Abstract
Background
Preclinical data have shown the immunomodulatory effects of metformin and dipeptidyl peptidase 4 (DPP4) inhibitors in patients with diabetes. However, its clinical impact remains unclear in lung cancer.
Methods
Between 2017 and 2021, 466 patients received ICI monotherapy. Patients were categorized into concurrent (MET; metformin or combination of metformin and DPP4 inhibitor) and without concomitant (NMET; nonmetformin/DPP4 inhibitors) administration of metformin and DPP4 inhibitors groups at least 8 weeks before and during ICI therapy. The primary objectives were the objective response rate (ORR) and progression‐free survival (PFS). The second objective was to evaluate the overall survival (OS) and the occurrence of immune‐related adverse events (irAEs).
Results
Among 466 patients, 89 (19.0%) and 377 (81%) were categorized into the MET and NMET groups, respectively. MET group had a significantly higher ORR (MET group: 24.7% vs. NMET group: 14.8%, p = 0.025) and longer PFS than those in the NMET group (MET group 5.1 month vs. NMET group 2.8 months, p = 0.018). After patients were stratified based on the prior line of therapy and PD L1 expression status, the PFS of the second‐line therapy and PD L1 ≥50 was significantly higher in the MET than in the NMET group. The proportion of patients experiencing all‐grade irAEs was numerically higher in the MET group (19.1%) than in the NMET group (14.3%), without statistical significance (p = 0.382).
Conclusions
Concurrent use of metformin and DPP4 inhibitors with ICIs significantly improved the clinical outcomes without increasing the incidence of irAEs in NSCLC.
Keywords: dipeptidyl peptidase 4 inhibitor, immune checkpoint inhibitor, metformin, non‐small cell lung cancer
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have become the standard treatment options for non‐small cell lung cancer (NSCLC) because they have significantly improved survival by enhancing the immune response against tumors. Recent global guidelines have recommended treatment with ICIs alone or combined with chemotherapy for lung cancer patients without targetable genetic alterations. 1 The results of early clinical trials involving agents targeting the programmed cell‐death protein 1 (PD‐1) or programmed cell‐death ligand 1 (PD‐L1) axis indicate the possibility for a long‐term, durable clinical benefit in 15%–20% of metastatic NSCLC patients. 2 Nonetheless, the median overall survival (OS) for patients with metastatic or recurrent NSCLC unresponsive to ICIs remains to be <3 years. 3 Therefore, selecting appropriate patients for current ICI therapy and enhancing ICI response pose a challenge.
In addition to recognized predictive factors such as PD‐L1 expression, tumor mutational burden and host immune status can affect the response to ICI therapy. 4 Similarly, in addition to the PD‐L1/PD‐1 axis, a patient's immune profile can be influenced by many factors, including age, sex, ethnicity, comorbidity, intake of immune‐modifying drugs, and gut microbiota. Previous studies have highlighted the effects of other factors such as obesity‐related immune suppression and promotion of antitumor immune signaling by antidiabetic drugs, especially metformin, in cancer patients. 5 , 6 , 7 Therefore, aside from predictive biomarkers, it is crucial to indicate the correlation between host factors, such as concomitant medications, and the efficacy of ICIs.
Metformin is a widely used therapeutic agent for type 2 diabetes mellitus and has recently been demonstrated as an immunomodulatory agent in preclinical studies. 8 It induces an anticancer immune response via an immunomodulatory pathway by activating AMP‐activated protein kinase (AMPK) signaling and promoting anti‐tumor immune activity through T cells and endoplasmic reticulum‐associated degradation of PD‐L1. 9 , 10
Dipeptidyl peptidase 4 (DPP4) inhibitors are used as an add‐on therapy to other classes of drugs for type 2 diabetes, such as metformin. 11 , 12 Furthermore, the cluster of differentiation 26/dipeptidyl peptidase 4 (CD26/DPP4) is a surface glycoprotein that affects glucose metabolism and immunomodulation within the tumor microenvironment (TME). 13 A preclinical study reported that DPP4 inhibition increases checkpoint blockade and lymphocyte trafficking via C‐X‐C motif chemokine ligand 10 (CXCL10) cleavage, consequently enhancing tumor immunotherapy. 14 However, the clinical effect of the concurrent use of metformin and DPP4 inhibitors on ICIs in NSCLC remains controversial.
The present study evaluated the clinical effects and immune‐related adverse events (irAEs) of combining metformin and DPP4 inhibitors with ICI monotherapy in patients with metastatic or recurrent NSCLC.
METHODS
Study design, study population, and methods
We retrospectively analyzed the clinical data of 466 patients with metastatic or recurrent NSCLC treated with ICI monotherapy between January 2017 and January 2021 at the Seoul National University Bundang Hospital. The eligible patients were aged 20 years or older and had histologically confirmed NSCLC, metastatic or recurrent disease, and ≥1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, with or without concurrent metformin and DPP4 inhibitors (administered separately or in combination) from at least 8 weeks before starting ICI and during ICI therapy (Figure 1). Patients with any systemic disease or malignancy that could influence the survival analysis and those taking oral hypoglycemic agents or insulin were excluded to eliminate the confounding effect. Data on baseline demographics, molecular profiles, and prior treatments were collected from electronic medical records.
FIGURE 1.
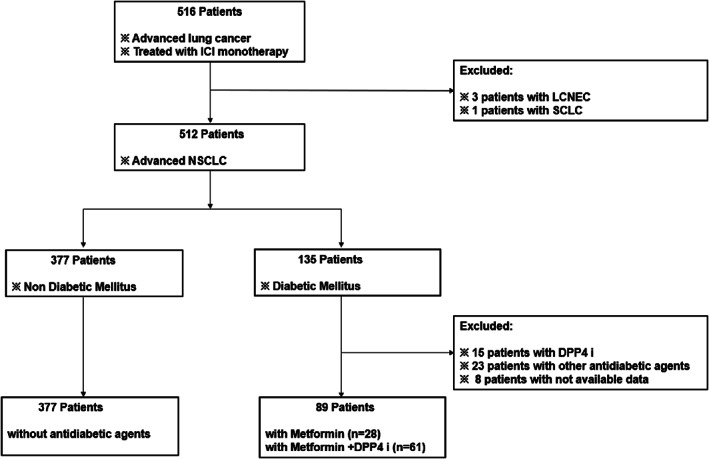
Consolidated standards of reporting trials (CONSORT) diagram. DPP4 i, DPP4 inhibitor; ICI, immune checkpoint inhibitor; LCNEC, large cell neuroendocrine carcinoma; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
All patients were treated with ICI monotherapy, including administration of anti PD‐1 (pembrolizumab or nivolumab) or anti‐PD L1 (atezolizumab) antibodies. Patients with concomitant administration of metformin and DPP4 (separately or in combination), and those without concurrent use of the same were categorized into the metformin/DDP‐4 inhibitors (MET) and nonmetformin/DPP4 inhibitors (NMET) groups, respectively. First, all patients were treated with ICI monotherapy until disease progression or cessation due to drug toxicity. Subsequently, PD‐L1 expression in the tumor samples was evaluated using the PD‐L1 IHC 22C3 pharmDx kit on the Dako Link 48 platform (Dako) or SP263 on the Ventana Benchmark Ultra platform (Ventana Medical Systems). Experienced pathologists manually estimated the percentage of tumor cells with membranous PD‐L1 staining of any intensity. Next, imaging with computed tomography was performed at baseline and every 8–12 weeks to assess the response during treatment until progression. In addition, laboratory tests (complete blood cell count, liver function chemistry, and renal function) were performed at baseline and before each cycle. Furthermore, a response assessment was performed using the RECIST criteria version 1.1. IrAEs were defined as adverse events with an immunological basis that required frequent follow‐up and potential intervention. 15 Lastly, irAEs were evaluated by clinicians before ICI administration and in case of new symptoms.
Notably, progression‐free survival (PFS) was defined as the time from the date of first ICI treatment to the date of disease progression or death. OS was defined as the time from the start date of ICI therapy until the date of death, irrespective of the cause. The overall response rate (ORR) was defined as the percentage of patients with complete response (CR) or partial response (PR). In contrast, disease control rate (DCR) was defined as the proportion of patients who experienced CR, PR, or stable disease (SD).
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board of the Seoul National University (IRB number B‐2110‐717‐101). The requirement for consent for participation was waived by the ethics board.
Statistical analysis
Continuous and categorical variables across groups were compared using the t‐test and the chi‐squared or Fisher's exact test, respectively. PFS and OS were estimated by Kaplan–Meier analysis and compared using the log‐rank test. Univariate and multivariate Cox proportional hazard regression analyses were performed to examine the relationship between the concomitant medication (MET) and survival, summarizing the hazard ratio (HR) and 95% confidence interval (CI) for each group. All p‐values were two‐sided, and p‐values of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS software version 25.0 (IBM Corporation).
RESULTS
Patient characteristics
A total of 466 patients with metastatic or recurrent NSCLC treated with ICI monotherapy were included in this study. Of these patients, 89 (19%) and 377 (81%) comprised the MET group (metformin, n = 28; metformin+DPP4 inhibitor, n = 61) and NMET group, respectively. Additionally, 419 patients were evaluated for PD‐L1 expression, with 252 and 167 patients showing PD‐L1 expressions <50% and ≥ 50%, respectively. All adult patients received ICI monotherapy, with 53.6% and 36.3% of patients receiving second‐ and third‐line therapies, respectively. The median age at treatment was higher in the MET group without being statistically significant (67.3 ± 10.1 vs. 65.3 ± 11.0, p = 0.179). In both groups, similar proportions of patients previously received chemotherapy (92.1% in the MET group vs. 92.8% in the NMET group, p = 0.818). Overall, 21 and 24.4% of patients in the MET and NMET groups, respectively, had brain metastasis (p = 0.543). Notably, no major differences in clinicopathological features were observed between the MET and NMET groups. Table 1 summarizes the baseline patient characteristics.
TABLE 1.
Baseline characteristics of patients
| Variables | MET group (n = 89) | NMET group (n = 377) | p‐value |
|---|---|---|---|
| Median age at ICI treatment | 67.3 ± 10.1 | 65.3 ± 11.0 | 0.179 |
| Sex | |||
| Male | 72 (80.9%) | 275 (72.9%) | 0.122 |
| Female | 17 (19.1%) | 102 (27.1%) | |
| Smoking history | |||
| Smoker (current or ex‐smoker) | 69 (77.5%) | 254 (67.4%) | 0.110 |
| Never smoker | 20 (22.5%) | 116 (30.8%) | |
| NA | 0 (0%) | 7 (1.8%) | |
| ECOG PS | |||
| 0–1 | 50 (56.2%) | 201 (53.3%) | 0.498 |
| ≥2 | 34 (38.2%) | 140 (37.1%) | |
| NA | 5 (5.6%) | 36 (9.5%) | |
| Previous treatment | |||
| Chemotherapy | |||
| Yes | 82 (92.1%) | 350 (92.8%) | 0.818 |
| No | 7 (7.9%) | 27 (7.2%) | |
| TKI | |||
| Yes | 17 (19.1%) | 96 (25.5%) | 0.208 |
| No | 72 (80.9%) | 281 (74.5%) | |
| PD‐L1 status | |||
| <1% | 24 (27.0%) | 114 (30.2%) | 0.334 |
| 1–49% | 25 (28.1%) | 89 (23.6%) | |
| ≥50 | 35 (39.3%) | 132 (35.0%) | |
| NA | 5 (5.6%) | 42 (11.2%) | |
| ICIs | |||
| PD‐1 Ab | 58 (65.2%) | 249 (66.0%) | 0.901 |
| PD‐L1 Ab | 31 (34.8%) | 128 (34.0%) | |
| ICI line | |||
| First‐line | 6 (6.7%) | 41 (10.9%) | 0.473 |
| Second‐line | 51 (57.3%) | 199 (52.8%) | |
| Third−/later‐line | 32 (36.0%) | 137 (36.3%) | |
| Median cycles of ICI | 8.9 ± 10.1 | 8.9 ± 10.8 | 0.654 |
| Driver mutation | |||
| (EGFR or ALK) | |||
| Yes | 17 (19.1%) | 97 (25.7%) | 0.191 |
| No | 72 (80.9%) | 280 (74.3%) | |
| Mean number of metastatic sites before starting therapy | 1.7 ± 1.1 | 1.69 ± 0.9 | 0.468 |
| Brain metastasis | |||
| Yes | 19 (21.3%) | 92 (24.4%) | 0.543 |
| Bo | 70 (78.7%) | 285 (75.6%) |
Abbreviations: Ab, antibody; ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; MET group, concomitant administration of metformin and DPP4 inhibitor (metformin or combination of metformin and DPP4 inhibitor); NA, not available; NMET group, without concomitant administration of metformin and DPP4 inhibitor; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; TKI, tyrosine kinase inhibitor.
Clinical outcomes
Efficacy of metformin and DPP4 inhibitors in ICI monotherapy
First, we evaluated the efficacy of metformin and DPP4 inhibitors (separately and in combination) during ICI treatment. The median follow‐up time was 11.6 (0.1–77.6) months. We found that the concomitant administration of the inhibitor was associated with improved ORR and PFS. A statistically significant higher ORR was observed in the MET group (24.7%) than in the NMET group (14.8%) (p = 0.025). Table 2 presents the detailed overall response data. Notably, patients in the MET group also experienced a longer PFS than those in the NMET group. As shown in Figure 2a, the median PFS in the MET and NMET groups was 5.1 and 2.8 months, respectively (HR: 0.72, 95% CI: 0.55–0.95, p = 0.018). Adding DPP4 inhibitors to metformin did not improve PFS, as compared with metformin alone with ICI treatment (PFS: 7.1 months with metformin alone vs. 4.9 months with metformin and DPP4 inhibitor; HR: 0.84, 95% CI: 0.50–1.42, p = 0.513) (Figure 2b). Second‐line ICI therapy was associated with longer PFS in the MET group than in the NMET group (HR: 0.74, 95% CI: 0.57–0.97, p = 0.028) (Figure 2c). In patients with PD‐L1 expression ≥50%, PFS was 9.4 and 4.1 months in the MET and NMET groups, respectively (HR: 0.57, 95% CI: 0.45–0.71, p < 0.001) (Figure 2d). However, no significant difference in OS was observed between the two groups (HR: 0.96, 95% CI: 0.62–1.49, p = 0.865) (Figure S1).
TABLE 2.
Overall best response
| MET group (n = 89) | NMET group (n = 377) | p‐value | |
|---|---|---|---|
| Best response | |||
| CR | 1 (1.1%) | 1 (0.2%) | 0.27 |
| PR | 21 (23.6%) | 55 (14.6%) | 0.039 |
| SD | 36 (40.5%) | 136 (36.1%) | 0.44 |
| PD | 31 (34.8%) | 185 (49.1%) | 0.015 |
| ORR | 22 (24.7%) | 56 (14.8%) | 0.025 |
| DCR | 58 (65.2%) | 192 (50.9%) | 0.015 |
Abbreviations: CR, complete response; DCR, disease control rate; MET group, concomitant administration of metformin and DPP4 inhibitor (metformin or combination of metformin and DPP4 inhibitor); NMET group, without concomitant administration of metformin and DPP4 inhibitor; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
FIGURE 2.
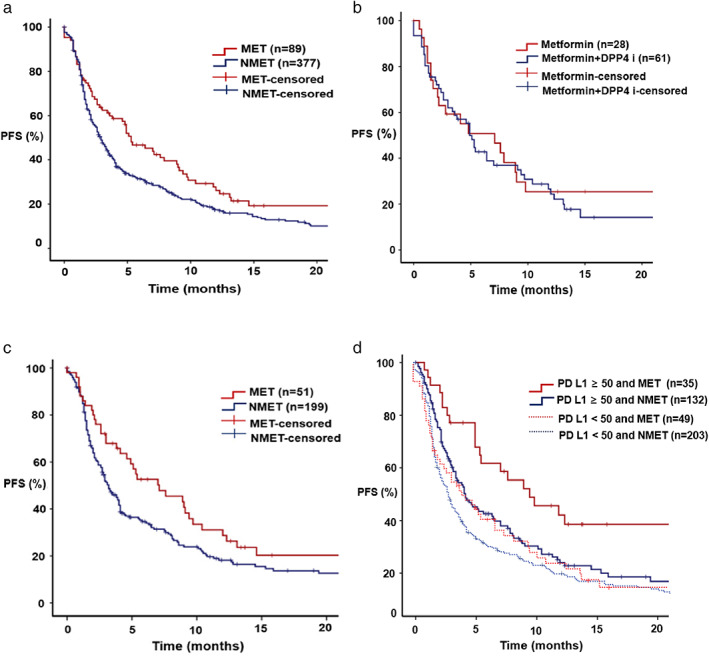
Kaplan–Meier curve for progression‐free survival (PFS). (a) PFS according to the group. Red line; MET group, blue line; NMET group, hazard ratio (HR): 0.72; 95% confidence interval (CI): 0.55–0.95, p = 0.018. (b) PFS according to the MET subgroup. Red line; metformin, blue line; metformin and DPP4 inhibitor, HR: 0.84; 95% CI: 0.50–1.42, p = 0.513. (c) PFS with second‐line therapy. Red line, MET group; blue line, NMET group; HR: 0.70; 95% CI: 0.49–0.99, p = 0.044. (d) PFS according to PD L1 expression. Red line: MET group and PD L1 ≥ 50, blue line; NMET group and PD L1 ≥ 50, HR: 0.57; 95% CI: 0.45–0.71, p < 0.001, red dotted line; MET group and PD L1 < 50, blue dotted line; NMET group and PD L1 < 50, HR: 0.94; 95% CI: 0.66–1.32, p = 0.708.
Univariate and multivariate analyses for PFS and OS
Univariate and multivariate analyses were conducted to identify the prognostic factors. The MET group had significantly longer PFS than the NMET group (HR: 0.72, 95% CI: 0.55–0.95, p = 0.018) in the former. PD‐L1 expression score ≥ 50 was associated with longer PFS (5% cut‐off, HR: 0.57, 95% CI: 0.45–0.71, p < 0.0001). However, Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 (p < 0.0001) and previous chemotherapy history (p = 0.018) were associated with shorter PFS.
Significantly, concomitant inhibitor use remained an independent prognostic factor for PFS (HR: 0.69, 95% CI: 0.52–0.93, p = 0.013) after adjusting for other confounding factors (Table 3). However, concomitant inhibitors were not associated with OS in the univariate and multivariate analyses (Table 4).
TABLE 3.
Univariate and multivariable analyses of the clinical features for progression‐free survival
| Univariate analysis Hazard ratio (95% CI) | p‐value | Multivariate analysis Hazard ratio (95% CI) | p‐value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 0.79 (0.62–1.01) | 0.059 | 0.97 (0.68–1.38) | 0.864 |
| Age, years | ||||
| <65 | 1 | 1 | ||
| ≥65 | 0.81 (0.65–1.00) | 0.051 | 0.80 (0.63–1.01) | 0.057 |
| Smoking | ||||
| Never smoker | 1 | 1 | ||
| Ever‐smoker | 0.74 (0.58–0.93) | 0.010 | 0.84 (0.60–1.18) | 0.312 |
| ECOG PS | ||||
| 0–1 | 1 | 1 | ||
| ≥2 | 1.62 (1.29–2.03) | <0.0001 | 1.84 (1.45–2.33) | <0.0001 |
| Previous chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 1.73 (1.10–2.72) | 0.018 | 1.18 (0.67–2.10) | 0.574 |
| Previous TKI | ||||
| No | 1 | 1 | ||
| Yes | 1.23 (0.97–1.57) | 0.089 | 1.10 (0.83–1.46) | 0.491 |
| PD‐L1 expression | ||||
| <50 | 1 | 1 | <0.0001 | |
| ≥50 | 0.57 (0.45–0.71) | <0.0001 | 0.62 (0.49–0.78) | |
| ICI line | ||||
| First‐line | 1 | 1 | 0.071 | |
| Second−/later‐line | 1.77 (1.17–2.66) | 0.006 | 1.62 (0.96–2.72) | |
| Driver mutation | ||||
| No | 1 | 1 | ||
| Yes | 1.12 (0.89–1.42) | 0.334 | 1.10 (0.83–1.47) | 0.523 |
| Brain metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.11 (0.86–1.43) | 0.442 | 1.15 (0.87–1.52) | 0.333 |
| Treatment | ||||
| NMET group | 1 | 1 | ||
| MET group | 0.72 (0.55–0.95) | 0.018 | 0.69 (0.52–0.93) | 0.013 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; MET, with concomitant administration of metformin and DDP4 inhibitor; NMET, without concomitant administration of metformin and DPP4 inhibitors (metformin or combination of metformin and DPP4 inhibitor); PD‐L1, programmed death‐ligand 1; TKI, tyrosine kinase inhibitor. Note: Statistically significant p values of multivariate analysis are in bold type.
TABLE 4.
Univariate and multivariable analyses of the clinical features for overall survival
| Univariate analysis Hazard ratio (95% CI) | p‐value | Multivariate analysis Hazard ratio (95% CI) | p‐value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 0.56 (0.34–0.91) | 0.018 | 0.41 (0.22–0.78) | 0.007 |
| Age, years | ||||
| <65 | 1 | 1 | ||
| ≥65 | 0.98 (0.69–1.41) | 0.925 | 0.89 (0.62–1.30) | 0.551 |
| Smoking | ||||
| Never smoker | 1 | 1 | ||
| Ever‐smoker | 0.84 (0.56–1.26) | 0.388 | 0.76 (0.44–1.33) | 0.335 |
| ECOG PS | ||||
| 0–1 | 1 | 1 | ||
| ≥2 | 2.76 (1.91–3.97) | <0.0001 | 2.94 (2.02–4.27) | <0.0001 |
| Previous chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 2.09 (0.91–4.78) | 0.083 | 1.44 (0.51–4.03) | 0.490 |
| Previous TKI | ||||
| No | 1 | 1 | ||
| Yes | 1.14 (0.75–1.74) | 0.546 | 1.02 (0.63–1.65) | 0.943 |
| ICI line | ||||
| First‐line | 1 | 1 | ||
| Second−/later‐line | 1.52 (0.76–3.03) | 0.234 | 1.45 (0.60–3.51) | 0.410 |
| Driver mutation | ||||
| No | 1 | 1 | ||
| Yes | 1.19 (0.78–1.83) | 0.416 | 1.13 (0.70–1.83) 0.608 | |
| Brain metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.20 (0.77–1.86) | 0.424 | 1.22 (0.77–1.94) | 0.398 |
| Treatment | ||||
| NMET group | 1 | 1 | ||
| MET group | 0.96 (0.62–1.49) | 0.865 | 0.79 (0.50–1.23) | 0.296 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; MET group, with concomitant administration of metformin and DDP4 inhibitor; NMET group, without concomitant administration of metformin and DPP4 inhibitors (metformin or combination of metformin and DPP4 inhibitor); PD‐L1, programmed death‐ligand 1; TKI, tyrosine kinase inhibitor.
Immune‐related adverse events
The irAEs are summarized in Table 5. The most common irAE was pneumonitis, which occurred in 12.4% and 8.2% of patients in the MET and NMET groups, respectively (p = 0.220), followed by thyroiditis (4.5% of patients in both MET and NMET groups, p = 1.000). The rates of all‐grade and grade 3–5 irAEs were slightly higher in the MET group than in the NMET group; however, irAE development showed no significant difference between the two groups (MET group: 19.1% vs. NMET group: 14.3%, p = 0.382).
TABLE 5.
Immune‐related adverse events
| Type of irAE | MET group (n = 89) | NMET group (n = 377) | p‐value | |||
|---|---|---|---|---|---|---|
| Any grade | Grades 3–5 | Any grade | Grades 3–5 | |||
| Pneumonitis | 11 (12.4%) | 3 (3.4%) | 31 (8.2%) | 10 (2.7%) | 0.220 | |
| Hepatitis | 2 (2.2%) | 2 (2.2%) | 3 (0.8%) | 2 (0.5%) | 0.244 | |
| Enterocolitis | 0 (0%) | 0 | 1 (0.3%) | 1 (0.3%) | 1.000 | |
| Hypothyroidism | 4 (4.5%) | 0 | 17 (4.5%) | 0 | 1.000 | |
| Hematological toxicity a | 0 (0%) | 0 | 1 (0.3%) | 1 (0.3%) | 1.000 | |
| Polyarthritis | 0 (0%) | 0 | 1 (0.3%) | 0 | 1.000 | |
| Total | 17 (19.1%) | 5 (5.6%) | 54 (14.3%) | 14 (3.7%) | 0.382 | |
Abbreviations: irAE, immune‐related adverse event; MET group, concomitant administration of metformin and DPP4 inhibitor (metformin or combination of metformin and DPP4 inhibitor); NMET group, without concomitant administration of metformin and DPP4 inhibitor.
neutropenia.
DISCUSSION
To the best of our knowledge, this is the largest retrospective study to evaluate the association between ICI monotherapy and antidiabetic agents, including metformin and DPP4 inhibitors, in advanced NSCLC. Although evidence suggesting that antidiabetic agents such as metformin act as immunomodulating agents in ICI therapy for lung cancer; the data for the same are limited. A recent study by Afzal et al. reported that metformin improved the clinical outcomes of ICI in NSCLC, although the difference was not statistically significant. 16 However, Jacobi et al. reported no significant correlation with PFS or OS in NSCLC patients with diabetes treated with a combination of metformin and ICI in a small‐sized study (nonmetformin, 20 patients; metformin, 37 patients). 17 A meta‐analysis (14 studies) by Luo et al. reported that metformin extended OS in various stages of lung cancer patients who received chemotherapy, epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitor (TKI) administration, and ICI. 18 This study supported the evidence that the concomitant use of metformin and DPP4 inhibitors improved the clinical outcomes of ICI compared with ICI alone. Therefore, this finding proposes that metformin and DPP4 inhibitors are associated with improved ORR and PFS without an increased occurrence of irAEs in patients treated with ICI monotherapy. The MET group did not extend OS in this study. However, the complications of diabetes mellitus, such as renal impairment and coronary artery occlusive disease, may have affected the OS rate. Moreover, the follow‐up duration was relatively short. Therefore, the difference did not reach statistical significance.
The increasing rate of immunotherapies for advanced NSCLC necessitates the establishment of factors associated with antitumor immune responses, including predictive biomarkers and clinical characteristics. Several clinical characteristics, including sex and body mass index (BMI), have been evaluated to identify their association with antitumor immune response. 19 , 20 In addition, a previous study reported that high BMI is independently associated with improved survival with atezolizumab in advanced NSCLC. 21
In addition to sex, age, and BMI, concomitant medication is associated with an antitumor immune response. 22 For example, metformin, which is commonly administered to treat type 2 diabetes, exerts anticancer effects through several mechanisms. In the TME, metformin decreases the infiltration of FOXP3+ T regulatory cells, increases the number of CD8+ tumor‐infiltrating lymphocytes, and protects them from apoptosis and exhaustion by decreasing interleukin 2, tumor necrosis factor‐alpha, and interferon‐gamma. 23 Notably, metformin also remodels the TME by inhibiting the oxygen intake of tumors and blocking the accumulation of myeloid‐derived suppressor cells. 24 , 25
Furthermore, the DPP4 inhibitor, which was the main potential candidate for immune enhancement in this study, showed no additional clinical benefit, as compared with metformin alone. In a previous study conducted by da Silva et al., a DPP4 inhibitor enhanced the antitumor response to ICIs in an in vivo model by regulating the CXCL10‐mediated lymphocyte trafficking. 14 The study also proposed that induced or expressed DPP4 in tumors can prohibit lymphocyte trafficking into the tumor site and that DPP4 inhibition restores and enhances CXCL10 chemokine‐mediated lymphocyte infiltration into the tumor parenchyma. 26 Therefore, they suggested that combining DPP4 inhibitors and immunotherapy will potentiate the anticancer response. Although lymphocyte infiltration is necessary, it is insufficient to induce an antitumor immune response in lung cancer, a more frequently inflamed tumor type than other malignancies.
The association between antidiabetic drugs and irAEs related to ICI has been scarcely reported. However, this study could not determine if the inhibitors significantly increased the incidence of all‐grade irAEs compared with the NMET group. One of the major pathophysiologies of irAEs is the uncontrolled active T cells, which can trigger an inflammatory response in normal tissues. 27 Metformin could reduce the inflammatory response by activating AMPK‐dependent and ‐independent pathways, which reduces nuclear factor‐kB (NF‐kB) signaling associated with inflammation. 28 However, DPP4 inhibitors promote the CXCL12‐CXCR4‐mTOR pathway, which reduces the production of proinflammatory cytokines by blocking NF‐kB activity. 29 In this study, metformin and DPP4 inhibitor addition to ICI did not significantly reduce irAEs.
This study had certain limitations. First, we used retrospective data that were deficient in comorbidities. Metformin is a medication that has different requirements depending on the patient's blood glucose level. Therefore, it was difficult to stratify the metformin dosage. Additionally, it was difficult to clearly determine the effectiveness of DPP4 inhibitor monotherapy because DPP4 inhibitors are usually administered as an add‐on therapy to glucose control. Second, this study could not evaluate the efficacy of metformin and DPP4 inhibitors in patients without diabetes. Third, this study could not analyze the effect of DPP‐4 inhibitors alone. Administration of DPP‐4 inhibitors is generally not used as initial therapy in cases of type 2 diabetes mellitus, unlike metformin. Especially, administration of DPP‐4 inhibitors is considered as monotherapy in cases where metformin cannot be provided to the patients (i.e., renal impairment and increased risk of hypoglycemia). 30 Fourth, a previous trial showed a clinical benefit of ICI monotherapy in previously treated advanced or metastatic NSCLC cases compared to cytotoxic chemotherapy, regardless of PD‐L1 expression. Therefore, we included patients with PD‐L1 expression <1%. 31 , 32
Despite the aforementioned limitations, this study demonstrated the clinical benefits of metformin and DPP4 inhibitors with ICIs in NSCLC patients without causing irAEs. Therefore, these results provide an effective and valuable approach for treating NSCLC patients, considering that these drugs are widely used. However, further prospective studies conducted on patients with and without diabetes are needed to validate the efficacy of combining metformin and DPP4 inhibitors in ICI therapy for patients with advanced NSCLC.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Figure S1 Kaplan–Meier curve of overall survival. Overall survival (OS) according to group. Red line, MET group; blue line, NMET group; hazard ratio (HR): 0.96, 95% confidence interval (CI): 0.62–1.49, p = 0.865
ACKNOWLEDGMENTS
None declare.
Yang J, Kim SH, Jung EH, Kim S‐A, Suh KJ, Lee JY, et al. The effect of metformin or dipeptidyl peptidase 4 inhibitors on clinical outcomes in metastatic non‐small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2023;14(1):52–60. 10.1111/1759-7714.14711
REFERENCES
- 1. Ettinger DS, Wood DE, Aisner DL, Arkerley W, Bauman JR, Bharat A, et al. Non–small cell lung cancer, version 3.2022. NCCN clinical guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. [DOI] [PubMed] [Google Scholar]
- 2. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non‐small‐cell lung cancer. Nat Med. 2021;27:1345–56. [DOI] [PubMed] [Google Scholar]
- 3. Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver‐negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18(10):625–44. [DOI] [PubMed] [Google Scholar]
- 4. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spyrou N, Vallianou N, Kadillari J, Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin Cancer Biol. 2021;73:356–76. [DOI] [PubMed] [Google Scholar]
- 6. Ciccarese C, Iacovelli R, Buti S, Primi F, Astore S, Massari F, et al. Concurrent Nivolumab and metformin in diabetic cancer patients: is it safe and more active? Anticancer Res. 2022;42(3):1487–93. [DOI] [PubMed] [Google Scholar]
- 7. Wabitsch S, McCallen JD, Kamenyeva O, Ruf B, McVey J, Kabat J, et al. Metformin treatment rescues CD8+ T‐cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatology. 2022;77(3):748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu W, Wang Y, Luo J, Liu M, Luo Z. Pleiotropic effects of metformin on the antitumor efficiency of immune checkpoint inhibitors. Front Immunol. 2021;11:586760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. Metformin promotes antitumor immunity via endoplasmic‐reticulum‐associated degradation of PD‐L1. Mol Cell. 2018;71(4):606–620.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune‐mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112(6):1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sesti G, Avogaro A, Belcastro S, Bonora BM, Croci M, Daniele G, et al. Ten years of experience with DPP‐4 inhibitors for the treatment of type 2 diabetes mellitus. Acta Diabetol. 2019;56(6):605–17. [DOI] [PubMed] [Google Scholar]
- 12. Verdura S, Cuyàs E, Martin‐Castillo B, Menendez JA. Metformin as an archetype immuno‐metabolic adjuvant for cancer immunotherapy. Onco Targets Ther. 2019;8(10):e1633235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohnuma K, Hatano R, Morimoto C. DPP4 in anti‐tumor immunity: going beyond the enzyme. Nat Immunol. 2015;16(8):791–2. [DOI] [PubMed] [Google Scholar]
- 14. Da silva RB, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol. 2015;16(8):850–8. [DOI] [PubMed] [Google Scholar]
- 15. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel‐Vinay S, et al. Immune‐related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. [DOI] [PubMed] [Google Scholar]
- 16. Afzal MZ, Dragnev K, Sarwar T, Shirai K. Clinical outcomes in non‐small‐cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 2019;8(2):LMT11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobi O, Landman Y, Reinhorn D, Icht O, Sternschuss M, Rotem O, et al. The relationship of diabetes mellitus to efficacy of immune checkpoint inhibitors in patients with advanced non‐small cell lung cancer. Oncology. 2021;99(9):555–61. [DOI] [PubMed] [Google Scholar]
- 18. Luo X, Chen X, Wang L, Yang B, Cai S. Metformin adjunct with antineoplastic agents for the treatment of lung cancer: a meta‐analysis of randomized controlled trials and observational cohort studies. Front Pharmacol. 2021;12:639016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta‐analysis. Lancet Oncol. 2018;19(6):737–46. [DOI] [PubMed] [Google Scholar]
- 20. Wu Q, Wang Q, Tang X, Xu R, Zhang L, Chen X, et al. Correlation between patients' age and cancer immunotherapy efficacy. Onco Targets Ther. 2019;8(4):e1568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non‐small cell lung cancer. JAMA Oncol. 2020;6(4):512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, et al. Effect of concomitant medications with immune‐modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2021;142:18–28. [DOI] [PubMed] [Google Scholar]
- 23. Murciano‐Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment‐targeting combinations. Cell Res. 2020;30(6):507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD‐1 blockade is potentiated by metformin‐induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Wang W, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin‐induced reduction of CD39 and CD73 blocks myeloid‐derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78(7):1779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almagthali AG, Alkhaldi EH, Alzahrani AS, Alghamdi AK, Alghamdi WY, Kabel AM. Dipeptidyl peptidase‐4 inhibitors: anti‐diabetic drugs with potential effects on cancer. Diabetes Metab Syndr. 2019;13(1):36–9. [DOI] [PubMed] [Google Scholar]
- 27. Alissafi T, Hatzioannou A, Legaki AI, Varveri A, Verginis P. Balancing cancer immunotherapy and immune‐related adverse events: the emerging role of regulatory T cells. J Autoimmun. 2019;104:102310. [DOI] [PubMed] [Google Scholar]
- 28. Postler TS, Peng V, Bhatt DM, Ghosh S. Metformin selectively dampens the acute inflammatory response through an AMPK‐dependent mechanism. Sci Rep. 2021;11(1):18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baggio LL, Varin EM, Koehler JA, Cao X, Lokhnygina Y, Stevens SR, et al. Plasma levels of DPP‐4 activity and DPP‐4 are dissociated from inflammation in mice and humans. Nat Commun. 2020;11(1):3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallwitz B. Clinical use of DPP‐4 inhibitors. Front Endocrinol (Lausanne). 2019;10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Kaplan–Meier curve of overall survival. Overall survival (OS) according to group. Red line, MET group; blue line, NMET group; hazard ratio (HR): 0.96, 95% confidence interval (CI): 0.62–1.49, p = 0.865


