Abstract
OBJECTIVE
To formulate a nomogram to predict the risk of one-year mortality after percutaneous coronary intervention (PCI) based on a large-scale real-world Asian cohort.
METHODS
This study cohort included consecutive patients undergoing PCI in the National Center for Cardiovascular Diseases of China. The endpoint was all-cause mortality. Least absolute shrinkage and selection operator Cox regression and backward stepwise regression were used to select potential risk factors. A nomogram based on the predictors was accordingly constructed to predict one-year mortality. The performance of the nomogram was evaluated. Patients were stratified into low-, intermediate- and high-risk groups according to the tertile points in the nomogram and compared by the Kaplan-Meier analysis.
RESULTS
A total of 9603 individuals were included in this study and randomly divided into the derivation cohort (60%) and the validation cohort (40%). Six variables were selected to formulate the nomogram, including age, renal insufficiency, cardiac dysfunction, previous cerebrovascular disease, previous PCI, and TIMI 0–1 before PCI. The area under the curve of this nomogram regarding one-year mortality risks were 0.792 and 0.754 in the derivation cohort and validation cohort, respectively. Kaplan-Meier curve successfully stratified the patients according to three risk groups. This nomogram calibrated well and exhibited satisfactory clinical utility in the decision curve analysis.
CONCLUSIONS
This study developed and validated a simple-to-use nomogram predicting one-year mortality risk in Asian patients undergoing PCI and could help clinicians make risk-dependent decisions.
Coronary heart disease (CHD) is a global health burden. The estimated prevalence of CHD is approximately 4%–6% among the general population.[1] CHD is the leading cause of death around the world. Approximately 10 million people die from CHD yearly, accounting for 16% of deaths worldwide.[2] The prognosis of CHD has improved as its therapeutic strategies developed. Percutaneous coronary intervention (PCI) is one of the most frequent treatments for CHD. Compared to thrombolytic therapy, PCI further decreases mortality. Nowadays, PCI is one of the first-line treatments for CHD in the guideline.[3] However, mortality after PCI still exists, especially in high-risk patients.[4] Therefore, evaluating the personalized condition of each patient and identifying high-risk individuals are essential and could prevent mortality after PCI. The risk prediction model will provide patients and clinicians with a personalized risk evaluation and help them make clinical decisions.
Several risk models predicting the prognosis of PCI patients have been developed,[5–9] most of which were derived from American and European people and were rarely validated in Asian populations. The coefficients and predictive values of the parameters in the model might vary between races, such as the thrombogenic and inflammatory activities.[10,11] The prognosis of PCI also altered between distinct races.[12,13] Therefore, risk models derived from American and European people may have limited applicability in Asian population. And it is meaningful to develop risk models based on the Asians. Besides, previous risk scores focused on the in-hospital or 30-day mortality after PCI. Few risk models have been established to evaluate long-term mortality in patients undergoing PCI.
Nomogram is a graphic prediction tool integrating selected risk factors.[14,15] It evaluates the risk probability by the total score, which is calculated according to the scores of each included variable. Compared to the traditional point-based risk models, a nomogram could evaluate the risk probability of each individual according to the length of the line segment corresponding to the variable, which is easier to use for clinicians and easier to understand for patients.
In current personalized and precision medicine, predicting mortality after PCI in accuracy is vital for the joint decision-making of physicians and patients. Therefore, this study aimed to establish and validate a nomogram predicting the one-year mortality risk of patients undergoing PCI based on the data from a large-scale real-world cohort.
METHODS
Study Population
A total of 10,724 patients who underwent PCI in the National Center of Cardiovascular Diseases of China were enrolled between January 2013 and December 2013. The patients were excluded if their angiographies were for follow-up. Finally, 9603 individuals were included in this study. The clinical data were prospectively collected, including body mass index, diastolic blood pressure, systolic blood pressure, age, gender, smoking, previous myocardial infarction, SYNTAX score, diabetes mellitus, hyperlipidemia, hypertension, previous cerebrovascular disease, previous vascular disease, chronic obstructive pulmonary disease, previous PCI, previous coronary artery bypass graft, PCI duration, radial or femoral access, intermediate to severe calcification, legion diameter, legion length, multivessel disease, left main artery disease, acute coronary syndrome, ST-segment elevation myocardial infarction, new-generation drug-eluting stent, chronic total occlusion, Thrombolysis in Myocardial Infarction (TIMI) 0–1 before PCI, TIMI 0–2 after PCI, left ventricular ejection fraction and estimated glomerular filtration rate. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg and/or the current use of antihypertensive medication. Type 2 diabetes mellitus was considered fasting plasma glucose ≥ 126 mg/dL, two-hour oral glucose tolerance test value ≥ 200 mg/dL, hemoglobin A1c ≥ 6.5%, or random plasma glucose ≥ 200 mg/dL with classic symptoms of hyperglycemia. Hyperlipidemia was defined as low-density lipoprotein cholesterol ≥ 140 mg/dL, high-density lipoprotein cholesterol < 40 mg/dL, or triglyceride ≥ 150 mg/dL. Left ventricular ejection fraction < 50% was defined as cardiac dysfunction,[16] and estimated glomerular filtration rate < 30 mL/min per 1.73 m2 was defined as renal insufficiency.[17] This study was approved by the Ethics Committee of the National Center of Cardiovascular Diseases (No.2012-431), and each individual signed the informed consent.
Follow-up and Endpoints
Follow-up was conducted by the professional staff by telephone calls and outpatient visits at 30 days, 6 months, and every year after PCI. All-cause mortality was considered the primary endpoint. If no events occurred during follow-up, the follow-up was considered complete at the two years juncture.
Model Establishment and Model Performance
A total of 9603 individuals were randomly divided into the derivation cohort (60%) and the validation cohort (40%). The least absolute shrinkage and selection operator (LASSO) method was adopted for variable selection. Variables with nonzero coefficients in the LASSO Cox regression model were selected as potential predictors. Then these potential predictors were included in multivariable Cox regression analysis followed by a backward stepwise regression, in which variables with P-value < 0.05 were identified and incorporated in the nomogram for predicting one-year mortality after PCI. And the nomogram was validated in the validation cohort. The C-index and area under the curve (AUC) of the receiver operating characteristic curves were calculated to assess the discrimination ability of the nomogram. The accuracy of the nomogram was evaluated by calibration plots. Patients were stratified into low-, intermediate-, and high-risk groups based on the tertile points in the nomogram, and the comparison between the three groups was evaluated by Kaplan-Meier analysis. Decision curve analysis (DCA) was performed to measure the clinical net benefit of the nomogram.[18]
Statistical Analysis
Continuous variables were presented as mean ± SD and compared with the independent Student’s t-test, and categorical variables were presented as counts (percentages) and compared by the Pearson’s chi-squared test. Two-sided P-value < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 24.0 (SPSS Inc., IBM, Chicago, IL, USA) and R statistical software 4.0.3 (https://www.r-project.org).
RESULTS
Baseline Characteristics
This study included 9603 individuals for the final analysis. All individuals were divided into the derivation cohort (60%) and the validation cohort (40%). Table 1 shows the baseline characteristics of the derivation cohort and the validation cohort. The derivation cohort comprised 5762 individuals with an average age of 58.6 years. The validation cohort consisted of 3841 individuals with an average age of 58.4 years. The two cohorts had no significant difference in baseline characteristics. There were 99 events (1.7%) in the derivation cohort and 44 events (1.1%) in the validation cohort.
Table 1. Baseline characteristics.
| Variables | Derivation cohort (n = 5762) | Validation cohort (n = 3841) | P-value |
| Data are presented as means ± SD or n (%). PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction. | |||
| Age, yrs | 58.6 ± 10.3 | 58.4 ± 10.3 | 0.361 |
| Male | 4415 (76.6%) | 2966 (77.2%) | 0.513 |
| Body mass index, kg/m2 | 25.9 ± 3.2 | 25.9 ± 3.2 | 0.728 |
| Systolic blood pressure, mmHg | 126.9 ± 16.6 | 126.9 ± 16.7 | 0.981 |
| Diastolic blood pressure, mmHg | 77.3 ± 10.4 | 77.5 ± 10.4 | 0.488 |
| Smoking | 3278 (56.9%) | 2188 (57.0%) | 0.959 |
| Hypertension | 3671 (63.7%) | 2495 (65.0%) | 0.22 |
| Hyperlipidemia | 3842 (66.7%) | 2590 (67.4%) | 0.456 |
| Diabetes mellitus | 1774 (30.8%) | 1146 (29.8%) | 0.332 |
| Previous cerebrovascular disease | 606 (10.5%) | 426 (11.1%) | 0.392 |
| Previous vascular disease | 149 (2.6%) | 102 (2.7%) | 0.885 |
| Chronic obstructive pulmonary disease | 120 (2.1%) | 104 (2.7%) | 0.055 |
| Previous PCI | 1454 (25.2%) | 932 (24.3%) | 0.292 |
| Previous coronary artery bypass grafting | 242 (4.2%) | 144 (3.7%) | 0.294 |
| Renal insufficiency | 102.6 (22.8%) | 102.3 (22.1%) | 0.545 |
| Cardiac dysfunction | 62.8 (7.4%) | 62.8 (7.1%) | 0.994 |
| SYNTAX score | 12.1 ± 7.8 | 12.1 ± 7.9 | 0.802 |
| PCI time, min | 35.0 ± 31.9 | 34.2 ± 28.0 | 0.235 |
| Lesion diameter, mm | 3.2 ± 2.3 | 3.2 ± 2.8 | 0.438 |
| Lesion length, mm | 28.8 ± 18.8 | 28.8 ± 19.8 | 0.888 |
| Radial artery access | 5265 (91.4%) | 3486 (90.8%) | 0.315 |
| Coronary artery calcification | 890 (15.4%) | 612 (15.9%) | 0.538 |
| New-generation drug-eluting stent | 4929 (85.5%) | 3320 (86.4%) | 0.23 |
| Chronic total occlusion | 440 (7.6%) | 321 (8.4%) | 0.214 |
| TIMI 0–1 before PCI | 1165 (20.2%) | 826 (21.5%) | 0.134 |
| TIMI 0–2 after PCI | 200 (3.5%) | 120 (3.1%) | 0.384 |
| Chronic total occlusion | 440 (7.6%) | 321 (8.4%) | 0.214 |
| Left main artery disease | 235 (6.1%) | 361 (6.3%) | 0.803 |
| Multivessel disease | 2844 (74.0%) | 4300 (74.6%) | 0.537 |
Feature Selection and Model Development
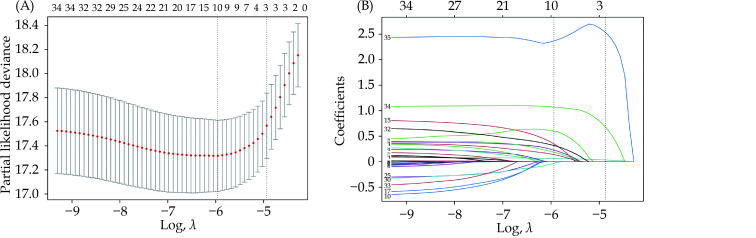
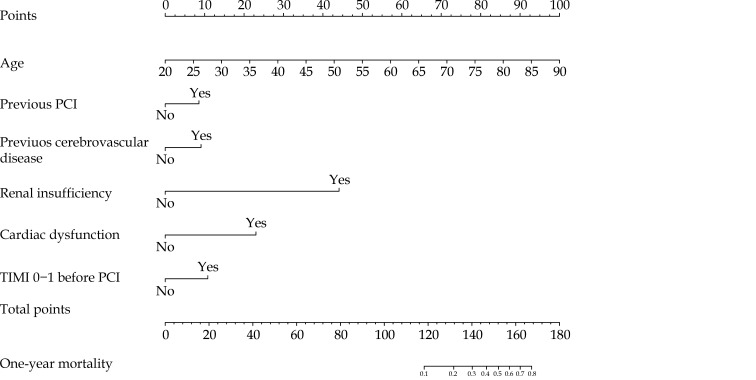
Thirty-five features were reduced to ten candidate predictors with non-zero coefficients in the LASSO Cox regression model (Figure 1). Ten candidate predictors included age, previous myocardial infarction, renal insufficiency, cardiac dysfunction, previous cerebrovascular disease, chronic obstructive pulmonary disease, previous PCI, bare-mental stent, new-generation drug-eluting stent, and TIMI 0–1 before PCI. These candidate variables were then included in a multivariable Cox regression analysis followed by a backward stepwise regression. Finally, six variables with P < 0.05 in multivariable analysis, including age, renal insufficiency, cardiac dysfunction, previous cerebrovascular disease, previous PCI, and TIMI 0–1 before PCI, were used to establish the nomogram to predict one-year mortality after PCI (Table 2 & Figure 2).
Figure 1.
LASSO Cox regression model to select candidate variables associated with one-year mortality after percutaneous coronary intervention.
(A): Plot of 10-fold cross-validation via minimum criteria for selection of the optimal value of tuning parameter (λ). Dotted vertical lines were drawn at the value with the minimum criteria and one standard error of the minimum criteria; and (B): LASSO coefficient profiles of 35 clinical features associated with one-year mortality after percutaneous coronary intervention. A dotted vertical line is drawn at the optimal λ value of ten nonzero coefficients through 10-fold cross-validation. LASSO: least absolute shrinkage and selection operator.
Table 2. Multivariable Cox regression analysis.
| Hazard ratio | 95% CI | P-value | |
| PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction. | |||
| Age | 1.08 | 1.06–1.11 | < 0.0001 |
| Renal insufficiency | 11.6 | 2.76–48.8 | 0.001 |
| Cardiac dysfunction | 3.59 | 2.18–5.91 | < 0.0001 |
| Previous cerebrovascular disease | 1.66 | 1.02–2.7 | 0.042 |
| Previous PCI | 1.61 | 1.07–2.42 | 0.022 |
| TIMI 0–1 before PCI | 1.82 | 1.19–2.8 | 0.006 |
Figure 2.
Nomogram predicting one-year mortality after PCI.
Each clinical variable has a certain number of points. The sum of points of each variable was related to the probability of mortality at specific timepoints. For each covariate, please draw a vertical line upwards and note down the corresponding points. This is repeated for each covariate ending with a total score that corresponds to a predicted one-year mortality at the bottom of the nomogram. PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction.
Predictive Ability and Performance of the Nomogram
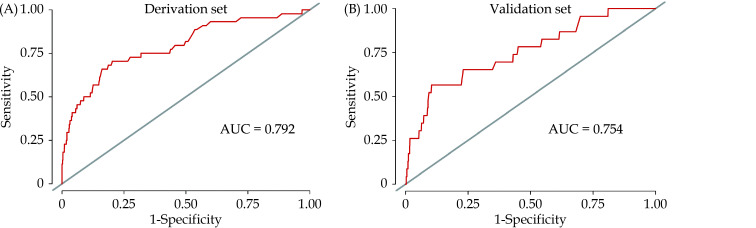
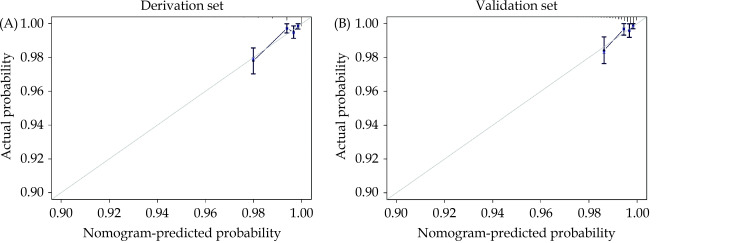
The predictive value of the nomogram was evaluated by the AUC. The AUC regarding one-year mortality was 0.792 (95% CI: 0.717–0.868) and 0.754 (95% CI: 0.648–0.861) in the derivation cohort (Figure 3A) and the validation cohort (Figure 3B), respectively. The calibration plots proved good reliability of the nomogram predicting survival rates at one year in the derivation cohort and the validation cohort (Figure 4).
Figure 3.
The AUC of the nomogram predicting one-year mortality after percutaneous coronary intervention in the derivation cohort (A) and the validation cohort (B).
AUC: area under the curve.
Figure 4.
Calibration plots of the nomogram predicting one-year mortality after percutaneous coronary intervention in the derivation cohort (A) and the validation cohort (B).
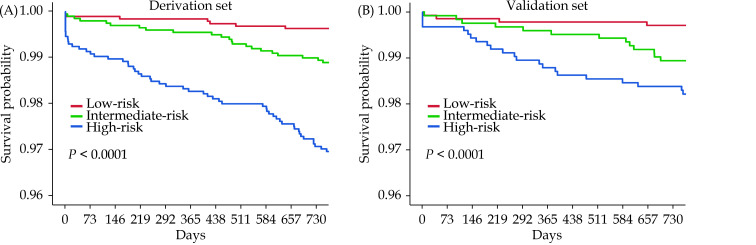
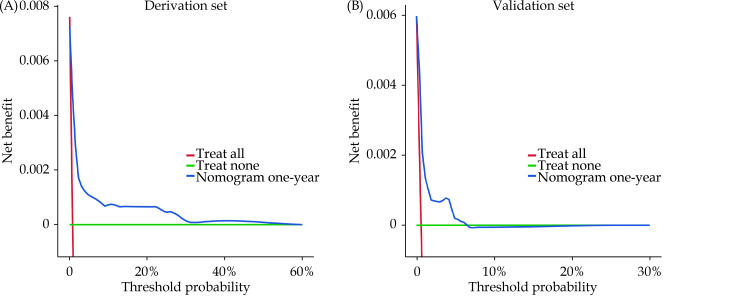
The cohorts were divided into low-, intermediate- and high-risk groups according to the tertile points (54 and 69) in the derivation cohort. The discrimination of the nomogram was assessed by Kaplan-Meier survival analysis, the survival rates were distinguishable between the three risk groups in the derivation cohort and the validation cohort (P < 0.0001, Figure 5). The DCA demonstrated that the nomogram predicting one-year mortality confers a net benefit than the “full treatment” and “no treatment” strategy in the derivation cohort and the validation cohort (Figure 6).
Figure 5.
Kaplan-Meier analysis of the nomogram predicting one-year mortality after percutaneous coronary intervention in the derivation cohort (A) and the validation cohort (B).
Figure 6.
Decision curve analysis of the nomogram predicting one-year mortality after percutaneous coronary intervention in the derivation cohort (A) and the validation cohort (B).
DISCUSSION
This study is the first nomogram explicitly derived from Asians to identify patients at risk of one-year death after PCI. This nomogram integrated six variables: age, renal insufficiency, cardiac function, previous PCI, previous cerebrovascular disease, and TIMI 0–1 before PCI. This graphic nomogram can evaluate the risk of each individual.
This nomogram exhibited good discrimination and calibration. The discrimination was assessed by an AUC of 0.779 for one-year mortality in the whole population. The calibration plots demonstrated a good consistency between our nomogram and actual predictive probability. The Kaplan-Meier survival curve successfully stratified the patients based on the tertile points in the derivation cohort and the validation cohort. The DCA ensured the clinical benefits in both derivation and validation sets. Based on the good performance of our nomogram, we advocated the use of this nomogram for evaluating one-year mortality after PCI.
Nomogram is an integral part of modern medical decision-making. It has been frequently used in predicting outcomes of cancer and surgery.[19–21] Nomogram is also gradually being used in the cardiovascular area for risk prediction. A nomogram was derived from the Multi-Ethnic Study of Atherosclerosis study to predict 10-year CHD risk.[22] Ó Hartaigh, et al.[23] established a nomogram predicting 5-, 10- and 15-year survival in asymptomatic adults undergoing cardiac risk factors screening. Previous studies have formulated nomograms predicting in-hospital mortality of elderly patients undergoing PCI,[24] and in-hospital major adverse cardiovascular and cerebrovascular events in acute coronary syndrome patients undergoing PCI.[25] However, current nomograms predicting mortality risk after PCI mainly concentrate on in-hospital mortality rather than long-term outcomes. This nomogram can evaluate the one-year mortality risk after PCI. As an example, an individual age 55 years who has renal insufficiency and cardiac dysfunction, has a history of cerebrovascular disease and PCI, with TIMI 0–1 before PCI, will have a total risk score of 143 points, which corresponds to a one-year mortality risk of 37%. This nomogram is easy to understand and use, and patients can calculate it themselves.
This nomogram included clinical characteristics (age, cardiac dysfunction, renal insufficiency, previous cerebrovascular disease, and previous PCI) and angiographic characteristics (TIMI 0–1 before PCI). These variables are routinely collected by clinical evaluation and can comprehensively evaluate the features of patients and reflect the actual status of patients. Age is almost included in all risk models predicting mortality after PCI, including EuroScore,[7] New York risk score,[6] Mayo risk score,[26] and National Cardiovascular Data Registry risk model.[27] It’s widely accepted that the risk of death after PCI is elevated as age increases. Among patients hospitalized for acute myocardial infarction, mortality was much higher in patients with age ≥ 65 years than patients with age < 65 years, both in males and females.[12] Cardiac dysfunction and renal insufficiency are closely related to the prognosis after PCI and are included in most previous risk models.[28,29] It has been proved that patients with reduced ejection fraction exhibited an increased rate of all-cause death compared to patients with preserved left ventricular ejection fraction. This result is consistent in patients with left main coronary artery disease, stable CHD, acute coronary syndrome, and chronic coronary occlusion.[30–35] Renal insufficiency can improve cardiovascular disease prediction accuracy beyond traditional risk factors.[36] Previous studies revealed that chronic kidney disease was associated with a solid and independent risk of major adverse cardiovascular events, not affected by lesion type, complication, stent type, and anti-platelet therapy patterns.[37–41] Previous PCI is still a standard variable presented in previous risk models, the NHLBI dynamic registry demonstrated that patients with prior PCI had more adverse baseline characteristics, cardiovascular risk factors, and increased risk of repeated revascularization than those without previous PCI.[42] Previous cerebrovascular disease is a prognostic effector after PCI as well. EXCEL study found that patients with left main coronary artery disease and previous cerebrovascular disease had higher stroke rates and reduced event-free survival after PCI compared to those without previous cerebrovascular disease.[43] Natsuaki, et al.[44] found that patients with previous cerebrovascular disease had an increased risk for intracranial hemorrhage and ischemic events compared to those with no previous cerebrovascular disease. TIMI 0–1 before PCI is an angiographic variable. Few studies incorporated angiographic variables.[6,45] A recent study found that all-cause death and cardiac death were significantly higher in pre-PCI TIMI 0–1 patients than in pre-PCI TIMI 2–3 patients with non-ST-segment elevation myocardial infarction.[46] All variants incorporating the nomogram are all risk factors for adverse events after PCI. Therefore, individuals with more risk factors would have higher risk scores. Kaplan-Meier analysis proved that high risk scores were associated with increased mortality risk after PCI. Therefore, compared to the low-risk patient, intermediate- and high-risk patients should be more serious about lifestyle change, standardized medication, and regularly revisit after PCI.
Previous risk models predicting short-term or long-term mortality after PCI,[24,28] such as Global Registry of Acute Coronary Events scores, were derived from the patients treated with therapeutic strategy at old time, which was different from current standard therapy.[47] Therefore, these models have less applicability because of the improvement of stent technology and the utility of dual antiplatelet therapy in the last twenty years. Some risk models are derived from updated cohorts, such as the EuroHeart score and New York risk score. However, they included more than ten variables. Too many variables may decrease the accuracy of coefficient estimation and response prediction, which leads to reduced utility.[6,7] NCDR CathPCI risk score was derived from 588,398 procedures in America. Therefore, data accuracy and quality may be affected by reporting bias and errors in data collection and center-level variation of different medical centers.[27] Sorin J Brener’s score was derived from 21 randomized controlled trials, but compared to our nomogram, Sorin J Brener’s score did not include cardiac function and renal function, which may abate the predictive value itself.[48]
Most importantly, no nomogram predicting mortality after PCI was specified for the Asian population. The risk models mentioned previously were all derived from American and European people. Geography differences in lifestyle, population genomics, medical standards, and clinical strategies contribute to the diversity of prognosis after PCI in distinct races. The performance of risk models derived from the American and European people is uncertain in Asians. This nomogram is derived from a large-scale real-world study in Asia, which may be more applicable to the Asian population. At the same time, we advocate the validation of our nomogram in the American and European people to improve its utility.
LIMITATIONS
The present study has some limitations that should be acknowledged. Firstly, this study is a single-center study, which may reduce the generalizability of the nomogram. The multi-center study is in need for further investigation. Secondly, although the internal validation exhibited good discrimination, a lack of external validation led to an uncertain generality of the nomogram in other populations. Therefore, external validation is necessary for the future utility of our nomogram.
CONCLUSIONS
This nomogram incorporated six routinely collected characteristics to predict one-year mortality of patients undergoing PCI. This nomogram provides clinicians with a simple-to-use clinical tool to identify individuals with high mortality risk after PCI and guideline preventive therapy.
ACKNOWLEDGMENTS
This study was supported by the National Key R&D Program of China (2020YFC2004705), the National Natural Science Foundation of China (No.81825003 & No.91957123 & No.82270376), the Beijing Nova Program from Beijing Municipal Science & Technology Commission (Z201100006820002). All authors had no conflicts of interest to disclose. The warmest thanks to all the participates in this study.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Yu Y, Mubarik S, et al Global burden of ischemic heart disease and attributable risk factors, 1990–2017: a secondary analysis based on the Global Burden of Disease Study 2017. Clin Epidemiol. 2021;13:859–870. doi: 10.2147/CLEP.S317787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, et al 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, National Center for Health Statistics Home Page. National Vital Statistics System: public use data file documentation: mortality multiple cause-of-death micro-data files. https://www.cdc.gov/nchs/nvss/mortality_public_use_data.htm (accessed March 8, 2022).
- 5.Hizoh I, Gulyas Z, Domokos D, et al A novel risk model including vascular access site for predicting 30-day mortality after primary PCI: the ALPHA score. Cardiovasc Revasc Med. 2017;18:33–39. doi: 10.1016/j.carrev.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Hannan EL, Farrell LS, Walford G, et al The New York State risk score for predicting in-hospital/30-day mortality following percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6:614–622. doi: 10.1016/j.jcin.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 7.de Mulder M, Gitt A, van Domburg R, et al EuroHeart score for the evaluation of in-hospital mortality in patients undergoing percutaneous coronary intervention. Eur Heart J. 2011;32:1398–1408. doi: 10.1093/eurheartj/ehr034. [DOI] [PubMed] [Google Scholar]
- 8.Castro-Dominguez YS, Wang Y, Minges KE, et al Predicting in-hospital mortality in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2021;78:216–229. doi: 10.1016/j.jacc.2021.04.067. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhary S, Ivanov J, Mackie K, et al The Toronto score for in-hospital mortality after percutaneous coronary interventions. Am Heart J. 2009;157:156–163. doi: 10.1016/j.ahj.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Kim HK, Tantry US, Smith SC Jr, et al The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. 2021;121:422–432. doi: 10.1055/s-0040-1718729. [DOI] [PubMed] [Google Scholar]
- 11.Kim HK, Tantry US, Park HW, et al Ethnic difference of thrombogenicity in patients with cardiovascular disease: a pandora box to explain prognostic differences. Korean Circ J. 2021;51:202–221. doi: 10.4070/kcj.2020.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez F, Foody JM, Wang Y, et al Young Hispanic women experience higher in-hospital mortality following an acute myocardial infarction. J Am Heart Assoc. 2015;4:e002089. doi: 10.1161/JAHA.115.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZM, Rautaharju PM, Prineas RJ, et al Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2016;133:2141–2148. doi: 10.1161/CIRCULATIONAHA.115.021177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balachandran VP, Gonen M, Smith JJ, et al Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iasonos A, Schrag D, Raj GV, et al How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Wang F, Wang L, et al Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 18.Vickers AJ, Elkin EB Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Margonis GA, Prescott JD, et al Nomograms to predict recurrence-free and overall survival after curative resection of adrenocortical carcinoma. JAMA Surg. 2016;151:365–373. doi: 10.1001/jamasurg.2015.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jehi L, Yardi R, Chagin K, et al Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14:283–290. doi: 10.1016/S1474-4422(14)70325-4. [DOI] [PubMed] [Google Scholar]
- 21.Cappellari M, Mangiafico S, Saia V, et al IER-SICH nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. 2019;50:909–916. doi: 10.1161/STROKEAHA.118.023316. [DOI] [PubMed] [Google Scholar]
- 22.McClelland RL, Jorgensen NW, Budoff M, et al 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study) J Am Coll Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ó Hartaigh B, Gransar H, Callister T, et al Development and validation of a simple-to-use nomogram for predicting 5-, 10-, and 15-year survival in asymptomatic adults undergoing coronary artery calcium scoring. JACC Cardiovasc Imaging. 2018;11:450–458. doi: 10.1016/j.jcmg.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein LW, Block P, Brindis RG, et al Percutaneous coronary interventions in octogenarians in the American College of Cardiology-National Cardiovascular Data Registry: development of a nomogram predictive of in-hospital mortality. J Am Coll Cardiol. 2002;40:394–402. doi: 10.1016/S0735-1097(02)01992-7. [DOI] [PubMed] [Google Scholar]
- 25.Bo X, Liu Y, Yang M, et al Development and validation of a nomogram of in-hospital major adverse cardiovascular and cerebrovascular events in patients with acute coronary syndrome. Front Cardiovasc Med. 2021;8:699023. doi: 10.3389/fcvm.2021.699023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh M, Rihal CS, Lennon RJ, et al Bedside estimation of risk from percutaneous coronary intervention: the new Mayo Clinic risk scores. Mayo Clin Proc. 2007;82:701–708. doi: 10.1016/S0025-6196(11)61190-7. [DOI] [PubMed] [Google Scholar]
- 27.Peterson ED, Dai D, DeLong ER, et al Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588, 398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halkin A, Singh M, Nikolsky E, et al Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol. 2005;45:1397–1405. doi: 10.1016/j.jacc.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 29.Serruys PW, Morice MC, Kappetein AP, et al Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 30.Galassi AR, Boukhris M, Toma A, et al Percutaneous coronary intervention of chronic total occlusions in patients with low left ventricular ejection fraction. JACC Cardiovasc Interv. 2017;10:2158–2170. doi: 10.1016/j.jcin.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 31.Daneault B, Généreux P, Kirtane AJ, et al Comparison of three-year outcomes after primary percutaneous coronary intervention in patients with left ventricular ejection fraction < 40% versus ≥ 40% (from the HORIZONS-AMI trial) Am J Cardiol. 2013;111:12–20. doi: 10.1016/j.amjcard.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Halkin A, Stone GW, Dixon SR, et al Impact and determinants of left ventricular function in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction. Am J Cardiol. 2005;96:325–331. doi: 10.1016/j.amjcard.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 33.Siontis GC, Branca M, Serruys P, et al Impact of left ventricular function on clinical outcomes among patients with coronary artery disease. Eur J Prev Cardiol. 2019;26:1273–1284. doi: 10.1177/2047487319841939. [DOI] [PubMed] [Google Scholar]
- 34.Toma A, Stähli BE, Gick M, et al Comparison of benefit of successful percutaneous coronary intervention for chronic total occlusion in patients with versus without reduced (≤ 40%) left ventricular ejection fraction. Am J Cardiol. 2017;120:1780–1786. doi: 10.1016/j.amjcard.2017.07.088. [DOI] [PubMed] [Google Scholar]
- 35.Thuijs DJFM, Milojevic M, Stone GW, et al Impact of left ventricular ejection fraction on clinical outcomes after left main coronary artery revascularization: results from the randomized EXCEL trial. Eur J Heart Fail. 2020;22:871–879. doi: 10.1002/ejhf.1681. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita K, Coresh J, Sang Y, et al Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baber U, Giustino G, Sartori S, et al Effect of chronic kidney disease in women undergoing percutaneous coronary intervention with drug-eluting stents: a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv. 2016;9:28–38. doi: 10.1016/j.jcin.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Généreux P, Giustino G, Witzenbichler B, et al Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. doi: 10.1016/j.jacc.2015.06.1323. [DOI] [PubMed] [Google Scholar]
- 39.Baber U, Chandrasekhar J, Sartori S, et al Associations between chronic kidney disease and outcomes with use of prasugrel versus clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a report from the PROMETHEUS study. JACC Cardiovasc Interv. 2017;10:2017–2025. doi: 10.1016/j.jcin.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 40.Giustino G, Mehran R, Serruys PW, et al Left main revascularization with PCI or CABG in patients with chronic kidney disease: EXCEL trial. J Am Coll Cardiol. 2018;72:754–765. doi: 10.1016/j.jacc.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 41.Naganuma T, Tsujita K, Mitomo S, et al Impact of chronic kidney disease on outcomes after percutaneous coronary intervention for chronic total occlusions (from the Japanese multicenter registry) Am J Cardiol. 2018;121:1519–1523. doi: 10.1016/j.amjcard.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 42.Bourassa MG, Detre KM, Johnston JM, et al Effect of prior revascularization on outcome following percutaneous coronary intervention; NHLBI Dynamic Registry. Eur Heart J. 2002;23:1546–1555. doi: 10.1053/euhj.2002.3262. [DOI] [PubMed] [Google Scholar]
- 43.Diamond J, Madhavan MV, Sabik JF 3rd, et al Left main percutaneous coronary intervention versus coronary artery bypass grafting in patients with prior cerebrovascular disease: results from the EXCEL trial. JACC Cardiovasc Interv. 2018;11:2441–2450. doi: 10.1016/j.jcin.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Natsuaki M, Morimoto T, Watanabe H, et al Ischemic and bleeding risk after percutaneous coronary intervention in patients with prior ischemic and hemorrhagic stroke. J Am Heart Assoc. 2019;8:e013356. doi: 10.1161/JAHA.119.013356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh M, Lennon RJ, Gulati R, et al Risk scores for 30-day mortality after percutaneous coronary intervention: new insights into causes and risk of death. Mayo Clin Proc. 2014;89:631–637. doi: 10.1016/j.mayocp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Kim YH, Her AY, Jeong MH, et al Two-year clinical outcomes according to pre-PCI TIMI flow grade and reperfusion timing in non-STEMI after newer-generation drug-eluting stents implantation. Angiology. 2022;73:152–164. doi: 10.1177/00033197211012537. [DOI] [PubMed] [Google Scholar]
- 47.Widera C, Pencina MJ, Meisner A, et al Adjustment of the GRACE score by growth differentiation factor 15 enables a more accurate appreciation of risk in non-ST-elevation acute coronary syndrome. Eur Heart J. 2012;33:1095–1104. doi: 10.1093/eurheartj/ehr444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brener SJ, Leon MB, Serruys PW, et al Derivation and external validation of a novel risk score for prediction of 30-day mortality after percutaneous coronary intervention. EuroIntervention. 2019;15:e551–e557. doi: 10.4244/EIJ-D-19-00262. [DOI] [PubMed] [Google Scholar]