Abstract
Background
The required distal margin in partial mesorectal excision (PME) is controversial. The aim of this systematic review was to determine incidence and distance of distal mesorectal spread (DMS).
Methods
A systematic search was performed using PubMed, Embase and Google Scholar databases. Articles eligible for inclusion were studies reporting on the presence of distal mesorectal spread in patients with rectal cancer who underwent radical resection.
Results
Out of 2493 articles, 22 studies with a total of 1921 patients were included, of whom 340 underwent long-course neoadjuvant chemoradiotherapy (CRT). DMS was reported in 207 of 1921 (10.8%) specimens (1.2% in CRT group and 12.8% in non-CRT group), with specified distance of DMS relative to the tumor in 84 (40.6%) of the cases. Mean and median DMS were 20.2 and 20.0 mm, respectively. Distal margins of 40 mm and 30 mm would result in 10% and 32% residual tumor, respectively, which translates into 1% and 4% overall residual cancer risk given 11% incidence of DMS. The maximum reported DMS was 50 mm in 1 of 84 cases. In subgroup analysis, for T3, the mean DMS was 18.8 mm (range 8–40 mm) and 27.2 mm (range 10–40 mm) for T4 rectal cancer.
Conclusions
DMS occurred in 11% of cases, with a maximum of 50 mm in less than 1% of the DMS cases. For PME, substantial overtreatment is present if a distal margin of 5 cm is routinely utilized. Prospective studies evaluating more limited margins based on high-quality preoperative magnetic resonance imaging and pathological assessment are required.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10151-022-02690-1.
Keywords: Distal mesorectal spread, Mesorectal cancer spread, Distal mesorectal resection margin, Partial mesorectal excision, PME
Introduction
Heald et al. popularized the concept of total mesorectal excision (TME) as curative surgical treatment for rectal cancer [1]. In both open and laparoscopic TME surgery, the 3-year local recurrence rate (LR) is approximately 5% as reported in large randomized trials [2–6]. Although the laparoscopic approach has shown decreased short-term morbidity compared to the open approach, minimally invasive TME is still associated with high rates of permanent colostomy, postoperative morbidity such as anastomotic leakage and long-term bowel, urinary and sexual dysfunction [7, 8].
Partial mesorectal excision (PME), compared to TME, is a less-extensive surgical procedure associated with less morbidity and better long-term functional outcomes. It has been shown to be associated with significantly lower anastomotic leak rates, increased restorative procedure rate, shorter median hospital stay, less long-term bowel dysfunction and better urinary and sexual function [7–9]. Currently, the indication of necessity for PME or formal TME for patients with proximal rectal cancer remains unclear. The recent consensus on magnetic resonance imaging (MRI) definition of the proximal border of the rectum has improved decision-making on the use of neoadjuvant therapy, but still no consensus exist when to perform a formal TME up to the puborectalis muscle or a PME leaving distal mesorectum with more length of the remaining rectal stump in place and still an adequate distal margin. Only a minority of guidelines consider that PME can be performed and the distal margin of the mesorectum proposed is usually 5 cm [10, 11]. There is a need for consensus on whether a PME or TME should be performed avoiding unnecessary functional impairment while maintaining enough distal margin to incorporate all cancer cells in the specimen.
It is important to have a distal mesorectal margin because of the potential presence of distal mesorectal spread (DMS) of the primary rectal cancer, presenting as mesorectal lymph-node metastases, as vascular or perineural invasion or as tumor deposits. The prevalence and extent of DMS are largely unknown, but these data are prerequisites to define a safe distal resection margin. If oncologically safe, preserving as much as (meso)rectum as possible is preferred for better short-term and long-term functional outcomes [7–9, 12].
The aim of this study was to review the reported pattern (presence and distal spread) of tumor cells in the mesorectum as a step toward reaching consensus about the distal resection margin for patients with rectal cancer undergoing PME.
Materials and methods
This systematic review was reported in accordance with the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (PRISMA) and A MeaSurement Tool to Assess systematic Reviews (AMSTAR) 2 was used as a critical appraisal tool [13, 14]. This study is registered in PROSPERO (ID: CRD42020153098) and the review protocol can be accessed.
Search strategy
Systematic searches were performed from inception in PubMed (up to October 7th 2019) and Embase.com (up to October 16th 2019) with the assistance of a medical information specialist. The full search strategies for both databases are provided in Table 1 and Table 2. The search query included index terms and free-text words for ‘rectal cancer’, ‘mesorectum’ and ‘metastasis’ or ‘seeding’. Conference abstracts from Embase.com were excluded. No limits on publication date were used. Google Scholar (on November 1st 2019) was also used to look for additional references, using the anonymous mode. References of included studies were checked for other eligible studies.
Table 1.
Search strategy for PubMed (7 October 2019)
| Search | Query | Items found |
|---|---|---|
| #1 | "Rectal Neoplasms" [Mesh:NoExp] OR (cancer[sb] AND ("Rectum"[Mesh] OR rectum[tiab] OR rectal*[tiab])) | 85,150 |
| #2 | mesorect*[tiab] | 3853 |
| #3 | (("Neoplasm Metastasis"[Mesh] OR "Neoplasm Invasiveness"[Mesh] OR metast*[tiab] OR micrometast*[tiab] OR seeding*[tiab] OR circulat*[tiab] OR spread*[tiab]) | 1,168,095 |
| #4 | #1 AND #2 AND #3 | 1107 |
Table 2.
Search strategy for Embase.com (16 October 2019)
| Search | Query | Items found |
|---|---|---|
| #1 | ‘rectum tumor’/de OR ‘rectum cancer’/de OR ‘rectum carcinoma’/exp OR ((‘neoplasm’/exp OR carcinoma*:ti,ab,kw OR neoplas*:ti,ab,kw OR tumour*:ti,ab,kw OR sarcoma*:ti,ab,kw OR adenocarcin*:ti,ab,kw OR tumor*:ti,ab,kw OR cancer*:ti,ab,kw OR oncolog*:ti,ab,kw OR malignan*:ti,ab,kw OR metasta*:ti,ab,kw OR carcinogen*:ti,ab,kw OR oncogene*:ti,ab,kw OR paraneoplastic:ti,ab,kw OR plasmacytoma*:ti,ab,kw OR carcinosarcoma*:ti,ab,kw) AND (‘rectum’/exp OR rectal*:ti,ab,kw OR rectum:ti,ab,kw)) | 114,481 |
| #2 | ‘mesorectum’/exp OR ‘mesorectal fascia’/exp OR mesorect*:ti,ab,kw | 6599 |
| #3 | ‘metastasis’/exp OR ‘tumor invasion’/de OR ‘tumor seeding’/exp OR metast*:ti,ab,kw OR micrometast*:ti,ab,kw OR seeding*:ti,ab,kw OR circulat*:ti,ab,kw OR spread*:ti,ab,kw | 1,651,540 |
| #4 | #1 AND #2 AND #3 | 2038 |
| #5 | #4 AND (‘conference abstract’/it OR ‘conference paper’/it OR ‘conference review’/it) | 667 |
| #6 | #4 NOT #5 | 1371 |
Inclusion and exclusion criteria
Studies eligible for inclusion were studied reporting on the presence of malignant cells in the mesorectum in any form in patients with rectal cancer who underwent radical resection. Studies were excluded if they reported solely the intramural spread of rectal cancer, if they on lateral lymph-node metastases instead of mesorectal spread and if they did not describe the localisation of the mesorectal spread in relation to the primary tumor. Reviews or narrative studies, comment letters or non-human studies were also excluded. In case of studies with suspected overlap of patients, the most recent study was included.
Selection process
After removal of duplicates, two reviewers (AG and SvO) independently selected the articles by screening on title and abstract using Rayyan QCRI (www.rayyan.ai) [15]. Discrepancies between the two reviewers were resolved by discussion and mutual agreement. If necessary, a third author was consulted in case of disagreement. Thereafter, the same two reviewers independently assessed the resulting articles in full text. References of the included studies were checked to identify further relevant studies.
Quality assessment and scoring
The Agency for Healthcare Research and Quality (AHRQ) recommends 11 items to assess methodological quality of cross-sectional studies [16, 17]. That checklist was used for the included studies. Every item was judged with the use of “Yes”, “No”, “Unclear” or “Not applicable”. If there was any discrepancy between the two reviewers, it was resolved by discussion and two-way agreement. If required, a third author was consulted to reach an agreement. There were no consequences for low-quality studies, because there are no abundance of data.
Outcomes of interest
Primary outcome was the distance of tumor cells in the mesorectum distal from the rectal tumor. All kinds of malignant spread in the mesorectum, in form of lymph nodes, tumor deposits, direct invasion or lymphatic permeation and extramural vascular invasion (EMVI), were extracted. The participants with DMS were classified in different subgroups depending on characteristics of the primary rectal cancer and whether or not long-course neoadjuvant chemoradiotherapy (CRT) was given. We intended to provide the DMS for every clinical distinct subgroup of rectal cancer based on, for example, TNM stage and tumor height. Low rectal cancer was defined as the lower edge of the tumor < 7 cm from the anal verge and high rectal cancer as a distance of ≥ 7 cm from the anal verge.
Quantitative analysis
Data analysis was performed using IBM SPSS Statistics 26. The mean and maximum DMS were calculated and displayed in tables and figures. The mean DMS was calculated only from patients where the distance of the DMS relative to the tumor was reported, so the primary data of the articles were used to calculate the means. In addition, two scatter plots were constructed to illustrate the distribution of DMS per subgroup of rectal cancer.
Results
Literature search
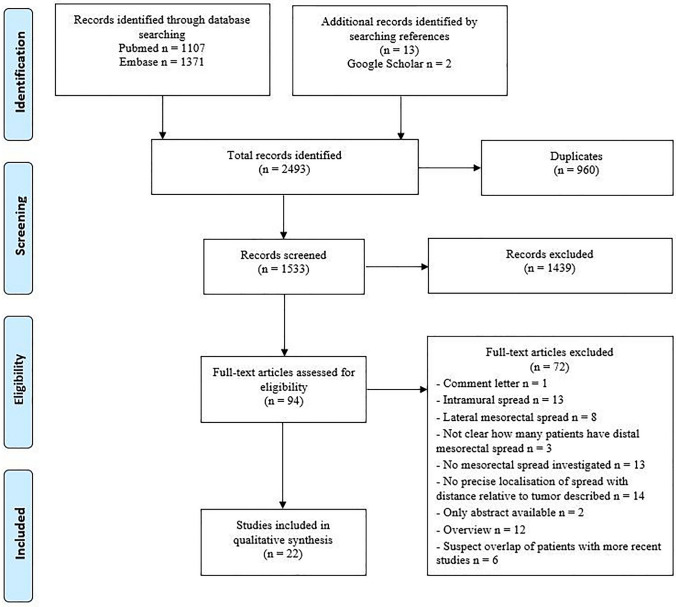
The literature search yielded 2493 records that resulted in 1533 unique articles after removal of duplicates. Thirteen studies were identified by crosschecking references of the included articles. After screening on title and abstract, 94 articles were assessed by full text. A total of 72 articles were excluded by reasons outlined in Fig. 1 [1, 18–88]. The 22 included studies reported a total number of 1921 patients [89–110]. Study design and characteristics are described in Table 3. Three studies were of retrospective design [94, 95, 103], twelve of prospective design [89, 90, 92, 96, 98–101, 104, 105, 109, 110], and of the remaining seven studies, the design was unclear [91, 93, 97, 102, 106–108].
Fig. 1.
Flowchart of the literature search
Table 3.
Characteristics of the included studies
| Study | Design | Total number of patients | Patients received long-course CRT (%) | Patients without long-course CRT with DMS of any extent (%) | Patients with CRT with DMS of any extent (%) | Mean DMS (mm) | Maximum DMS (mm) | Mode of spread (LN/TD/EMVI/DI/LP) |
|---|---|---|---|---|---|---|---|---|
| Choi [89] | Prospective | 53 | NM | 11 (20.8%) | NA | NA | NM | LN and TD |
| Girona [90] | Prospective | 47 | NM | 5 (10.6%) | NA | NA | NM | LN |
| Grinnell [91] | NM | 118 | NM | 5 (4.2%) | NA | NA | 20 | LN |
| Guedj [92] | Prospective | 124 | 124 (100%) | NA | 1 (0.8%) | NA | 20 | TD |
| Guo [93] | NM | 23 | 0 (0%) | 3 (13%) | NA | NA | NM | LN |
| Heijnen [94] | Retrospective | 61 | 61 (100%) | NA | 0 (0%) | NA | 0 | NM |
| Hida [95] | Retrospective | 198 | NM | 40 (20%) | NA | 20.8 | 40 | LN |
| Joh [96] | Prospective | 72 | NM | 11 (15.3%) | NA | NA | NM | LN and TD |
| Kiss [97] | NM | 50 | NM | 12 (24%) | NA | NA | 50 | LN and TD |
| Koh [98] | Prospective | 16 | 0 (0%) | 0 (0%) | NA | NA | 0 | NM |
| Langman [99] | Prospective | 244 | 74 (30.3%) | 5 (2.9%) | 3 (4.1%) | NA | 35 | LN |
| Ono [100] | Prospective | 40 | NM | 3 (7.5%) | NA | 14 | 20 | LN |
| Scott [101] | Prospective | 20 | NM | 4 (20%) | NA | 20 | 30 | LN |
| Shan [102] | NM | 62 | 0 (0%) | 15 (24%) | NA | NA | 40 | LN and TD and EMVI |
| Shimada [103] | Retrospective | 381 | 0 (0%) | 31 (8.1%) | NA | NA | 38 | LN and TD |
| Sprenger [104] | Prospective | 81 | 81 (100%) | NA | 0 (0%) | NA | 0 | NM |
| Tocchi [105] | Prospective | 53 | NM | 15 (28.3%) | NA | NA | NM | LN and TD |
| Wang [106] | NM | 31 | 0 (0%) | 4 (12.9%) | NA | NA | 35 | NM |
| Wang [107] | NM | 60 | NM | 15 (25%) | NA | NA | 40 | NM |
| Yu [108] | NM | 96 | 0 (0%) | 6 (6.3%) | NA | NA | 35 | LN |
| Zhang [109] | Prospective | 46 | 0 (0%) | 10 (21.7) | NA | 15.5 | 40 | LN and TD and EMVI |
| Zhao [110] | Prospective | 45 | 0 (0%) | 8 (17.8%) | NA | 12.2 | 36 | LN and DI and LP |
NM = not mentioned, NA= not applicable, CRT= chemoradiotherapy, DMS= distal mesorectal spread, LN= lymph node, TD= tumor deposit, EMVI= extramural vascular invasion, DI= direct invasion, LP= lymphatic permeation
Quality assessment: agency for healthcare research and quality (AHRQ) methodological checklist
The quality assessment is shown in Supplementary Table 1. The AHRQ scores ranged from 0 to 7. Thirteen of the included studies were considered to be of low quality (0–4) [90, 91, 93, 95–101, 107–109], and the remaining nine studies to be of moderate quality (5–8) [89, 92, 94, 102–106, 110]. Items 5, 7 and 9 were not applicable for any of the included studies, because in all patients, an intended radical resection was performed. Therefore, none of the patients were excluded from analysis and missing data were not handled in the analysis. In addition, item 11 was not applicable in most of the studies. Only 4 studies included patient follow-up [102, 103, 105, 110].
Outcomes
Overall DMS
DMS was found in 207 of the total of 1921 examined specimens (10.8%). In 84 of 207 cases, the distance of the DMS relative to the tumor was reported. The maximum reported DMS was 50 mm, which was found in one of the evaluable 84 specimens [97].
The overall median DMS was 20.0 mm and the overall mean DMS was 20.2 mm. DMS less than 10 mm was reported in 8.3% of the examined specimens, DMS from 10 till 20 mm was reported in 38.1% of the cases, DMS from 20 till 30 mm was reported in 21.4%, DMS from 30 till 40 was reported in 22.6%, and more than 40 mm DMS was reported in 9.5% of the 84 cases (see Table 4).
Table 4.
The amount of specimens per distance of distal mesorectal spread (DMS)
| Distance of DMS | Amount of specimens |
|---|---|
| > 0 mm and < 10 mm | 7 |
| ≥ 10 and < 20 mm | 32 |
| ≥ 20 and < 30 mm | 18 |
| ≥ 30 and < 40 mm | 19 |
| ≥ 40 mm | 8 |
DMS after long-course neoadjuvant chemoradiotherapy
Four of the included articles [92, 94, 99, 104] performed pathological assessment of the specimens after long-course neoadjuvant CRT and radical resection (see Table 3). In total, 340 of the 1921 included patients (17.7%) received long-course neoadjuvant CRT and DMS was found in 4 of those 340 examined specimens (1.2%). The distance was noted in only 1 of those 4 patients with DMS and it was 20 mm. Two hundred and three of the remaining 1581 patients, who did not receive long-course neoadjuvant CRT, had DMS (12.8%).
DMS and T-stage
Subgroup analysis for the different T categories are provided in Fig. 2 and Supplementary Fig. 1. The mean DMS for T3 rectal tumors was 18.8 mm (range: 8–40 mm), based on 36 patients. Corresponding outcome for 17 patients with T4 tumors was 27.2 mm with a range of 10–40 mm. The T-stage was not reported in 27 patients.
Fig. 2.
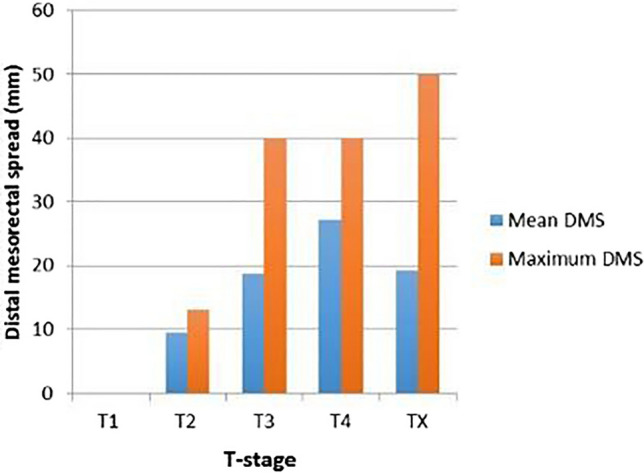
The mean and maximum distal mesorectal spread (DMS) per T-stage
DMS and tumor height
Results regarding high and low tumors are presented in Supplementary Figs. 2 and 3. The mean DMS in the high rectal cancer group was 22.8 mm (range: 10–40 mm). The mean DMS in the low rectal cancer group was 18.5 mm with a range of 8–30 mm.
DMS per T-stage and tumor height together
The mean DMS of the high located T3 and T4 rectal tumors was 23.4 mm (range: 10–40 mm). The mean DMS for the T3 and T4 tumors localized low was 18.4 mm with a range of 8–30 mm.
Type of DMS
Type of DMS was reported in 77 cases. In the vast majority of the cases, DMS comprised a lymph-node metastasis, namely in 65 of the 77 cases (84.4%). The mean DMS in this subgroup was 20.9 mm, with a range of 8–40 mm. In 8 cases, the spread was a tumor deposit (10.3%) with a DMS between 20 and 38 mm, in two cases, there was direct invasion distal to the mesorectum (2.6%) with a DMS of 6 and 10 mm, respectively, in one case, there was lymphatic invasion (1.3%) with a DMS of 8 mm, and in the remaining case, there was both direct invasion and a lymph-node distal in the mesorectum (1.3%) with a DMS of 10 mm.
Discussion
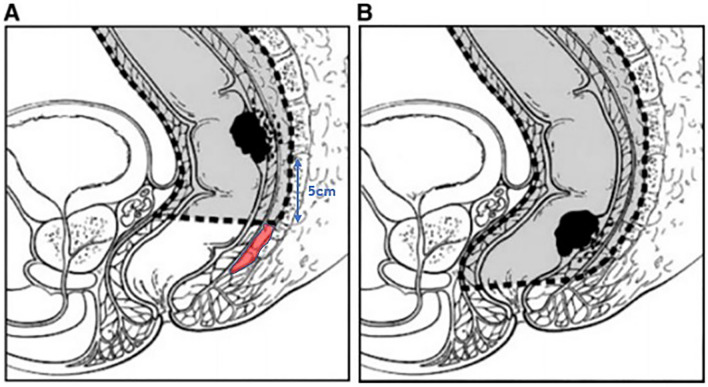
To the best of our knowledge, this is the first report that has systematically reviewed the published data about DMS in rectal cancer. The incidence was 10.8%, whereas the mean reported DMS was 20.2 mm. Using a distal margin of 30 mm would have resulted in residual tumor in 32% of cases with DMS and 10% of the patients with a distal margin of 40 mm would have had residual tumor. The maximum DMS was 50 mm. However, this was only recorded in less than 1% of the specimens with DMS (1 of 84 evaluable cases). The available data suggested that DMS increased with higher T-stage. These data imply that a PME can be safely executed if a distal margin of at least 50 mm can be obtained for T3 and T4 tumors, indicating that TME should be performed for tumors located up to 5 cm proximal from the most distal part of the mesorectum as measured on preoperative MRI, see Fig. 3. This also implies that not all patients with rectal cancer based on the new consensus definition (sigmoid take-off) [111] should be treated with a formal TME. This would result in a better quality of life, since PME is associated with less morbidity and better functional outcomes than TME.
Fig. 3.
On the left side (A), a partial mesorectal excision is illustrated with a distal mesorectal resection margin of 5 cm. The red shaded area is residual mesorectum. On the right side (B), a total mesorectal excision is illustrated
Tailoring the distal margin based on stage of disease and neoadjuvant treatment has the potential to increase the chance of tumor free distal mesorectal margins, potentially improving functional outcomes. Reducing the distal margin to 1 cm has a risk of residual DMS of 11%, which translates to an absolute risk of 1% given the fact that DMS is only found in 11% of the patients overall in this systematic review. If a distal margin of 2 cm is aimed for, a 32% risk of residual DMS results in an absolute increase of 4% for the whole group. For patients with non-locally advanced disease (T1-3a), these risks may be acceptable, given the balance between oncological safety and functional outcome. It is important to highlight that the data are not robust enough to allow firm conclusions regarding tailoring to specific patients groups, since the DMS is poorly reported.
Neoadjuvant CRT has been shown to decrease the number of (positive) lymph nodes available for pathologic assessment [112, 113]. Only 1.2% of the present examined specimens, from patients who had long-course neoadjuvant CRT and radical resection, had DMS (versus 12.8% after radical resection only). However, whether a PME with a distal margin of less than 5 cm in radiated patients is safe based on the current available data is not clear. This would require properly designed studies with a high sample size and with both DMS and local recurrence (LR) rate as endpoints.
Several studies have reported distal intramural spread in 5% of TME specimens, and this rarely exceeds 1 cm [79, 84, 110]. One cohort study from 2011 showed no significant difference in LR rates after 5 years between patients who had a distal resection margin of ≤ 1 cm and patients who had a distal resection margin of > 1 cm following TME [114]. Data from the Dutch TME trial showed that in patients with nodal disease and a distal margin of 2 cm or less, TME with radiotherapy was associated with lower recurrence rates compared to TME without radiotherapy. It was suggested that for node negative patients, a distal margin of 1 cm is sufficient, and for node positive patients, a margin of more than 2 cm is required [115].
The current review shows that DMS beyond 2 cm can occur in a proportion of patients. Individual studies showed DMS in 0–30% of patients with rectal cancer [95, 100, 105], with a pooled proportion of DMS in the present review of 10.8%. Of course, underreporting might be present, since the distal spread is not always mentioned in standard pathology reports. Furthermore, an inadequate distal mesorectal margin might result in false negative findings. Finally, there might be only 1 or 2 cm mesorectum distal to the tumor in patients with low rectal cancer, and therefore, DMS cannot occur beyond 2 cm by definition. Applying the 5 cm rule in clinical practice can be challenging. It is important to stress that the distal border of the mesorectum is often located above the level of the anorectal junction due to tapering of the mesorectum toward the pelvic floor. Surgical decision-making should be based on detailed assessment of the preoperative MRI, with extent of the resection tailored to individual anatomy. In practice, the 5 cm distal margin can be obtained in tumors located at a distance of more than 5 cm from the distal edge of the mesorectum, approximately 7 cm from the anorectal junction. Another 3 to 5 cm have to be added if the anal verge is used as a reference for tumor height, but this is less accurate and not recommended for clinical decision-making in the era of detailed preoperative staging using MRI.
Oncological safety and morbidity may be inversely related when it comes to the treatment of rectal cancer. On the one hand, morbidity should be kept as low as possible. On the other hand, treatment should be oncologically safe with the lowest chance of LR. Several centers have reported similar LR rates when TME is compared to PME with a 5 cm distal mesorectal resection margin (excluding patients receiving neoadjuvant therapy) [9, 116–118]. However, studies published from 2010 to 2015 demonstrate a concerning high rate of 10–16% LR in patients with proximal rectal cancer after PME [119–124]. Bondeven et al. found that inadvertent residual mesorectal tissue was often visualized on postoperative MRI, especially after PME (63%) [119]. Moreover, they showed that the distal mesorectal resection margin after PME, measured by postoperative MRI, was less than 5 cm in 80% and less than 3 cm in 52%. These findings could be a possible explanation for the relatively high rate of local recurrence after PME. Moreover, the quality of surgery was questionable and probably the major reason for local recurrence. Subsequently, Bondeven and co-workers investigated the impact of a multidisciplinary training program on outcomes of high rectal cancer by critical appraisal of the extent of mesorectal excision on postoperative MRI in another study [125]. The 3-year LR rate fell from 12.9% to 5.0%, and none of the patients treated with PME developed local recurrence when a distal resection margin of at least 3.5 cm was achieved. This illustrates the importance of a good quality of distal mesorectal excision and demonstrates that local recurrence is comparable to TME when PME is performed with adequate margins.
A good quality of mesorectal excision is not only about the distal mesorectal resection margin. Some studies suggest that the integrity of the surgical plane is more important. Jiménez-Toscano et al. recently found no significant difference in local-recurrence-free survival, DFS and OS between patients with ≤ 10 mm, 11–20 mm, 21–30 mm or ≥ 31 mm distal mesorectal resection margin [126]. In agreement with Quirke et al., classification of the integrity of the planes together with stage of disease were the most important factors for LR rates [127].
This systematic review has several limitations. First, there are paucity of data in the literature and this combined with the heterogeneity and lack of detail in the existing data in terms of T-stage and neoadjuvant therapy use limits the strength of the conclusions that can be drawn. In particular, DMS is not always included in datasets as an outcome, since routine pathologic examination of the entire mesorectal specimen for individual tumor cells is not standard practice. This could explain why DMS was found in only approximately 11% of the specimens. Second, postoperative MRI data from Bondeven et al. found residual mesorectum in many cases where a formal TME was intended, potentially resulting in underreporting of DMS [119, 128]. A further limitation relates to quality. More than half of the included studies were considered to be of low quality. One of the included studies is old (1950) and the surgical and histological reporting standards in that time were less accurate. Furthermore, similarly to refinements of surgical technique, neoadjuvant CRT regimens have likely been adjusted over time. Another limitation is that most of the included studies did not take into account the fact that shrinkage occurs in fixed specimens. The length of the distal resection margin may reduce by up to 30% after fixation [23, 129]. Bearing in mind that most of the included studies investigated fixed specimens, the DMS might potentially be higher than reported. In addition, inter-observer variability between pathologists can occur, as is shown in the study of Mekenkamp et al. [130]. Finally, the included studies did not report long-term oncological outcomes such as local recurrence..
These limitations mean that definite indications about the distal mesorectal resection margin should be in PME, and when a PME is safe or when a TME should be performed, it is not possible. Clearly, there is a need for a properly designed and quality controlled (international) study to prospectively evaluate the incidence of distal mesorectal tumor spread by precise pathologic assessment, with and without neoadjuvant treatment. This could be combined with a modified Delphi study to determine a core outcome set for pathology and MRI. In the meantime, we recommend a detailed assessment of the preoperative MRI taking into account individual variability in anatomy of the distal mesorectum, combined with a discussion of the balance between oncological safety and functional outcome with the patient, to guide the decision on the level of mesorectal transection in rectal cancer.
Conclusions
This systematic review shows that PME is a safe procedure in those patients where a margin of 5 cm can be obtained. The data revealed an incidence of DMS in rectal cancer of 11% overall, which was 1% and 13% with and without long-course neoadjuvant CRT. The maximum reported DMS was 50 mm based on a single case, with a risk of residual DMS of 1% using a 40 mm distal margin and this risk increases to 4% with a 30 mm distal margin. Prospective studies evaluating margins based on high-quality preoperative MRI and pathological assessment are required.
Supplementary Information
Below is the link to the electronic supplementary material.
Scatter plot with the individual patients with distal mesorectal spread (DMS) per T-stage.
Supplementary file1 Scatter plot with the individual patients with distal mesorectal spread (DMS) per T-stage (JPG 40 KB)
The mean and maximum distal mesorectal spread (DMS) per level of rectal tumor
Supplementary file2 The mean and maximum distal mesorectal spread (DMS) per level of rectal tumor (JPG 61 KB)
Scatter plot with the individual patients with distal mesorectal spread (DMS) per level of rectal tumor.
Supplementary file3 Scatter plot with the individual patients with distal mesorectal spread (DMS) per level of rectal tumor (JPG 54 KB)
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AAJG and SEVO. The first draft of the manuscript was written by AAJG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The ethical approval was waived by ethical committee of Amsterdam UMC, location VUmc.
Informed Consent
Informed consent is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg. 1982;69(10):613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 2.van der Pas MH, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson ARL, et al. Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg. 2019;269(4):596–602. doi: 10.1097/SLA.0000000000003021. [DOI] [PubMed] [Google Scholar]
- 4.Fleshman J, et al. Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg. 2019;269(4):589–595. doi: 10.1097/SLA.0000000000003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayne D, et al. Robotic-assisted surgery compared with laparoscopic resection surgery for rectal cancer: the ROLARR RCT. Efficacy Mech Eval. 2019 doi: 10.3310/eme06100. [DOI] [PubMed] [Google Scholar]
- 6.Park JW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021 doi: 10.1016/S2468-1253(21)00094-7. [DOI] [PubMed] [Google Scholar]
- 7.Nesbakken A, et al. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg. 2000;87(2):206–210. doi: 10.1046/j.1365-2168.2000.01357.x. [DOI] [PubMed] [Google Scholar]
- 8.Bregendahl S, et al. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15(9):1130–1139. doi: 10.1111/codi.12244. [DOI] [PubMed] [Google Scholar]
- 9.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240(2):260–268. doi: 10.1097/01.sla.0000133185.23514.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynne-Jones R, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl_4):iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 11.Luzietti E, et al. Comparison of guidelines for the management of rectal cancer. BJS Open. 2018;2(6):433–451. doi: 10.1002/bjs5.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmertsen KJ, Laurberg S, G. Rectal Cancer Function Study Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013;100(10):1377–1387. doi: 10.1002/bjs.9223. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Shea BJ, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng X, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 17.Rostom ADC, Cranney A, et al. Quality assessment forms. Celiac disease. Evid Rep Technol Assess. 2005;104:1–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Akagi Y, et al. Benefit of the measurement of mesorectal extension in patients with pT3N1-2 rectal cancer without pre-operative chemoradiotherapy: post-operative treatment strategy. Exp Ther Med. 2013;5(3):661–666. doi: 10.3892/etm.2012.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akagi Y, et al. Predicting oncologic outcomes by stratifying mesorectal extension in patients with pT3 rectal cancer: a Japanese multi-institutional study. Int J Cancer. 2012;131(5):1220–1227. doi: 10.1002/ijc.27315. [DOI] [PubMed] [Google Scholar]
- 20.Andreola S, et al. Adenocarcinoma of the lower third of the rectum surgically treated with a <10-MM distal clearance: preliminary results in 35 N0 patients. Ann Surg Oncol. 2001;8(7):611–615. doi: 10.1245/aso.2001.8.7.611. [DOI] [PubMed] [Google Scholar]
- 21.Andreola S, et al. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40(1):25–29. doi: 10.1007/BF02055677. [DOI] [PubMed] [Google Scholar]
- 22.Autschbach F. The pathological assessment of total mesorectal excision: what are the relevant resection margins? Recent Results Cancer Res. 2005;165:30–39. doi: 10.1007/3-540-27449-9_5. [DOI] [PubMed] [Google Scholar]
- 23.Bondeven P, et al. Objective measurement of the distal resection margin by MRI of the fresh and fixed specimen after partial mesorectal excision for rectal cancer: 5 cm is not just 5 cm and depends on when measured. Acta Radiol. 2016;57(7):789–795. doi: 10.1177/0284185115604007. [DOI] [PubMed] [Google Scholar]
- 24.Brown G, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227(2):371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 25.Canessa CE, et al. Anatomic study of the lymph nodes of the mesorectum. Dis Colon Rectum. 2001;44(9):1333–1336. doi: 10.1007/BF02234794. [DOI] [PubMed] [Google Scholar]
- 26.Cawthorn SJ, et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer. Lancet. 1990;335(8697):1055–1059. doi: 10.1016/0140-6736(90)92631-Q. [DOI] [PubMed] [Google Scholar]
- 27.Cserni G, Tarjan M, Bori R. Distance of lymph nodes from the tumor: an important feature in colorectal cancer specimens. Arch Pathol Lab Med. 2001;125(2):246–249. doi: 10.5858/2001-125-0246-DOLNFT. [DOI] [PubMed] [Google Scholar]
- 28.Debove C, et al. What happens after R1 resection in patients undergoing laparoscopic total mesorectal excision for rectal cancer? A study in 333 consecutive patients. Colorectal Dis. 2015;17(3):197–204. doi: 10.1111/codi.12849. [DOI] [PubMed] [Google Scholar]
- 29.Demetter P, et al. Review of the quality of total mesorectal excision does not improve the prediction of outcome. Colorectal Dis. 2016;18(9):883–888. doi: 10.1111/codi.13254. [DOI] [PubMed] [Google Scholar]
- 30.Di Matteo G, et al. Histological study of the distal spread of rectal cancer. Implications for surgical tactics and technique. Chirurgia. 1989;2(9):452–457. [Google Scholar]
- 31.Ding PR, et al. Depth of tumor invasion independently predicts lymph node metastasis in T2 rectal cancer. J Gastrointest Surg. 2011;15(1):130–136. doi: 10.1007/s11605-010-1353-1. [DOI] [PubMed] [Google Scholar]
- 32.Elezkurtaj S, et al. Histopathological regression grading matches excellently with local and regional spread after neoadjuvant therapy of rectal cancer. Pathol Res Pract. 2013;209(7):424–428. doi: 10.1016/j.prp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Farinella E, et al. In vivo lymph node mapping and pattern of metastasis spread in locally advanced mid/low rectal cancer after neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2013;28(11):1523–1529. doi: 10.1007/s00384-013-1727-4. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, et al. Colorectal cancer: lymphatic metastasis and choice of operation. Zhonghua Wai Ke Za Zhi. 1999;37(12):721–723. [PubMed] [Google Scholar]
- 35.Greco P, Magro G. Pathologic examination and staging of rectal carcinoma: a critical review. Pathologica. 2010;102(1):12–27. [PubMed] [Google Scholar]
- 36.Guillem JG, et al. A prospective pathologic analysis using whole-mount sections of rectal cancer following preoperative combined modality therapy: implications for sphincter preservation. Ann Surg. 2007;245(1):88–93. doi: 10.1097/01.sla.0000232540.82364.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han XY, et al. Reevaluation resection margin rectal cancer by flow cytometry and pathological examination. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10(3):226–229. [PubMed] [Google Scholar]
- 38.Hayden DM, et al. Tumor scatter after neoadjuvant therapy for rectal cancer: are we dealing with an invisible margin? Dis Colon Rectum. 2012;55(12):1206–1212. doi: 10.1097/DCR.0b013e318269fdb3. [DOI] [PubMed] [Google Scholar]
- 39.Hermanek P, Klimpfinger M. Sphinktererhaltende radikale Resektion des Rektumkarzinoms aus der Sicht der Pathologie. Acta chirurgica Austriaca. 1994;26(3):126–130. doi: 10.1007/BF02620011. [DOI] [Google Scholar]
- 40.Hida J, Okuno K, Tokoro T. Distal dissection in total mesorectal excision, and preoperative chemoradiotherapy and lateral lymph node dissection for rectal cancer. Surg Today. 2014;44(12):2227–2242. doi: 10.1007/s00595-013-0811-2. [DOI] [PubMed] [Google Scholar]
- 41.Joyce W, Dolan J, Hyland J. The mesorectum: re-appraisal of its morphology and its unique importance in rectal cancer. Int J Colorectal Dis. 1993;8(2):235–237. [Google Scholar]
- 42.Junginger T, Hermanek P. Problems in the treatment of upper rectal carcinoma. Chirurg. 2008;79(4):327–339. doi: 10.1007/s00104-008-1467-0. [DOI] [PubMed] [Google Scholar]
- 43.Kanso F, et al. Partial mesorectal excision for rectal adenocarcinoma: morbidity and oncological outcome. Clin Colorectal Cancer. 2016;15(1):82–90e1. doi: 10.1016/j.clcc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Komori K, et al. Adequate length of the surgical distal resection margin in rectal cancer: from the viewpoint of pathological findings. Am J Surg. 2012;204(4):474–480. doi: 10.1016/j.amjsurg.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Law WL, Chu KW. Local recurrence following total mesorectal excision with double-stapling anastomosis for rectal cancers: analysis of risk factors. World J Surg. 2002;26(10):1272–1276. doi: 10.1007/s00268-002-6560-9. [DOI] [PubMed] [Google Scholar]
- 46.Leblanc F, Laurent C, Rullier E. Can lymph node dissection for rectal cancer ever be omitted? J Chir (Paris) 2008;145(4):12S40–12S43. [PubMed] [Google Scholar]
- 47.Lee KY. Factors influencing oncologic outcomes after tumor-specific mesorectal excision for rectal cancer. J Korean Soc Coloproctol. 2012;28(2):71–72. doi: 10.3393/jksc.2012.28.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lelong B, Moutardier V, Delpero JR. Colorectal cancer: what should be the management of primary tumour? Rev Prat. 2004;54(2):155–166. [PubMed] [Google Scholar]
- 49.Leong AF. Total mesorectal excision (TME)–twenty years on. Ann Acad Med Singap. 2003;32(2):159–162. [PubMed] [Google Scholar]
- 50.Linter Kapisinska M, et al. Distribution of metastases in mesorectum is unpredictable: metastases do not respect tumor localization even in small non-circumferential rectal cancers. Eur J Surg Oncol. 2018;44(1):87–92. doi: 10.1016/j.ejso.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Maeda K, et al. Indications for and limitations of low anterior resection. Nihon Geka Gakkai Zasshi. 2000;101(6):449–453. [PubMed] [Google Scholar]
- 52.Marks R, et al. What determines the outcome after total mesorectal excision for rectal carcinoma-15 years experience of a specialist surgical unit. Colorectal Dis. 2000;2(5):270–276. doi: 10.1046/j.1463-1318.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 53.Maurer CA, et al. Rectal carcinoma. Optimizing therapy by partial or total mesorectum removal. Zentralbl Chir. 1999;124(5):428–435. [PubMed] [Google Scholar]
- 54.Mezhir JJ, et al. Presence of distal intramural spread after preoperative combined-modality therapy for adenocarcinoma of the rectum: what is now the appropriate distal resection margin? Surgery. 2005;138(4):658–663. doi: 10.1016/j.surg.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 55.Moore HG, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10(1):80–85. doi: 10.1245/ASO.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Morikawa E, et al. Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis Colon Rectum. 1994;37(3):219–223. doi: 10.1007/BF02048158. [DOI] [PubMed] [Google Scholar]
- 57.Nagtegaal I, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology. 2007;51(2):141–149. doi: 10.1111/j.1365-2559.2007.02720.x. [DOI] [PubMed] [Google Scholar]
- 58.Nash GM, et al. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon Rectum. 2010;53(10):1365–1373. doi: 10.1007/DCR.0b013e3181f052d4. [DOI] [PubMed] [Google Scholar]
- 59.Palasciano N, et al. Effect of the resection margin and the extrarectal spread on the recurrence following 2 types of surgical procedures in rectal cancer. Rev Esp Enferm Dig. 1991;80(1):28–32. [PubMed] [Google Scholar]
- 60.Park IJ, Kim JC. Adequate length of the distal resection margin in rectal cancer: from the oncological point of view. J Gastrointest Surg. 2010;14(8):1331–1337. doi: 10.1007/s11605-010-1165-3. [DOI] [PubMed] [Google Scholar]
- 61.Picon AI, et al. Prognostic significance of depth of gross or microscopic perirectal fat invasion in T3 N0 M0 rectal cancers following sharp mesorectal excision and no adjuvant therapy. Int J Colorectal Dis. 2003;18(6):487–492. doi: 10.1007/s00384-003-0504-1. [DOI] [PubMed] [Google Scholar]
- 62.Pirro N, et al. What do we know about the lymphatic drainage of the rectum? Gastroenterol Clin Biol. 2009;33(2):138–146. doi: 10.1016/j.gcb.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Pricolo VE, Abodeely A, Resnick M. Distal margins in radical resections for rectal cancer after chemoradiation therapy: how short is long enough? Dig Surg. 2010;27(3):185–189. doi: 10.1159/000274464. [DOI] [PubMed] [Google Scholar]
- 64.Ratto C, et al. Mesorectal microfoci adversely affect the prognosis of patients with rectal cancer. Dis Colon Rectum. 2002;45(6):733–742. doi: 10.1007/s10350-004-6288-8. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds JV, et al. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg. 1996;83(8):1112–1115. doi: 10.1002/bjs.1800830826. [DOI] [PubMed] [Google Scholar]
- 66.Rutkowski A, et al. Distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: is it safe? Ann Surg Oncol. 2008;15(11):3124–3131. doi: 10.1245/s10434-008-0125-6. [DOI] [PubMed] [Google Scholar]
- 67.Sadahiro S, et al. Optimal lymph node dissection for colorectal cancer. Nihon Geka Gakkai Zasshi. 2001;102(6):497–500. [PubMed] [Google Scholar]
- 68.Shepherd NA. Mesorectal spread and involvement of lateral resection margin in rectal cancer. Lancet. 1990;335(8702):1402–1403. doi: 10.1016/0140-6736(90)91281-E. [DOI] [PubMed] [Google Scholar]
- 69.Shin R, et al. Depth of mesorectal extension has prognostic significance in patients with T3 rectal cancer. Dis Colon Rectum. 2012;55(12):1220–1228. doi: 10.1097/DCR.0b013e31826fea6a. [DOI] [PubMed] [Google Scholar]
- 70.Shirouzu K, et al. Clinical significance of the mesorectal extension of rectal cancer: a Japanese multi-institutional study. Ann Surg. 2011;253(4):704–710. doi: 10.1097/SLA.0b013e3182119331. [DOI] [PubMed] [Google Scholar]
- 71.Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76(3):388–392. doi: 10.1002/1097-0142(19950801)76:3<388::AID-CNCR2820760307>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 72.Socha J, et al. A systematic review and meta-analysis of pT2 rectal cancer spread and recurrence pattern: implications for target design in radiation therapy for organ preservation. Radiother Oncol. 2019;133:20–27. doi: 10.1016/j.radonc.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 73.Syk E, et al. Tumour budding correlates with local recurrence of rectal cancer. Colorectal Dis. 2011;13(3):255–262. doi: 10.1111/j.1463-1318.2009.02119.x. [DOI] [PubMed] [Google Scholar]
- 74.Tian ZSS-L. Detection of CD44v6 in mesorectum from total mesorectal excision: an analysis of 47 cases. World Chin J Dig. 2005;13(20):2514–2516. [Google Scholar]
- 75.Wan J, et al. Mesorectal metastasis of middle and lower rectal cancer. Zhonghua Wai Ke Za Zhi. 2006;44(13):894–896. [PubMed] [Google Scholar]
- 76.Wang C, et al. Nodal spread and micrometastasis within mesorectum. World J Gastroenterol. 2005;11(23):3586–3590. doi: 10.3748/wjg.v11.i23.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang C, et al. Mesorectal spread and circumferential margin involvement of rectal cancer studied on large slice. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35(5):723–726. [PubMed] [Google Scholar]
- 78.Wang C, et al. Mesorectal spread and micrometastasis of rectal cancer studied with large slice technique and tissue microarray. J Surg Oncol. 2005;91(3):167–172. doi: 10.1002/jso.20278. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z, et al. Microscopic spread of low rectal cancer in regions of the mesorectum: detailed pathological assessment with whole-mount sections. Int J Colorectal Dis. 2005;20(3):231–237. doi: 10.1007/s00384-004-0674-5. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, et al. Distribution of micrometastatic nodules of low rectal cancer in mesorectum: a pathological study using whole-mount sections. Zhonghua Zhong Liu Za Zhi. 2006;28(5):361–363. [PubMed] [Google Scholar]
- 81.Wang Z, et al. Microscopic spread of low rectal cancer in regions of mesorectum: pathologic assessment with whole-mount sections. World J Gastroenterol. 2004;10(20):2949–2953. doi: 10.3748/wjg.v10.i20.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, et al. Regional micrometastasis of low rectal cancer in mesorectum: a study utilizing HE stain on whole-mount section and ISH analyses on tissue microarray. Cancer Invest. 2006;24(4):374–381. doi: 10.1080/07357900600705300. [DOI] [PubMed] [Google Scholar]
- 83.Wasserberg N, Gutman H. Resection margins in modern rectal cancer surgery. J Surg Oncol. 2008;98(8):611–615. doi: 10.1002/jso.21036. [DOI] [PubMed] [Google Scholar]
- 84.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients' survival. Br J Surg. 1983;70(3):150–154. doi: 10.1002/bjs.1800700305. [DOI] [PubMed] [Google Scholar]
- 85.Yegen G, et al. The effect of neoadjuvant therapy on the size, number, and distribution of mesorectal lymph nodes. Ann Diagn Pathol. 2016;20:29–35. doi: 10.1016/j.anndiagpath.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Zhang HG, Tian SL, Zhang RP. Significance of detection for matrix metalloproteinase-7 in rectal cancer tissues and mesorectum after total mesorectal excision. World Chin J Digestol. 2005;13:2497–2500. [Google Scholar]
- 87.Zheng Y-C, et al. Anatomic pathology of tumor cell spread through lymph nodes in the mesorectum of rectal cancer. World Chin J Digestol. 2004 doi: 10.3748/wjg.v10.i22.3369. [DOI] [Google Scholar]
- 88.Zheng YC, et al. Distribution and patterns of lymph nodes metastases and micrometastases in the mesorectum of rectal cancer. J Surg Oncol. 2007;96(3):213–219. doi: 10.1002/jso.20826. [DOI] [PubMed] [Google Scholar]
- 89.Choi JS, et al. Nodal metastasis in the distal mesorectum: need for total mesorectal excision of rectal cancer. Yonsei Med J. 1996;37(4):243–250. doi: 10.3349/ymj.1996.37.4.243. [DOI] [PubMed] [Google Scholar]
- 90.Girona J. The mesorectum in surgery of rectal cancer. Chirurg. 1993;64(7):549–551. [PubMed] [Google Scholar]
- 91.Grinnell RS. Lymphatic metastases of carcinoma of the colon and rectum. Ann Surg. 1950;131(4):494–506. doi: 10.1097/00000658-195004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guedj N, et al. Distal intramural and tumor spread in the mesorectum after neoadjuvant radiochemotherapy in rectal cancer: about 124 consecutive patients. Hum Pathol. 2016;52:164–172. doi: 10.1016/j.humpath.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 93.Guo X, et al. Metastasis and micrometastasis in ultra-low rectal cancer. Chin-Ger J Clin Oncol. 2010;9(9):524–527. doi: 10.1007/s10330-010-0608-3. [DOI] [Google Scholar]
- 94.Heijnen LA, et al. Good and complete responding locally advanced rectal tumors after chemoradiotherapy: where are the residual positive nodes located on restaging MRI? Abdom Radiol (NY) 2016;41(7):1245–1252. doi: 10.1007/s00261-016-0640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hida J, et al. Lymph node metastases detected in the mesorectum distal to carcinoma of the rectum by the clearing method: justification of total mesorectal excision. J Am Coll Surg. 1997;184(6):584–588. [PubMed] [Google Scholar]
- 96.Joh NS, et al. Lymph node metastases and tumor deposits in the mesorectum distal to rectal cancer: a need of total mesorectal excision. J Korean Soc Coloproctol. 1999;15(4):273. [Google Scholar]
- 97.Kiss L, et al. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Chirurgia (Bucur) 2011;106(3):347–352. [PubMed] [Google Scholar]
- 98.Koh DM, et al. Distribution of mesorectal lymph nodes in rectal cancer: in vivo MR imaging compared with histopathological examination. Initial Obs Eur Radiol. 2005;15(8):1650–1657. doi: 10.1007/s00330-005-2751-8. [DOI] [PubMed] [Google Scholar]
- 99.Langman G, Patel A, Bowley DM. Size and distribution of lymph nodes in rectal cancer resection specimens. Dis Colon Rectum. 2015;58(4):406–414. doi: 10.1097/DCR.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 100.Ono C, et al. Discontinuous rectal cancer spread in the mesorectum and the optimal distal clearance margin in situ. Dis Colon Rectum. 2002;45(6):744–749. doi: 10.1007/s10350-004-6290-1. [DOI] [PubMed] [Google Scholar]
- 101.Scott N, et al. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82(8):1031–1033. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 102.Shan KS, et al. Usefulness of large-section cytokeratin 20 in the detection of intestinal wall infiltration and mesangial metastasis in patients with middle and lower rectal cancer. J Cancer Res Ther. 2019;15(2):437–441. doi: 10.4103/jcrt.JCRT_405_18. [DOI] [PubMed] [Google Scholar]
- 103.Shimada Y, et al. Intramural and mesorectal distal spread detected by whole-mount sections in the determination of optimal distal resection margin in patients undergoing surgery for rectosigmoid or rectal cancer without preoperative therapy. Dis Colon Rectum. 2011;54(12):1510–1520. doi: 10.1097/DCR.0b013e318233fc4a. [DOI] [PubMed] [Google Scholar]
- 104.Sprenger T, et al. Lymph node metastases in rectal cancer after preoperative radiochemotherapy: impact of intramesorectal distribution and residual micrometastatic involvement. Am J Surg Pathol. 2013;37(8):1283–1289. doi: 10.1097/PAS.0b013e3182886ced. [DOI] [PubMed] [Google Scholar]
- 105.Tocchi A, et al. Total mesorectal excision and low rectal anastomosis for the treatment of rectal cancer and prevention of pelvic recurrences. Arch Surg. 2001;136(2):216–220. doi: 10.1001/archsurg.136.2.216. [DOI] [PubMed] [Google Scholar]
- 106.Wang C, et al. Patterns of neoplastic foci and lymph node micrometastasis within the mesorectum. Langenbecks Arch Surg. 2005;390(4):312–318. doi: 10.1007/s00423-005-0562-7. [DOI] [PubMed] [Google Scholar]
- 107.Wang XS, et al. Study on retrograde metastasis rule of middle-low rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2008;11(2):128–131. [PubMed] [Google Scholar]
- 108.Yu YY, et al. Mesorectal and lateral node metastasis and micrometastasis in lower rectal cancer. Hepatogastroenterology. 2011;58(107–108):745–748. [PubMed] [Google Scholar]
- 109.Zhang WJ, Chen JP. Spread of rectal cancer in the distal mesorectum. Chin J Cancer. 2008;27(7):74–76. [PubMed] [Google Scholar]
- 110.Zhao GP, et al. Pathological study of distal mesorectal cancer spread to determine a proper distal resection margin. World J Gastroenterol. 2005;11(3):319–322. doi: 10.3748/wjg.v11.i3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.D'Souza N, et al. Definition of the rectum: an international, expert-based Delphi consensus. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003251. [DOI] [PubMed] [Google Scholar]
- 112.Rullier A, et al. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32(1):45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 113.Wichmann MW, et al. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137(2):206–210. doi: 10.1001/archsurg.137.2.206. [DOI] [PubMed] [Google Scholar]
- 114.Kiran RP, Lian L, Lavery IC. Does a subcentimeter distal resection margin adversely influence oncologic outcomes in patients with rectal cancer undergoing restorative proctectomy? Dis Colon Rectum. 2011;54(2):157–163. doi: 10.1007/DCR.0b013e3181fc9378. [DOI] [PubMed] [Google Scholar]
- 115.Kusters M, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36(5):470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 116.Bokey EL, et al. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: role of total anatomical dissection. Br J Surg. 1999;86(9):1164–1170. doi: 10.1046/j.1365-2168.1999.01216.x. [DOI] [PubMed] [Google Scholar]
- 117.Lopez-Kostner F, et al. Total mesorectal excision is not necessary for cancers of the upper rectum. Surgery. 1998;124(4):612–618. doi: 10.1067/msy.1998.91361. [DOI] [PubMed] [Google Scholar]
- 118.Nesbakken A, et al. Local recurrence after mesorectal excision for rectal cancer. Eur J Surg Oncol. 2002;28(2):126–134. doi: 10.1053/ejso.2001.1231. [DOI] [PubMed] [Google Scholar]
- 119.Bondeven P, et al. Extent and completeness of mesorectal excision evaluated by postoperative magnetic resonance imaging. Br J Surg. 2013;100(10):1357–1367. doi: 10.1002/bjs.9225. [DOI] [PubMed] [Google Scholar]
- 120.Bondeven P, et al. Suboptimal surgery and omission of neoadjuvant therapy for upper rectal cancer is associated with a high risk of local recurrence. Colorectal Dis. 2015;17(3):216–224. doi: 10.1111/codi.12869. [DOI] [PubMed] [Google Scholar]
- 121.Kodeda K, et al. Regional differences in local recurrence rates after rectal cancer surgery. Colorectal Dis. 2010;12(10 Online):e206–e215. doi: 10.1111/j.1463-1318.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 122.Rosenberg R, et al. Does a rectal cancer of the upper third behave more like a colon or a rectal cancer? Dis Colon Rectum. 2010;53(5):761–770. doi: 10.1007/DCR.0b013e3181cdb25a. [DOI] [PubMed] [Google Scholar]
- 123.Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 124.Tiefenthal M, et al. The effects of short-course preoperative irradiation on local recurrence rate and survival in rectal cancer: a population-based nationwide study. Dis Colon Rectum. 2011;54(6):672–680. doi: 10.1007/DCR.0b013e318210c067. [DOI] [PubMed] [Google Scholar]
- 125.Bondeven P, et al. Impact of a multidisciplinary training programme on outcome of upper rectal cancer by critical appraisal of the extent of mesorectal excision with postoperative MRI. BJS Open. 2019 doi: 10.1002/bjs5.50242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jimenez-Toscano M, et al. Oncological outcome of wide anatomic resection with partial mesorectal excision in patients with upper and middle rectal cancer. Colorectal Dis. 2021;23(7):1837–1847. doi: 10.1111/codi.15690. [DOI] [PubMed] [Google Scholar]
- 127.Quirke P, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373(9666):821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Veltcamp Helbach M, et al. Residual mesorectum on postoperative magnetic resonance imaging following transanal total mesorectal excision (TaTME) and laparoscopic total mesorectal excision (LapTME) in rectal cancer. Surg Endosc. 2019;33(1):94–102. doi: 10.1007/s00464-018-6279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuzu MA, Maretto I, Pucciarelli S. Comprehensive step-by-step training package on rectal cancer surgery (3 DVD Set) Tech Coloproctol. 2013;17:337. doi: 10.1007/s10151-013-1010-2. [DOI] [Google Scholar]
- 130.Mekenkamp LJ, et al. Lymph node retrieval in rectal cancer is dependent on many factors–the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33(10):1547–1553. doi: 10.1097/PAS.0b013e3181b2e01f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plot with the individual patients with distal mesorectal spread (DMS) per T-stage.
Supplementary file1 Scatter plot with the individual patients with distal mesorectal spread (DMS) per T-stage (JPG 40 KB)
The mean and maximum distal mesorectal spread (DMS) per level of rectal tumor
Supplementary file2 The mean and maximum distal mesorectal spread (DMS) per level of rectal tumor (JPG 61 KB)
Scatter plot with the individual patients with distal mesorectal spread (DMS) per level of rectal tumor.
Supplementary file3 Scatter plot with the individual patients with distal mesorectal spread (DMS) per level of rectal tumor (JPG 54 KB)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.