Abstract
Human epidermal growth factor receptor 2 (HER2) possesses tyrosine kinase activity and participates in cell growth, differentiation and migration, and survival. Its alterations, mainly including mutations, amplifications, and overexpression are associated with poor prognosis and are one of the major drivers in non–small cell lung cancer (NSCLC). Several clinical trials had been investigating on the treatments of HER2‐altered NSCLC, including conventional chemotherapy, programmed death 1 (PD‐1) inhibitors, tyrosine kinase inhibitors (TKIs) and antibody‐drug conjugates (ADCs), however, the results were either disappointing or encouraging, but inconsistent. Trastuzumab deruxtecan (T‐DXd) was recently approved by the Food and Drug Administration as the first targeted agent for treating HER2‐mutant NSCLC. Effective screening of patients is the key to the clinical application of HER2‐targeted agents such as TKIs and ADCs. Various testing methods are nowadays available, including polymerase chain reaction (PCR), next‐generation sequencing (NGS), fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), etc. Each method has its pros and cons and should be reasonably assigned to appropriate patients for diagnosis and guiding treatment decisions. To help standardize the clinical workflow, our expert group reached a consensus on the clinical management of HER2‐altered NSCLC, focusing on the diagnosis and treatment strategies.
Keywords: antibody‐drug conjugate, non–small cell lung cancer, precision medicine, targeted therapy, tyrosine receptor kinase
To improve the diagnosis and treatment of HER2‐altered non‐small cell lung cancer in China, we reached consensus on detection strategy based on alteration type, sample selection, detection method, quality control, and treatment suggestion. Moreover, we systematically reviewed the clinical efficacy, resistance mechanisms, and development status of HER2‐ targeting drugs.
INTRODUCTION
Non–small cell lung cancer (NSCLC) with human epidermal growth factor receptor 2 (HER2) alterations that mainly include mutations, amplifications, and overexpression is considered to be a unique molecular subtype and tends to have poor prognosis. Up to now, conventional chemotherapy has shown limited efficacy in HER2‐mutant NSCLC. Combining angiogenesis inhibitors may be superior to chemotherapy alone, whereas combining programmed death 1 (PD‐1) inhibitors may be inferior to chemotherapy alone. 1 Non‐selective tyrosine kinase inhibitors (TKIs) have shown moderate antitumor activity, but are less effective against the most common HER2 exon 20 insertion mutations. In contrast, novel selective TKIs (e.g., poziotinib and pyrotinib) have shown promising activity in NSCLC patients with HER2 mutations or amplifications. 2 , 3 The most encouraging data came from the phase II clinical trials of two antibody‐drug conjugates (ADCs): ado‐trastuzumab emtansine (T‐DM1) and trastuzumab deruxtecan (T‐DXd). 4 , 5 Based on the excellent data from DESTINY‐Lung02, T‐DXd has been approved under accelerated approval from the Food and Drug Administration (FDA) and becomes the first targeted agent approved for treating HER2‐mutant NSCLC. 6 Moreover, there is still a lack of effective treatments targeting HER2 amplifications and overexpression in NSCLC.
Considering the diversity of the currently available testing platforms and the complexity of the testing processes, it is important to standardize the detection workflow of HER2 alterations and the application of targeted agents. In this expert consensus, we (1) summarized the types of HER2 alterations and their treatment options in NSCLC, (2) suggested recommendations on the choice of methods and platforms in performing genetic testing, and (3) developed a universal strategy for the diagnosis and treatment of HER2‐altered NSCLC patients.
HER2 ALTERATIONS AND THEIR MECHANISMS OF PATHOGENESIS
HER2 gene locating on the long arm of chromosome 17 (17q21) is a member of the ERBB family and has tyrosine kinase activity. It consists of three parts: an extracellular ligand‐binding region, a single‐stranded transmembrane region, and an intra‐cellular protein tyrosine kinase region. 7 HER2 protein mainly activates the RAS/RAF/MAPK pathway, phosphoinositide 3‐kinase/protein kinase B (PI3K/AKT) and several other signaling pathways through forming heterodimers with other members of the family including epidermal growth factor receptor (EGFR) (HER1/ERBB1), EGFR3 (HER3/ERBB3), and EGFR4 (HER4/ERBB4). 8 , 9 , 10 HER2 abnormalities are closely associated with the development of various malignancies, such as breast cancer, gastric cancer, and lung cancer. 11 , 12 HER2 mutations and amplifications are also one of the mechanisms of acquired resistance in NSCLC patients receiving EGFR‐TKIs. 13 , 14 HER2 mutations, HER2 amplifications and HER2 overexpression are the main types of HER2 alterations observed in NSCLC. As shown in Figure 1, most of the HER2 mutation types are present in ~2%–4% of Chinese NSCLC patients. 15 , 16 HER2 exon 20 insertions, including A772_G775dup (55.0%), G776delinsVC (8.3%), G776delinsLC (2.1%), and G778_P780dup (5.6%), are the most common mutation type, accounting for ~71% of HER2 mutations. Other HER2 mutations include L755P (1.9%) in exon 19, V842I (0.7%) in exon 21, V659E (4.1%), and G660D (0.9%) in the transmembrane domain and D277Y (1.9%), S310F (7.7%), S310Y (1.9%), and A466V (1.4%) in the extracellular domain. 17 A list of real‐world HER2 hotspots in NSCLC is shown in Table S1. The frequency of HER2 amplification varies between populations (1.4%–22%; 1.7% in Chinese). 18 The frequency of HER2 overexpression also varies between populations (7.7%–23%; 15.4% in Chinese). 19 In addition, HER2 fusions (0.29%) 20 and tyrosine kinase domain duplication (KDD) (0.01%) are also observed in Chinese, which may benefit from HER2 inhibitors. 21 It has been well documented that HER2 amplification is poorly correlated with HER2 overexpression in NSCLC. Only rare cases harbor HER2 mutation, HER2 amplification, and/or HER2 overexpression simultaneously, suggesting that different alterations may be associated with different clinicopathological characteristics and are unique drug targets. 22 , 23 , 24 , 25 At present, clinical studies of HER2‐targeted ADCs and TKIs mainly focus on patients with HER2 mutations and amplifications, and the evidence on treating NSCLC patients with HER2 overexpression is insufficient.
FIGURE 1.
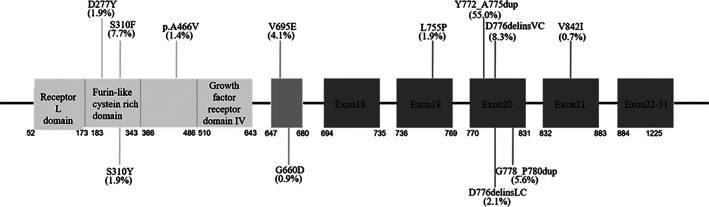
Frequency and location of HER2 hotspots in non–small cell lung cancer.
HER2 ALTERATION TESTING METHODS
Currently, common methods for testing HER2 alterations (Table 1) include next‐generation sequencing (NGS), immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and amplification refractory mutation system‐polymerase chain reaction (ARMS‐PCR). Other platforms such as droplet digital PCR (ddPCR) are also under development and supporting clinical data need to be accumulated. Different testing methods have different limitations (e.g., types and quantities of gene alterations capable of detection), requirements (e.g., specimen types, quantity and quality of specimens, and laboratory conditions) and performance (e.g., sensitivity/specificity). Appropriate testing methods should be selected based on the clinical scenario. When necessary, multi‐platform testing should be performed to obtain complementary and validated results. 26 , 27 , 28 , 29
TABLE 1.
Comparison of HER2 alteration testing methods and recommendation level in NSCLC
| Methods | Alterations | Types of alteration | Sample type | Sensitivity | Specificity | TAT | Advantages and disadvantages | Recommendation level |
|---|---|---|---|---|---|---|---|---|
| Sanger | Point mutation, small insertions/deletions | Known/unknown alterations | FFPE cytology | Moderate | Moderate | 4–5 days | High requirement for sample input and low testing throughput | Recommended |
| qRT‐PCR | Point mutation, small insertions/deletions, amplification | Known alterations | FFPE cytology liquid | High | High | 2–3 days | Widely used platform, relatively fast turnaround time, but unable to detect unknown alterations | Recommended |
| FISH | Amplification | Known/unknown alterations | FFPE cytology | Moderate | Moderate | 2–3 days | Intuitive results, high requirement for sample quality, complicated testing and analysis procedures | Recommended |
| IHC | Overexpression | Protein level | FFPE | Moderate | Moderate | 1–2 days | Cheap, fast, and highly accessible platform, but poor antibody accessibility and risk of false negatives and false positives | Recommended |
| NGS | Point mutation, small insertions/deletions, fusion, amplification | Known/unknown alterations, dependent on panel coverage | FFPE cytology liquid | High | High | 5–7 days | High throughput, cover multiple alteration types, high sensitivity and specificity, but highly dependent on probe design and bioinformatic analysis | Strongly recommended |
Abbreviations: HER2, human epidermal growth factor receptor 2; FFPE, formalin‐fixed paraffin‐embedded; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next‐generation sequencing; NSCLC, non–small cell lung cancer; qRT‐PCR, quantitative real time PCR; TAT, turnaround time.
HER2 mutations
Methods for HER2 mutation testing mainly include NGS, ARMS‐PCR, and Sanger sequencing. Because the differences in sensitivity, specificity, sample requirements, test turnaround time, and technical complexity among different methods, it is recommended to select appropriate testing methods based on laboratory conditions, sample types, sample size, and clinical needs. Sanger sequencing can directly identify known and unknown HER2 mutations, but requires high tumor cell content in samples. 30 , 31 ARMS‐PCR has high sensitivity and specificity and is simple to perform, but only allows the testing of known mutations. When simultaneously detecting various mutation loci, a higher DNA input is required and the probability of non‐specific binding increases. 32 NGS platforms are capable of testing both known and unknown alterations, including clinically actionable mutations such as exon 20 insertions, missense mutations, and gene amplifications, with high sensitivity and specificity and a relatively lower requirement of DNA input. 33 , 34 , 35 Therefore, NGS is recommended for HER2 mutation testing.
HER2 amplification
Both NGS, quantitative real‐time PCR (qRT‐PCR) and FISH can be used to test HER2 amplifications. NGS is a more commonly used test in clinical practice for NSCLC. NGS possesses advantages in classifying focal gene amplifications and alterations in chromosomal ploidy because it can simultaneously test alterations in several hundred genes. Although qRT‐PCR has also been used to test HER2 amplification, the threshold for defining HER2 amplification remains unclear. The mechanism of FISH is to hybridize fluorescent‐labeled nucleic acid probes with DNA target sequences in the nucleus. Fluorescence signals emitting from the probes hybridized to DNA target sequences in the nucleus are analyzed under fluorescence microscope and gene copy number is calculated by counting signals and ratios. Generally, dual probes is used to test HER2 amplifications, which contains both the HER2 gene sequence and the centromere sequence of chromosome 17 (CEP17) where the gene is located. A single probe containing only the HER2 gene can also be used. 36 HER2 copy number can be judged by calculating the mean HER2 gene copy number or the HER2 to CEP17 signal count ratio. Adding CEP17 probe to the HER2 FISH system allows the detection of the number of chromosome 17, which ensure the distinguishment of aneuploidy of chromosome 17 from pure HER2 gene amplification, especially in cases of low‐level amplification.
The judgment criteria for HER2 amplification testing in NSCLC by two‐probe in situ hybridization can be referred to the guidelines for HER2 testing in breast cancer, 37 as detailed in Figure 2.
FIGURE 2.
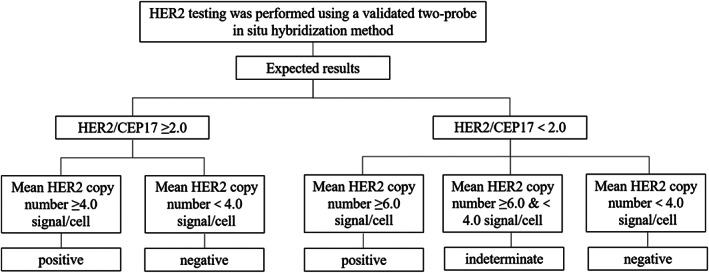
Judgment criteria of HER2 dual probe in situ hybridization testing.
HER2 overexpression
IHC is the standard method to measure HER2 overexpression levels. It is widely used in breast and gastric cancers, in which well‐established testing and outcome interpretation guidelines are available. The level of HER2 overexpression by IHC is not a routine test in NSCLC, and the judgment criteria can refer to the guidelines for HER2 testing in breast cancer. 37 IHC scores of 0, 1+, 2+, or 3+ were determined according to the degree of cell membrane staining. As shown in Figure 3, cases that are 0/1+ and 3+ will be diagnosed as HER2 overexpression negative and positive, respectively. Cases that are 2+ should be further tested with in situ hybridization or retested with another specimen. Notably, HER2 low expression (HER2 1+ or 2+) is important for selecting patients for HER2 ADC drugs.
FIGURE 3.
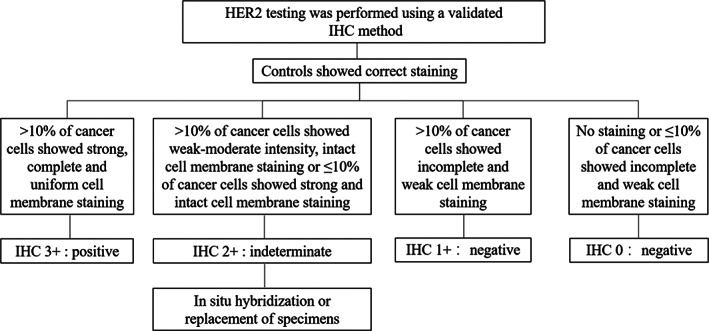
Judgment criteria of HER2 overexpression testing. HER2, human epidermal growth factor receptor 2.
CONTENT REQUIREMENTS OF HER2 TESTING REPORT
It is recommended to include the following contents in NGS or RT‐PCR report of HER2 testing, (1) patient's information: including name, gender, age, outpatient/inpatient identification number, name of the responsible physician, and clinical indications; (2) sample information: including sample type, date and site of sample collection, sample identification number, date of submission and report generation, and key quality control (QC) parameters such as tumor cell content, DNA quality assessment, as well as sequencing quality assessment; (3) test details: including instruments and reagents used, detection methods, panel coverage, limit of detection (LoD), etc.; (4) test results and interpretations: including the genotype, details of alteration, related drug information, and supporting evidence of each alteration detected; and (5) description of the testing limitations.
It is recommended to include the following contents in FISH report of HER2 testing: patient's information (including name, gender, age, outpatient/inpatient identification number), name of the responsible physician, date of submission, pathology report identification number, site of sample collection, specimen type, probe information, detection method, whether image analysis is used, control setting, whether the sample size is sufficient for evaluation, interpretation of results (including the number of cells assessed, mean HER2 copies/cell, mean CEP17 copies/cell, and ratio of mean HER2 copies/mean CEP17 copies), and test results (i.e., positive, negative, required to validate with IHC, and indeterminate). 37
It is recommended to include the following contents in IHC report of HER2 testing: patient's information (including name, gender, age, outpatient/inpatient identification number), name of the responsible physician, date of submission, pathology report identification number, site of sample collection, specimen type, antibody information, detection method, whether image analysis is used, control setting, whether the sample size is sufficient for evaluation, and interpretation of results (i.e., 0, 1+, 2+, 3+). 37
HER2 TESTING PROCEDURE
Several HER2 testing methods have been applied in clinical practice. Differences in sensitivities and specificities exist between methods, and each method possesses unique advantages and limitations. In real‐world settings, physicians have to select appropriate testing platform based on the type of specimens, sample size, tumor cell content, sample quality, clinical needs, and laboratory testing capability. Tests can be simultaneously performed in multiple platforms to ensure the accuracy of results if conditions allowed. According to the clinical experience and relevant literatures, the following testing strategy is recommended to efficiently and accurately detect HER2 alterations (Figure 4). In clinical practice, HER2 is usually not tested independently in NSCLC. For the detection of HER2 mutations and amplifications, it is recommended to simultaneously test with EGFR, ALK, ROS1, and other driver genes based on NGS when NGS platform is available. When NGS platform is not available, ARMS‐PCR can be an alternative method to test HER2 point mutations and small insertions/deletions, whereas FISH can be used to test HER2 amplifications. For the detection of HER2 overexpression, IHC can be used. It should be noted that the use of FISH and IHC are restricted to tissue samples, in contrast, NGS and PCR can be applied to circulating tumor DNA (ctDNA) samples when tissue samples are insufficient.
FIGURE 4.
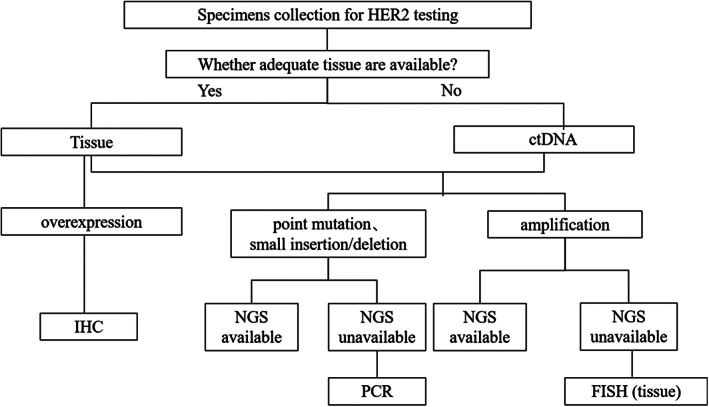
Strategy of HER2 testing in NSCLC. HER2, human epidermal growth factor receptor 2; NSCLC, non–small cell lung cancer.
HER2 TESTING QUALITY CONTROL
Sample selection and processing method
To conserve patient's tumor tissue specimens, we should try our best to obtain the samples adequate for both pathologic and molecular diagnoses with a single cut. If patients develop resistance and require re‐biopsy sampling to test gene alterations or explore the mechanism of resistance, NGS multi‐gene testing is recommended to obtain more gene alterations information with limited specimens. This approach can minimize the need of invasive sampling and guide subsequent treatment decision. Tumor cell content assessment by experienced pathologists is required for tumor tissue and cytology samples. To select appropriate testing method, the laboratory circumstances, sample types, sample size, and QC results should be considered. Liquid biopsy samples such as plasma, cerebrospinal fluid (CSF) supernatant, and thoracoabdominal effusion supernatant can be used as a complementary test to tumor tissue and cytology samples, but the limitations of testing with such samples need to be clearly stated in the test report.
Tumor tissue samples
Tumor tissue samples include fresh tissue samples and formalin‐fixed paraffin‐embedded (FFPE) samples. The cellular content of tumor tissue samples should be evaluated by pathologists before proceeding with HER2 testing. A tumor cell content of at least 20% is recommended. 38 If the tumor tissue sample has <20% tumor cell content, tumor cell enrichment is recommended before testing and the sample limitations should be clearly stated in the report.
FFPE samples should be processed according to the pathological specifications. It is recommended to fix the tumor tissue through immersion in neutral buffered formalin as soon as possible after tissue isolation (within 1 hour) or removal from liquid nitrogen (within 10 minutes). 39 , 40 Surgical tissue samples require 12–48 hours to be fixed, but not more than 72 hours; biopsy tissue samples require 6–12 hours to be fixed. The duration of preservation of FFPE samples is recommended to be no more than 24 months.
Cytology samples
For advanced lung cancer patients whose tumor tissues cannot be retrieved, sputum, alveolar lavage fluid, thoracoabdominal effusion, endobronchial ultrasound‐guided fine‐needle aspiration (EBUS‐FNA) biopsy can be used for testing. For these sample types, wax block should be prepared according to the standard protocols before testing. Tumor cell content should meet the testing criteria.
Liquid biopsy
For advanced lung cancer patients whose tumor tissue or cytology samples cannot be obtained, blood ctDNA, CSF supernatant, and thoracoabdominal effusion supernatant can be used for testing. However, the rareness of tumor cells in these sample types may lead to false‐negative results. Therefore, more sensitive testing method is recommended.
If disposable ethylene‐diamine‐tetraacetic acid vacutainers are used to collect patient's peripheral blood, it is recommended to perform plasma isolation within 2 to 4 hours. If immediate extraction is not possible, samples should be stored at −20°C ± 5°C for no longer than 12 months, or long‐term storage below −70°C (avoiding repeated freeze thawing). It is recommended to perform testing immediately after DNA extraction; if not, samples should be stored at −20°C ± 5°C for no longer than 12 months.
Internal and external quality control of HER2 testing
In clinical practice, a standard QC system is crucial to the reliability of pathological diagnosis and evaluation. To ensure the accuracy of HER2 testing results, laboratories should establish their own standard operating procedure (SOP) and QC system. For internal QC, HER2 testing SOP should be established and optimized before clinical test, through comparing between different methods and different technicians. Negative and positive control samples should be applied. The performance of new reagents should be validated. Regular sampling inspection should be performed. If NGS is used to test HER2 alterations, the NGS laboratories should meet the national and international quality standards. Special attention should be paid to the possible cross contamination between different samples during the NGS experiment operation. For example, when automated instrument is used, library construction of single sample under sealed condition can be considered to reduce the risk of cross contamination. In addition, laboratory technicians should be regularly trained for experimental skills, and test results should be regularly summarized and analyzed. Laboratories should participate in annual quality control programs such as the Pathology Quality Control Center (PQCC), College of American Pathologists (CAP), or Clinical Laboratory Improvement Amendments (CLIA) or other inter‐laboratory quality assessment. The reliability of test results can also be determined by comparing the results with those from qualified laboratories applying the same testing methods. For inconsistent results, it is necessary to ensure that alternative methods are available for verification and review.
TREATMENTS FOR NSCLC PATIENTS WITH HER2 ALTERATIONS
Before the advent of targeted therapy, the standard treatment option for HER2‐altered patients is chemotherapy. Results from early clinical trials are listed in Table 2. EUHER2, a retrospective study investigating conventional chemotherapy in advanced NSCLC patients with HER2 mutations, demonstrated an objective response rate (ORR) of 43.5% and a median progression‐free survival (mPFS) of 6.0 months in first‐line setting, and an ORR of 10% and an mPFS of 4.3 months in second‐line setting. 41 Wang et al. 42 showed that HER2‐mutant NSCLC patients receiving pemetrexed‐based first‐line chemotherapy had poor efficacy compared to those with ALK/ROS1 rearrangement, with an mPFS of only 5.1 months. POLISH, a retrospective real‐world study performed in China, showed that first‐line chemotherapy plus angiogenesis inhibitors might have greater survival benefits than chemotherapy alone (mPFS, 5.63 vs. 4.03 months, p = 0.006) in HER2‐altered NSCLC, whereas combining immunotherapy might not be superior to chemotherapy alone (mPFS, 5.20 vs. 4.03 months, p = 0.20). 1
TABLE 2.
Efficacy of anti‐HER2 agents in NSCLC with HER2 alterations
| Class | Drug | Study type | Number of patients | HER2 alterations | ORR, % | Median PFS, months | Median OS, months | Reference |
|---|---|---|---|---|---|---|---|---|
| Chemotherapy | Platinum‐based doublet with pemetrexed | Retrospective | 93 (first‐line) | HER2 exon‐20 mutation | 43.5 | 6.0 | NA | 31 |
| 52 (second‐line) | HER2 exon‐20 mutation | 10 | 4.3 | NA | ||||
| Platinum/pemetrexed | Retrospective | 29 | HER2 mutation | 36 | 5.1 | NA | 32 | |
| Platinum/pemetrexed | Retrospective | 83 | HER2 mutation or amplification | 16.9 | 4.03 | 31.67 | 33 | |
| Chemotherapy + angiogenesis inhibitors | Platinum/pemetrexed+ bevacizumab | Retrospective | 81 | HER2 mutation or amplification | 23.8 | 5.63 | 36.27 | |
| Chemotherapy + immune checkpoint inhibitors | Platinum/pemetrexed+ PD‐1 inhibitors | Retrospective | 46 | HER2 mutation or amplification | 28.9 | 5.20 | NA | |
| TKI | Afatinib | Global named patient use program | 28 | HER2 mutation | 18.8 | NA | NA | 34 |
| Observational study | 10 | HER2 mutation (G778_P780dup, G776delinsVC) | 40 | 7.6 | NA | 35 | ||
| Dacomitinib | II | 30 | HER2 mutation or amplification | 12 | 3.0 | 9.0 | 37 | |
| Neratinib | II | 26 | HER2 mutation | 3.8 | 5.5 | NA | 41 | |
| Poziotinib | II | 90 | HER2 mutation | 27.8 | 5.5 | NA | 42 | |
| II | 48 | HER2 mutation | 43.8 | 5.6 | NA | 43 | ||
| Pyrotinib | II | 60 | HER2 mutation | 30 | 6,9 | 14.4 | 44 | |
| II | 78 | HER2 mutation | 19.2 | 5.6 | 10.5 | 45 | ||
| II | 27 | HER2 amplification | 22.2 | 6.3 | 12.5 | 46 | ||
| Mobocertinib | I/II | 14 | HER2 mutation | 21.4 | NA | NA | 48 | |
| TKI + angiogenesis inhibitors | Pyrotinib + apatinib | II | 33 | HER2 mutation or amplification | 51.5 | 6.9 | 14.8 | 47 |
| ADC | T‐DM1 | II | 15 | HER2 mutation or overexpression | 6.7 | 2.0 | 10.9 | 49 |
| II | 18 | HER2 mutation | 44 | 5.0 | NA | 50 | ||
| II | 29 | HER2 overexpression (IHC2+) | 0 | 2.6 | 12.2 | 51 | ||
| II | 20 | HER2 overexpression (IHC3+) | 20 | 2.7 | 15.3 | |||
| T‐DXd | I | 11 | HER2 mutation | 72.7 | 11.3 | NA | 52 | |
| II | 49 | HER2 overexpression | 24.5 | 5.4 | NA | 53 | ||
| II | 91 | HER2 mutation | 54.9 | 8.2 | 17.8 | 54 | ||
| II | 52 | HER2 mutation | 58 | NA | NA | 55 |
Abbreviations: ADC, antibody‐drug conjugates; HER2, human epidermal growth factor receptor 2; NA, not available; NSCLC, non–small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; T‐DM1, ado‐trastuzumab emtansine; T‐DXd, trastuzumab deruxtecan; TKI, tyrosine kinase inhibitors.
Non‐selective TKIs are the first attempt of targeted therapy in treating NSCLC patients with HER2 mutations. In a global named patient use program, 28 patients with HER2 mutations were treated with afatinib, with an ORR of 18.8% and a disease control rate (DCR) of 68.8%. 43 In contrast, another observational study revealed that patients with G778_P780dup and G776delinsVC mutations were the most responsive to afatinib (ORR, 40%; mPFS, 7.6 months), whereas patients with A775_G776insYVMA mutation did not respond. 44 A recently published study showed that YVMA insertion of the HER2 kinase domain changes the secondary structure of HER2 protein, forming a steric block that binds to afatinib, therefore, causing resistance. 45 Dacomitinib is an irreversible pan‐HER2 TKI. A phase II trial showed that the ORR of dacomitinib in treating NSCLC patients with HER2 mutations was 12%. The median PFS and overall survival (OS) were 3 and 9 months, respectively. No response was observed in G776insYVMA mutated patients, indicating that YVMA insertion may also be a potential mechanism of drug resistance to dacomitinib. 46 SUMMIT, a phase II basket study evaluating the efficacy of neratinib, had only one patient with partial response. Although the ORR (3.8%) was low, the mPFS was 5.5 months, and the treatment duration of six patients exceeded 1 year. 47 At present, non‐selective TKIs have shown moderate antitumor activity, but poor efficacy in exon 20 insertions, the most common type of HER2 mutations.
Recently, novel TKIs with advantages in selectivity and structure have been developed. Based on the initial results of the ZENITH20 study (ORR of 27.8%, DCR of 70.0%, and mPFS of 5.5 months), 48 the FDA granted a fast track designation for poziotinib in March 2021 for previously treated NSCLC patients with HER2 exon 20 mutations. Updated results from cohort 4 of ZENITH20 further demonstrated promising antitumor activity of poziotinib in treatment‐naive patients, with an ORR of 43.8%, a DCR of 75.0%, and an mPFS of 5.6 months. 2 In Chinese, a phase II study of pyrotinib in treating patients with HER2‐mutant NSCLC showed an ORR of 30%, a median duration of response (mDOR) of 6.9 months, and median PFS and OS of 6.9 and 14.4 months, respectively. 3 In another phase II prospective study, the ORR was 19.2%, the mDOR was 9.9 months, and the median PFS and OS were 5.6 and 10.5 months, respectively. In patients with disease progression, HER2 copy number alteration (CNA) and EGFR CNA co‐existence and HER2 deletions were found, as well as EGFR E330K, KRAS G12D, MET CNA, and BRAF CNA. Further studies revealed that genetic alterations in the RAS/RAF pathway may be a potential mechanism of pyrotinib resistance. 49 Another study that enrolled 27 NSCLC patients with HER2 amplification showed an ORR of 22.2%, an mPFS of 6.3 months, and a median OS of 12.5 months with pyrotinib. 50 Recently, the first phase II study exploring the combination of HER2‐TKI with antiangiogenic agent in the treatment of NSCLC patients with HER2 mutations or amplifications has reached the primary endpoint, with an ORR of 51.5% and a median PFS and OS of 6.9 and 14.8 months, respectively, suggesting that the feasibility of combining pyrotinib with apatinib. 51 Mobocertinib is an EGFR/HER2 inhibitor targeting exon 20 insertions with preclinical data comparable to that of pyrotinib. The results of a preliminary study showed objective responses in 21.4% (3/14) patients with HER2 exon 20 insertions. 52 Further efficacy data of mobocertinib in HER2‐mutant subtypes is still waiting.
ADCs are emerging antitumor agents that covalently couple cytotoxic drugs and monoclonal antibodies to specifically target and kill tumor cells. T‐DM1 is an ADC that consists of the anti‐HER2 antibody trastuzumab and the microtubule‐targeted cytotoxic agent. A small‐scale phase II trial with 15 patients showed limited efficacy of T‐DM1 monotherapy, with an ORR of 6.7%, median PFS and OS of 2.0 and 10.9 months, respectively. No remission was achieved in the HER2 amplified or overexpressed subgroup. The study was terminated early because of limited efficacy. 53 However, in another phase II basket trial, T‐DM1 treatment achieved ORR of 44% and mPFS of 5 months, with responses seen across all subtypes. 4 Peters et al. 54 studied the efficacy of T‐DM1 in treating HER2 overexpressing NSCLC. The ORR was 0% in the IHC 2+ subgroup and 20% in the IHC 3+ subgroup. The two subgroups showed comparable median PFS (2.6 vs. 2.7 months) and OS (12.2 vs. 15.3 months), 54 suggesting that HER2 IHC alone is not enough to serve as predictive biomarker. Compared to T‐DM1, T‐DXd, as a third‐generation ADC drug, has higher membrane permeability and higher drug antibody ratio, and allows stable delivery of topoisomerase I inhibitors under conditions of low HER2 expression. The first‐in‐human phase I trial of T‐DXd showed an ORR of 72.7%, an mDOR of 9.9 months, and an mPFS of 11.3 months in 11 previously treated NSCLC patients with HER2 mutations. 55 The multicenter trial: DESTINY‐Lung01 showed an ORR of 24.5% and an mPFS of 5.4 months in the HER2 overexpressing cohort. In subgroups of patients with IHC3+ and IHC2+, the ORR was comparable (20.0% and 25.6%, respectively). 56 In the HER2‐mutant cohort, the ORR was 54.9%, and the median PFS and OS were 8.2 and 17.8 months, respectively. 5 The latest data from the DESTINY‐Lung02 phase II trial showed an ORR of 58% and a mDOR of 8.7 months in the primary efficacy cohort (n = 52). 6 Based on this results, the FDA has granted accelerated approval to T‐DXd for HER2‐mutant NSCLC, which was the first agent approved for HER2‐mutant NSCLC. Ongoing clinical trials of anti‐HER2 agents in NSCLC patients are shown in Table S2. The quick development of HER2‐altered NSCLC treatments further raises the importance of standardizing HER2 testing in advanced NSCLC.
SUMMARY AND PROSPECT
Based on the currently available evidence, several clinical guidelines including National Comprehensive Cancer Network and Chinese Society of Clinical Oncology are already recommending HER2 testing in clinical diagnosis and treatment or clinical trials. We established this expert consensus and suggested practical guidelines for the management of HER2‐altered NSCLC (Table 3). We systematically summarized the prevalence of different types of HER2 alterations (including mutations, amplifications, overexpression, fusions, and tyrosine kinase domain duplications), testing methods, QC criteria, testing report requirements, and treatment regimens. Notably, HER2 ADCs, especially the third generation ADC: T‐DXd, have led to a revolutionary change in the diagnosis and treatment of HER2‐related tumors. In principle, HER2 ADCs are “biological bullets” with HER2 protein as target. T‐DXd has reported great success in treating breast cancer with HER2 low expression. However, that was not the case in NSCLC, no matter in subgroups with high or low HER2 expression. The mechanisms needs to be further investigated in clinical trials. Meanwhile, it is worth noting that the absence of HER2 expression is not an absolute contraindication to T‐DXd. The vast majority of NSCLC patients with HER2 mutations, but without IHC expression and gene amplification, showed a response to T‐DXd treatment. This phenomenon may possibly be explained by the enhancement of the receptor internalization and the endocytosis of HER2 receptor‐ADC complexes by HER2 mutations. 5 Additionally, based on data from previous studies, pyrotinib (a HER2‐TKI) could be recommended as a new treatment option in patients with HER2 mutations and amplifications. 3 , 49 , 50
TABLE 3.
Expert consensus on the diagnosis and treatment of NSCLC patients with HER2 alterations in China.
| Consensus number | Key points | Recommendation level | |
|---|---|---|---|
| Detection time point | Consensus1 | HER2 mutation testing is recommended in patients with stage III/IV lung adenocarcinoma or squamous cell carcinoma, with or without a smoking history, especially in women | Strongly recommended |
| Consensus2 | HER2 amplification testing is recommended in patients with EGFR‐TKI resistance | Strongly recommended | |
| Consensus3 | HER2 mutations are recommended to be tested simultaneously with other driver genes including EGFR, ALK, ROS1, RET, NTRK, etc. | Strongly recommended | |
| Consensus4 | EGFR, ALK, and ROS1 should be tested first; when negative, HER2 mutations should be tested | Recommended | |
| Consensus5 | Patients with HER2 mutations, amplifications, and overexpression are recommended to participate in clinical trials | Recommended | |
| Consensus6 | HER2 mutations or amplifications should be tested independently | Not recommended | |
| Detection method | Consensus7 | HER2 mutation testing is preferentially performed by NGS | Strongly recommended |
| Consensus8 | When NGS is unavailable, HER2 mutation testing can be performed by other methods (ARMS‐PCR, ddPCR, and Sanger sequencing) | Recommended | |
| Consensus9 | HER2 amplifications can be tested by NGS and FISH | Recommended | |
| Consensus10 | HER2 protein expression can be tested by IHC | Recommended | |
| Detection strategy | Consensus11 | HER2 testing is preferentially performed in tumor tissue samples (surgical, biopsy, etc.) | Strongly recommended |
| Consensus12 | HER2 testing can be performed in cytology samples (fine needle aspiration, pleural effusion, alveolar lavage fluid, EBUS‐FNA, etc.) | Recommended | |
| Consensus13 | HER2 testing can be performed in liquid biopsy samples (blood, thoracoabdominal effusion supernatant, cerebrospinal fluid supernatant, etc.) | Recommended | |
| Detection quality control | Consensus14 | Pathologists should try their best to obtain the samples adequate for both pathologic and molecular diagnoses with a single cut | Strongly recommended |
| Consensus15 | For tumor tissue and cytology samples, the tumor cell content should be assessed by experienced pathologists. When testing amplifications by NGS, the tumor cell content of tissue and cytology samples should be above 20%. Appropriate testing method should be selected based on the laboratory circumstances, sample types, sample size and QC results; liquid biopsy samples such as plasma, cerebrospinal fluid supernatant and thoracoabdominal effusion supernatant can be used as a complementary test to tumor tissue and cytology samples, but the limitations of testing with such samples need to be clearly stated in the test report | Strongly recommended | |
| Consensus16 | For internal QC, HER2 testing SOP should be established and optimized before clinical test, through comparing between different methods and different technicians; negative and positive control samples should be applied; the performance of new reagents should be validated; regular sampling inspection should be performed; laboratory technicians should be regularly trained for experimental skills, and test results should be regularly summarized and analyzed | Strongly recommended | |
| Consensus17 | Laboratories should participate in annual quality control programs, such as the PQCC, CAP, CLIA or other inter‐laboratory quality assessment; the reliability of test results can also be determined by comparing the results with those from qualified laboratories applying the same testing methods; for inconsistent results, it is necessary to ensure that alternative methods are available for verification and review | Strongly recommended | |
| Treatment suggestion | Consensus18 | For patients with HER2 mutations, T‐DXd (an ADC) has been approved by the FDA and is currently under clinical trials in China | Recommended |
| Consensus19 | For patients with HER2 mutations or amplifications, pyrotinib (a TKI) can be a novel treatment option | Recommended | |
| Consensus20 | For patients developed resistance to EGFR‐TKI, if T790M is not detected, resistance mechanisms such as HER2 alternations can be tested to guide subsequent treatment | Recommended |
Abbreviations: ADC, antibody‐drug conjugates; ARMS‐PCR, amplification refractory mutation system‐polymerase chain reaction; CAP, College of American Pathologists; CLIA, Clinical Laboratory Improvement Amendments; ddPCR, droplet digital polymerase chain reaction; EBUS‐FNA, endobronchial ultrasound‐guided fine‐needle aspiration; EGFR‐TKI, epidermal growth factor receptor tyrosine kinase inhibitors; FDA, Food and Drug Administration; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NGS, next‐generation sequencing; NSCLC, non–small cell lung cancer; PQCC, Pathology Quality Control Center QC, quality control; SOP, standard operating procedure; T‐DXd, trastuzumab deruxtecan; TKI, tyrosine kinase inhibitors.
AUTHOR CONTRIBUTIONS
Shengli Ma, Wenfeng Fang and Yuanzhi Lu participated in the design of the expert consensus. Shirong Zhang, Wenxian Wang and Chunwei Xu conceived of the expert consensus, and participated in its design and other authors coordination and helped to draft the expert consensus. All authors read and approved the final manuscript.
Supporting information
Table S1. List of real‐world HER2 (ERBB2) cancer hotspots in NSCLC
Table S2. Ongoing clinical trials of anti‐HER2 agents in NSCLC patients
Zhang S, Wang W, Xu C, Zhang Y, Cai X, Wang Q, et al. Chinese expert consensus on the diagnosis and treatment of HER2‐altered non–small cell lung cancer. Thorac Cancer. 2023;14(1):91–104. 10.1111/1759-7714.14743
Shirong Zhang, Wenxian Wang, Chunwei Xu, and Yongchang Zhang contributed equally.
Contributor Information
Shenglin Ma, Email: mashenglin@medmail.com.cn.
Wenfeng Fang, Email: fangwf@sysucc.org.cn.
Yuanzhi Lu, Email: yuanzhi.lu@jnu.edu.cn.
REFERENCES
- 1. Yang G, Yang Y, Liu R, et al. First‐line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2‐altered NSCLC: a retrospective real‐world POLISH study. Ther Adv Med Oncol. 2022;14:17588359221082339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cornelissen R, Sun S, Wollner M, Garassino MCC, Prelaj A, Haura EB, et al. LBA46‐efficacy and safety of poziotinib in treatment‐naïve NSCLC harboring HER2 exon 20 mutations: a multinational phase II study (ZENITH20‐4) Ann. Oncologia. 2021;32:S1283–346. [Google Scholar]
- 3. Zhou C, Li X, Wang Q. Pyrotinib in HER2‐mutant advanced lung adenocarcinoma after platinum‐based chemotherapy: a multicenter, open‐label, single‐arm, phase II study. J Clin Oncol. 2020;38(24):2753–61. [DOI] [PubMed] [Google Scholar]
- 4. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado‐Trastuzumab Emtansine for patients with HER2‐mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36:2532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab Deruxtecan in HER2‐mutant non‐Small‐cell lung cancer. N Engl J Med. 2022;386:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grants‐accelerated‐approval‐fam‐trastuzumab‐deruxtecan‐nxki‐her2‐mutant‐non‐small‐cell‐lung.
- 7. Wang T, Amemiya Y, Hsieh ET, et al. Multiplex ligation‐dependent probe amplification can clarify HER2 status in gastric cancers with “Polysomy 17”. J Cancer. 2015;6(5):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Saba NF, Chen GZ, Shin DM. Targeting HER (ERBB) signaling in head and neck cancer: an essential update. Mol Aspects Med. 2015;45:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shepard HM, Brdlik CM, Schreiber H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. J Clin Invest. 2008;118(11):3574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arkhipov A, Shan Y, Kim ET, Dror RO, Shaw DE. Her2 activation mechanism reflects evolutionary preservation of asymmetric ectodomain dimers in the human EGFR family. Elife. 2013;2:e00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermeulen Z, Segers VF, De Keulenaer GW. ErbB2 signaling at the crossing between heart failure and cancer. Basic Res Cardiol. 2016;111(6):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, et al. LBA50 ‐ mechanisms of acquired resistance to first‐line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29:viii740. [Google Scholar]
- 14. Papadimitrakopoulou VA, Wu YL, Han JY, Ahn MJ, Ramalingam SS, John T, et al. LBA51 ‐ analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29:viii741. [Google Scholar]
- 15. Jebbink M, de Langen AJ, Boelens MC, Monkhorst K, Smit EF. The force of HER2: a druggable target in NSCLC? Cancer Treat Rev. 2020;86:101996. [DOI] [PubMed] [Google Scholar]
- 16. Song Z, Yu X, Shi Z, Zhao J, Zhang Y. HER2 mutations in Chinese patients with non‐small cell lung cancer. Oncotarget. 2016;7(47):78152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu F, Li Q, Li W, Zhang S, Zhao Y, Guo D, et al. Molecular characteristics of ERBB2‐activating mutations in Chinese patients with NSCLC. J Clin Oncol. 2022;40:8546–6. [Google Scholar]
- 18. Song Z, Lv D, Chen S, Huang J, Wang L, Xu S, et al. Efficacy and resistance of afatinib in Chinese non‐small cell lung cancer patients with HER2 alterations: a multicenter retrospective study. Front Oncol. 2021;11:657283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Zhao C, Su C, Ren S, Chen X, Zhou C. Epidemiological study of HER‐2 mutations among EGFR wild‐type lung adenocarcinoma patients in China. BMC Cancer. 2016;16(1):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu CW, Wang WX, et al. Abstract e13002: real‐world large‐scale study of ERBB2 gene fusions and its response to afatinib in Chinese non‐small cell lung cancer (NSCLC): a multicenter study. J Clin Oncol. 2019;37(15_suppl):e13002–2. [Google Scholar]
- 21. Xu CW, Wang WX, Wang XJ, Zhu YC, Zhang QX, Fang Y, et al. Abstract 1597: real‐world large‐scale study kinase domain duplications across diverse tumor types in Chinese populations. Cancer Res. 2019;79(13_Supplement):1597. [Google Scholar]
- 22. Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11(3):414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431(7008):525–6. [DOI] [PubMed] [Google Scholar]
- 24. Hainsworth JD, Meric‐Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open‐label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6):536–42. [DOI] [PubMed] [Google Scholar]
- 25. Arcila ME, Chaft JE, Nafa K, Roy‐Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non‐Small Cell Lung Cancer. Version 5. 2022‐September 26,2022. Available at www.nccn.org/patients.
- 27. Guidelines for non‐small cell lung cancer 2022.V1.
- 28. Mosele F, Remon J, et al. Recommendations for the use of next‐generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. 2020;31(11):1491–505. [DOI] [PubMed] [Google Scholar]
- 29. Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology clinical practice guideline update. J Clin Oncol. 2018;6(9):911–9. [DOI] [PubMed] [Google Scholar]
- 30. Fernandes MGO, Jacob M, Martins N, Moura CS, Guimarães S, Reis JP, et al. Targeted gene nextgeneration sequencing panel in patients with advanced lung adenocarcinoma: paving the way for clinical implementation. Cancers. 2019;11(9):1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jing C, Mao X, Wang Z, Sun K, Ma R, Wu J, et al. Next‐generation sequencing‐based detection of EGFR, KRAS, BRAF, NRAS, PIK3CA, her‐2 and TP53 mutations in patients with non‐small cell lung cancer. Mol Med Rep. 2018;18(2):2191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong J, Li B, Lin D, Zhou Q, Huang D. Advances in targeted therapy and immunotherapy for non‐small cell lung cancer based on accurate molecular typing. Front Pharmacol. 2019;10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li BT, Janku F, Jung B, Hou C, Madwani K, Alden R, et al. Ultra‐deep next‐generation sequencing of plasma cell‐free DNA in patients with advanced lung cancers: results from the actionable genome consortium. Ann Oncol. 2019;30(4):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Nimwegen KJ, van Soest RA, Veltman JA, et al. Is the $1000 genome as near as we think? A cost analysis of next‐generation sequencing. Clin Chem. 2016;62(11):1458–64. [DOI] [PubMed] [Google Scholar]
- 35. Drilon A, Wang L, Arcila ME, Balasubramanian S, Greenbowe JR, Ross JS, et al. Broad, hybrid capture‐based nextgeneration sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res. 2015;21(16):3631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Halilovic A, Verweij DI, Simons A, Stevens‐Kroef MJPL, Vermeulen S, Elsink J, et al. HER2, chromosome 17 polysomy and DNA ploidy status in breast cancer; a translational study. Sci Rep. 2019;9(1):11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang W, Bu H. Guideline for HER2 detection in breast cancer, the 2019 version. Zhonghua Bing Li Xue Za Zhi. 2019;48(3):169–74. [DOI] [PubMed] [Google Scholar]
- 38. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 40. Buttitta F, Barassi F, Fresu G, Felicioni L, Chella A, Paolizzi D, et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer. 2006;119(11):2586–91. [DOI] [PubMed] [Google Scholar]
- 41. Mazières J, Barlesi F, Filleron T, Besse B, Monnet I, Beau‐Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2‐targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27:281–6. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Zhang S, Wu F, Zhao J, Li X, Zhao C, et al. Outcomes of Pemetrexed‐based chemotherapies in HER2‐mutant lung cancers. BMC Cancer. 2018;18:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peters S, Curioni‐Fontecedro A, Nechushtan H. Activity of afatinib in heavily pretreated patients with ERBB2 mutation–positive advanced NSCLC: findings from a global named patient use program. J Thorac Oncol. 2018;13(12):1897–905. [DOI] [PubMed] [Google Scholar]
- 44. Fang W, Zhao S, Liang Y. Mutation variants and co‐mutations as genomic modifiers of response to afatinib in HER2‐mutant lung adenocarcinoma. Oncologist. 2020;25(3):e545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang G, Xu H, Hu J, Liu R, Hu P, Yang Y, et al. Specific HER2 exon 20 Gly776 deletion‐insertions in non‐Small cell lung cancer: structural analysis and sensitivity to HER2‐targeted tyrosine kinase inhibitors. Front Pharmacol. 2022;13:806737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kris MG, Camidge DR, Giaccone G. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan‐HER tyrosine kinase inhibitor dacomitinib in patients with HER2‐mutant or amplified tumors. Ann Oncol. 2015;26(7):1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hyman DM, Piha‐Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2‐ and HER3‐mutant cancers. Nature. 2018;554:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le X, Cornelissen R, Garassino M, Clarke JM, Tchekmedyian N, Goldman JW, et al. Poziotinib in non‐Small‐cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20‐2 trial. Oncol: J. Clin; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Song Z, Li Y, Chen S, Ying S, Xu S, Huang J, et al. Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: a multicenter, single‐arm, phase II trial. BMC Med. 2022;20(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song Z, Lv D, Chen SQ, Huang J, Li Y, Ying S, et al. Pyrotinib in patients with HER2‐amplified advanced non‐Small cell lung cancer: a prospective, multicenter. Single‐Arm Trial Clin Cancer Res. 2022;28(3):461–7. [DOI] [PubMed] [Google Scholar]
- 51. Yang G, Xu H, Yang Y, Zhang S, Xu F, Hao X, et al. Pyrotinib combined with apatinib for targeting metastatic non‐small cell lung cancer with HER2 alterations: a prospective, open‐label, single‐arm phase 2 study (PATHER2). BMC Med. 2022;20(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riely GJ, Neal JW, Camidge DR, Spira A, Piotrowska Z, Horn L, et al. Updated results from a phase I/II study of Mobocertinib (TAK‐788) in NSCLC with EGFR exon 20 insertions (Exon20ins). Ann Oncol. 2020;31(suppl_4):S815–6. [Google Scholar]
- 53. Hotta K, Aoe K, Kozuki T, Ohashi K, Ninomiya K, Ichihara E, et al. A phase II study of Trastuzumab Emtansine in HER2‐positive non‐Small cell lung cancer. J Thorac Oncol. 2018;13:273–9. [DOI] [PubMed] [Google Scholar]
- 54. Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM, et al. Trastuzumab Emtansine (T‐DM1) in patients with previously treated HER2‐overexpressing metastatic non‐Small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res. 2019;25:64–72. [DOI] [PubMed] [Google Scholar]
- 55. Tsurutani J, Iwata H, Krop I. Targeting HER2 with trastuzumab deruxtecan: a dose‐expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakagawa K, Nagasaka M, Felip E, Pacheco J, Baik C, Goto Y, et al. Trastuzumab Deruxtecan in HER2‐overexpressing metastatic non‐Small cell lung cancer: interim results of Destiny‐Lung01. J Thorac Oncol. 2021;16:S109–S10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of real‐world HER2 (ERBB2) cancer hotspots in NSCLC
Table S2. Ongoing clinical trials of anti‐HER2 agents in NSCLC patients


