Abstract
Sampling of bile juice during endoscopic retrograde cholangiopancreatography (ERCP) has potential benefit of being amenable to the identification of novel biomarkers in liquid biopsy. This study reports the results of a global investigation of exosomal microRNAs (miRNAs) in bile to identify potential biomarkers for biliary tract cancers (BTCs). Eighty‐eight bile samples collected during ERCP (45 BTC and 43 noncancer control samples) were enrolled in this study. Eleven BTC samples and nine control samples were assigned as the discovery set. Exosomes in bile and serum samples were collected using a glass membrane column with size‐controlled macroporous glass (MPG), and exosomal miRNA expression profiles were evaluated using comprehensive miRNA microarray analysis (3D‐Gene). For validation, exosomal miRNA in the bile samples of 34 BTCs and 34 controls were comprehensively evaluated using 3D‐Gene. In the discovery set, eight exosomal miRNAs in bile were identified as significant aberrant expression markers, while no miRNA with aberrant expression in serum was identified. In a comparison of the discovery and validation sets, miR‐451a and miR‐3619‐3p were identified as reproducible upregulated markers, and the combination of the two bile miRNAs showed an excellent area under the curve (0.819) value for diagnosing BTCs. In addition, high miR‐3619‐3p expression in bile reflects poorer prognosis of BTCs (hazard ratio = 2.89). The MPG‐extracted exosomal miRNAs in bile aspirated during ERCP provide a convenient new approach for diagnosing biliary diseases. Bile‐derived miRNA analysis with miR‐451a and miR‐3619‐3p represents a potentially valuable diagnostic strategy for identifying BTCs as well as a predictive indicator of BTC prognosis.
Keywords: bile, biliary tract cancer, exosome, liquid biopsy, miRNA
In a comparison of the discovery and validation sets, miR‐451a and miR‐3619‐3p were identified as reproducible upregulated markers, and the combination of the two bile miRNAs showed an excellent area under the curve (0.819) value for diagnosing BTCs. In addition, high miR‐3619‐3p expression in bile reflects poorer prognosis of BTCs (hazard ratio = 2.89). Bile‐derived miRNA analysis with miR‐451a and miR‐3619‐3p represents a potentially valuable diagnostic strategy for identifying BTCs as well as a predictive indicator of BTC prognosis.
![]()
Abbreviations
- AGC
advanced glass membrane column
- AUC
area under the ROC curve
- BTC
biliary tract cancer
- CA19‐9
carbohydrate antigen 19‐9
- CBD
common bile duct
- CCA
cholangiocarcinoma
- CI
confidence interval
- Ct
cycle threshold
- CTRL
control
- dCCA
distal cholangiocarcinoma
- ERCP
endoscopic retrograde cholangiopancreatography
- GBC
gallbladder cancer
- HR
hazard ratio
- miRNA
microRNA
- MPG
macroporous glass
- PC
pancreatic cancer
- pCCA
perihilar cholangiocarcinoma
- qRT‐PCR
quantitative real‐time RT‐PCR
- ROC
receiver operating characteristic
- T‐bil
total bilirubin
- UC
ultracentrifugation
1. INTRODUCTION
Biliary tract cancers are highly heterogenous malignancies derived from bile duct cells, and consist of CCA and GBC. In addition, CCA is anatomically classified into intrahepatic CCA and extrahepatic cholangiocarcinoma, followed by subclassification as pCCA and dCCA. 1 , 2 , 3 Despite the recent development of outstanding diagnostic imaging tools, such as computed tomography and MRI, 4 imaging‐based differential diagnosis of BTCs remains challenging. Moreover, the acquisition of tissue samples from the primary target could provide pathological evidence for differential diagnosis. Endoscopic retrograde cholangiopancreatography is an acknowledged approach for direct access to gallbladder and biliary tract diseases. Moreover, the use of brush cytology and intraductal biopsies from the bile duct during ERCP are considered ideal methods to obtain a tissue diagnosis. However, because of the nature of biliary systems, which are anatomically narrow and long tube‐like structures opening to the duodenum, these methods fall short of satisfactory outcomes for adequate diagnosis. 5 Thus, new approaches for differential diagnosis of bile tract diseases are urgently required.
In recent years, liquid biopsy has drawn attention by providing opportunities for cancer detection in various body fluids, such as peripheral blood and urine. Bile juice is a specific fluid that is secreted from the liver and drains into the duodenum through the bile ducts, and it plays a special role in the emulsion of lipids to facilitate fat digestion. As a microenvironment for the development of tumors in the biliary tract, bile is expected to contain abundant tumor‐related factors directly secreted from the primary tumor compared to peripheral blood. Sampling of bile juice during ERCP is a simple but not widely used method, and could represent an additional approach for biological‐based diagnosis.
Many studies have shown that exosomes can be utilized as appropriate and highly specific biomarkers in liquid biopsy. Exosomes are small vesicles of 30–100 nm in diameter that are secreted from cells, and are considered to deliver diverse contents including DNA, protein, and miRNAs to target cells. 6 MicroRNAs are small noncoding RNAs approximately 19–25 nt in length, which act as translational suppressors by regulating protein‐coding genes. 7 , 8 The aberrant expression of miRNAs necessitates close attention for the identification of novel clinical biomarkers for various diseases.
In this study, we aimed to determine a global analysis of exosomal miRNAs in ERCP‐based collection of bile juice to identify potential biomarkers for BTCs.
2. MATERIALS AND METHODS
2.1. Patients and study design
From September 2016 to December 2021, bile and serum samples were prospectively collected at four Japanese institutions (Nagoya City University Hospital, Nagoya City West Medical Center, Gifu Prefectural Tajimi Hospital, and Toyokawa City Hospital). In total, 44 patients with BTC were initially enrolled in this study. In addition, 44 samples of sex‐ and age‐matched noncancer controls were randomly selected from our bile bank containing 189 noncancer patients. Patients with BTCs (BTC group) were histologically confirmed to have adenocarcinoma using endoscopic biopsy or surgical specimen. Patients who underwent ERCP for biliary benign diseases were enrolled as the control (CTRL group). To exclude contamination of bile samples with infection or hemorrhage, patients with apparent cholangitis or hemobilia were excluded in this study. In addition, to exclude possible contamination of bile samples with reflux enteric fluid, patients with naïve papilla without previous endoscopic procedures were enrolled in this study. All patients in the CTRL group were carefully screened for evidence of neoplasms by a follow‐up care check. Originally, among the 88 total samples, 10 BTC samples and 10 noncancer control samples were assigned to the discovery set. The follow‐up care check revealed that one case with CBD stones in the CTRL group of the discovery set had GBC following cholecystectomy for gallbladder stones; this case was subsequently assigned to the BTC group of the discovery set. After the discovery analysis, 34 BTC samples and 34 noncancer control samples were assigned to the validation set (Figure 1).
FIGURE 1.
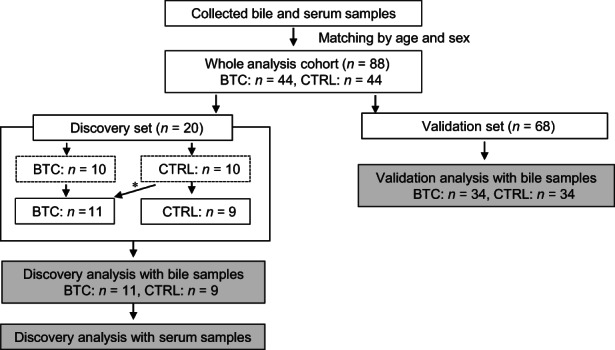
Flowchart of the study, which included 44 samples each from biliary tract cancer (BTC) and control (CTRL) patients. *Cholecystectomy for gallbladder stones revealed the presence of gallbladder cancer in one case with common bile duct stones. This case was consequently reassigned to the BTC group.
The ethics committee at each institution approved our study protocol (#60‐16‐0069), which conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). Each patient provided written, informed consent for this study.
2.2. Samples
Bile samples were obtained by ERCP. Bile samples (1–5 ml) were aspirated after cannulation to the bile duct without contrast agent injection. Following collection, the samples were centrifuged at 2000 g for 10 min to remove debris. One milliliter of the collected bile samples was used for the following analysis. Peripheral blood was collected for serum samples on the same day as bile sampling. Serum was separated by centrifugation at 1500 g for 5 min. The purified samples were stored at −80°C until use. All patients with BTCs were classified based on TNMs staging and the UICC version 8.
2.3. Exosome collection and miRNA extraction using the AGC device
To collect exosomes efficiently from a limited volume liquid sample, we utilized an AGC device in this study, as has been published previously. 9 One milliliter of the liquid samples, that is, bile juice, serum, and cell culture supernatants, was filtered through a 0.22 μm filter; 200 μl of the filtered samples was transferred to the upper part of the AGC device and centrifuged at 6000 g for 5 min. Total RNA was then isolated from the exosomes captured in the AGC device using a miRNeasy Serum/Plasma kit (Qiagen).
2.4. MicroRNA array analysis
Extracted RNA was measured by capillary electrophoresis using a 2100 Bioanalyzer (Agilent Technologies). A comprehensive miRNA microarray analysis was performed using a 3D‐Gene Human miRNA Oligo Chip version 21 (Toray Industries, Inc.). The miRNA signal values were normalized using a global normalization method. To identify robust miRNAs, average signal values with a normalized signal value exceeding 100 in either/or group were selected, as previously reported. 10 , 11
2.5. Overall survival using the Kaplan–Meier estimator
Based on the median expression value of miRNAs as the cut‐off value, the 45 BTC patients in this study were classified to either a high expression group (n = 22) or a low expression group (n = 23). Survival was estimated using the Kaplan–Meier method, and the survival distributions in association with each miRNA expression were compared between the two groups using the log–rank test.
2.6. Quantitative real‐time RT‐PCR
In an in vitro study, exosomes were also isolated from cell culture supernatants using the AGC device, as mentioned above. Total RNAs including miRNAs in exosomes were purified with a miRNeasy Serum/Plasma kit (Qiagen) following the manufacturer’s instructions. Specifically, total RNA was extracted from 2 ml of the supernatant from each subject, to which 1.6 × 108 copies of Caenorhabditis elegans cel‐miR‐39‐3p (cel‐miR‐39‐3p) was added as spike‐in RNA for subsequent normalization, and total RNA was then eluted from each column with 20 μl nuclease‐free water. The concentration of total RNA was quantified using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific).
MicroRNA levels were determined by qRT‐PCR with StepOnePlus (Thermo Fisher Scientific) and TaqMan MicroRNA Assays: hsa‐miR‐451a (assay ID 001141), hsa‐miR‐3619‐3p (assay ID 464743), and cel‐miR‐39‐3p (assay ID 000200; Thermo Fisher Scientific). Two microliters of total RNA was subjected to reverse transcription with a TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) and the respective TaqMan MicroRNA Assay reagents for the target molecules, in a total volume of 15 μl, followed by qRT‐PCR in a total volume of 20 μl, according to the manufacturer’s protocol. Amplification was carried out as follows: 95°C for 10 min, 45 cycles at 95°C for 15 s, and 60°C for 60 s. All reactions were carried out in duplicate. Cycle threshold values were calculated using StepOne Software version 2.3 (Thermo Fisher Scientific). Expression levels of miRNAs were normalized to those of the spike‐in cel‐miR‐39‐3p. The expression levels were determined by the method, in which ΔCt was calculated as: ΔCt = Ct (miRNA [miR‐451a and miR‐3619‐3p] – Ct [cel‐miR‐39‐3p]). The relative expression levels were calculated based on the expression level of MMNK‐1. These experiments were undertaken as four independent procedures.
2.7. Statistical analysis
The Mann–Whitney U‐test, Student’s t‐test, and χ2‐test were used to identify significant differences as appropriate. In terms of the statistical analysis of the microarray, the data were globally normalized per array, and the significantly differentially expressed miRNAs were obtained by comparing the BTC groups with the CTRL groups using a t‐test. Receiver operating characteristic curve analysis was used to calculate the AUC for each biomarker, and the AUC value with a 95% CI was shown as the representative value. Logistic regression models were used to estimate the HR with 95% CI. All of the statistical analyses were undertaken using JMP software (version 16; JMP Statistical Discovery). Differences were considered statistically significant at p < 0.05. Data are expressed as the mean ± SD.
Fundamental materials and methods (cell cultures, western blot analysis, and ELISA) are described in Document S1.
3. RESULTS
3.1. Exosomal RNAs in bile
To confirm the captured exosomes and exosome RNAs by the AGC device, we first quantified the exosomes by western blotting. In addition, standard exosome markers, CD9 and CD63, were detected by a CD9/CD63 ELISA kit to quantify the exosomes, as previously reported. 9 Subsequently, the amount of RNA in exosomes was quantified. As shown in Figure 2A, three independent samples processed from the bile of three BTC patients using the AGC device were confirmed to contain clearly significant amounts of exosomes. Moreover, it was shown that the processed bile contained a significantly greater quantity of RNA than the serum (median, 13.314 vs. 0.533 ng/μl, p < 0.001) in the discovery set (Figure 2B). We also investigated the relationship between cholestasis and the quantity of exosomal RNA in bile, using regression analysis. This analysis showed that the amount of RNA in bile did not show any positive correlation with T‐bil level that indicates the status of cholestasis within the same disease group (BTC: r 2 = 0.0114, p = 0.485; CTRL: r 2 = 0.0001, p = 0.941; Figure S1). These data showed that bile exosomes could be appropriately collected and the exosomal miRNAs in bile were more abundant compared to the serum, which were independent of the status of cholestasis, and it was considered suitable for the following miRNA analysis.
FIGURE 2.
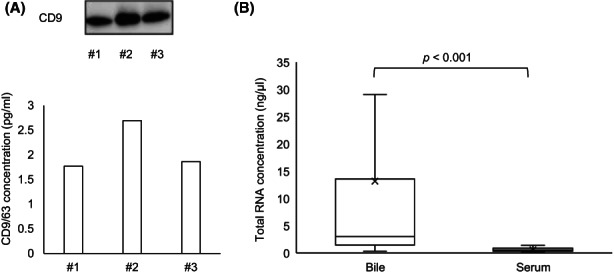
Exosome extraction from bile and exosomal RNAs in bile. (A) Exosomes captured by the advanced glass membrane column device. Upper panel, Immunoblotting showing the CD9 expression in three independent bile samples of biliary tract cancer patients. Lower panel, Standard exosome markers, CD9 and CD63, were detected by a CD9/CD63 ELISA kit to quantify the exosomes. (B) Concentration of total RNA purified from captured exosomes in bile and serum was calculated using the discovery set samples (n = 20).
3.2. Clinical characteristics of study participants
Clinical and laboratory characteristics of all patients enrolled in this study are shown in Tables S1 and S2, and a summary of the patient characteristics is given in Table 1. Age, sex, comorbidity (history of hyperlipidemia), and markers for systemic inflammation (white blood cell count, C‐reactive protein) did not significantly differ between the BTC and CTRL groups. Compared to the CTRL group, T‐bil was significantly increased in the BTC group of both the discovery (5.82 ± 3.85 vs. 1.56 ± 0.83 mg/dl, p = 0.002) and validation sets (6.88 ± 5.47 vs. 1.24 ± 0.73 mg/dl, p < 0.001). In terms of the tumor markers, CA19‐9 was significantly higher in the BTC group of the validation set than in the CTRL group (410.7 ± 686.4 vs. 19.5 ± 12.9 U/ml, p < 0.001), as expected.
TABLE 1.
Characteristics of patients with biliary tract cancers (BTC) or controls (CTRL)
| Variable | Discovery set | n = 20 | p value | Validation set | n = 68 | p value |
|---|---|---|---|---|---|---|
| BTC | CTRL | BTC | CTRL | |||
| n = 11 | n = 9 | n = 34 | n = 34 | |||
| Age (years) | ||||||
| Median (range) | 77 (61–88) | 74 (66–84) | 0.676 | 75.5 (40–97) | 73.5 (40–90) | 0.792 |
| Sex | ||||||
| M:F | 7:4 | 5:4 | 0.535 | 25:9 | 25:9 | 1.000 |
| Disease | ||||||
| dCCA | 5 | N/A | N/A | 19 | N/A | N/A |
| GBC | 4 | N/A | N/A | 8 | N/A | N/A |
| pCCA | 2 | N/A | N/A | 7 | N/A | N/A |
| CBD stone | N/A | 9 | N/A | N/A | 32 | N/A |
| SOD | N/A | 0 | N/A | N/A | 2 | N/A |
| Comorbidity | ||||||
| HL | 3 (27.3%) | 4 (44.4%) | 0.423 | 10 (29.4%) | 11 (32.4%) | 0.793 |
| WBC, /μl | 6800 ± 2465 | 5288 ± 1584 | 0.068 | 6467 ± 2262 | 6485 ± 1640 | 0.536 |
| CRP, mg/dl | 2.08 ± 1.44 | 1.09 ± 1.00 | 0.087 | 1.70 ± 1.91 | 1.68 ± 1.80 | 0.995 |
| T‐bil, mg/dl | 5.82 ± 3.85 | 1.56 ± 0.83 | 0.002 | 6.88 ± 5.47 | 1.24 ± 0.73 | <0.001 |
| CEA, ng/ml | 4.44 ± 3.70 | 2.81 ± 1.77 | 0.323 | 5.83 ± 12.86 | 2.33 ± 1.05 | 0.051 |
| CA19‐9, U/ml | 1877.4 ± 5212.0 | 118.8 ± 262.8 | 0.087 | 410.7 ± 686.4 | 30.97 ± 34.24 | 0.002 |
| Stage | ||||||
| I | 1 | N/A | N/A– | 3 | N/A | N/A– |
| II | 5 | N/A | N/A | 18 | N/A | N/A |
| III | 2 | N/A– | N/A | 5 | N/A– | N/A– |
| IV | 3 | N/A– | N/A– | 8 | N/A– | N/A |
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CBD, common bile duct; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; dCCA, distal cholangiocarcinoma; F, female; GBC, gallbladder cancer; HL, hyperlipidemia; M, male; N/A, not applicable; pCCA, perihilar cholangiocarcinoma; SOD, sphincter of Oddi dysfunction; T‐bil, total bilirubin; WBC, while blood cell.
The italic values, statistically significant.
3.3. Discovery analysis for miRNA expression in bile
To identify bile miRNA biomarkers, we first undertook a comprehensive miRNA microarray analysis using a 3D‐Gene Human miRNA Oligo Chip to compare bile miRNA expression levels of the BTC group (n = 11) and CTRL group (n = 9) in the discovery set. Among 2632 miRNAs examined, we focused on miRNAs that showed a log2‐fold change of ≥1 with mean signal intensity of ≥100, as previously reported. 10 , 11 Among 403 miRNAs with a normalized mean signal value of ≥100, eight upregulated miRNAs (miR‐451a, miR‐6835‐5p, miR‐6826‐5p, miR‐3619‐3p, miR‐5195‐3p, miR‐514b‐5p, miR‐4442, and miR‐4675) were identified as significant differential expression markers with a log2‐fold change of ≥1 in the BTC group (Table 2).
TABLE 2.
Bile and serum exosomal microRNA (miRNA) expression in the discovery set of 11 biliary tract cancer (BTC) samples and nine control (CTRL) samples
| Name | Bile exosomal miRNAs | Serum exosomal miRNAs | ||||||
|---|---|---|---|---|---|---|---|---|
| Relative expression level | p value | Expression intensity | Relative expression level | p value | Expression intensity | |||
| (BTC/CTRL, log2) | BTC | CTRL | (BTC/CTRL, log2) | BTC | CTRL | |||
| High: 8 miRNAs | ||||||||
| hsa‐miR‐451a | 5.16 | 0.005 | 311 | 9 | −0.55 | 0.592 | 152 | 223 |
| hsa‐miR‐6835‐5p | 2.38 | 0.018 | 242 | 46 | 0.78 | 0.014 | 69 | 40 |
| hsa‐miR‐6826‐5p | 1.99 | 0.012 | 690 | 174 | 0.39 | 0.164 | 116 | 89 |
| hsa‐miR‐3619‐3p | 1.66 | 0.049 | 660 | 209 | 0.17 | 0.586 | 202 | 179 |
| hsa‐miR‐5195‐3p | 1.44 | 0.019 | 344 | 127 | 0.3 | 0.547 | 89 | 72 |
| hsa‐miR‐514b‐5p | 1.25 | 0.001 | 102 | 43 | −0.01 | 0.87 | 46 | 46 |
| hsa‐miR‐4442 | 1.23 | 0.002 | 1977 | 841 | 0.26 | 0.406 | 600 | 502 |
| hsa‐miR‐4675 | 1.23 | 0.019 | 500 | 213 | 0.32 | 0.283 | 132 | 106 |
| Low: 0 miRNA | ||||||||
| None | ||||||||
The italic values, statistically significant.
3.4. Comparative analysis between bile and serum samples by miRNA microarray
To compare miRNA profiles of bile and serum samples in the same cohort, we also undertook a microarray analysis using the serum samples obtained on the same day as the bile collection to compare serum miRNA expression levels of the BTC and CTRL groups. In the analysis of serum samples, none of the eight miRNAs from the bile analysis showed a log2‐fold change of ≥1 between the BTC and CTRL groups (Table 2). Furthermore, none of the other miRNAs were identified as differential expression markers with a log2‐fold change of ≥1 between the two groups (Table S3). These data indicated that for BTCs, miRNA analysis using bile samples showed significant differences that were not observed in the serum analysis.
3.5. Validation analysis of bile miRNAs by miRNA microarray
To validate the specificity of bile miRNAs as diagnostic biomarkers of BTCs, we evaluated bile miRNA expression levels in the validation set (BTC group, n = 34; CTRL group, n = 34) by microarray analysis with 2632 miRNAs. Among 354 miRNAs with a normalized mean signal value of ≥100, five upregulated miRNAs (miR‐451a, miR‐21‐5p, miR‐3619‐3p, miR‐6778‐5p, and miR‐1246) and one downregulated miRNA (miR‐5189‐5p) were identified as significant differential expression markers between the two groups (Table 3). Intriguingly, in a comparison of the discovery and validation analyses, miR‐451a and miR‐3619‐3p were identified as reproducible upregulated markers (miR‐451a: log2‐fold change, 4.31, p < 0.001; miR‐3619‐3p: 1.74, p = 0.001). These two miRNAs showed significant differences between the BTC and CTRL groups in the ROC analysis: miR‐451a, AUC = 0.746 (95% CI, 0.613–0.844); and miR‐3619‐3p, 0.726 (0.584–0.833). The combination of the two miRNAs showed excellent AUC (0.819, 0.692–0.902), which was higher than that of the tumor markers CA19‐9 (0.762, 0.612–0.867) and carcinoembryonic antigen (0.662, 0.501–0.793; Figure 3).
TABLE 3.
Bile exosomal miRNA expression in the validation set of bile samples of 34 patients with biliary tract cancer (BTC) and 34 controls (CTRL)
| Name | Relative expression level (BTC/CTRL, log2) | p Value | Expression intensity | |
|---|---|---|---|---|
| BTC | CTRL | |||
| High: 5 miRNAs | ||||
| hsa‐miR‐451a | 4.31 | 0.000 | 396 | 20 |
| hsa‐miR‐21‐5p | 1.95 | 0.001 | 112 | 29 |
| hsa‐miR‐3619‐3p | 1.74 | 0.001 | 855 | 256 |
| hsa‐miR‐6778‐5p | 1.34 | 0.004 | 747 | 295 |
| hsa‐miR‐1246 | 1.06 | 0.014 | 1474 | 708 |
| Low: 1 miRNA | ||||
| hsa‐miR‐5189‐5p | −1.43 | 0.01 | 59 | 159 |
Abbreviation: miRNA, microRNA.
FIGURE 3.
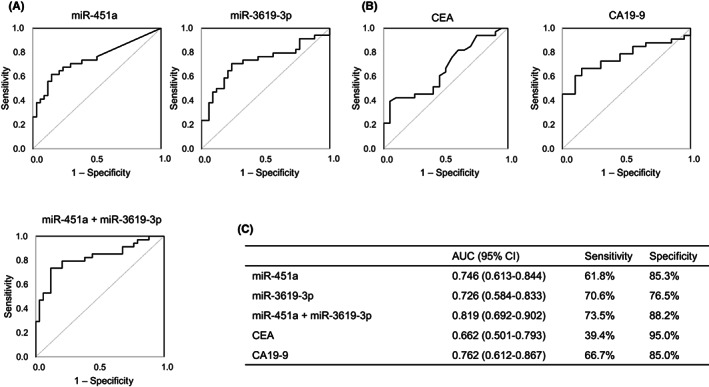
Receiver operating characteristic (ROC) curves in the validation set of patients with biliary tract cancer (BTC) and controls (CTRL). (A) Ability of each microRNA (miR‐451a and miR‐3619‐3p) and their combination to distinguish between BTC and CTRL samples in the validation set. (B) Ability of tumor markers (carcinoembryonic antigen [CEA] and carbohydrate antigen 19‐9 [CA19‐9]) to distinguish between BTC and CTRL samples in the validation set. (C) ROC analysis was used to determine the area under the ROC curve (AUC). CI, confidence interval.
3.6. Correlation of bile exosomal miR‐451a and miR‐3619‐3p with clinical features of BTC patients
We next analyzed the association between bile exosomal miR‐451a/miR‐3619‐3p and the clinical status of BTC patients. The median expression level of each miRNA was used as the cut‐off point to divide the 45 BTC patients into two groups: high expression (n = 22) and low expression (n = 23; Table 4). In the miR‐451a analysis, no significant difference was noted between these two groups in age, sex, tumor site, or clinical stage. However, a majority of patients in the high expression group underwent palliative care without operative treatment or chemotherapy, a majority of patients in the low expression group underwent operative treatment for BTCs, and a significant treatment difference was observed (p = 0.03). In the miR‐3619‐3p analysis, no significant difference was noted between these two groups for the parameters of age, sex, or tumor site. However, a significant difference in clinical stage between the two groups was observed for miR‐3619‐3p (p = 0.03). Thirteen of the high miR‐3619‐3p expressing patients were in advanced (III or IV) stages (59.1%), while nine were in an earlier (I or II) stage (40.9%). In contrast, the low miR‐3619‐3p group consisted of 17 patients in the I/II stage (73.9%) and six in III/IV stages (26.1%). MicroRNA‐3619‐3p showed the exact same tendency as miR‐451a in terms of medical treatment (p = 0.03). Overall survival is shown in Figure 4. For miR‐3619‐3p, the high expression group showed significantly poorer prognosis than the low expression group (HR = 2.89; 95% CI, 1.25–6.86; p = 0.010), whereas miR‐451‐a did not show a difference between the high and low expression groups (HR = 1.05; 95% CI, 0.43–2.40; p = 0.920). Collectively, these results suggest that high miR‐3619‐3p expression in bile is more prevalent in advanced stages of BTCs and reflects poorer prognosis of BTCs.
TABLE 4.
Characteristics of 45 patients with biliary tract cancer
| Clinical status | Bile exosomal miR‐451a level | p value | Bile exosomal miR‐3619‐3p level | p value | ||
|---|---|---|---|---|---|---|
| High | Low | High | Low | |||
| n = 22 | n = 23 | n = 22 | n = 23 | |||
| Age | 76 | 76 | 0.67 | 76.5 | 76.0 | 0.10 |
| Gender | ||||||
| M:F | 15:7 | 17:6 | 0.67 | 13:9 | 19:4 | 0.08 |
| Diseases | ||||||
| dCCA | 10 | 13 | 10 | 13 | ||
| GBC | 5 | 7 | 0.35 | 8 | 4 | 0.35 |
| pCCA | 7 | 3 | 4 | 6 | ||
| Stage | ||||||
| I–II | 10 | 16 | 0.10 | 9 | 17 | 0.03 |
| III–IV | 12 | 7 | 13 | 6 | ||
| Treatment | ||||||
| Surgery | 8 | 17 | 8 | 17 | ||
| Chemotherapy | 5 | 3 | 0.03 | 5 | 3 | 0.03 |
| BSC | 9 | 3 | 9 | 3 | ||
Abbreviations: BSC, best supportive care; dCCA, distal cholangiocarcinoma; F, female; GBC, gallbladder cancer; M, male; miR, microRNA; pCCA, perihilar cholangiocarcinoma.
The italic values, statistically significant.
FIGURE 4.
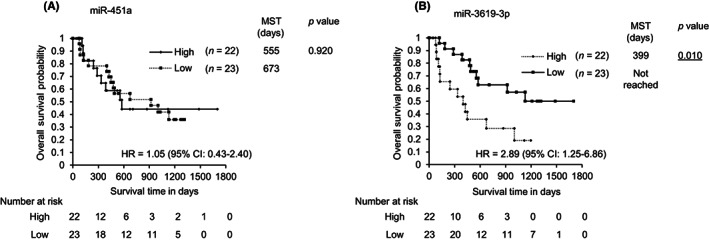
Overall survival of patients with biliary tract cancer according to microRNA (miRNA) expression levels. (A) miR‐451a. (B) miR‐3619‐3p. High, high expression group of each exosomal miRNA in bile; Low, low expression group of each exosomal miRNA in bile. CI, confidence interval; HR, hazard ratio; MST, median survival time.
3.7. Additional validation analysis with cell lines
We undertook additional in vitro analyses to further validate the miRNA biomarkers from BTCs. Using the conditioned media from BTC cell lines and a normal biliary duct epithelial cell line, we investigated expression levels of miR‐451a and miR‐3619‐3p in exosomes released from these cells. Compared to MMNK‐1 (relative expression level, 1.00 ± 0.98), which is a normal bile duct epithelial cell line, miR‐451a was significantly more abundant in the cell culture supernatant of the two BTC cell lines HuCCT‐1 (4.25 ± 2.90, p < 0.05) and SSP‐25 (5.96 ± 3.85, p < 0.05). Moreover, miR‐3619‐3p showed significantly greater levels in SSP‐25 (2.98 ± 0.65 vs. 1.00 ± 0.33, p < 0.01) and TFK‐1 (7.98 ± 5.11 vs. 1.00 ± 0.33, p < 0.05) (Figure 5). These results revealed that miR‐451a and miR‐3619‐3p are more abundant in exosomes from BTC cell lines compared to the normal bile duct cell line, which supported the observation from the bile analysis.
FIGURE 5.
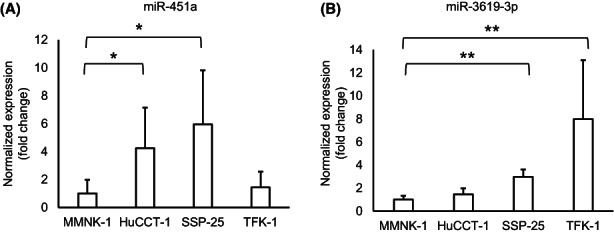
Expression levels of microRNAs (miRNAs) in conditioned media from biliary tract cancer (BTC) cell lines. Expression levels of (A) miR‐451a and (B) miR‐3619‐3p in three BTC cell lines (HuCCT‐1, SSP‐25, and TFK‐1) is shown relative to that in a normal bile duct epithelial cell line (MMNK‐1). Data are shown as mean ± SD (n = 4). *p < 0.05; **p < 0.01.
4. DISCUSSION
For biliary diseases, ERCP has significant advantages, enabling not only examination by direct imaging but also sampling of cells for cytologic assessment. However, cell sampling from the biliary target remains challenging in light of the anatomical nature of biliary systems, that is, the accurate diagnosis of malignancy in patients with primary sclerosing cholangitis and IgG4‐related sclerosing cholangitis is more challenging. For endoscopists, these current limitations have motivated the investigation of bile juice, obtained during ERCP, as a possible liquid biopsy, which is expected to contain abundant factors sourced directly from the primary cancer site compared to peripheral blood samples. Indeed, the data presented herein showed that bile contains more abundant RNAs stably capsulized in exosomes than serum, an encouraging indicator that the analysis of exosomal miRNAs in bile could enable the discovery of novel biomarkers for BTCs. In this study, the independent comprehensive investigation with discovery and validation analyses identified two reproducible diagnostic markers, miR‐451a and miR‐3619‐3p. High expression of exosomal miR‐451a and miR‐3619‐3p in bile was shown to be strongly associated with the presence of BTCs. In our study, bile analysis targeting the two miRNAs showed better diagnostic value than the current serum gold standard, CA19‐9 (AUC, 0.819 vs. 0.762). Moreover, miR‐3619‐3p expression in bile is more prevalent in advanced stages of BTCs and high expression levels of miR‐3619 in bile exosomes reflect poorer prognosis of BTCs. Additionally, our clinical findings were supported by an in vitro study, which showed that exosomal miR‐451a and miR‐3619‐3p are more prominent in BTC cell lines than in a normal bile duct cell line.
Using the same protocol, the miRNA microarray analysis with serum samples could not detect any obvious miRNA markers distinguishing BTC patients from nonmalignant patients in the same discovery cohort, which appears to be inconsistent with previous studies. 12 , 13 , 14 This result might be attributed to the present small sample size for serum analysis; however, we strongly emphasize that the present findings could indicate that bile analysis has a stronger impact on liquid biopsy for BTC diagnosis. Due to the microenvironment in the biliary tract, bile must contain abundant tumor‐related miRNA in exosomes directly secreted from the primary tumor compared to peripheral blood. To the best of our knowledge, this is the first report to perform comparative verification between bile and serum samples from the same patients. Even though it is moderately invasive to collect bile samples endoscopically compared with serum sampling, we believe that bile sampling itself does not bring additional invasiveness during the ERCP procedure, and these findings could actively encourage endoscopists to investigate bile juice obtained during ERCP as a possible liquid biopsy, which is expected to be more useful for both BTC diagnosis and prognostic prediction than peripheral blood samples.
MicroRNAs are short noncoding RNAs that are associated with cancer regulation. In recent decades, numerous studies have focused on the roles of miRNAs in various cancers. Previous reports about miR‐451a and miR‐3619‐3p provide context for our new findings of bile‐derived miRNAs. Specifically, miR‐451a is recognized to be involved in a variety of cancers. It has been reported to participate in cancer‐related biological functions, such as proliferation, apoptosis, and metastasis. 15 MicroRNA‐451a is reported to act with conflicting functional genes depending on the cancer type. Some cancer types involved miR‐451a as a tumor suppressor. 16 , 17 , 18 , 19 , 20 In pancreaticobiliary diseases, miR‐451a has been mainly categorized as an oncogene. Goto et al. reported that exosomal miR‐451a in serum was upregulated in patients with PC and intraductal papillary mucinous neoplasm compared to controls. 21 Takahashi et al. and Kawamura et al. independently reported that exosomal miR‐451a was related to poor prognosis in patients with PC. 22 , 23 Chen et al. also reported that exosomal miR‐451a in serum is a diagnostic marker of PC. 24 Indeed, plasma miR‐451a is also known as a hemolytic marker. 25 However, neither the discovery nor validation analysis of this study identified other hemolytic markers (e.g., miR‐16) as significant differential expression markers. Moreover, validation analysis with cell lines revealed that miR‐451a is more abundant in exosomes from BTC cell lines compared to the normal bile duct cell line. These data supported the conclusion showing that miR‐451a is a tumor‐derived marker, rather than a hemolytic marker in this study. MicroRNA‐3619‐3p, which was the focus of attention as a diagnostic and prognostic marker for BTCs in our study, is a relative newcomer as a biomarker compared to miR‐451a. Indeed, miR‐3619‐3p was previously reported as an oncogene in some cancers, which is in line with our findings. MicroRNA‐3619‐3p was found to be more abundant in tumors of the esophagus, indicating its potential as a predictive marker for postoperative outcomes in small‐cell carcinoma of the esophagus. 26 It was also reported as an oncogene in the promotion of cancer progression by activating the Wnt/β‐catenin pathway in papillary thyroid carcinoma. 27
Accumulating evidence has shown that development of BTCs also involves dysregulation of miRNAs, 28 , 29 which has spurred clinical studies to identify novel miRNAs as biomarkers for BTCs. 30 , 31 , 32 , 33 However, although bile has been previously proven to contain extracellular vesicles, which regulate cholangiocyte proliferation, 34 studies focusing on exosomal miRNAs in human bile for pancreaticobiliary cancers are limited. Li et al. undertook qRT‐PCR analyses targeting 11 miRNAs in 96 bile samples (46 cases of cholangiocarcinoma and 50 controls), and five markers were identified (miR‐191, miR‐486‐3p, miR‐1274b, miR‐16, and miR‐484). 35 Ge et al. 36 undertook an RNA‐sequencing analysis with 10 samples (five cases of malignant biliary obstruction and five controls), followed by validation analysis using qRT‐PCR with 82 patients (37 malignant cases and 45 nonmalignant), which included cases of PC in malignant biliary obstructions. They identified two markers: miR‐483‐5p and miR‐126‐3p.
These studies used UC. Ultracentrifugation is regarded as a conventional and standard method for exosome isolation; however, multiple parameters can alter the consistency of a differential UC protocol, which would affect the consistency and reproducibility of exosomal miRNA purification under various sample conditions. 37 , 38 In our study, we utilized the AGC device for exosome collection from bile with a volume of only 200 μl, which could purify a suitable amount of exosome‐derived RNAs for the following microarray analysis. The AGC column is a useful tool for the simple and quick collection of exosomes and the extraction of miRNA from exosomes. The glass membrane column with size‐controlled MPG can simply, quickly, and efficiently collect exosomes using general equipment. Furthermore, we previously showed that the AGC column with an MPG membrane could collect exosomes simply and quickly (<10 min) from various liquid samples. 9 In addition, the AGC device could extract miRNAs from the exosomes captured in the MPG membrane with high efficiency using the miRNA extraction solution. We have also shown that the number of miRNAs detected by miRNA array in the AGC device was greater than those collected by three major methods, UC, magnetic beads, and precipitation reagent. 9 This isolation protocol could be expected to help standardize the sample quality for the following analysis targeting exosomal miRNAs.
Our study focused on the methodology for miRNA analysis. In the present study, miRNA microarray analysis by 3D‐Gene was first carried out to identify candidates among the more than 2600 genes in the discovery set composed of 11 BTCs and 9 CTRL samples. Indeed, a comprehensive analysis with this sample size (n = 20) could identify various candidates through coincidence and produce false‐negative results for some candidates. To overcome such limitations and achieve reliable markers, we undertook a validation analysis with a larger sample size (68 patients) using the same miRNA microarray analysis (3D‐Gene). These independent comprehensive investigations with discovery and validation analyses revealed reproducible diagnostic markers (miR‐451a and miR‐3619‐3p).
There are some limitations in the present study because of its small sample size. In the validation analysis for miRNAs, qRT‐PCR is considered a standard procedure. However, the outcomes of qRT‐PCR analysis rely on the choice for the internal‐control. Currently, there is no consensus in terms of an internal‐control for bile samples, which could be a critical issue for data reproducibility. In the future, validated qRT‐PCR studies based on a consensus housekeeping gene in bile would be required. In addition, heterogeneity was observed with respect to the enrolled patients. In this study for biliary diseases, although PC patients were excluded for the case group of malignant diseases, the BTC group was comprised of a discrete number of pCCA, GBC, and dCCA patients. The CTRL group consisted entirely of patients with CBD stones (n = 41) or sphincter of Oddi dysfunction (n = 2). These limitations based on the small sample size could be overcome by future analysis with a larger sample size. Clinical benefit beyond the differential diagnosis, such as treatment selection and treatment efficiency prediction, would be also expected to be clarified by future research.
In conclusion, using the convenient MPG method, the present study established novel and reliable exosomal miRNA biomarkers in bile for malignant biliary tract diseases. Using ERCP procedures to collect bile juice, which contains abundant miRNA in exosomes, bile‐derived miRNA analysis with miR‐451a and miR‐3619‐3p might become a valuable diagnostic strategy for biliary diseases and a predictive indicator for prognosis of BTCs.
FUNDING INFORMATION
Japan Society for the Promotion of Science (KAKENHI), Grant/Award Number: KK20K08291; Research Program on Hepatitis from the Japan Agency for Medical Research and Development (AMED), Grant/Award Number: 21fk0210048, 22fk0210103.
DISCLOSURE
The authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: The ethics committee at each institution approved our study protocol (#60‐16‐0069), which conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008).
Informed consent: Each patient provided written, informed consent for this study.
Animal studies: N/A.
Supporting information
Figure S1
Document S1
Table S1
ACKNOWLEDGMENTS
This study was supported by grants from the Japan Society for the Promotion of Science (KAKENHI Grant Number KK20K08291) and from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development (AMED 21fk0210048, 22fk0210103). We thank Shuji Yamazaki for supporting sample preparations and data analysis, and Kyoko Ito for performing qRT‐PCR using the samples of cell culture supernatant.
Yoshida M, Yukawa H, Hayashi K, et al. Clinical impact of bile‐derived exosomal microRNAs as novel diagnostic and prognostic biomarkers for biliary tract cancers. Cancer Sci. 2023;114:295‐305. doi: 10.1111/cas.15597
REFERENCES
- 1. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma ‐ evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428‐444. [DOI] [PubMed] [Google Scholar]
- 4. Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology. 2018;288:7‐13. [DOI] [PubMed] [Google Scholar]
- 5. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta‐analysis. Gastrointest Endosc. 2015;81:168‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raimondo F, Morosi L, Chinello C, Magni F, Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011;11:709‐720. [DOI] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281‐297. [DOI] [PubMed] [Google Scholar]
- 8. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yukawa H, Yamazaki S, Aoki K, et al. Co‐continuous structural effect of size‐controlled macro‐porous glass membrane on extracellular vesicle collection for the analysis of miRNA. Sci Rep. 2021;11:8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arakawa F, Kimura Y, Yoshida N, et al. Identification of miR‐15b as a transformation‐related factor in mantle cell lymphoma. Int J Oncol. 2016;48:485‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muramatsu‐Maekawa Y, Kawakami K, Fujita Y, et al. Profiling of serum extracellular vesicles reveals miRNA‐4525 as a potential biomarker for advanced renal cell carcinoma. Cancer Genomics Proteomics. 2021;18:253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kishimoto T, Eguchi H, Nagano H, et al. Plasma miR‐21 is a novel diagnostic biomarker for biliary tract cancer. Cancer Sci. 2013;104:1626‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang W, Deng X, Zhu T, et al. Identification of cholangiocarcinoma associated with hepatolithiasis via the combination of miRNA and ultrasound. Cancer Manag Res. 2020;12:1845‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun C, Zhu J, Wu B, et al. Diagnostic and prognostic value of microRNAs in cholangiocarcinoma: a systematic review and meta‐analysis. Cancer Manag Res. 2018;10:2125‐2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai H, Wu S. miR‐451: a novel biomarker and potential therapeutic target for cancer. Onco Targets Ther. 2019;12:11069‐11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun H, Jiang P. MicroRNA‐451a acts as tumor suppressor in cutaneous basal cell carcinoma. Mol Genet Genomic Med. 2018;6:1001‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan X, Zhao Y. miR‐451a inhibits cancer growth, epithelial‐mesenchymal transition and induces apoptosis in papillary thyroid cancer by targeting PSMB8. J Cell Mol Med. 2019;23:8067‐8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei GY, Hu M, Zhao L, Guo WS. MiR‐451a suppresses cell proliferation, metastasis and EMT via targeting YWHAZ in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2019;23:5158‐5167. [DOI] [PubMed] [Google Scholar]
- 19. Xu K, Han B, Bai Y, et al. MiR‐451a suppressing BAP31 can inhibit proliferation and increase apoptosis through inducing ER stress in colorectal cancer. Cell Death Dis. 2019;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woo JW, Choi HY, Kim M, Chung YR, Park SY. miR‐145, miR‐205 and miR‐451: potential tumor suppressors involved in the progression of in situ to invasive carcinoma of the breast. Breast Cancer. 2022;29:814‐824. [DOI] [PubMed] [Google Scholar]
- 21. Goto T, Fujiya M, Konishi H, et al. An elevated expression of serum exosomal microRNA‐191, − 21, −451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. 2018;18:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi K, Iinuma H, Wada K, et al. Usefulness of exosome‐encapsulated microRNA‐451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2018;25:155‐161. [DOI] [PubMed] [Google Scholar]
- 23. Kawamura S, Iinuma H, Wada K, et al. Exosome‐encapsulated microRNA‐4525, microRNA‐451a and microRNA‐21 in portal vein blood is a high‐sensitive liquid biomarker for the selection of high‐risk pancreatic ductal adenocarcinoma patients. J Hepatobiliary Pancreat Sci. 2019;26:63‐72. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Yao D, Chen W, et al. Serum exosomal miR‐451a acts as a candidate marker for pancreatic cancer. Int J Biol Markers. 2022;37:74‐80. [DOI] [PubMed] [Google Scholar]
- 25. Shah JS, Soon PS, Marsh DJ. Comparison of methodologies to detect low levels of hemolysis in serum for accurate assessment of serum microRNAs. PLoS One. 2016;11:e0153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okumura T, Shimada Y, Omura T, Hirano K, Nagata T, Tsukada K. MicroRNA profiles to predict postoperative prognosis in patients with small cell carcinoma of the esophagus. Anticancer Res. 2015;35:719‐727. [PubMed] [Google Scholar]
- 27. Yu S, Cao S, Hong S, et al. miR‐3619‐3p promotes papillary thyroid carcinoma progression via Wnt/β‐catenin pathway. Ann Transl Med. 2019;7:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salati M, Braconi C. Noncoding RNA in cholangiocarcinoma. Semin Liver Dis. 2019;39:13‐25. [DOI] [PubMed] [Google Scholar]
- 29. Shi T, Morishita A, Kobara H, Masaki T. The role of microRNAs in cholangiocarcinoma. Int J Mol Sci. 2021;22:7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salem PES, Ghazala RA, El Gendi AM, Emara DM, Ahmed NM. The association between circulating MicroRNA‐150 level and cholangiocarcinoma. J Clin Lab Anal. 2020;34:e23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu C, Huang F, Deng G, Nie W, Huang W, Zeng X. miR‐31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Ther Med. 2013;6:1265‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down‐regulation of miR‐214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting twist. FEBS J. 2012;279:2393‐2398. [DOI] [PubMed] [Google Scholar]
- 33. Chen L, Yan HX, Yang W, et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50:358‐369. [DOI] [PubMed] [Google Scholar]
- 34. Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990‐G999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L, Masica D, Ishida M, et al. Human bile contains microRNA‐laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60:896‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ge X, Tang L, Wang Y, et al. The diagnostic value of exosomal miRNAs in human bile of malignant biliary obstructions. Dig Liver Dis. 2021;53:760‐765. [DOI] [PubMed] [Google Scholar]
- 37. Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3:23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347‐8545327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Document S1
Table S1


