouk et al. [21] | 31 | 33 | - | - | - | - | - | - | - | - | - | - | 25 | 20 |
| von Minckwitz et al. [36] | - | - | - | - | - | - | 619 | 379 | - | - | - | - | - | - |
Of the included studies, 10,238 patients received BPs, among whom, the number of postmenopausal women was 388, whereas 10,743 patients did not receive BPs, or received another treatment, and were categorized as the control group, with a total 3,879 cases of postmenopausal women. In five studies, the authors did not report the data of the postmenopausal women. The follow-up period ranged from 12 to 120 months.
These studies were performed in six countries (USA, Germany, England, Italy, Sweden, and Japan). The earliest study began in 1997, whereas the most recent study was conducted in 2021.
The most commonly used BPs in the case group were zoledronic acid, clodronate, and ibronate. The control group received hormone therapy, cytostatic treatment, endocrine therapy, chemotherapy, goserelin, tamoxifen, or anastrozole.
Review findings
Twenty-one studies were identified as eligible for this systematic review and meta-analysis. We categorized and analyzed whether the study designs were RCTs or others. The results of this study are summarized in Tables 2 and 3.
HR with corresponding 95% CIs for each study and the combination of all included studies are presented in Figures 2, 3, 4, 5, 6, and Supplementary Table 1, respectively.
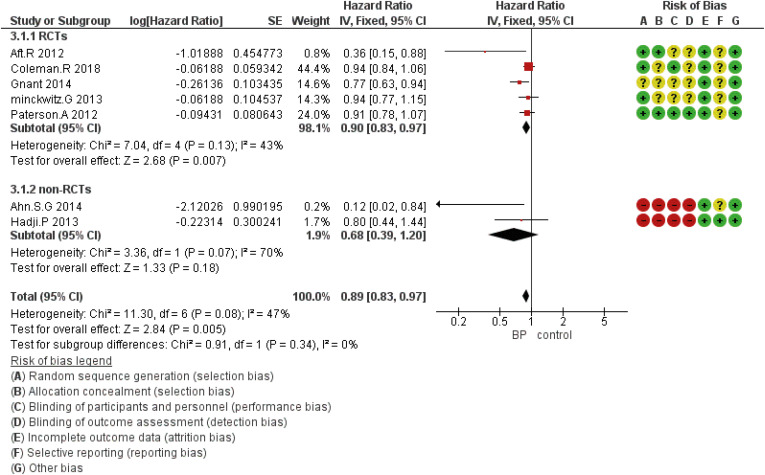
Figure 2. Forest plot for the bisphosphonates’ role in DFS. Square markers indicate effect sizes of each study; horizontal lines, the 95% CI. The diamond data marker indicates the summarized effect size.
DFS = disease-free survival; CI = confidence interval; HR = hazard ratio; SE = standard error; IV = generic inverse variance method; RCT = randomized controlled trial.
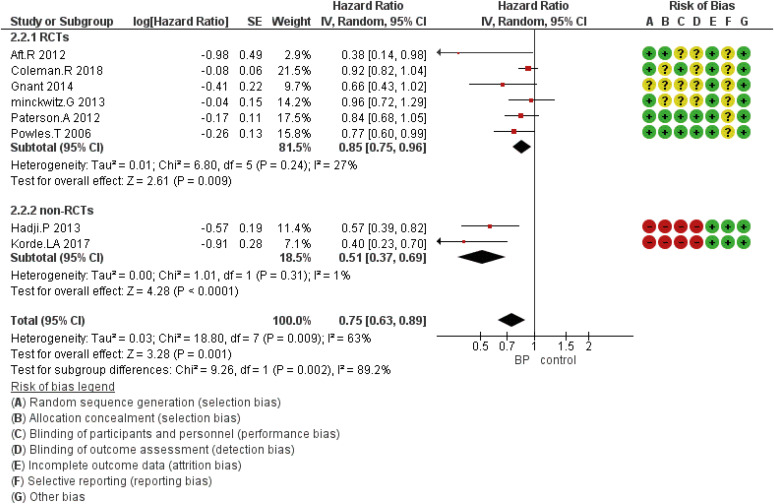
Figure 3. Forest plot for the bisphosphonates’ role in OS. Square markers indicate effect sizes of each study; horizontal lines, the 95% CI. The diamond data marker indicates the summarized effect size.
OS = overall survival; CI = confidence interval; HR = hazard ratio; SE = standard error; IV = generic inverse variance method; RCT = randomized controlled trial.
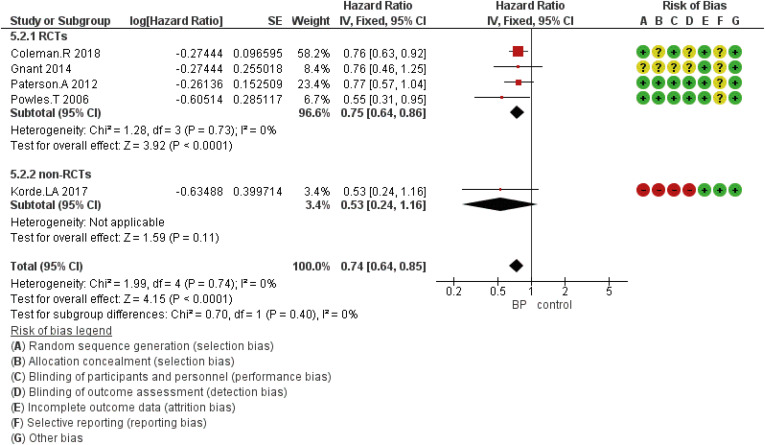
Figure 4. Forest plot for the BPs’ role in the prevention of bone metastasis of breast cancer in the perimenopausal period. Square markers indicate effect sizes of each study; horizontal lines, the 95% CI. The diamond data marker indicates the summarized effect size.
BP = bisphosphonate; CI = confidence interval; HR = hazard ratio; SE = standard error; IV = generic inverse variance method; RCT = randomized controlled trial.
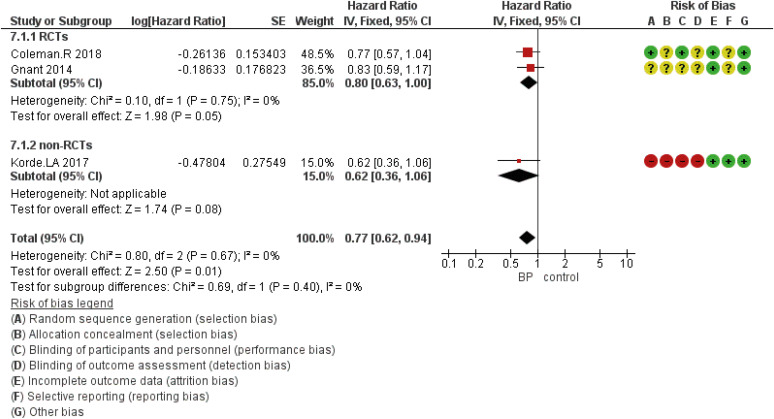
Figure 5. Forest plot for the BPs’ role in the prevention of distant metastasis of breast cancer in the perimenopausal period. Square markers indicate effect sizes of each study; horizontal lines, the 95% CI. The diamond data marker indicates the summarized effect size.
BP = bisphosphonate; CI = confidence interval; HR = hazard ratio; SE = standard error; IV = generic inverse variance method; RCT = randomized controlled trial.
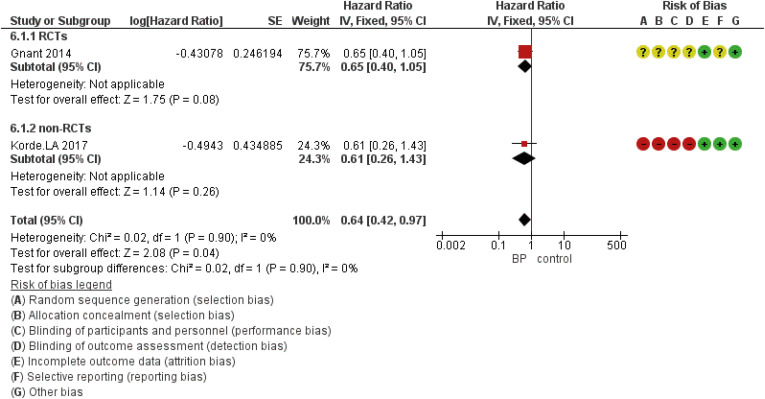
Figure 6. Forest plot for the BPs’ role in the prevention of locoregional metastasis in perimenopausal women with breast cancer. Square markers indicate the effect sizes of each study; horizontal lines, the 95% CI. The diamond data marker indicates the summarized effect size.
BP = bisphosphonate; CI = confidence interval; HR = hazard ratio; SE = standard error; IV = generic inverse variance method; RCT = randomized controlled trial.
According to the study findings, seven studies reported DFS, eight studies discussed OS, and two, three, and five studies investigated other outcomes including locoregional, bone, or distant metastasis, respectively.
Meta-analysis findings
DFS based on the study design was analyzed using five eligible RCTs and two non-randomized (cohort) studies. Although the results showed that the HR was statistically significant at 0.89 (95% CI, 0.83–0.97; p = 0.005), in subgroup analysis based on the study design, there was no statistically significant difference for included non-RCT studies (Figure 2). The HR for OS was 0.75 (95% CI, 0.63–0.89; p = 0.001) (Figure 3). The HR for bone metastases was shown in Figure 4. According to the results, the overall HR was 0.74 (95% CI, 0.64–0.85; p < 0.0001). The HR was significant (HR, 0.75; 95% CI, 0.64–0.86; p < 0.0001) for bone metastasis in the included RCTs, only (Figure 4). The HR for locoregional recurrence in women who received BPs was 0.64 (95% CI, 0.42–0.97; p = 0.04) (Figure 5). In addition, the analysis results showed that the HR was 0.77 (95% CI, 0.62–0.94; p = 0.01) for distant metastasis (Figure 6).
DISCUSSION
The results of this systematic review and meta-analysis showed that among 21 studies, BPs had a promising effect on DFS, OS, and bone metastasis among perimenopausal women survivors of breast cancer.
Evidently, breast cancer is a concern not only in the Western world but is one of the most common cancers among women in the developing world because of increased life expectancy, urbanization, and adoption of Western lifestyles. With early diagnosis and improvement in the treatment of breast cancer, survival has increased significantly. The overall survival rate of patients with breast cancer varies worldwide. Generally, it shows improvement due to early diagnosis at an earlier and localized stage, improved surgical techniques, and the availability of adjuvant treatment regimens. Stage I/II (only spreading to the tissues or nodes under the arm) breast cancer has a higher rate of five-year survival rate of approximately 80%–90%. However, in the distant stage (spreading to the distant lymph nodes or organs), this rate decreases to 24% [40].
BPs are analogs of endogenous pyrophosphates that replace carbon atoms instead of the central atom of oxygen. In vivo studies have shown that BPs are potent inhibitors of osteoclast-mediated bone resorption, which bind strongly to hydroxyapatite on the bone surface, and decrease the serum calcium concentrations in malignancy-related hypercalcemia.
The mechanisms by which BPs prevent osteoclast-mediated bone resorption involve the inhibition of osteoclast formation from immature precursor cells, apoptosis induction of mature osteoclasts, or direct inhibition of resorption. Furthermore, they inhibited the progression and development of bone metastases in a mouse model of breast cancer [41]. In recent years, the role of BPs in skeletal-related events in patients with breast cancer and metastatic bone disease has been investigated [42,43]. BPs increase the bone mineral density of lumbar and hip joints in premenopausal and postmenopausal women with breast cancer [44,45]. Other roles of BPs have been reported in previous studies, including antitumor effects, apoptosis, reduced proliferation, and inhibition of tumor cell migration and invasion [46].
Recently, the administration of BPs as adjuvant therapy has been recommended for postmenopausal patients with breast cancer. However, the final decision of its administration should be made during consultation between the patient and oncologist, considering the recurrence risk and the adverse effects [47].
A previous EBCTCG meta-analysis found a beneficial effect of BPs in all subgroups of postmenopausal patients; however, the absolute benefit was small [48], and reportedly, in patients with a low risk of recurrence, the administration of BPs has a low clinically meaningful effect [47].
The most recommended BPs for adjuvant therapy in breast cancer are zoledronic acid and clodronate. In the studies included in our meta-analysis, these two agents were commonly used.
According to the results of the SWOG S0307 trial, clodronate, ibandronate, and zoledronic acid may provide similar DFS and OS benefits [49]. Owing to the limited number of included studies, we could not perform a subgroup analysis on the type of administered BPs.
The EBCTCG meta-analysis found that clodronate in postmenopausal patients (1,600 mg/d for 2–3 years) (4.6% vs. 7.0%; relative risk [RR], 0.57; 95% CI, 0.41–0.79; p = 0.0007) and zoledronic acid (3.4% vs. 4.5%; RR, 0.73; 99% CI, 0.53–1.00) significantly reduced bone recurrence [48]. However, we did not observe any significant reduction in bone recurrence in our meta-analysis. This may be attributed to the absence of a subgroup analysis in our study, including women in the perimenopausal period.
Regarding the study inclusion criteria in the current study, BPs show promising effects on DFS, OS, preventing mortality, and bone metastasis among perimenopausal women survivors of breast cancer. However, we could not identify any role of BPs in the subgroup analysis of only RCTs, except for that in bone metastasis. The present study had certain limitations. First, we analyzed the data of RCTs and cohort studies. We limited our keywords to the postmenopausal period; however, our results showed that in some of the included studies, the authors did not report the recurrence rate or metastasis in the pre-or postmenopausal period. Therefore, we manually searched again to retrieve other studies on the perimenopausal period. We did not perform a subgroup analysis for the post-menopausal period, duration of follow-up, and the dosage used, or regarding the hormone receptors or HER2 status, owing to the limited number of studies that reported them for our desirable outcomes, and only mentioned them in a narrative form, which may affect the results of our meta-analysis. Another limitation was the lack of subgroup analysis for the type of BPs or the study design.
In conclusion, the results of this meta-analysis suggest a promising effect of BPs on DFS, OS, locoregional, distant, and bone metastasis among perimenopausal women survivors of breast cancer. This study might help convey information to clinicians and patients regarding the rational use of BPs in the prevention of breast cancer recurrence.
Appendix 1
Search Strategy of Embase
('breast cancer'/exp OR 'advanced breast cancer':ti,ab OR 'breast cancer':ti,ab OR 'breast cancer recurrence':ti,ab OR 'breast gland cancer':ti,ab OR 'breast gland neoplasm':ti,ab OR 'mamma cancer':ti,ab OR 'mammary cancer':ti,ab OR 'mammary gland cancer':ti,ab OR 'breast tumor'/exp OR 'breast gland tumor':ti,ab OR 'breast gland tumour':ti,ab OR 'breast mass':ti,ab OR 'breast tumor':ti,ab OR 'breast tumour':ti,ab OR 'female breast neoplasm':ti,ab OR 'female breast tumor':ti,ab OR 'female breast tumour':ti,ab OR 'male breast neoplasms':ti,ab OR 'mamma tumor':ti,ab OR 'mamma tumour':ti,ab OR 'mammary gland tumor':ti,ab OR 'mammary gland tumour':ti,ab OR 'mammary neoplasms':ti,ab OR 'mammary tumor':ti,ab OR 'mammary tumor cell':ti,ab OR 'mammary tumour':ti,ab OR 'mammary tumour cell':ti,ab OR 'unilateral breast neoplasms':ti,ab OR 'breast neoplasm*':ti,ab OR 'postmenopausal breast cancer'/exp OR 'postmenopausal breast cancer':ti,ab) AND ('bisphosphonic acid derivative'/exp OR 'biphosphonate':ti,ab OR 'biphosphonates':ti,ab OR 'bisphosphonate':ti,ab OR 'bisphosphonate derivative':ti,ab OR 'bisphosphonates':ti,ab OR 'bisphosphonic acid derivative':ti,ab OR 'diphosphonate derivative':ti,ab OR 'diphosphonate series':ti,ab OR 'diphosphonates':ti,ab OR 'diphosphonic acid derivative':ti,ab) AND ('recurrence risk'/exp OR 'recidivation risk':ti,ab OR 'recidivism risk':ti,ab OR 'recurrence rate':ti,ab OR 'recurrence risk':ti,ab OR 'relapse rate':ti,ab OR 'risk recidivism':ti,ab OR 'risk, recurrence':ti,ab OR recurrence:ti,ab)
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Sanaat Z.
- Data curation: Sanaat Z, Khanzadeh M, Kabiri N, Salehi-Pourmehr H.
- Formal analysis: Salehi-Pourmehr H.
- Methodology: Sanaat Z, Nouri O, Vahed N, Ali Akbari Khoei R, Salehi-Pourmehr H.
- Supervision: Salehi-Pourmehr H.
- Writing - original draft: Nouri O, Salehi-Pourmehr H.
- Writing - review & editing: Sanaat Z, Mostafaei H.
SUPPLEMENTARY MATERIAL
The amount of reported HR of the included studies
References
- 1.Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13:1387–1397. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1–7. doi: 10.1007/978-3-030-20301-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharm Nanotechnol. 2019;7:3–23. doi: 10.2174/2211738507666190122111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis MD, Russell RG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science. 1969;165:1264–1266. doi: 10.1126/science.165.3899.1264. [DOI] [PubMed] [Google Scholar]
- 5.Billington EO, Reid IR. Benefits of bisphosphonate therapy: beyond the skeleton. Curr Osteoporos Rep. 2020;18:587–596. doi: 10.1007/s11914-020-00612-4. [DOI] [PubMed] [Google Scholar]
- 6.van Hellemond IEG, Smorenburg CH, Peer PGM, Swinkels ACP, Seynaeve CM, van der Sangen MJC, et al. Breast cancer outcome in relation to bone mineral density and bisphosphonate use: a sub-study of the DATA trial. Breast Cancer Res Treat. 2020;180:675–685. doi: 10.1007/s10549-020-05567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou YJ, Chiu HF, Wong YH, Yang CC, Yang YH. Bisphosphonate use and the risk of breast cancer: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2017;26:1286–1295. doi: 10.1002/pds.4302. [DOI] [PubMed] [Google Scholar]
- 8.Clezardin P. Potential anticancer properties of bisphosphonates: insights from preclinical studies. Anticancer Agents Med Chem. 2012;12:102–113. doi: 10.2174/187152012799014977. [DOI] [PubMed] [Google Scholar]
- 9.Strobl S, Korkmaz B, Devyatko Y, Schuetz M, Exner R, Dubsky PC, et al. Adjuvant bisphosphonates and breast cancer survival. Annu Rev Med. 2016;67:1–10. doi: 10.1146/annurev-med-053014-103600. [DOI] [PubMed] [Google Scholar]
- 10.Suarez-Almazor ME, Herrera R, Lei X, Chavez-MacGregor M, Zhao H, Giordano SH. Survival in older women with early stage breast cancer receiving low-dose bisphosphonates or denosumab. Cancer. 2020;126:3929–3938. doi: 10.1002/cncr.33035. [DOI] [PubMed] [Google Scholar]
- 11.Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Vidula N, Greenberg S, Petrillo L, Hwang J, Melisko M, Goga A, et al. Evaluation of disseminated tumor cells and circulating tumor cells in patients with breast cancer receiving adjuvant zoledronic acid. NPJ Breast Cancer. 2021;7:113. doi: 10.1038/s41523-021-00323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sing CW, Wong AY, Kiel DP, Cheung EY, Lam JK, Cheung TT, et al. Association of alendronate and risk of cardiovascular events in patients with hip fracture. J Bone Miner Res. 2018;33:1422–1434. doi: 10.1002/jbmr.3448. [DOI] [PubMed] [Google Scholar]
- 14.Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J Cancer. 2012;131:E717–E725. doi: 10.1002/ijc.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranenburg G, Bartstra JW, Weijmans M, de Jong PA, Mali WP, Verhaar HJ, et al. Bisphosphonates for cardiovascular risk reduction: a systematic review and meta-analysis. Atherosclerosis. 2016;252:106–115. doi: 10.1016/j.atherosclerosis.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Hue TF, Cummings SR, Cauley JA, Bauer DC, Ensrud KE, Barrett-Connor E, et al. Effect of bisphosphonate use on risk of postmenopausal breast cancer: results from the randomized clinical trials of alendronate and zoledronic acid. JAMA Intern Med. 2014;174:1550–1557. doi: 10.1001/jamainternmed.2014.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn Z, Aromataris E, Tufanaru C, Stern C, Porritt K, Farrow J, et al. The development of software to support multiple systematic review types: the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI) Int J Evid-Based Healthc. 2019;17:36–43. doi: 10.1097/XEB.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 19.Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol. 1997;15:955–962. doi: 10.1200/JCO.1997.15.3.955. [DOI] [PubMed] [Google Scholar]
- 20.Hadji P, Frank M, Jakob A, Siebers JW. Effect of adjuvant bisphosphonates on disease-free survival in early breast cancer: retrospective analysis results in an unselected single-center cohort. J Bone Oncol. 2013;2:2–10. doi: 10.1016/j.jbo.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jallouk AP, Paravastu S, Weilbaecher K, Aft RL. Long-term outcome of (neo)adjuvant zoledronic acid therapy in locally advanced breast cancer. Breast Cancer Res Treat. 2021;187:135–144. doi: 10.1007/s10549-021-06100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rack B, Jückstock J, Genss EM, Schoberth A, Schindlbeck C, Strobl B, et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30:1807–1813. [PubMed] [Google Scholar]
- 23.Ahn SG, Kim SH, Lee HM, Lee SA, Jeong J. Survival benefit of zoledronic acid in postmenopausal breast cancer patients receiving aromatase inhibitors. J Breast Cancer. 2014;17:350–355. doi: 10.4048/jbc.2014.17.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banys M, Solomayer EF, Gebauer G, Janni W, Krawczyk N, Lueck HJ, et al. Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer. 2013;13:480. doi: 10.1186/1471-2407-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 26.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26:313–320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa T, Akazawa K, Hasegawa Y, Tanino H, Horiguchi J, Miura D, et al. Survival outcomes of neoadjuvant chemotherapy with zoledronic acid for HER2-negative breast cancer. J Surg Res. 2017;220:46–51. doi: 10.1016/j.jss.2017.05.066. [DOI] [PubMed] [Google Scholar]
- 28.Korde LA, Doody DR, Hsu L, Porter PL, Malone KE. Bisphosphonate use and risk of recurrence, second primary breast cancer, and breast cancer mortality in a population-based cohort of breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2018;27:165–173. doi: 10.1158/1055-9965.EPI-17-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13:734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone F, De Laurentiis M, De Placido S, Orditura M, Cinieri S, Riccardi F, et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer. 2019;118:178–186. doi: 10.1016/j.ejca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04) J Bone Oncol. 2018;13:123–135. doi: 10.1016/j.jbo.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11:421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 34.Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 36.von Minckwitz G, Möbus V, Schneeweiss A, Huober J, Thomssen C, Untch M, et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol. 2013;31:3531–3539. doi: 10.1200/JCO.2012.47.2167. [DOI] [PubMed] [Google Scholar]
- 37.Body JJ, Diel IJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol. 2003;14:1399–1405. doi: 10.1093/annonc/mdg367. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen B, Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Bjerregaard B, et al. Bisphosphonate treatment in primary breast cancer: results from a randomised comparison of oral pamidronate versus no pamidronate in patients with primary breast cancer. Acta Oncol. 2008;47:740–746. doi: 10.1080/02841860801964988. [DOI] [PubMed] [Google Scholar]
- 39.Aft RL, Naughton M, Trinkaus K, Weilbaecher K. Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breast cancer. Br J Cancer. 2012;107:7–11. doi: 10.1038/bjc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Breast cancer now most common form of cancer: WHO taking action. 2021. [Accessed March 2nd, 2022]. https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common-form-of-cancer-who-taking-action .
- 41.Senaratne SG, Colston KW. Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res. 2002;4:18–23. doi: 10.1186/bcr412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadji P, Coleman RE, Wilson C, Powles TJ, Clézardin P, Aapro M, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016;27:379–390. doi: 10.1093/annonc/mdv617. [DOI] [PubMed] [Google Scholar]
- 43.Baba K, Kaida H, Hattori C, Muraki K, Kugiyama T, Fujita H, et al. Tumoricidal effect and pain relief after concurrent therapy by strontium-89 chloride and zoledronic acid for bone metastases. Hell J Nucl Med. 2018;21:15–23. doi: 10.1967/s002449910702. [DOI] [PubMed] [Google Scholar]
- 44.Sun S, Wang F, Dou H, Zhang L, Li J. Preventive effect of zoledronic acid on aromatase inhibitor-associated bone loss for postmenopausal breast cancer patients receiving adjuvant letrozole. Onco Targets Ther. 2016;9:6029–6036. doi: 10.2147/OTT.S115058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyvernitakis I, Kann PH, Thomasius F, Hars O, Hadji P. Prevention of breast cancer treatment-induced bone loss in premenopausal women treated with zoledronic acid: final 5-year results from the randomized, double-blind, placebo-controlled ProBONE II trial. Bone. 2018;114:109–115. doi: 10.1016/j.bone.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 46.von Minckwitz G, Rezai M, Tesch H, Huober J, Gerber B, Zahm DM, et al. Zoledronate for patients with invasive residual disease after anthracyclines-taxane-based chemotherapy for early breast cancer - The Phase III NeoAdjuvant Trial Add-oN (NaTaN) study (GBG 36/ABCSG 29) Eur J Cancer. 2016;64:12–21. doi: 10.1016/j.ejca.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Dhesy-Thind S, Fletcher GG, Blanchette PS, Clemons MJ, Dillmon MS, Frank ES, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 48.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 49.Gralow JR, Barlow WE, Paterson AH, M’iao JL, Lew DL, Stopeck AT, et al. Phase III randomized trial of bisphosphonates as adjuvant therapy in breast cancer: S0307. J Natl Cancer Inst. 2020;112:698–707. doi: 10.1093/jnci/djz215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The amount of reported HR of the included studies