Abstract
To determine the burden of vision impairment (VI) and ocular conditions, women (n=254, mean age 66.0 years) participated in a comprehensive vision assessment. Visual acuity (VA) and ocular disorders (diabetic retinopathy, macular degeneration, hypertensive retinopathy, glaucoma and cataracts) were defined clinically. Race, economic strain and education were self-reported. The prevalence of presenting VI (VA 20/40 or worse) was 11.0% and 75% was correctable (best-corrected VI 2.8%). Black women and those with greater economic strain or less education had a higher prevalence of presenting VI. These disparities were no longer present after considering best-corrected VI. Ocular disease prevalence ranged from 3.3% (age-related macular degeneration) to 30.2% (hypertensive retinopathy), but most participants were unaware of their ocular diagnosis. The discordance of presenting versus best-corrected VI and lack of knowledge of ocular conditions suggests a need for increased vision services. Access to optimal vision correction may attenuate differences across sociodemographic groups.
Keywords: Prevalence, women, vision impairment, disparities
INTRODUCTION
Vision impairment (VI) and the prevalence of eye diseases increase with advancing age.1,2 An estimated 11.4 million United States (US) adults are visually impaired and the number of people with VI is anticipated to increase substantially in the coming years due to aging of the population.3 VI adversely affects a wide range of outcomes in aging adults including physical4-7 and cognitive functioning8, mental health status9, and increased risk of falls and fractures.10,11 Population-based studies in the US, Australia and Europe show that the prevalence of VI increases dramatically between mid-life (ages 40-65) and late adulthood (ages 65 and older)1,12, thereby suggesting that this transition may be an optimal time for interventions. The vast majority of VI is correctable12,13, yet vision services are under-utilized.14 Thus, identifying groups most in need of vision intervention services and those most vulnerable to adverse outcomes associated with VI is needed to inform research and clinical care.
Studies suggest that women have a disproportionate burden of VI as compared to age-matched men.3,15 Further, women experience more rapid declines in physical functioning16,17, fall more often at every age18,19, and have worse fall-related outcomes including injury and fractures as compared to men.18,20 While the reasons for these sex differences are not fully understood, some studies suggest that women experience more vision-related adverse impacts on quality of life than do men21,22, thereby suggesting that a greater understanding of the burden and correlates of VI in this population is highly relevant.
As the population ages, the public health burden of vision loss will continue to grow substantially unless corrective measures are optimally implemented. Accurate population-based estimates are essential to inform health care professionals and policy makers about the burden of VI and potential health disparities, as clinic-based estimates are inherently biased. In spite of availability of effective interventions to treat many causes of VI, its prevalence is increasing.23 Nationally and internationally, there is a strong impetus to curtail VI and prioritize vision health at the population level.24-26 To do so, investigations within well-evaluated and high-risk or vulnerable groups must be conducted, including consideration of the sociodemographic and pathophysiological correlates of poor vision. Thus, the purpose of this study was to estimate the prevalence of VI, eye diseases, and use of appropriate optical correction in a population-based cohort of older women. Correlates of VI and awareness of ocular disease diagnosis were also assessed.
MATERIALS and METHODS
Study Population.
The Study of Women's Health Across the Nation (SWAN) is an ongoing, 25-year multi-site longitudinal cohort study. The design and recruitment procedures have been published.27 In brief, eligible women were 42-52 years at study baseline (1996), had an intact uterus and at least one menstrual period with no use of reproductive hormones in the previous 3 months. Originally conceptualized to study the natural history of the menopausal transition, women have been followed since baseline with near-annual follow-up visits to collect information on a variety of sociodemographic, health behavior, and health outcome information. Since its inception, the southeastern Michigan SWAN site, including 543 women (60% African American, 40% White) has included a site-specific protocol focused on sensory health and physical functioning. At the most recent in-person visit for SWAN (follow-up visit 16, 2016-2017), the Michigan SWAN site included a vision assessment as part of its site-specific protocol. As of follow-up visit 16, 308 of the still-living Michigan SWAN participants were active in the study (64%); of those, 255 women (83%) participated in the vision assessment. One woman did not have data on distance vision or ocular disorders, and so she was excluded from all analyses, leaving a final analytic sample size of 254 women. Some women were missing vision/ocular disorders data elements including best-corrected visual acuity (n=2), glaucoma (n=2), hypertensive retinopathy (n=9), diabetic retinopathy (n=4), and age-related macular degeneration (n=11). There were no statistically significant differences in age, race/ethnicity, body mass index, diabetes or hypertension status between women who participated in the vision assessment and those who were still active in the study but did not participate in the vision assessment. The University of Michigan Institutional Review Board approved the study protocol and written informed consent was obtained from all participants. This study conformed to the principles of the Declaration of Helsinki.
Vision Assessment.
The vision assessment included measurement of presenting distance visual acuity (VA) using habitual correction. Distance VA was tested using a Snellen chart displayed by means of a projection system (M&S Technologies Inc., Skokie, Illinois). Best-corrected VA (BCVA) was assessed following objective, non-cyloplegic refraction using an autorefractometer (Topcon Auto Keratorefractor, model KR800), and a subsequent subjective refraction using a phoropter or sometimes a trial frame and lenses. Use of eyeglasses for distance vision was noted. Vision impairment, based on presenting VA as well as BCVA was defined as VA 20/40 or worse in the better-seeing eye.
In addition, participants underwent a comprehensive eye examination by an ophthalmologist (SM) or a residency-trained optometrist (SDW) that included assessment of the anterior segment using a slit lamp microscope and dilated eye examination with appropriate lenses to assess the posterior segment. Intraocular pressure was measured using Goldmann applanation tonometry and visual fields were tested using the frequency doubling technique. Anterior chamber angle was assessed by gonioscopy. Optical coherence tomography (OCT, Heidelberg Spectralis) of the macula and optic nerves was also performed. Diagnoses of ocular disease and conditions were based on recommendations by the American Academy of Ophthalmology (AAO) Preferred Practice Pattern guidelines for diabetic retinopathy, macular degeneration, hypertensive retinopathy, glaucoma, and cataracts.28-33
Covariates.
Age was calculated based on date of birth and visit date. Race was self-reported as African American or White. Difficulty paying for basics was categorized as very/some vs. none. Education was categorized as high school or less, some college, and college or post-college. Height measured in centimeters (cm) and weight measured in kilograms (kg) were assessed using a stadiometer and calibrated balance beam scale, respectively, and used to calculate body mass index (BMI) in kg/m2. Health insurance status (yes/no), alcohol consumption (dichotomized as ≥1 serving/month vs. <1 serving/month) and smoking status (current vs. former/never) were based on self-report. Diabetes was defined as a fasting blood glucose ≥ 126 mg/dL, self-reported use of anti-diabetic medication, or self-reported doctor-diagnosed diabetes. Hypertension was defined as a measured systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or self-reported use of anti-hypertensive medication. Participants were asked to indicate on the questionnaire if they had doctor-diagnosed macular degeneration, glaucoma, cataract, or diabetic retinopathy. All self-reported information was sought prior to the clinical vision assessment. If an eye disease that required treatment was detected and the participant was not receiving medical care for the condition, a referral to specialist care was made to initiate appropriate treatment for the eye condition.
Statistical Analysis.
The prevalence of VI was estimated based on presenting VA and BCVA using the better eye measurement. Means and standard deviations were calculated for continuous covariates and frequencies were calculated for categorical covariates, overall and by VI, defined by presenting VA and BCVA. Crude associations of VI with socioeconomic and clinical factors were assessed using the Student t-test, Chi-square test or Fisher exact test depending on the nature of the variable. The prevalence of VI and each ocular condition were calculated and 95% confidence intervals (CI) were computed; simultaneous CIs for multinomial proportions were computed for cataract and glaucoma due to each having three categories. Bar plots were created to display use of appropriate glasses overall and among those with VI, as well as the prevalence of ocular conditions based upon comprehensive clinical exam and self-report. Logistic regression models were used to evaluate unadjusted and adjusted associations between VI and ocular conditions and race, education, and financial strain. In these models, glaucoma was dichotomized into yes vs. suspect/none and cataract was dichotomized into mild/early vs. visually significant, with all cases of prior cataract surgery excluded from those models. All covariates were assessed concurrently with vision with the exception of education, which was collected at SWAN study baseline in 1996. For any instances of missing any concurrent covariate data, information from the closest previous visit was used. All statistical analyses were performed using SAS 9.3 (SAS institute, Cary, NC).
RESULTS
Of the 254 Michigan SWAN women who participated in the vision assessment, 158 (62.2%) were African American and 96 (37.8%) were white. The mean age of the population was 66.0 years (standard deviation (SD) 2.7). Just over one-third of women reported very/some difficulty paying for basics (n=91, 35.8%) and only one-quarter had completed college or had post-college education. The majority of women (95.2%) reported having some type of health insurance (Table 1).
Table 1:
Characteristics of Michigan Study of Women’s Health Across the Nation participants, by vision impairment status.
| Total | Presenting visual acuity-defined vision impairment |
P | Best corrected visual acuity-defined vision impairmenta |
P | |||
|---|---|---|---|---|---|---|---|
| N=254 | Yes (n=28) | No (n=226) | Yes (n=7) | No (n=245) | |||
| Age (years), mean (SD) | 66.0 (2.7) | 66.0 (2.6) | 66.0 (2.8) | 0.98b | 65.0 (3.1) | 66.0 (2.8) | 0.35b |
| BMI (kg/m2), mean (SD) | 32.6 (7.6) | 33.8 (7.7) | 32.5 (7.6) | 0.41b | 34.7 (9.4) | 32.6 (7.6) | 0.51b |
| Race/Ethnicity, n (%) | |||||||
| African American | 158 (62.2%) | 22 (13.9%) | 136 (86.1%) | 0.06c | 5 (3.2%) | 153 (96.8%) | 0.99d |
| White | 96 (37.8%) | 6 (6.3%) | 90 (93.8%) | 2 (2.1%) | 92 (97.9%) | ||
| Difficulty paying for basics, n (%) | |||||||
| Very/some | 91 (35.8%) | 17 (18.7%) | 71 (81.3%) | 0.004c | 5 (5.6%) | 85 (94.4%) | 0.10d |
| None | 163 (64.2%) | 11 (6.8%) | 152 (93.3%) | 2 (1.2%) | 160 (98.8%) | ||
| Education, n (%) | |||||||
| ≤ High school | 68 (27.6%) | 11 (16.2%) | 57 (83.8%) | 0.05c | 3 (4.5%) | 64 (95.5%) | 0.70d |
| Some college | 115 (46.8%) | 14 (12.2%) | 101 (87.8%) | 3 (2.6%) | 111 (97.4%) | ||
| College degree / post college | 63 (25.6%) | 2 (3.2%) | 61 (96.8%) | 1 (1.6%) | 62 (98.4%) | ||
| Health insurance, n (%) | 0.63d | 0.99d | |||||
| Yes | 236 (95.2%) | 25 (10.6%) | 211 (89.4%) | 6 (2.6%) | 228 (97.4%) | ||
| No | 12 (4.8%) | 2 (16.7%) | 10 (83.3%) | 0 (0.0%) | 12 (100.0%) | ||
| Smoking status, n (%) | 0.19d | 0.13d | |||||
| Current | 27 (10.9%) | 5 (18.5%) | 22 (81.5%) | 2 (7.4%) | 25 (92.6%) | ||
| Former/never | 221 (89.1%) | 22 (10.0%) | 199 (90.1%) | 4 (1.8%) | 215 (98.2%) | ||
| Alcohol use, n (%) | 0.16c | 0.41d | |||||
| ≥1 serving/month | 95 (38.3%) | 7 (7.4%) | 88 (92.6%) | 1 (1.1%) | 94 (98.9%) | ||
| <1 serving/month | 153 (61.7%) | 20 (13.1%) | 133 (86.9%) | 5 (3.3%) | 146 (96.7%) | ||
| Diabetes, n (%) | 0.66c | 0.67d | |||||
| Yes | 72 (28.6%) | 9 (12.5%) | 63 (87.5%) | 3 (4.3%) | 67 (95.7%) | ||
| No | 180 (71.4%) | 19 (10.6%) | 161 (89.4%) | 4 (2.2%) | 176 (95.7%) | ||
| Hypertension, n (%) | 0.67c | 0.10d | |||||
| Yes | 169 (67.9%) | 20 (11.8%) | 149 (88.2%) | 7 (4.2%) | 160 (95.8%) | ||
| No | 80 (32.1%) | 8 (10.0%) | 72 (90.0%) | 0 (0.0%) | 80 (10.0%) | ||
Two participants were missing data on best-corrected visual acuity.
P-value based upon T-Test
P-value based upon Chi-Square Test
P-value based upon Fisher’s Exact Test
The prevalence of VI based upon presenting VA (20/40 or worse in better-seeing eye) was 11.0% (95% confidence interval (CI) 7.5, 15.5). The burden of VI based upon presenting VA was greater among African American women than white women (13.9% vs. 6.3%, respectively, p=0.06) and among women reporting very/some difficulty paying for basics vs. none (18.7% vs. 6.8%, respectively, p=0.004). Further, presenting VA-defined VI was inversely associated with education (p=0.05) (Table 1). After correction, the burden of VI (defined as BCVA worse than 20/40 in the better-seeing eye) declined to 2.8% (95% CI 1.1, 5.6), suggesting that three-quarters of the presenting VI in this population is correctable. Of the 7 women with VI defined based upon BCVA, three had cataract, one had cataract and diabetic retinopathy, two had glaucoma, and one had Fuchs endothelial dystrophy. Notably, despite differences in presenting VI by race/ethnicity, financial strain and education, there were no statistically significant differences in BCVA-defined VI by sociodemographic characteristics (Table 1). There were no statistically significant differences in presenting or BCVA-defined VI by BMI, insurance, diabetes, hypertension, smoking or alcohol use status. In models including age, race/ethnicity, difficulty paying for basics, and education, only economic strain was associated with VI defined by presenting VA (odds ratio (OR)=3.05, 95% CI 1.28, 7.26) after adjustment for the other variables (Table 3).
Table 3:
Relationship between sociodemographic variables and vision impairment and ocular conditions, the Michigan site of the Study of Women’s Health Across the Nation.
| Presenting visual acuity- defined vision impairment n=246 |
Best- corrected visual acuity- defined vision impairment n=244 |
Cataract n=210 |
Glaucoma n=244 |
Hypertensive retinopathy n=238 |
Diabetic retinopathy n=242 |
Age-related macular degeneration n=236 |
|
|---|---|---|---|---|---|---|---|
| OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
| Age | |||||||
| 0.99 (0.84, 1.16) |
0.73 (0.51, 1.06) |
1.35 (1.12, 1.64) |
1.03 (0.84, 1.27) |
1.02 (0.92, 1.14) |
0.98 (0.81, 1.20) |
1.17 (0.91,1.50) |
|
| African American (vs. White) | |||||||
| 1.71 (0.63, 4.67) |
1.21 (0.16, 8.86) |
1.29 (0.44, 3.78) |
4.03 (0.80, 20.34) |
1.73 (0.91, 3.30) |
0.81 (0.26, 2.47) |
0.46 (0.10, 2.17) |
|
| Very/some difficulty paying for basics (vs. none) | |||||||
|
3.05
(1.28, 7.26) |
4.70 (0.75, 29.34) |
0.99 (0.34, 2.82) |
0.79 (0.22, 2.82) |
2.50
(1.37, 4.55) |
4.39
(1.41, 13.65) |
0.30 (0.03, 2.69) |
|
| Education: ≤ High school vs. college / post-college | |||||||
| 3.16 (0.63, 15.91) |
1.02 (0.07, 15.13) |
7.04
(1.26, 39.35) |
0.61 (0.11, 3.42) |
1.00 (0.43, 2.31) |
1.35 (0.29, 6.24) |
1.26 (0.17, 9.29) |
|
| Education: Some college vs. college degree / post-college | |||||||
| 3.04 (0.64, 14.39) |
0.92 (0.07, 11.62) |
3.32 (0.62, 17.73) |
0.77 (0.18, 3.41) |
1.04 (0.49, 2.22) |
1.15 (0.27, 4.87) |
0.80 (0.15, 4.33) |
|
Bold values represent p<0.05.
OR = odds ratio; 95% CI = 95% confidence interval
All models include age, race/ethnicity, difficulty paying for basics, and education
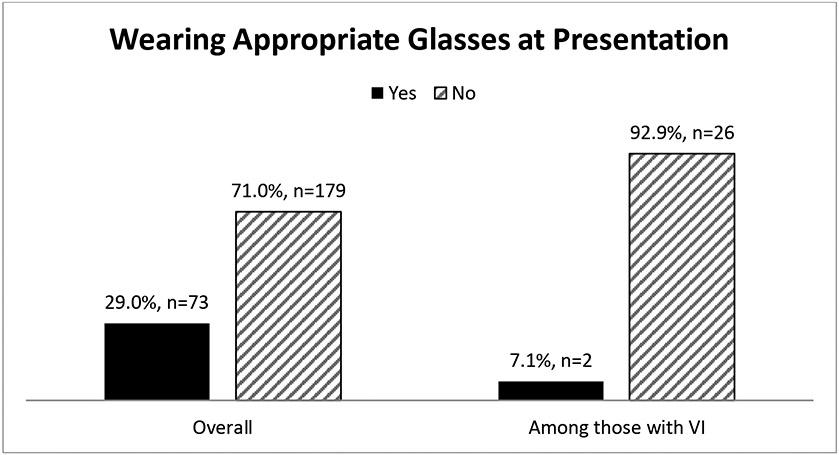
To explore the potential need for correction-based interventions for VI in this population, we examined whether participants were wearing the correct glasses prescription at presentation to their vision exam. Correct prescription was defined as no difference in presenting and BCVA. As shown in Figure 1, 71% (n=179) of women in the Michigan SWAN cohort were not currently wearing the correct vision prescription. Among those with VI defined as presenting VA 20/40 or worse, only 7% (n=2) of women were wearing the correct prescription (Figure 1).
Figure 1.
Comparison of women wearing (black bars) and not wearing (grey bars) correct glasses prescription at presenting for vision assessment, overall and among those with vision impairment based upon presenting visual acuity – Michigan Study of Women’s Health Across the Nation, 2016-2017. The figure is based upon the n=252 women with data on presenting and best corrected visual acuity.
In addition to measurement of VA, the Michigan SWAN participants underwent a comprehensive clinical vision exam for the characterization of ocular conditions including cataract, glaucoma, hypertensive retinopathy, diabetic retinopathy, and age-related macular degeneration. As shown in Table 2, the most common ocular condition was hypertensive retinopathy (30.2%), followed by dense cataract affecting vision (8.3%), diabetic retinopathy (6.4%), glaucoma (4.8%) and age-related macular degeneration (3.3%). Beyond the cases of clinically-observed dense cataract, an additional 14.2% of women had already undergone cataract surgery. Similarly, in addition to the women with clinically-defined glaucoma, an additional 28.6% had suspect glaucoma. To examine the relationship of these ocular conditions with the sociodemographic variables of interest, unadjusted and adjusted logistic regression models were examined (Table 3), with separate models run for each ocular condition. In fully adjusted models, age was statistically significantly associated with cataract; a one year increase in age was associated with 35% higher odds of having visually significant cataract (OR=1.35, 95% CI 1.12, 1.64), after adjustment for race/ethnicity, difficulty paying for basics, and education. Age was not associated with any of the other ocular conditions. Economic strain was associated with both hypertensive retinopathy and diabetic retinopathy, even after adjustment for age, race/ethnicity and education. Women reporting very/some difficulty paying for basics had 2.5-fold higher odds (95% CI 1.37, 4.55) of having hypertensive retinopathy and more than 4-fold higher odds (OR=4.39, 95% CI 1.41, 13.65) of having diabetic retinopathy as compared to women with no difficulty paying for basics. Economic strain was not statistically significantly associated with cataract, glaucoma or age-related macular degeneration. Women with a high school degree or less had 7-fold higher odds (OR=7.04, 95% CI 1.26, 39.35) of visually significant cataract as compared to women with college or post college education, even after adjustment for age, race/ethnicity and economic strain. None of the sociodemographic characteristics were statistically significantly associated with glaucoma or age-related macular degeneration.
Table 2:
Ocular disorders among Michigan Study of Women’s Health Across the Nation participants.
| Prevalence (n, %) | 95% Confidence Interval | |
|---|---|---|
| Cataract | ||
| Dense, affecting vision | 21 (8.3%) | 3.5 – 13.4% |
| Mild | 197 (77.6%) | 72.8 – 82.6% |
| Post-cataract surgery | 36 (14.2%) | 9.4 – 19.3% |
| Glaucoma a | ||
| Yes | 12 (4.8%) | 0.0 – 10.9% |
| Suspect | 72 (28.6%) | 23.0 – 34.7% |
| No | 168 (66.7%) | 61.1 – 72.8% |
| Diabetic Retinopathy a | 16 (6.4%) | 3.7 – 10.2% |
| Hypertensive Retinopathy a | 74 (30.2%) | 24.5 – 36.4% |
| Age-related Macular Degeneration a | 8 (3.3%) | 1.4 – 6.4% |
Some women were missing vision/ocular disorders data elements including glaucoma (n=2), hypertensive retinopathy (n=9), diabetic retinopathy (n=4), and age-related macular degeneration (n=11).
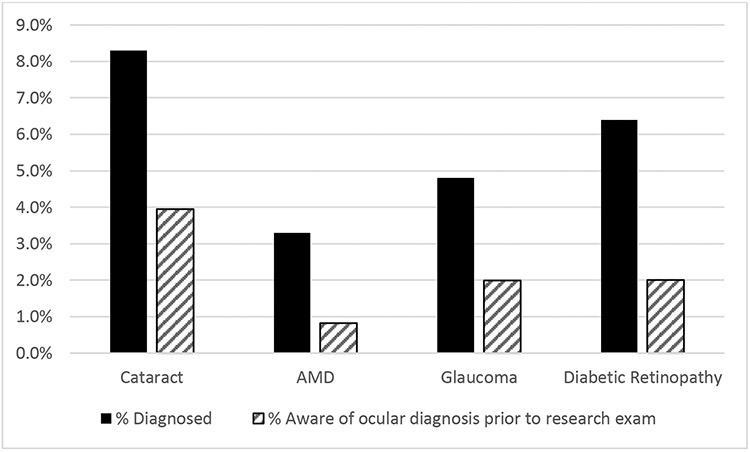
Among women with both vision assessments and self-reported questionnaire data, the concordance of observed vs. known ocular conditions was examined. As shown in Figure 2, women who had ocular conditions identified based upon the comprehensive clinical eye exam were largely unaware of their condition. When asked to self-report the presence of ocular conditions prior to the exam, more than half of the women subsequently identified with a given condition did not report it on their questionnaire. For example, while nearly 8% of women had visually significant cataract identified on clinical exam, only 4% of women reported on the questionnaire that they had cataract. Findings are similar for age-related macular degeneration, glaucoma, and diabetic retinopathy (Figure 2).
Figure 2.
Prevalence of ocular disease based upon comprehensive clinical eye exam (black bars) versus self-reported conditions (grey bars) among Michigan Study of Women’s Health Across the Nation participants, 2016-2017.
DISCUSSION
In this population-based sample of 254 women (mean age 66.0 years), nearly 75% of VI (based on presenting VA) was due to uncorrected or inadequately corrected refractive error. Upon providing correction for refractive error, cataract was the most common cause of VI. Both of these conditions are largely correctable. This study found significant disparities in VI based upon presenting VA whereby African American women and those with greater economic strain and less education had higher burden of VI. Notably, these disparities were no longer observed after provision of optical correction as there were no differences in VI based upon BCVA. This finding demonstrates racial and socioeconomic disparities underlying correctable VI and reinforces the potential for minimizing VI among older women with appropriate attention to at-risk groups.
We found that financial strain was an independent risk factor for VI (based on presenting VA) and for eye diseases. Older women who reported financial strain had more than three-fold higher odds of VI (based on presenting VA). Cost associated with seeking vision care and purchasing eyeglasses is a deterrent to effectively addressing VI34-39, especially among women, who report greater difficulty affording eyeglasses compared to men.40 Eye diseases that tend to require treatment and follow-up care for early detection and optimal control, namely diabetic retinopathy and hypertensive retinopathy, were associated with financial strain. Vision problems contribute to considerable economic burden, costing 65 billion dollars in direct medical costs.41 Tailored public health interventions to improve access and affordability of eye care should be considered to alleviate VI and promote optimal eye health.
Wearing an appropriate optical correction is an effective way to address the majority of VI.3 This study found that only 29% of women wore appropriate eyeglasses while the rest needed a new eyeglasses prescription. This need was even greater among those with presenting VA defined vision impairment where only 7% of women were wearing the correct prescription. Thus, a substantial gap exists between availability of effective treatment options and access to these treatment measures for those in need of treatment. Understanding barriers in accessing vision care and devising innovative strategies to overcome the barriers are necessary to bridge this gap and to achieve the eventual goal of reducing VI.38,42,43
Regular eye exams or vision screenings are not routinely performed for older adults, and the US Preventive Services Task Force stated that they did not find sufficient evidence to recommend vision screening for older adults.44 However, more than 50% of our Michigan SWAN cohort who were identified as having an eye disease were unaware of their diagnosis, and thus had an undetected eye disease. In addition, the majority of our cohort who presented with VI needed a new glasses prescription to achieve their best visual potential. These data suggest the presence of a large unmet need in this population for regular eye examination and vision screening to rectify correctable VI and to aid early diagnosis of uncorrectable VI before irreversible changes set in. Improving eye care utilization by promoting regular eye examination is a crucial step to identify and treat eye diseases in a timely fashion.
Strengths of this study include use of a population-based sample of older women residing in southeast Michigan to derive prevalence estimates, and use of standardized objective methods for assessing visual acuity and diagnosis of eye diseases. Further, this sample represents a racially and economically diverse population, as more than 60% of our population was African American and more than one-third reported very or some difficulty paying for basic needs. Examination of diverse populations is critical to fully elucidate the magnitude of the burden of VI and ocular conditions and to identify those groups in which intervention is most needed and may be most efficacious. A limitation of this cross-sectional study includes lack of opportunity to assess temporality of association and causation. In addition, the narrow age range (61.6-72.9 years) of the study participants may have limited our ability to fully examine the effect of age on VI and ocular disorders. Finally, because our sample includes only women from one geographic locale within the United States, our findings cannot be generalized to men or to women in other areas of the country.
In conclusion, this study reports a VI prevalence of 11.0% based on presenting VI, for which the majority could be improved with optical correction. Race, economic hardship and education were important correlates of VI (based on presenting VA) as well as ocular conditions. We posit that our findings support a need for regular eye examination among similar populations of women during early late adulthood, given the high proportion of participants needing new glasses and the detection of ocular conditions that were previously unknown to many of the participants. Community-based programs to promote public awareness, coordination of primary and eye care providers to detect and treat eye diseases, and tailored interventions for at-risk populations are essential to promote vision and eye health among older women.
ACKNOWLEDGEMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016-present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
This work was additionally supported by a grant from the National Institutes of Health (NIH), DHHS, through the National Eye Institute (R21EY030363 (DCM, SEM), by NIA R01AG017104 (Michigan SWAN Strength & Functioning Study), and University of Michigan MCubed (SEM, CK-G).
Footnotes
DECLARATION of INTEREST STATEMENT
The authors have no conflicts of interest to report.
REFERENCES
- 1.Congdon N, O'Colmain B, Klaver CCW, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 2.Chan T, Friedman DS, Bradley C, Massof R. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the United States. JAMA Ophthalmol. 2018;136(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma R, Vajaranant TS, Burkemper B, et al. Visual impairment and blindness in adults in the United States: Demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016;134(7):802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147(3):156–164. [DOI] [PubMed] [Google Scholar]
- 5.Salive ME, Guralnik J, Glynn RJ, Christen W, Wallace RB, Ostfeld AM. Association of visual impairment with mobility and physical function. J Am Geriatr Soc. 1994;42(3):287–292. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry SI, McAvay G, Ning Y, Allore HG, Newman AB, Gill TM. Geriatric impairments and disability: the cardiovascular health study. J Am Geriatr Soc. 2010;58(9):1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein BEK, Moss SE, Klein R, Lee KE, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology. 2003;110(4):644–650. [DOI] [PubMed] [Google Scholar]
- 8.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135(9):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Bullard KM, Cotch MF, et al. Association between depression and functional vision loss in persons 20 years of age or older in the United States, NHANES 2005-2008. JAMA Ophthalmol. 2013;131(5):573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46(1):58–64. [DOI] [PubMed] [Google Scholar]
- 11.Hong T, Mitchell P, Burlutsky G, Samarawickrama C, Wang JJ. Visual impairment and the incidence of falls and fractures among older people: longitudinal findings from the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2014;55(11):7589–7593. [DOI] [PubMed] [Google Scholar]
- 12.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. JAMA. 2006;295(18):2158–2163. [DOI] [PubMed] [Google Scholar]
- 13.Zebardast N, Friedman DS, Vitale S. The prevalence and demographic associations of presenting near-vision impairment among adults living in the United States. Am J Ophthalmol. 2017;174:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey RN, Indian RW, Zhang X, Geiss LS, Duenas MR, Saaddine JB, Centers for Disease Control and Prevention. Visual impairment and eye care among older adults – five States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(49):1321–1325. [PubMed] [Google Scholar]
- 15.Clayton JA, Davis AF. Sex/gender disparities and women’s eye health. Curr Eye Res. 2015;40(2):102–109. [DOI] [PubMed] [Google Scholar]
- 16.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond). 1993;84(1):95–98. [DOI] [PubMed] [Google Scholar]
- 17.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29(3):235–242. [DOI] [PubMed] [Google Scholar]
- 18.Ylitalo KR, Karvonen-Gutierrez CA. Body mass index, falls, and injurious falls among U.S. adults: Findings from the 2014 Behavioral Risk Factor Surveillance System. Prev Med. 2016;91:217–223. [DOI] [PubMed] [Google Scholar]
- 19.Timsina LR, Willetts JL, Brennan MJ, et al. Circumstances of fall-related injuries by age and gender among community-dwelling adults in the United States. PLoS One. 2017;12(5):e0176561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Rivera Drew JA. Cause, nature and care-seeking behaviour for injuries among community-dwelling older adults, USA, 2004-2013. Inj Prev. 2016;22(1):46–51. [DOI] [PubMed] [Google Scholar]
- 21.Esteban JJN, Solera Martinez M, Garcia Navalon P, et al. Visual impairment and quality of life: gender differences in the elderly in Cuenca, Spain. Qual Life Res. 2008;17(1):37–45. [DOI] [PubMed] [Google Scholar]
- 22.Crews JE, Chou CF, Zhang X, Zack MM, Saaddine JB. Health-related quality of life among people aged ≥65 years with self-reported visual impairment: findings from the 2006-2010 behavioral risk factor surveillance system. Ophthalmic Epidemiol. 2014;21(5):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. [DOI] [PubMed] [Google Scholar]
- 24.Welp A, Woodbury RB, McCoy MA, Teutsch SM, eds. Making Eye Health a Population Health Imperative. Washington DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 25.World Health Organization. Vision 2020. https://www.iapb.org/about/vision-2020/. Accessed February 2021. [Google Scholar]
- 26.Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services. 2020 Topics & Objectives – Vision. https://www.healthypeople.gov/2020/topics-objectives/topic/vision. Accessed February 2021. [Google Scholar]
- 27.Sowers M, Crawford S, Sternfeld B. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey L, Marcus R, al e., editors. Menopause: Biology and pathology. Academic Press; New York: 2000. pp. 175–188. [Google Scholar]
- 28.American Academy of Ophthalmology. Preferred Practice Pattern guidelines. https://www.aao.org/about-preferred-practice-patterns. Accessed February 2019. [Google Scholar]
- 29.Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336–345. [DOI] [PubMed] [Google Scholar]
- 30.Prum BE Jr, Lim MC, Mansberger SL, et al. Primary open-angle glaucoma suspect Preferred Practice Pattern(®) guidelines. Ophthalmology. 2016;123(1):P112–51. [DOI] [PubMed] [Google Scholar]
- 31.Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma Preferred Practice Pattern(®) guidelines. Ophthalmology. 2016;123(1):P41–P111. [DOI] [PubMed] [Google Scholar]
- 32.Prum BE Jr, Herndon LW Jr, Moroi SE, et al. Primary angle closure Preferred Practice Pattern(®) guidelines. Ophthalmology. 2016;123(1):P1–P40. [DOI] [PubMed] [Google Scholar]
- 33.Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. Arch Ophthalmol. 1993;111(6):831–836. [DOI] [PubMed] [Google Scholar]
- 34.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 35.McGwin G, Khoury R, Cross J, Owsley C. Vision impairment and eye care utilization among Americans 50 and older. Curr Eye Res. 2010;35(6):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeganathan VSE, Robin AL, Woodward MA. Refractive error in underserved adults: causes and potential solutions. Curr Opin Ophthalmol. 2017;28(4):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naël V, Moreau G, Monfermé S, et al. Prevalence and associated factors of uncorrected refractive error in older adults in a population-based study in France. JAMA Ophthalmol. 2019;137(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elam AR, Lee PP. Barriers to and suggestions on improving utilization of eye care in high-risk individuals: focus group results. Int Sch Res Notices. 2014;527831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otte B, Woodward MA, Ehrlich JR, Stagg BC. Self-reported eyeglass use by US Medicare beneficiaries aged 65 years or older. JAMA Ophthalmol. 2018;136(9):1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varadaraj V, Frick KD, Saaddine JB, Friedman DS, Swenor BK. Trends in eye care use and eyeglasses affordability: The US National Health Interview Survey, 2008-2016. JAMA Ophthalmol. 2019;137(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rein DB. Vision problems are a leading source of modifiable health expenditures. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF18–22. [DOI] [PubMed] [Google Scholar]
- 42.Alexander RL Jr., Miller NA, Cotch MF, Janiszewski R. Factors that influence the receipt of eye care. Am J Health Behav. 2008;32(5):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou CF, Sherrod CE, Zhang X, et al. Barriers to eye care among people aged 40 Years and older with diagnosed diabetes, 2006–2010. Diabetes Care. 2014;37(1):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Preventive Services Task Force (USPSTF), Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for impaired visual acuity in older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(9):908–914. [DOI] [PubMed] [Google Scholar]