Abstract
Introduction
“Other oncocytic renal tumors of the kidney” is a new category constituted by 2022 WHO classification and different in the point of morphology and immunohistochemistory from typical oncocytic/eosinophilic renal tumors including chromophobe renal cell carcinoma and oncocytoma.
Case presentation
The patient was an 84‐year‐old woman in whom a left renal tumor was incidentally discovered. She underwent left nephrectomy, and the pathological specimens showed a borderline eosinophilic renal tumor between chromophobe renal cell carcinoma and renal oncocytoma. After all recognized oncocytic tumors were excluded, we diagnosed the tumor as other oncocytic renal tumor of the kidney.
Conclusion
Other oncocytic renal tumor of the kidney is a provisional category. Therefore, further research and accumulation of similar cases are necessary.
Keywords: kidney neoplasms, renal cell carcinoma, renal oncocytoma, surgical pathology, urologic neoplasms
Abbreviations & Acronyms
- BHD syndrome
Birt-Hogg-Dube syndrome
- ChRCC
chromophobe renal cell carcinoma
- CK7
cytokeratin 7
- CT
computed tomography
- GUPS
the Genitourinary Pathology Society
- H&E
hematoxylin and eosin
- HOCT
hybrid oncocytic chromophobe tumor
- LOT
low-grade oncocytic tumor
- oChRCC
chromophobe renal cell carcinoma, oncocytic variant
Keynote message.
Recently, 2022 WHO classification proposed a new category, other oncocytic renal tumors of the kidney. The present case is included in this entity. This information will assist pathologists who encounter renal eosinophilic tumors that do not fit the criteria of oncocytoma and chromophobe renal cell carcinoma.
Introduction
Chromophobe renal cell carcinoma (ChRCC) is a primary renal malignant tumor that can be histopathologically classified into conventional and eosinophilic variants. 1 It is crucial to differentiate the latter from other renal eosinophilic tumors, especially oncocytoma, which is a benign tumor. 2 Therefore, immunohistochemistry is often used for their differentiation. Usually, ChRCC is positive for c‐kit (CD117) and cytokeratin 7 (CK7), whereas oncocytoma is positive for c‐kit but negative or focally positive for CK7. Here, we experienced a case of renal eosinophilic tumor that required immunohistochemistry to differentiate ChRCC and oncocytoma but led to an unusual immunoprofile: negative for c‐kit and positive for CK7. Recently, a new category “Other oncocytic renal tumors of the kidney” has been proposed for such cases. 3 We report a case of this category with a review of the literature.
Case presentation
The patient was an 84‐year‐old woman who underwent surgery for lumbar canal stenosis at the nearest orthopedic hospital. Six months after the operation, a follow‐up computed tomography (CT) scan incidentally showed a mass in the left upper part of the kidney. She was referred to the Urology Department at our hospital for further examination and treatment. She had a medical history of lumbar canal stenosis and hypertension but no significant family history. No notable findings were observed on physical examinations and blood tests. Microscopic hematuria (red blood cells 5–9/high power field) was present on the urine test. Contrast‐enhanced dynamic CT showed a 31‐mm mass without early enhanced lesions in the upper part of the left kidney (Fig. 1). In addition, no remarkable finding was noted in the lungs. Consequently, the patient was diagnosed with renal cell carcinoma, clinical‐stage T1aN0M0, and underwent laparoscopic radical left nephrectomy.
Fig. 1.

(a) Plain CT showing a 31‐mm mass in the upper part of the left kidney. (b)–(e) Four‐phase contrast‐enhanced CT. (b) Arterial phase. (c) Corticomedullary phase. (d) Nephrographic phase. (e) Excretory phase. Early enhanced lesions were not seen.
Pathological findings
Grossly, the mass was a well‐circumscribed 3.3 × 2.3 × 1.5 cm tumor without a fibrous capsule, and the cut surface showed a brown and exophytic appearance (Fig. 2). Histologically, hematoxylin and eosin (H&E) staining showed that tumor cells had round nuclei and eosinophilic cytoplasm with tubular, microcystic growth patterns (Fig. 3a). Perinuclear halo and capillary networks were not prominent (Fig. 3b). Venous invasion was recognized at the fat tissue in the renal pelvis (Fig. 3c). On immunohistochemical staining, tumor cells were positive for CK7, AE1/AE3, and vimentin, and negative for c‐kit, CD10, CA9, AMACR, PAX8, HMB45, and CK20 (Fig. 3d). SDHB and FH expression were retained (positive). Ki‐67 labeling index was less than 1%.
Fig. 2.

(a) The mass was a well‐circumscribed 3.3 × 2.3 × 1.5 cm tumor. (b) The cut surface showed a brown and exophytic appearance.
Fig. 3.
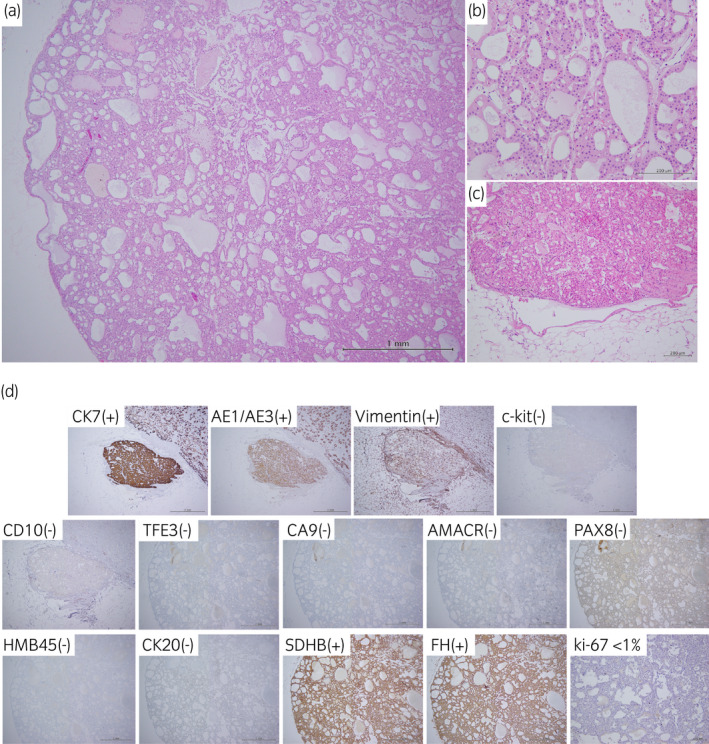
(a) The tumor showed tubular, microcystic growth patterns. (b) Tumor cells had round nuclei and eosinophilic cytoplasm. Perinuclear halo and capillary networks were not prominent (H&E staining). (c) Venous invasion was detected at the fat tissue in the renal pelvis (H&E staining). (d) Tumor cells were positive for CK7, AE1/AE3, and vimentin, and negative for c‐kit, CD10, TFE3, CA9, AMACR, PAX8, HMB45, and CK20. SDHB and FH expression were retained (positive). Ki‐67 labeling index was less than 1%.
Discussion
It is sometimes difficult to differentiate a primary renal tumor with eosinophilic cytoplasm in daily diagnostic practice because it has a wide‐ranging differential diagnosis 1 , 2 , 5 , 6 (Table 1).
Table 1.
| Diagnosis | HE | Immunohistochemistry |
|---|---|---|
| Chromophobe renal cell carcinoma | Solid sheets, separated by often hyalinized vascular septa, large pale cells with prominent cell membranes and perinuclear haloes | c‐kit(+), CK7(+) |
| Oncocytoma of the kidney | Sharply demarcated tumor with oncocytic cells forming solid nests, tubules, or microcysts; absence of raisinoid nuclei and perinuclear haloes | c‐kit(+), CK7(−) |
| Clear cell renal cell carcinoma | Nested, tubular, or alveolar growth pattern, composed of cells with optically clear or eosinophilic cytoplasm | c‐kit(−), CA9(+), CD10(+), Vimentin(+) |
| Papillary renal cell carcinoma | Variable proportions of papillary and tubular architecture lined by cuboidal to columnar cells, having a clear or vacuolated appearance or being voluminous and eosinophilic | AMACR(+), CD10(+), Vimentin(+) |
| Epithelioid angiomyolipoma/epithelioid PEComa of the kidney | Nests of atypical large eosinophilic cells with prominent nucleoli and intranuclear inclusions or epithelioid and plump spindle cells in diffuse growth, consists of sheets of voluminous cells and spindle cells with atypia and pale cytoplasm | AE1/AE3(−), CK7(−), PAX8(−), HMB45(+) |
| Eosinophilic solid and cystic renal cell carcinoma | Solid and cystic architecture; voluminous eosinophilic cytoplasm with coarse basophilic stippling | c‐kit(−), CK7(−), CK20(+) |
| Fumarate hydratase‐deficient renal cell carcinoma | Papillary, solid, tubulocystic, cribriform/sieve‐like, and cystic pattern with eosinophilic macronucleoli | FH(−) |
| Succinate dehydrogenase‐deficient renal cell carcinoma | sheets or compact nests of bland cells with eosinophilic cytoplasm, which may have a pale, bubbly appearance | SDHB(−) |
| TFE3‐rearranged renal cell carcinomas | papillary neoplasm composed of clear to densely granular and eosinophilic cells with abundant psammoma bodies | TFE3(+) |
| The present case | tubular, microcystic growth patterns with round nuclei and eosinophilic cytoplasm; without raisinoid nuclei and perinuclear haloes | c‐kit(−), CK7(+), CA9(−), CD10(−), Vimentin(+), AMACR(−), AE1/AE3(+), PAX8(−), HMB45(−), CK20(−), FH(+), SDHB(+), TFE3(−) |
The present case (the bottom of the list) is not consistent with the other entities.
ChRCC usually shows a well‐circumscribed cut surface and varies in color from light tan to brown macroscopically. The tumor cells are typically arranged in a solid sheet‐like pattern, and other architectural patterns include small nests and tubular, microcystic, and trabecular patterns. Conventional ChRCC shows predominantly large pale cells with reticular cytoplasm and a prominent cell membrane that is plant cell‐like. The eosinophilic variant of ChRCC has small cells with a fine oxyphilic granular cytoplasm. The cells often have an irregular wrinkled (so‐called raisinoid) nuclei with a perinuclear halo. 1 On the other hand, oncocytoma has a cut surface that varies from mahogany brown to tan or yellow, with an occasional central scar. Microscopically, it has a solid‐nested architecture and small islands of oncocytic cells with round and regular nuclei. 2 The present case resembled oncocytoma more than eosinophilic variant of ChRCC in H&E staining but showed unusual immunoprofile (negative for c‐kit and positive for CK7) inconsistent with oncocytoma. Other differential diagnoses include clear cell renal cell carcinoma, papillary renal cell carcinoma, epithelioid angiomyolipoma/epithelioid PEComa of the kidney, eosinophilic solid and cystic renal cell carcinoma, fumarate hydratase‐deficient renal cell carcinoma, succinate dehydrogenase‐deficient renal cell carcinoma, and TFE3‐rearranged renal cell carcinomas as shown in Table 1. However, the immunoprofile in the present case did not fit these entities. Hybrid oncocytic chromophobe tumor (HOCT), associated with hereditary Birt–Hogg–Dube (BHD) syndrome and multiple lung cysts, has the borderline features of ChRCC and oncocytoma. 2 HOCT is characterized by the presence of oncocytoma and chromophobe cells that are found coexisting within the same nests. The case we experienced had no familial history of hereditary tumor syndrome and no pulmonary lesion on chest CT and showed predominantly oncocytoma‐like cells, not chromophobe cells.
In Japan, Kuroda et al. reported a renal oncocytic tumor case, which showed predominantly a tubular growth pattern without perinuclear halo and was negative for c‐kit and positive for CK7 in immunohistochemistry. They named the tumor “chromophobe renal cell carcinoma, oncocytic variant (oChRCC)”. 4 On the other hand, Trpkov et al. reported a case series of renal oncocytic tumors named “low‐grade oncocytic tumor (LOT)”. 5 LOT is a c‐kit negative and CK7 positive tumor. Therefore, it has a similarity with oChRCC in immunoprofile. However, LOT has predominantly a solid or nested growth pattern and often exhibits perinuclear halo. 5 , 6 , 7 Therefore, the present case has more similarity with the case presented by Kuroda et al. (oChRCC) than Trpkov et al. (LOT).
Recently, 2022 WHO classification constituted a new category, “Other oncocytic tumours of the kidney”. 3 This is a heterogeneous group of renal eosinophilic/oncocytic tumors that show overlapping or borderline features between ChRCC and oncocytoma and have an indolent clinical course. This category is not a specific entity and requires the exclusion of ChRCC, oncocytoma, and other well‐defined oncocytic tumors. On the other hand, the Genitourinary Pathology Society (GUPS) proposed a similar category, “Oncocytic renal neoplasm of low malignant potential, not further classified,” reserved for borderline cases after excluding other differential diagnoses. 8 Therefore, it is appropriate to diagnose the present case as other oncocytic tumor of the kidney or oncocytic renal neoplasm of low malignant potential, not further classified. Accumulating more knowledge about these borderline tumors is desirable to clarify the heterogeneous group and re‐classify distinct entities. Finally, the present case was diagnosed as pathological‐stage T3a for the venous invasion at the fat tissue in the renal pelvis (Fig. 3c). No adjuvant therapy was performed, and the patient is still alive without recurrence 11 months after the surgery.
Conclusion
We experienced a case of other oncocytic tumor of the kidney, a tumor that morphologically resembled oncocytoma but showed unusual immunoprofile. When the immunoprofile is not consistent with conventional renal eosinophilic tumors, other oncocytic tumor of the kidney should be considered after excluding other renal oncocytic tumors. However, this is a provisional category. Therefore, further research and accumulation of similar cases are necessary.
Author contributions
Kenta Matsui: Conceptualization; data curation; project administration; writing – original draft. Katsunori Uchida: Data curation; supervision. Masahiro Kanai: Data curation. Kana Asakawa: Data curation; supervision. Miki Usui: Data curation; supervision. Tetsuya Murata: Conceptualization; methodology; project administration; supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Review Board
Not applicable.
Informed consent
Not applicable.
Registry and the Registration No. of the study/trial
Not applicable.
References
- 1. Paner G, Amin MB, Moch H, Storkel S. Chromophobe renal cell carcinoma. In: Moch H, Humphrey PA, Ulbright TM, Reuter VE (eds) WHO Classification of Tumours of the Urinary System and Male Genital Organs. International Agency for Research on Cancer, Lyon; 2016: 27–8. [Google Scholar]
- 2. Hes O, Moch H, Reuter VE. Oncocytoma. In: Moch H, Humphrey PA, Ulbright TM, Reuter VE (eds) WHO Classification of Tumours of the Urinary System and Male Genital Organs. International Agency for Research on Cancer, Lyon; 2016: 43–4. [Google Scholar]
- 3. Hartman A, Gill A, He H et al. Other oncocytic tumours of the kidney. In: Moch H, Amin M, Tickoo S, Turajic S (eds) WHO Classification of Tumours Editorial Board. Urinary and male genital tumours [Internet]. Lyon (France), International Agency for Research on Cancer; 2022. [cited 2022/05/06]. (WHO classification of tumours series, 5th ed.; vol. 8). Available from:. https://tumourclassification.iarc.who.int/chapters/36.https://tumourclassification.iarc.who.int/chapters/36. [Google Scholar]
- 4. Kuroda N, Tanaka A, Yamaguchi T et al. Chromophobe renal cell carcinoma, oncocytic variant: a proposal of a new variant giving a critical diagnostic pitfall in diagnosing renal oncocytic tumors. Med. Mol. Morphol. 2013; 46: 49–55. [DOI] [PubMed] [Google Scholar]
- 5. Trpkov K, Williamson SR, Gao Y et al. Low‐grade oncocytic tumour of kidney (CD117‐negative, cytokeratin 7‐positive): a distinct entity? Histopathol 2019; 75: 174–84. [DOI] [PubMed] [Google Scholar]
- 6. Trpkov K, Hes O. New and emerging renal entities: a perspective post‐WHO 2016 classification. Histopathol 2019; 74: 31–59. [DOI] [PubMed] [Google Scholar]
- 7. Trpkov K, Williamson SR, Gill AJ et al. Novel, emerging and provisional renal entities: the genitourinary pathology society (GUPS) update on renal neoplasia. Mod. Pathol. 2021; 34: 1167–84. [DOI] [PubMed] [Google Scholar]
- 8. Trpkov K, Hes O, Williamson SR et al. New developments in existing WHO entities and evolving molecular concepts: the genitourinary pathology society (GUPS) update on renal neoplasia. Mod. Pathol. 2021; 34: 1392–424. [DOI] [PubMed] [Google Scholar]


