Abstract
Background
To investigate the correlation between the preoperative systemic immune‐inflammation index (pSII) and postoperative pneumonia (POP) in surgical non‐small cell lung cancer patients.
Methods
Patients who underwent lung cancer surgery at West China Hospital of Sichuan University were retrospectively included. The indicators were collected, including basic information of patients, surgery‐related variables and POP rate. The predictive value of the pSII in the occurrence of POP was analyzed.
Results
A total of 1486 patients (male: 748, 50.3%; female: 738, 49.7%; mean age: 58.2 ± 9.7 years; median age: 59 years old, interquartile range: 51–65 years old) were finally included in the study, of which 142 patients had POP with an incidence of 9.5% (142/1486), 9.2% (69/748) in males, and 9.9% (73/738) in females. The proportion of patients with diabetes in the pneumonia group was significantly higher than that in the nonpneumonia group (9.8%, 14/142 vs. 5.6%, 75/1344, p = 0.041). Compared with the nonpneumonia group, the level of the preoperative body mass index (24.2 [21.9, 26.1] vs. 23.1 [21.1, 25.2], p = 0.003) and SII (487 [350, 673] vs. 345 [230, 500], p < 0.001) in the pneumonia group were significantly higher. Multiple factor analysis showed that the pSII (odds ratio: 1.001, 95% confidence interval: 1.000–1.001, p < 0.001) was an independent risk factor for POP (487 [350, 673] vs. 345 [230, 500], p < 0.001); receiver operating characteristic curve analysis showed that the pSII was effective in predicting POP (area under curve: 0.751, p < 0.001).
Conclusion
The pSII is closely related to and can effectively predict the occurrence of POP after lung cancer surgery.
Keywords: non‐small cell lung cancer, postoperative pneumonia, predictive value, surgery, systemic immune‐inflammation index
SII had better efficiency in predicting POP (AUC: 0.751, p < 0.001), and SII = 483 was the optimal cutoff point (Youden index: 0.41, sensitivity: 68.3%, specificity: 72.7%), compared to preoperative NLR and PLR which showed NLR (AUC: 0.567) and PLR (AUC: 0.582). The SII level could also effectively predict the severity of pulmonary infection (AUC: 0.793, p < 0.001).

INTRODUCTION
Lung cancer is a malignant tumor with the largest growing morbidity and death globally, 1 and it poses a significant risk to human health and life. Currently, surgery continues to be a key therapy for lung cancer, particularly for individuals with early‐stage non‐small cell lung cancer (NSCLC). 2 As the primary cause or contributing factor of mortality following pulmonary resection, postoperative pulmonary complications have a direct impact on the recovery of postoperative cardiopulmonary function, unavoidably extending hospital stays and increasing medical expenses. 3 , 4 After lung cancer surgery, the postoperative pneumonia (POP) is the most prevalent complication. Although measures such as chest drainage, anti‐infective therapy, encouraging patients to cough and expectorate actively after surgery, and perioperative pulmonary rehabilitation training have been shown to be effective in preventing postoperative infection, the incidence of pulmonary infection remains relatively high, and is also a common cause of perioperative death in lung cancer patients. 5 , 6 , 7 , 8 Therefore, the ability to effectively detect and avoid POP is a crucial component of lung cancer surgery perioperative treatment. Clinically, various inflammatory peripheral hematological indicators, such as white blood cell (WBC) count, procalcitonin (PCT), C‐reactive protein (CRP), and so forth, may represent the postoperative inflammatory condition of lung cancer patients to a certain degree, which have certain diagnostic and monitoring significance for pulmonary infection. In recent years, a number of studies have demonstrated that inflammation‐based markers, such as the neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and lymphocyte‐to‐monocyte ratio (LMR) in serum, are used to evaluate the inflammation level of patients, which is closely related to the postoperative prognosis of lung cancer. 9 , 10 , 11 Recent research has shown that the systemic immune‐inflammation index (SII) comprised of the platelet (PLT) count, neutrophil (Neu) count, and lymphocyte (Lym) count may be used to completely assess the status of systemic inflammation (SII = PLT × Neu/Lym). It has a high clinical utility in assessing the surgical prognosis for lung cancer. 12 , 13 SII has not yet been shown to accurately predict the likelihood of POP in lung cancer patients. This research retrospectively reviewed the clinical data of 1486 patients who had lung cancer surgery at the Lung Cancer Center of West China Hospital of Sichuan University between January 2019 and December 2020, and then investigated the connection between the preoperative SII (pSII) and POP. The aim of the study was to investigate the predictive value of pSII for the development of POP after pulmonary resection of non‐small cell lung cancer.
METHODS
Grouping and inclusion/exclusion criteria
Patients who had surgery for lung cancer at the Lung Cancer Center, West China Hospital of Sichuan University between January 2019 to December 2020 were included retrospectively. The following inclusion requirements were: (1) patients had a postoperative pathological diagnosis of NSCLC; (2) no preoperative radiotherapy, chemotherapy, immunotherapy, targeted therapy or others; and (3) pulmonary resection. Exclusion criteria included: (1) patients who underwent stage IV or palliative surgery; (2) had been diagnosed with lung pneumonia or/and received antibiotics or hormone therapy within a month prior to surgery; and (3) complicated with autoimmune diseases, such as ulcerative colitis and Crohn's disease. In addition, blood for evaluation of pSII was collected on the day before surgery for all the patients included.
Observation indicators
(1) Primary observation indicators.
The primary observation indicator was the incidence of POP, and the diagnosis met at least three of the following criteria: (1) chest plain film or chest CT showed lung exudation and consolidation; (2) temperature > 38°C; (3) WBC > 10 000/mm3 or < 3000/mm3; and (4) pathogens were detected in sputum, or purulent secretions were detected by bronchoscopy. The Clavien–Dindo (CD) complication grading evaluation system was used to classify POP as follows: level 2: requiring antibiotics and other drugs; level 3: requiring invasive intervention such as fiberoptic aspiration and endotracheal intubation; level 4: serious life threatening, even requiring ICU treatment; and level 5: resulting in death.
(2) Secondary observation indicators.
Indicators for hospitalization and surgery, and postoperative length of stay were included.
Statistical analysis
Continuous variables are expressed as the mean and standard deviation (mean ± SD), non‐normal distribution data are expressed as the median and interquartile range (IQR), and categorical variables are expressed as proportion (N%). An unpaired Student's t‐test was used to test the metric variables accord with normal distribution, while the Mann–Whitney U‐test was used to test the metric variables accord with non‐normal distribution. Categorical variables were tested using the chi‐square test or Fisher's exact test. Multiple factor analysis of risk factors for pulmonary complications within 30 days after surgery was performed using binary logistic regression. Variables with p < 0.1 of single factor analysis were included in the final multiple factor analysis, and p < 0.05 was considered statistically significant. The optimal cutoff value of SII was evaluated by receiver‐operating characteristic (ROC) curve analysis. Statistical tests were conducted using two‐sided tests, and p < 0.05 was considered statistically significant. An area under the curve (AUC) ≥ 0.7 was considered clinically effective. Statistical analysis was performed using SPSS version 21.0.
RESULTS
Comparison of clinicopathological characteristics between patients with POP and patients without POP
A total of 1921 surgical patients in our department were screened between January 2019 and December 2020, and finally 1486 patients were enrolled into the analysis (males: 748, 50.3%; females: 738 cases, 49.7%; mean age: 58.2 ± 9.7 years old; median age: 59 years old, IQR: 51–65 years old) (Figure 1). POP occurred in 142 patients (9.5% (142/1486), 9.2% (69/748) in males, and 9.9% (73/738) in females). Among them, 86.6% (123/142) were for CD 1–2, 11.3% (16/142) for CD 3, and 2.1% (3/142) for CD 4–5. Comparison of clinical data showed that the proportion of patients with pneumonia complicated with diabetes was significantly higher than that of patients without pneumonia (9.8%, 14/142 vs. 5.6%, 75/1344, p = 0.041). In addition, the preoperative body mass index (BMI) of patients with pneumonia (24.2 [21.9, 26.1] vs. 23.1[21.1, 25.2], p = 0.003) was significantly higher compared with that of patients without pneumonia, and SII also higher greatly (487 [350, 673] vs. 345 [230, 500], p < 0.001). (Table 1).
FIGURE 1.
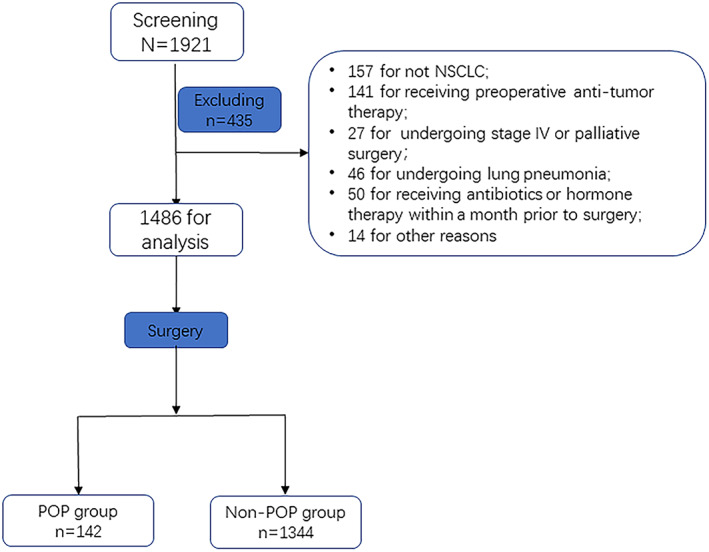
Study flow
TABLE 1.
Comparison of the clinicopathological features between POP group and nonpneumonia group in lung cancer patients
| Items | Pneumonia group (N = 142) | Nonpneumonia group (N = 1344) | p‐value |
|---|---|---|---|
| Age (mean ± SD) | 57.8 ± 10.4 | 58.2 ± 9.6 | 0.591 |
| Sex | 0.662 | ||
| Male | 69 (48.6) | 679 (50.5) | |
| Female | 73 (51.4) | 665 (49.5) | |
| Smoking | 0.297 | ||
| Yes | 18 (12.7) | 133 (9.9) | |
| No | 124 (87.3) | 1211 (90.1) | |
| Smoking index (pack/year) | 0.562 | ||
| 0 | 124 (87.3) | 1211 (90.1) | |
| 0–20 | 11 (7.7) | 85 (6.3) | |
| >20 | 7 (5.0) | 48 (3.6) | |
| Complication (Yes/No) | |||
| Diabetes | 14/128 | 75/1269 | 0.041 |
| Hypertention | 38/104 | 277/1067 | 0.088 |
| CHD | 7/135 | 61/1283 | 0.832 |
| COPD | 7/135 | 88/1256 | 0.454 |
| Tumor stage | 0.228 | ||
| Stage I | 96 (67.6) | 986 (73.4) | |
| Stage II | 39 (27.5) | 285 (21.2) | |
| Stage III | 7 (4.9) | 73 (5.4) | |
| Tumor histology | 0.798 | ||
| Adenocarcinoma | 100 (70.4) | 910 (67.7) | |
| Squamous cell carcinoma | 34 (23.9) | 352 (26.2) | |
| Other | 8 (5.7) | 84 (6.1) | |
| BMI (kg/m2) (median, IQR) | 24.2 (21.9, 26.1) | 23.1 (21.1, 25.2) | 0.003 |
| SII (median, IQR) | 487 (350, 673) | 345 (230, 500) | <0.001 |
| Preoperative lung function | |||
| FEV1% (median, IQR) | 99.0 (82.8, 110.5) | 101.5 (89.0, 112.2) | 0.090 |
| DLco% (median, IQR) | 93.0 (83.3, 107.3) | 94.7 (85.1, 106.7) | 0.414 |
| Operative time (median, IQR) | 120 (115, 130) | 120 (110, 130) | 0.370 |
| Operative blood loss (median, IQR) | 50 (30, 130) | 50 (20, 100) | 0.047 |
| VATS | 0.282 | ||
| No | 29 (20.4) | 329 (24.5) | |
| Yes | 113 (79.6) | 1015 (75.5) | |
| Postoperative in‐hospital stay (median, IQR) | 8 (7, 12) | 6 (5, 8) | <0.001 |
Abbreviations: CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DLco, diffusing capacity of the lungs for carbon monoxide; VATS, video‐assisted thoracic surgery.
Logistic regression analysis of risk factors for POP in patients undergoing lung cancer surgery
The univariate logistic regression analysis included variables with p < 0.1 of single factor analysis. It was found that diabetes (p = 0.044), absolute value of forced expiratory volume in 1 s (FEV1) (p = 0.068), BMI (p = 0.002) and SII (p = 0.002) were finally included in the univariate logistic regression analysis. Multifactor logistic regression analysis showed that BMI (OR = 1.091, 95% CI: 1.033–1.152, p = 0.002) and pSII (OR = 1.000, 95% CI: 1.000–1.001, p < 0.001) was an independent risk factor for POP (Table 2).
TABLE 2.
Logistic regression analysis of risk factors for POP in patients having lung cancer surgery
| Variables | Single factor analysis | Multiple factor analysis | ||||
|---|---|---|---|---|---|---|
| OR value | 95% CI | p‐value | OR value | 95% CI | p‐value | |
| Diabetes | 2.41 | 1.107–3.368 | 0.044 | 1.565 | 0.835–2.933 | 0.162 |
| FEV1 | 0.992 | 0.983–1.001 | 0.068 | 0.993 | 0.984–1.003 | 0.155 |
| BMI | 1.091 | 1.034–1.151 | 0.002 | 1.091 | 1.033–1.152 | 0.002 |
| SII | 1.001 | 1.000–1.001 | <0.001 | 1.000 | 1.000–1.001 | <0.001 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 s; SII, systemic immune‐inflammation index.
ROC curve analysis of the efficacy of PSII, NLR and PLR levels in predicting the occurrence of POP
ROC curve analysis showed that SII had better efficiency in predicting POP (AUC: 0.751, p < 0.001), and SII = 483 was the optimal cutoff point (Youden index: 0.41, sensitivity: 68.3%, specificity: 72.7%), compared to preoperative NLR and PLR which showed NLR (AUC: 0.567) and PLR (AUC: 0.582) (Figure 2).
FIGURE 2.
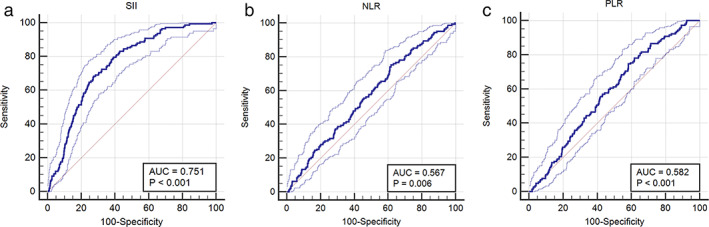
A receiver‐operating characteristic (ROC) curve was used to analyze the efficacy of pSII (a), NLR (b) and PLR (c) levels in predicting the occurrence of POP in lung cancer patients. pSII, preoperative systemic immune‐inflammation index; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio
Subgroup analysis of patients with POP
Further subgroup analysis of patients in the pneumonia group (n = 142) showed that CD grade above 2 of pneumonia accounted for 13.4% (19/142). In addition, the pSII level in the CD >2 group was significantly higher than that in the CD ≤2 group (476 [337, 611] vs. 1125 [429, 1369], p < 0.001). The ROC curve results showed that the SII level could effectively predict the severity of pulmonary infection (AUC: 0.793, p < 0.001). (Table 3) (Figure 3).
TABLE 3.
Comparison of clinicopathological features between different Clavien‐Dindo (CD) grade subgroups with pneumonia in lung cancer patients after surgery
| Items | CD ≤2 (N = 123) | CD >2 (N = 19) | p‐value |
|---|---|---|---|
| Age (mean ± SD) | 57.8 ± 10.5 | 57.6 ± 9.9 | 0.939 |
| Sex | 0.172 | ||
| Male | 57 (46.3) | 12 (63.2) | |
| Female | 66 (53.7) | 7 (36.8) | |
| Smoking | 1.000 | ||
| Yes | 16 (13.0) | 2 (10.5) | |
| No | 107 (87.0) | 17 (89.5) | |
| Smoking index (packayear) | 1.000 | ||
| 0 | 108 (87.8) | 16 (84.2) | |
| 0 ~ 20 | 9 (7.3) | 2 (10.5) | |
| >20 | 6 (4.9) | 1 (5.3) | |
| Complication (Yes/No) | |||
| Diabetes | 10/113 | 4/15 | 0.096 |
| Hypertension | 32/91 | 6/13 | 0.610 |
| CHD | 7/116 | 0/19 | 0.594 |
| COPD | 6/117 | 1/18 | 1.000 |
| Tumor stage | 0.797 | ||
| Stage I | 82 (66.7) | 14 (73.4) | |
| Stage II | 35 (28.5) | 4 (21.1) | |
| Stage III | 6 (4.9) | 1 (5.3) | |
| Tumor histology | 0.657 | ||
| Adenocarcinoma | 85 (69.1) | 15 (78.9) | |
| Squamous cell carcinoma | 31 (25.2) | 3 (15.8) | |
| Other | 7 (5.7) | 1 (5.3) | |
| BMI (kg/m2) (median, IQR) | 24.2 (22.0, 26.1) | 24.4 (20.9, 26.2) | 0.621 |
| SII (median, IQR) | 476 (337, 611) | 1125 (429, 1369) | <0.001 |
| Preoperative lung function | |||
| FEV1% | 99.0 (83.8, 110.5) | 101.7 (79.3, 112.6) | 0.943 |
| DLco% | 93.8 (83.5, 108.7) | 90.9 (81.6, 96.6) | 0.177 |
| Operative time (median, IQR) | 120 (115, 130) | 120 (115, 135) | 0.857 |
| Operative blood loss (median, IQR) | 50 (30, 150) | 50 (20, 100) | 0.440 |
| VATS | 0.764 | ||
| No | 26 (21.1) | 3 (15.8) | |
| Yes | 97 (78.9) | 16 (84.2) | |
|
Postoperative in‐hospital stay (median, IQR) |
8 (7, 12) | 10 (8, 12) | 0.129 |
Abbreviations: CHD, coronary heart disease, COPD, chronic obstructive pulmonary disease, DLco, diffusing capacity of the lungs for carbon monoxide; VATS, video‐assisted thoracic surgery.
FIGURE 3.
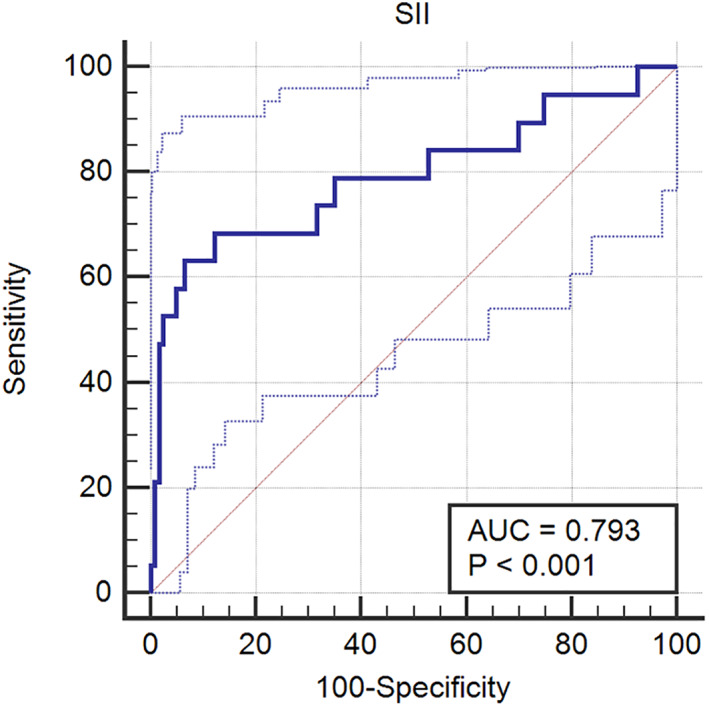
A receiver‐operating characteristic (ROC) curve analysis of the efficacy of SII in predicting postoperative pulmonary infection grade in lung cancer patients. pSII, preoperative systemic immune‐inflammation index
DISCUSSION
As the treatment option of choice for resectable lung cancer, surgery is also the most effective lung cancer treatment, particularly for early‐stage NSCLC, and its significance is obvious. With the advancement of thoracoscopic surgical equipment and technology and the development of perioperative management ideas, the inpatient experience and postoperative rehabilitation of patients have received increasing attention. However, the occurrence of postoperative pulmonary complications, such as pulmonary infection, increase the average postoperative in hospital‐stay, medical expenses, and mortality risk of patients undergoing surgical treatment for lung cancer, and seuqantially the workload of medical personnel, and so forth. Therefore, accurately predicting the occurrence of pulmonary complications after lung cancer surgery at an early stage is of tremendous therapeutic importance. Clinically, some biochemical indicators are often used to aid in the diagnosis and prediction of POP. Changes in peripheral blood cell count, for instance, might alter the proportions of certain cells, such as NLR, LMR and PLR. These ratios may represent changes in systemic and local inflammatory responses, and it has been observed in recent years that such indicators have some therapeutic relevance in predicting postoperative complications. 9 , 10 , 11 The retrospective study presented here examined the link between pSII level and POP in 1486 patients who had surgical therapy for NSCLC from 2019 to 2020 in Lung Cancer Center of West China Hospital of Sichuan University. The results demonstrated that the pSII was an independent risk factor for pulmonary infection after surgery in patients with lung cancer and had a strong predictive effect on the occurrence of postoperative pulmonary infection, indicating that SII may have clinical application value in predicting the occurrence of the POP.
Hu et al. 14 first reported SII in 2014. Based on PLR and NLR, it combines neutrophils, platelets and lymphocytes to more precisely depict patients' inflammation and immunological condition and to predict the prognosis of patients with NSCLC. 12 , 13 , 14 , 15 , 16 , 17 , 18 In recent years, the close relationship between SII and infectious disorders has also been highlighted. High SII levels are independently related to in‐hospital mortality, and SII may be a valid prognostic indicator for patients with infective endocarditis. 19 Domestic and international research on the association between the pSII and POP in NSCLC has not yet been published. The SII as a cellular immune inflammation marker that integrates the kinetics of NLR and PLR into one single parameter, could better indicate the inflammatory status of the patients, and the inflammatory status might be closely associated with infection. 20 This study revealed that the pSII of the pneumonia group was significantly higher than that of the nonpneumonia group, and multifactor logistic regression analysis of risk factors for POP revealed that SII was an independent risk factor, indicating that the pSII was significantly associated with POP. Consequently, ROC curve analysis was used in this research, and it was determined that, compared to preoperative NLR and PLR, SII was more predictive of the incidence of POP. In addition, a subgroup analysis of patients with pneumonia revealed that SII might predict the severity of POP more accurately. This data implies that SII has clinical use as a reliable predictor of pulmonary infection after lung cancer surgery.
It should also be mentioned that this research is a retrospective study with some flaws and restrictions. In contrast to prospective studies, the majority of research data is derived from clinical medical records, which presents some constraints in terms of the validity and reliability of data gathering. Future research will need to confirm the reliability and clinical importance of SII in predicting the POP in lung cancer patients. This will require a multicenter, prospective controlled investigation with a high sample size.
In conclusion, the pSII levels in NSCLC patients correlate strongly with the frequency of POP, and the pSII levels may effectively predict the incidence and severity of pulmonary infection following surgical treatment in lung cancer patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Jiang R, Li P, Shen W, Deng H, Qin C, Qiu X, et al. The predictive value of the preoperative systemic immune‐inflammation index in the occurrence of postoperative pneumonia in non‐small cell lung cancer: A retrospective study based on 1486 cases. Thorac Cancer. 2023;14(1):30–35. 10.1111/1759-7714.14691
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Altorki NK, Wang X, Wigle D, Gu L, Darling G, Ashrafi AS, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early‐stage non‐small‐cell lung cancer: post‐hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med. 2018;6(12):915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lugg ST, Agostini PJ, Tikka T, Kerr A, Adams K, Bishay E, et al. Long‐term impact of developing a postoperative pulmonary complication after lung surgery. Thorax. 2016;71:171–6. [DOI] [PubMed] [Google Scholar]
- 4. Ha D, Choi H, Zell K, Raymond DP, Stephans K, Wang XF, et al. Association of impaired heart rate recovery with cardiopulmonary complications after lung cancer resection surgery. J Thorac Cardiovasc Surg. 2015;149(4):1168–73. [DOI] [PubMed] [Google Scholar]
- 5. Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157(2):362–80. [DOI] [PubMed] [Google Scholar]
- 6. Schuetz P, Muller B, Christ‐Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10(10):CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kradin RL, Digumarthy S. The pathology of pulmonary bacterial infection. Semin Diagn Pathol. 2017;34(6):498–509. [DOI] [PubMed] [Google Scholar]
- 8. Mizgerd JP. Pathogenesis of severe pneumonia: advances and knowledge gaps. Curr Opin Pulm Med. 2017;23(3):193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative peripheral blood neutrophil‐to‐lymphocyte ratios (NLR) and platelet‐to‐lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited‐stage small‐cell lung cancer. Transl Lung Cancer Res. 2021;10(2):866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia L, Huang H, Xiao H, Wang D, Yang Z. Utilization of combined PD‐L1 expression and neutrophil‐to‐lymphocyte ratio prior to surgery as a prognostic factor in non‐small cell lung cancer with brain metastasis. Transl Cancer Res. 2019;8(8):2864–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Li H, Xu R, et al. The MLR, NLR, PLR and D‐dimer are associated with clinical outcome in lung cancer patients treated with surgery. BMC Pulm Med. 2022;22(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu F, Deng C, Wen Z, Gao Z, Zhao Y, Han H, et al. Systemic immune‐inflammation index is a stage‐dependent prognostic factor in patients with operable non‐small cell lung cancer. Transl Lung Cancer Res. 2021. Jul;10(7):3144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He H, Guo W, Song P, Liu L, Zhang G, Wang Y, et al. Preoperative systemic immune‐inflammation index and prognostic nutritional index predict prognosis of patients with pulmonary neuroendocrine tumors after surgical resection. Ann Transl Med. 2020;8(10):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- 15. Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134(1):230–4. [PubMed] [Google Scholar]
- 16. Stanger BZ, Kahn ML. Platelets and tumor cells: a new form of border control. Cancer Cell. 2013;24(1):9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: a systematic review with meta‐analysis. Br J Cancer. 2011;105(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jia Q, Yang Y, Wan Y. Tumor‐infiltrating memory T‐lymphocytes for prognostic prediction in cancer patients: a meta‐analysis. Int J Clin Exp Med. 2015;8(2):1803–13. [PMC free article] [PubMed] [Google Scholar]
- 19. Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren H, et al. Prognostic value of systemic immune‐inflammation index in patients with gastric cancer. Chin J Cancer. 2017;36(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karimi A, Shobeiri P, Kulasinghe A, Rezaei N. Novel systemic inflammation markers to predict COVID‐19 prognosis. Front Immunol. 2021;12:741061. [DOI] [PMC free article] [PubMed] [Google Scholar]


