Abstract
The association between germline BRCA1 and BRCA2 pathogenic variants (mutations: gBRCAm) and ovarian cancer risk is well established. Germline testing alone cannot detect somatic BRCA1/2 pathogenic variants (sBRCAm), which is calculated based on the proportion of tumor BRCAm (tBRCAm) from tumor samples and gBRCAm. Homologous recombination deficiency (HRD) results mainly from genetic/epigenetic alterations in homologous recombination repair‐related genes and can be evaluated by genomic instability status. In Japan, the prevalence of tBRCAm, sBRCAm, and HRD remains unclear. This multicenter, cross‐sectional, observational study, CHaRacterIzing the croSs‐secTional approach to invEstigate the prevaLence of tissue BRCA1/2 mutations in newLy diagnosEd advanced ovarian cancer patients (CHRISTELLE), evaluated the prevalence of tBRCAm, sBRCAm, and HRD in tumor specimens from newly diagnosed patients with ovarian cancer who underwent gBRCA testing. Of the 205 patients analyzed, 26.8% had a tBRCAm, including tBRCA1m (17.6%) and tBRCA2m (9.3%). The overall prevalence of tBRCAm, gBRCAm, sBRCAm, and HRD‐positive status was 26.8%, 21.5%, 6.3%, and 60.0%, respectively. The calculated sBRCAm/tBRCAm ratio was 23.6% (13/55), and the prevalence of gBRCA variant of uncertain significance was 3.9%. These results suggest gBRCA testing alone cannot clearly identify the best course of treatment, highlighting the importance of sBRCA testing in Japan. The present results also suggest that testing for tBRCA and HRD should be encouraged in advanced ovarian cancer patients to drive precision medicine.
Keywords: BRCA, epithelial ovarian cancer, genetic testing, germline pathogenic variant, homologous recombination
In Japan, the prevalence of tumor BRCA variants (tBRCA mutation [tBRCAm]), somatic BRCAm (sBRCAm), and homologous recombination deficiency (HRD) remains unclear. We conducted a multicenter, cross‐sectional, observational study to evaluate the prevalence of tBRCAm, sBRCAm, and HRD in tumor specimens from newly diagnosed patients with ovarian cancer who underwent germline BRCA (gBRCA) testing. Of the 205 patients analyzed, 26.8% had a tBRCAm and 6.3% had sBRCAm, and the prevalence of HDR‐positive status was 60.0%, suggesting that testing for tBRCAm and HRD should be encouraged in advanced ovarian cancer patients to drive precision medicine.
Abbreviations
- CI
confidence interval
- FAS
full analysis set
- FFPE
formalin‐fixed paraffin‐embedded
- FIGO
International Federation of Gynecology and Obstetrics
- gBRCAm
germline BRCA mutation (pathogenic variant)
- HRD
homologous recombination deficiency
- LGR
large genomic rearrangement
- PARP
poly(ADP‐ribose) polymerase
- PPS
per‐protocol set
- Q1
first quartile
- Q3
third quartile
- sBRCAm
somatic BRCA mutation
- SD
standard deviation
- tBRCAm
tumor BRCA mutation
- VUS
variant of uncertain significance
1. INTRODUCTION
Globally, ovarian cancer is the third most frequent gynecologic malignancy and the second most frequent cause of gynecologic cancer death. 1 Despite the considerable advances in cancer screening and therapeutic and surgical treatment methods in the past few decades, the improvements in survival among patients with ovarian cancer have not been remarkable. 2 , 3 , 4 Important reasons for the lack of improvement in survival and prognosis among women with ovarian cancer are ineffective screening methods and late‐stage diagnosis due to the absence of specific symptomatology. Furthermore, recent trends observed in Japan indicate that the age‐adjusted incidence rate of ovarian cancer has increased (from 4.0 to 15.0 per 100,000 women between 1975 and 2013). 5
The association between germline BRCA1 and BRCA2 (BRCA1/2) pathogenic variants (gBRCA mutation [gBRCAm]) and ovarian cancer risk is well established. 6 These germline pathogenic variants result in a risk of developing ovarian cancer of 40%–60% with BRCA1m and 11%–27% with BRCA2m. 7 Although gBRCAm have been associated with hereditary breast and ovarian cancer syndrome, 8 roughly 35%–40% of women with gBRCAm lack any relevant family history. 9 , 10 , 11 , 12 , 13 The CHARLOTTE study found that among Japanese women with newly diagnosed ovarian cancer, the prevalence of gBRCA1/2m was 14.7%, with a higher proportion of gBRCA1m (9.9%) than gBRCA2m (4.7%). This study also reported a prevalence of gBRCAm of 4.9% in stage I–II patients and 24.1% in stage III–IV patients. The prevalence of gBRCAm was 28.5% in high‐grade serous ovarian cancer. 14 The presence of gBRCAm is associated with enhanced sensitivity to platinum‐based chemotherapy 15 , 16 and poly(ADP‐ribose) polymerase (PARP) inhibitors, as well as improved survival. 15
BRCA mutations are either germline or somatic pathogenic variants (sBRCAm). Germline pathogenic variants in BRCA1 or BRCA2 are associated with an inherited susceptibility to epithelial ovarian cancer, present in approximately 15% of patients. 17 Somatic pathogenic variants of the BRCA1 and BRCA2 genes are responsible for nonhereditary epithelial ovarian cancer. 17 Given the differences in the origin of these pathogenic variants, sBRCAm cannot be detected by germline testing methods alone. 18 , 19 The proportion of sBRCAm can be calculated by subtracting the proportion of gBRCAm obtained by germline testing from the proportion of tumor BRCAm (tBRCAm) obtained by tumor tissue sample testing. 15 , 18 , 19
Several studies have shown that ovarian cancers with sBRCAm respond similarly to platinum‐based chemotherapy and PARP inhibitors 15 , 20 , 21 , 22 , 23 as ovarian cancers with gBRCAm. For these reasons, identifying patients with ovarian cancer and sBRCAm is critical. Homologous recombination deficiency (HRD) is caused by various types of genetic/epigenetic alterations, including gBRCAm and sBRCAm, as well as promoter hypermethylation of BRCA1 and RAD51C, and other HRD‐related genetic disorders. 24 It has been reported that roughly 50% of epithelial ovarian cancers present defective DNA repair by HRD. This distinctive characteristic of epithelial ovarian cancers, particularly the high‐grade serous subtype, has important implications for disease management, as targeting HRD‐positive cells allows for cancer‐specific lethality without affecting normal cells. 24 Furthermore, HRD induces genomic instability status with extensive chromosomal copy number variations, and the HRD score can be a predictive biomarker for PARP inhibitors in ovarian cancer.
The phase III PAOLA‐1 trial in patients who were being treated with platinum chemotherapy and bevacizumab followed by bevacizumab for newly diagnosed advanced ovarian cancer recently reported that a new maintenance regimen consisting of olaparib and bevacizumab showed survival benefits in patients with HRD‐positive tumors. 25 Of note, the prevalence of different BRCAm statuses, specifically tBRCAm and sBRCAm status, and HRD scores of patients with ovarian cancer in Japan have not been clarified.
This study, CHaRacterIzing the croSs‐secTional approach to invEstigate the prevaLence of tissue BRCA1/2 mutations in newLy diagnosEd advanced ovarian cancer patients (CHRISTELLE), aimed to determine the prevalence of tBRCAm, sBRCAm, and HRD scores in tumor specimens from newly diagnosed patients with ovarian cancer. The tBRCAm‐positive status was identified using the Myriad myChoice test (Myriad Genetics, Inc.), and gBRCAm‐positive status was identified by BRACAnalysis (Myriad Genetics, Inc.). The sBRCAm‐positive status was defined as tBRCAm‐positive status without the presence of gBRCAm, that is, when BRCAm is present only in tumor cells, and not in normal cells: this was calculated by subtracting the number of patients with gBRCAm from the number of patients with tBRCAm.
2. MATERIALS AND METHODS
2.1. Study design
This was a multicenter, cross‐sectional, observational study (UMIN000039226) undertaken at 20 sites throughout Japan between March 2020 and December 2020. The study sites were selected from every region of Japan to minimize locational bias. This study consecutively enrolled patients with newly diagnosed International Federation of Gynecology and Obstetrics (FIGO) stage III–IV ovarian cancer who had undergone or were planning to undergo Myriad BRACAnalysis to detect gBRCA status. This study was carried out in accordance with the Declaration of Helsinki, Ethical Guidelines for Medical and Health Research Involving Human Subjects, and Ethical Guidelines for Human Genome and Genetic Analysis Research. The protocol was approved by the institutional review board or ethics committee at each individual study site as well as a central ethics committee (Non‐Profit Organization MINS Institutional Review Board, approval ID: MINS‐REC‐190248).
2.2. Patients
Patients enrolled in this study were women who: (i) were aged 20 years or older at the time of providing written informed consent (the age at the time of death, if the patient was deceased); (ii) had been newly diagnosed with FIGO stage III–IV epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer (or a combination of these) after January 2019; (iii) had archived formalin‐fixed paraffin‐embedded (FFPE) samples of primary or peritoneal metastatic tumors collected after 1 January 2019; (iv) had undergone or planned to undergo BRACAnalysis for gBRCA testing; and (v) provided written informed consent to participate in this study (opt‐out was applicable if the patient had died).
2.3. Data collection and measurements
Archived FFPE samples from enrolled patients were forwarded to the central laboratory for tumor BRCAm testing using the Myriad myChoice test, recently approved in Japan (November 2020). 26 Possible test results were deleterious/suspected deleterious variants, uncertain/variant of uncertain significance (VUS), or mutation absent. Histopathology was assessed by the central pathologists using serial sections of the submitted FFPE samples.
BRAC Analysis was undertaken using a blood sample to determine the number and percentage of patients with gBRCAm, and the result was obtained from patient charts. Patients' clinical background information, sample collection information, and other relevant data were also collected from their medical records. The calculation to determine the number of sBRCAm consisted of subtracting the number of deleterious variants or suspected deleterious variants obtained from BRACAnalysis (gBRCAm) from the number of deleterious variants or suspected deleterious variants according to Myriad myChoice (tBRCAm). That is, sBRCAm = tBRCAm measured by Myriad myChoice − gBRCAm measured by BRACAnalysis.
BRCA variants and HRD status were also assessed by Myriad myChoice. The HRD score (genomic instability score) was defined as the unweighted numeric sum of the loss of heterozygosity, telomeric allelic imbalance, and large‐scale state transitions. The HRD score cut‐off value was 42 based on a previous study. 27 The HRD status was considered positive if the score was ≥42 or tBRCA1m or tBRCA2m was detected by mutation analysis, and negative if the score was <42 and no tBRCA1m or tBRCA2m or unknown (failed) was detected. The HRD status was reported as unknown (failed) when the HRD score was not determined and no tBRCA1m or tBRCA2m was detected by mutation analysis.
2.4. Study end‐points
2.4.1. Primary end‐point
The primary end‐point was the prevalence of tBRCAm, including tBRCA1 and tBRCA2. A patient was considered as having a tBRCA1m and/or tBRCA2m if the gene test results showed a deleterious mutation or suspected deleterious mutation (i.e., mutation present). A patient was considered to have no tBRCAm if the gene test results showed a VUS or mutation absent.
2.4.2. Secondary end‐points
The secondary end‐points were the prevalence of gBRCAm, the prevalence of sBRCAm, and the ratio of sBRCAm to tBRCAm. A patient was considered as having a BRCA1m and/or BRCA2m if the gene test results showed a deleterious mutation or suspected deleterious mutation (i.e., mutation present). A patient was not considered to have BRCAm if the gene test results showed a VUS or uncertain clinical significance/favor polymorphism or mutation absent or not specified (i.e., no mutation present).
2.4.3. Exploratory end‐points
As exploratory end‐points, tBRCAm variants, HRD score (positive/negative), and prevalence of tBRCAm, gBRCAm, and HRD by subgroup (age, menopausal status, cancer type, histological classification, FIGO stage, medical history, family history of cancer, and smoking history) were assessed. Furthermore, concordance between tBRCAm, gBRCAm, and HRD status was assessed.
2.5. Rationale for sample size and statistical analysis
Considering that roughly 50% of the 10,000 newly diagnosed ovarian cancer patients per year 28 , 29 would be diagnosed at an advanced stage (FIGO III–IV) and an estimated prevalence of tBRCAm of 30% among patients newly diagnosed with ovarian cancer in Japan, 14 , 30 the enrollment of at least 166 patients was estimated to yield a ≥90% probability of obtaining a point estimate with an exact 95% confidence interval (CI) of ±7.5%. After considering patient withdrawal and loss to follow‐up, the planned sample size was 200 patients.
The analytical populations of the study were the full anaysis set (FAS) and the per‐protocol set (PPS). The FAS was defined as enrolled patients who underwent gBRCAm and tBRCAm tests and had histological specimens available for central pathologist confirmation. The PPS consisted of enrolled patients with valid gBRCAm and tBRCAm results and underwent histopathological assessment by the central pathologist.
Continuous variables were summarized using mean (SD), median, first quartile (Q1), and third quartile (Q3), and categorical variables were summarized using n (%). No missing data or unknown responses were counted for percentage calculations, and missing data were not imputed. The statistical software package used was SAS version 9.4 or greater (SAS Institute Inc.).
3. RESULTS
3.1. Patients
Figure 1 shows the patient disposition. Of the 217 patients enrolled, 11 were excluded, and 206 were included in the FAS. Of the patients included in the FAS, one patient with a cancer type that was not confirmed as eligible by central pathology was excluded. Thus, 205 patients comprised the PPS. The regional distributions of the patients and the numbers enrolled at each site are shown in Figure S1 and Table S1.
FIGURE 1.
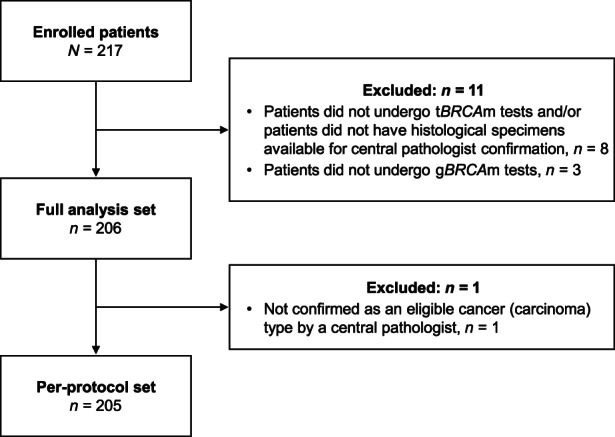
Patient disposition among study participants with advanced ovarian cancer. gBRCAm, germline BRCA mutation; tBRCAm, tumor BRCA mutation.
Table S2 summarizes the main characteristics of patients. Patients in the PPS had a mean (SD) age of 59.4 (10.9) years, with 80.0% (164/205) of patients aged 50 years or older, 73.7% (151/205) having postmenopausal status, and 19.0% (39/205) having received neoadjuvant chemotherapy. Of note, 83.4% (171/205) had epithelial ovarian cancer, followed by primary peritoneal cancer (9.8%, 20/205) and fallopian tube cancer (6.8%, 14/205). Histologically, 79.5% (163/205) had high‐grade serous ovarian carcinoma, followed by endometrioid carcinoma (12.7%, 26/205), clear cell carcinoma (6.3%, 13/205), and others (1.5%, 3/205). All patients had advanced ovarian cancer; specifically, 66.8% (137/205) had FIGO stage III, and 33.2% (68/205) had FIGO stage IV.
3.2. Study end‐points
3.2.1. Primary end‐point
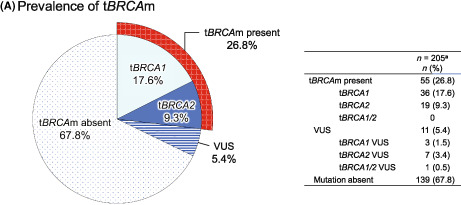
In the PPS, 26.8% (55/205) of patients had a tBRCAm. Of these, 17.6% (36/205) of patients had a tBRCA1m, and 9.3% (19/205) had tBRCA2m. However, 73.2% (150/205) had no tBRCAm present (VUS or mutation absent). Gene test results of 11/205 patients showed VUS, with a prevalence of 5.4%, and 139/205 were mutation absent, with a prevalence of 67.8%. The prevalence of tBRCA1 VUS was 1.5% (3/205), that of tBRCA2 VUS was 3.4% (7/205), and that of tBRCA1/2 VUS was 0.5% (1/205) (Figure 2A).
FIGURE 2.
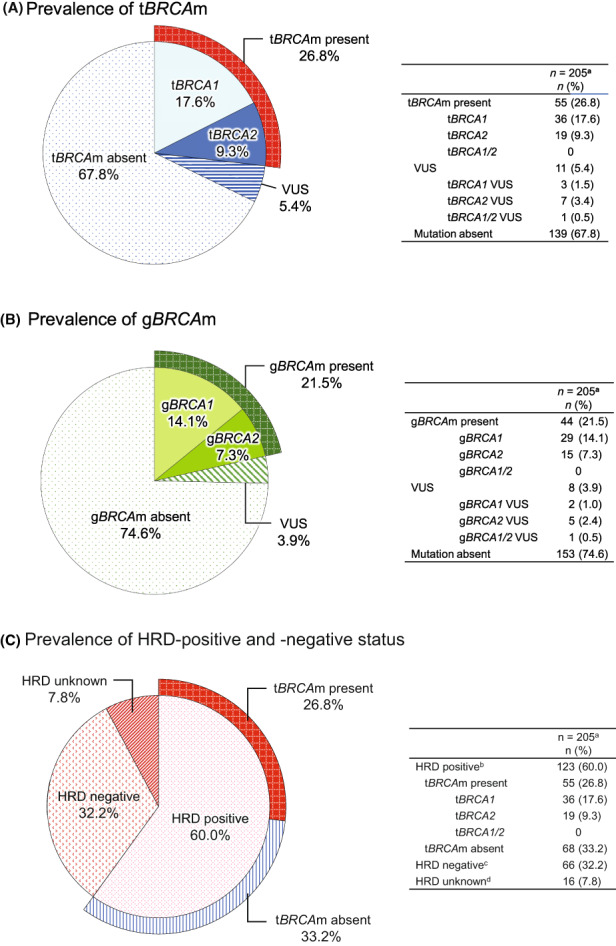
Prevalence of (A) tumor BRCA mutation (tBRCAm), (B) germline BRCA mutation (gBRCAm), and (C) homologous recombination deficiency (HRD)‐positive and ‐negative status among Japanese patients with advanced ovarian cancer. aPer‐protocol set. bHRD status was defined as positive if the HRD score was ≥42 or mutated tBRCA1 or tBRCA2 was present. cHRD status was defined as negative if the HRD score was <42, and there was no mutated tBRCA1 or tBRCA2. dHRD status was defined as unknown (failed) if the HRD score could not be determined, and tBRCA1 or tBRCA2 was not mutated or was canceled. VUS, variant of uncertain significance
3.2.2. Secondary end‐points
The prevalence of gBRCAm was 21.5% (44/205), of which gBRCA1m was 14.1% (29/205) and gBRCA2m was 7.3% (15/205); gBRCAm was absent in 74.6% (153/205). The prevalence of gBRCA VUS was 3.9% (8/205), that of gBRCA1 VUS was 1.0% (2/205), that of gBRCA2 VUS was 2.4% (5/205), and that of gBRCA1/2 VUS was 0.5% (1/205) (Figure 2B).
The concordance between gBRCAm and tBRCAm is shown in Table 1. Of the patients with tBRCA1m (n = 36), 29 had gBRCA1m. None had gBRCA2m or gBRCA1/2m, but 2 had gBRCA VUS, and 5 had gBRCAm absent status. Therefore, 7 had sBRCA1m. Of those with tBRCA2m (n = 19), 13 were positive for gBRCA2m, and 6 had gBRCAm absent status. Therefore, 6 had sBRCA2m. tBRCA VUS and gBRCAm absent was documented in 2.4% (5/205) of patients, and tBRCA absent and gBRCAm absent was documented in 66.8% (137/205) of patients.
TABLE 1.
Concordance between germline BRCA1/2 (gBRCA1/2) and tumor BRCA1/2 (tBRCA1/2) mutation status in Japanese patients with advanced ovarian cancer
| gBRCAm present | No gBRCAm present | Total | ||||
|---|---|---|---|---|---|---|
| gBRCA1 | gBRCA2 | gBRCA1/2 | gBRCA VUS | gBRCAm absent | ||
| tBRCAm present | ||||||
| tBRCA1 | 29 (14.1) | 0 (0.0) | 0 (0.0) | 2 (1.0) a | 5 (2.4) a | 36 (17.6) |
| tBRCA2 | 0 (0.0) | 13 (6.3) | 0 (0.0) | 0 (0.0) | 6 (2.9) a | 19 (9.3) |
| tBRCA1/2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| tBRCAm negative (VUS or absent) | ||||||
| tBRCA VUS | 0 (0.0) | 1 (0.5) b | 0 (0.0) | 5 (2.4) | 5 (2.4) | 11 (5.4) |
| tBRCAm absent | 0 (0.0) | 1 (0.5) b | 0 (0.0) | 1 (0.5) | 137 (66.8) | 139 (67.8) |
| Total | 29 (14.1) | 15 (7.3) | 0 (0.0) | 8 (3.9) | 153 (74.6) | 205 (100.0) |
Note: Values are n (%).
Abbreviations: VUS, variant of uncertain significance.
Somatic BRCAm (n = 13).
gBRCAm without the detection of tBRCAm (n = 2).
Overall, the prevalence of sBRCAm was 6.3% (13/205), of which sBRCA1m accounted for 3.4% (7/205), and sBRCA2m, 2.9% (6/205). The percentage of sBRCAm/tBRCAm was 23.6% (13/55), that of sBRCA1m/tBRCA1m was 19.4% (7/36), and that of sBRCA2m/tBRCA2m was 31.6% (6/19). Of note, two patients were positive for gBRCA2m without tBRCAm detection (one tBRCA VUS and one tBRCAm absent).
3.2.3. Exploratory end‐points
Table S3 summarizes the variant description of tBRCAm. Among the 36 (17.6%) patients with variant type tBRCA1m, 34 (16.6%) had sequencing variants, of which the most common variants were nonsense variants (7.3%, 15/205) and frameshift variants (6.8%, 14/205). Among the 19 (9.3%) patients with tBRCA2m, 19 (9.3%) had sequencing variants, of which the most common variants were nonsense variants (3.9%, 8/205) and frameshift variants (3.9%, 8/205).
Regarding the HRD prevalence, 60.0% (123/205) of patients were HRD positive, 32.2% (66/205) were HRD negative, and 7.8% (16/205) were HRD unknown (failed) in the PPS (Figure 2C).
A swarm plot with box chart shows the distribution of the genomic instability score for patients with tBRCAm present, absent, or VUS (Figure 3). The median genomic instability score was 66.0 in patients with tBRCAm present, 42.0 in those with tBRCAm absent, and 61.0 in those with tBRCA VUS. Figure S2 shows the distribution of the genomic instability score for patients with gBRCAm present, absent, or VUS. The median genomic instability score was 65.5 in patients with gBRCAm present, 45.0 in those with gBRCAm absent, and 54.0 in those with gBRCA VUS.
FIGURE 3.
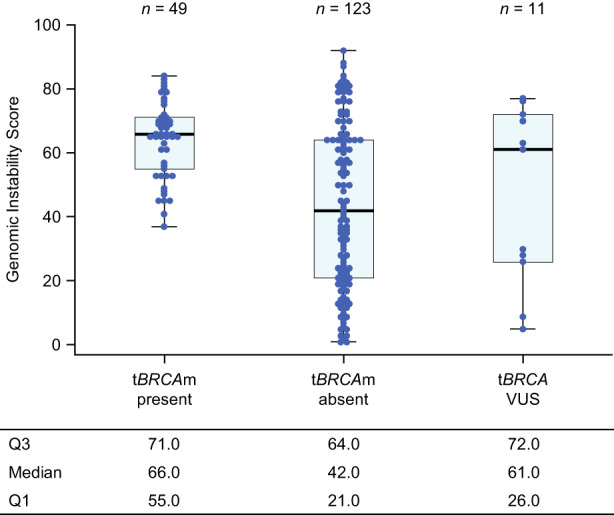
Genomic instability score distribution by tumor BRCA mutation (tBRCAm) present, absent, or variant of uncertain significance (VUS) status in the per‐protocol set of Japanese patients with advanced ovarian cancer. Circles indicate each patient by mutation. In the box plot, the box height indicates the interquartile range, with the first quartile (Q1) at the bottom and third quartile (Q3) at the top. The midline indicates the median.
Table 2 shows the prevalence of tBRCAm, gBRCAm, and HRD by patient and ovarian cancer characteristics. Regarding patient characteristics, numerically higher proportions of patients aged less than 50 years had tBRCAm and gBRCAm and were HRD positive. In this age group, the prevalence of tBRCAm was 36.6% (15/41), and that of gBRCAm was 29.3% (12/41), while 68.3% (28/41) were HRD positive. Among premenopausal women, the prevalence of tBRCAm, gBRCAm, and HRD positivity was 37.3% (19/51), 31.4% (16/51), and 66.7% (34/51), respectively, which were numerically higher than in postmenopausal women. By type of cancer, the prevalence of tBRCAm was highest among patients with epithelial ovarian cancer (28.1%, 48/171), followed by fallopian tube cancer (21.4%, 3/14) and primary peritoneal cancer (20.0%, 4/20) with similar prevalence. gBRCAm was more prevalent among patients with epithelial ovarian cancer (22.8%, 39/171) and fallopian tube cancer (21.4%, 3/14) than those with primary peritoneal cancer (10.0%, 2/20). No numerical differences were observed in the prevalence of HRD‐positive status across all three cancer types. By cancer histology, the prevalence of tBRCAm, gBRCAm, and HRD‐positive status were highest in high‐grade serous carcinoma (30.1% [49/163], 24.5% [40/163], and 66.9% [109/163], respectively), followed by endometrioid carcinoma (15.4% [4/26], 11.5% [3/26], and 34.6% [9/26]), and clear cell carcinoma (7.7% [1/13], 7.7% [1/13], and 23.1% [3/13]). No numerical differences were observed in the prevalence of tBRCAm, gBRCAm, or HRD‐positive status between FIGO stages III and IV.
TABLE 2.
Prevalence of tumor BRCA mutation (tBRCAm), germline BRCA mutation (gBRCAm), and homologous recombination deficiency (HRD) by patient and ovarian cancer characteristics in the per‐protocol set
| n = 205 a | tBRCAm | gBRCAm | HRD | |||||
|---|---|---|---|---|---|---|---|---|
| Present | Not present b | Present | Not present b | Positive | Negative | Failed | ||
| Patient factors | ||||||||
| Age, years | ||||||||
| <50 | 41 | 15 (36.6) | 26 (63.4) | 12 (29.3) | 29 (70.7) | 28 (68.3) | 10 (24.4) | 3 (7.3) |
| ≥50 to <65 | 84 | 24 (28.6) | 60 (71.4) | 21 (25.0) | 63 (75.0) | 54 (64.3) | 23 (27.4) | 7 (8.3) |
| ≥65 | 80 | 16 (20.0) | 64 (80.0) | 11 (13.8) | 69 (86.3) | 41 (51.3) | 33 (41.3) | 6 (7.5) |
| Menopausal status | ||||||||
| Postmenopausal | 151 | 36 (23.8) | 115 (76.2) | 28 (18.5) | 123 (81.5) | 89 (58.9) | 52 (34.4) | 10 (6.6) |
| Premenopausal | 51 | 19 (37.3) | 32 (62.7) | 16 (31.4) | 35 (68.6) | 34 (66.7) | 11 (21.6) | 6 (11.8) |
| Unknown | 3 | 0 (0.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) |
| Ovarian cancer factors | ||||||||
| Cancer type | ||||||||
| Epithelial ovarian | 171 | 48 (28.1) | 123 (71.9) | 39 (22.8) | 132 (77.2) | 106 (62.0) | 52 (30.4) | 13 (7.6) |
| Primary peritoneal | 20 | 4 (20.0) | 16 (80.0) | 2 (10.0) | 18 (90.0) | 10 (50.0) | 8 (40.0) | 2 (10.0) |
| Fallopian tube | 14 | 3 (21.4) | 11 (78.6) | 3 (21.4) | 11 (78.6) | 7 (50.0) | 6 (42.9) | 1 (7.1) |
| Histology | ||||||||
| High‐grade serous carcinoma | 163 | 49 (30.1) | 114 (69.9) | 40 (24.5) | 123 (75.5) | 109 (66.9) | 41 (25.2) | 13 (8.0) |
| Endometrioid carcinoma | 26 | 4 (15.4) | 22 (84.6) | 3 (11.5) | 23 (88.5) | 9 (34.6) | 15 (57.7) | 2 (7.7) |
| Clear cell carcinoma | 13 | 1 (7.7) | 12 (92.3) | 1 (7.7) | 12 (92.3) | 3 (23.1) | 9 (69.2) | 1 (7.7) |
| Others | 3 | 1 (33.3) | 2 (66.7) | 0 (0.0) | 3 (100) | 2 (66.7) | 1 (33.3) | 0 (0.0) |
| FIGO stage | ||||||||
| III | 137 | 36 (26.3) | 101 (73.7) | 28 (20.4) | 109 (79.6) | 85 (62.0) | 42 (30.7) | 10 (7.3) |
| IV | 68 | 19 (27.9) | 49 (72.1) | 16 (23.5) | 52 (76.5) | 38 (55.9) | 24 (35.3) | 6 (8.8) |
Note: Values are n (%) unless otherwise stated.
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Per‐protocol set.
No mutation present was defined as having a gene test result of variant of uncertain significance or mutation absent.
Table S4 shows the prevalence of tBRCAm by patients with a medical history. Among 18 patients with a medical history of breast cancer, 50.0% (9/18) had tBRCA1m and 5.6% (1/18) had tBRCA2m.
Table S5 shows the prevalence of tBRCAm by family history of cancer. Of 30 patients with a family history of breast cancer, 40.0% (12/30) had tBRCA1m and 16.7% (5/30) had tBRCA2m. Of 17 patients with a family history of ovarian cancer, 58.8% (10/17) had tBRCA1m and 17.6% (3/17) had tBRCA2m.
4. DISCUSSION
To the best of our knowledge, this is the first observational study to determine the prevalence of tBRCAm, sBRCAm, and HRD‐positive status among Japanese patients with advanced ovarian cancer. In this study, the prevalence of tBRCAm was 26.8%, that of gBRCAm was 21.5%, and that of sBRCAm was 6.3%; the calculated sBRCAm / tBRCAm ratio was 23.6%. The HRD prevalence among Japanese patients with advanced ovarian cancer was 60.0%; HRD was negative in 32.2% and unknown in 7.8% of patients. The median HRD score in HRD‐positive and tBRCAm‐negative patients was 42.0, indicating that half of this cohort was HRD positive, which would be missed by tBRCAm testing alone. Therefore, it is important to assess HRD status regardless of the presence or absence of tBRCAm. The prevalence of tBRCAm (26.8%) found in this study is consistent with previously reported prevalence data ranging from 19% to 24%. 15 , 31 , 32 Of note, these studies had heterogeneous patient populations in terms of stage and primary or recurrent disease. Additionally, the cohort in this study had already undergone gBRCA testing in the real‐world setting, and selection bias for gBRCA testing is possible. A similar prevalence was reported in other phase III multinational trials, such as the PAOLA‐1 trial (prevalence of tBRCAm of 29.9%), 25 the PRIMA trial (prevalence of tBRCAm of 30.4%), 33 and the VELIA trial (prevalence of tBRCAm of 26.1%), 34 which included a large number of patients with stage III and IV ovarian cancer. However, the inclusion criteria in some of these studies were defined by platinum sensitivity and other factors that might have affected the rate of HRD and tBRCAm. The prevalence of gBRCAm (21.5%) was also consistent with previous reports, including our Japanese study, the advanced ovarian cancer cohort in the CHARLOTTE study (24.1%), 14 and the PAOLA‐1 trial (19.3%) in a French cohort, 18 among others. 15 , 32
The prevalence of sBRCAm (6.3%) observed in the present Japanese cohort is also supported by the prevalence reported in previous overseas studies, ranging from 3% to 7.5%. 15 , 18 , 19 , 30 , 32 Overall, 13 of 55 patients (23.6%) with a tBRCAm had the mutation in somatic tumor tissues.
Results from the tumor tissue testing show that HRD‐positive status was 60.0%; this is consistent with the results of the PRIMA and PAOLA‐1 trials, 25 , 33 which reported HRD‐positive status in approximately 50% of patients. Of note, the percentage of patients with both HRD‐positive and tBRCAm‐negative status overall was 33.2%, which is higher than previously reported (PRIMA: niraparib, 19.5% [95/487] and placebo, 22.4% [55/246]; PAOLA‐1: olaparib + bevacizumab, 19.4% [104/537] and placebo + bevacizumab, 23.4% [63/269]). 25 , 33 Although our study includes patients regardless of platinum sensitivity, this suggests that the ratio of tBRCAm‐negative/HRD‐positive patients might be higher in Japan. As two‐thirds of the patients with HRD‐positive status were not identified by gBRCA testing, these patients would likely not receive PARP inhibitors, even though they are recommended in the first‐line setting for ovarian cancer with gBRCAm and HRD‐positive status and tBRCAm.
For two patients, gBRCA testing was positive, whereas tBRCA testing was negative. As per the concordance of data between tBRCA and gBRCA status in the PAOLA‐1 trial, 18 one patient had a positive gBRCA test, but a negative tBRCA test. The reason for this discrepancy could be that different methods were used to assess the status of each; gBRCAm status was analyzed by BRACAnalysis using Sanger sequencing and multiplex PCR, whereas tBRCAm and HRD score were analyzed using Myriad myChoice, which is based on next‐generation sequencing.
The data from this patient revealed the presence of a large genomic rearrangement (LGR) consisting of the deletion of exons 1 and 2 of the BRCA1 gene. In the CHRISTELLE study, an uncharacterized LGR of BRCA2 was observed in one of the patients who was gBRCA2m positive. Additionally, one other patient had an LGR of BRCA1. A previous study suggested that chemotherapy can affect intratumoral heterogeneity and HRD prevalence. 35 However, these patients did not receive neoadjuvant chemotherapy, and it is unlikely that chemotherapy affected their tBRCA status. Therefore, the presence of LGR might affect the detection of tBRCAm, and the presence of gBRCAm could be overlooked without gBRCA testing. Again, this might be because of the different methods used in each test. Additionally, the timings of sample collections were not always simultaneous for gBRCAm and tBRCAm, and the blood sample collection for gBRCAm was flexible in this study.
In 7.8% of patients in this study, HRD presence was unknown or failed. The failure rate was relatively lower than reported previously, with 18% in the PAOLA‐1 trial 25 and 15% in the PRIMA trial. 33 This finding suggests that stocked samples are appropriate for HRD testing in a real‐world setting.
The distribution of the genomic instability score sorted by patients with mutations, without mutations, or VUS in tBRCA and gBRCA showed some tendencies in each group. Among patients with tBRCAm present (median HRD score, 66.0), those with a genomic instability score ≥42 represented the majority of the population, which is consistent with previously reported data. 27 Among patients with tBRCAm absent, the distribution of genomic instability scores was bimodal and varied widely. It could be that several patients were categorized as HRD negative due to having a genomic instability score just below the cut‐off value. Furthermore, some patients with tBRCAm absent had high genomic instability scores, indicating that a high HRD prevalence did not necessarily predict BRCAm. For patients with gBRCAm absent, the genomic instability scores also varied widely and had a bimodal distribution. Furthermore, some samples presented a high genomic instability score despite gBRCA being negative. These high HRD scores in patients with no BRCAm could indicate genomic instabilities in genes other than BRCA, or instabilities that might not yet be detected as mutations in BRCA. Based on these findings, determining HRD prevalence might lead to the treatment of advanced ovarian cancer patients despite the absence of gBRCAm.
One of the main strengths of this study is that it achieved consecutive enrollment at 20 sites with well‐balanced locations across Japan, which helps minimize bias due to location. Furthermore, 94.9% of patients who were enrolled in this study were included in analysis. As 99.0% of cases were surviving patients, there was no bias toward death cases. In the central pathology assessment, approximately 70% of the cases were consistent among the three pathologists, and the remaining cases were consistent as a result of their consultation. In terms of the external validity, the demographics and background characteristics of patients enrolled in the CHRISTELLE study were consistent with those reported by the National Cancer Registration. 36 Furthermore, the target sample size was met, ensuring an accurate estimation of the results.
However, this study has some limitations. Selection bias could have occurred as physicians may have suspected hereditary breast and ovarian cancer syndrome for many of the patients enrolled, because eligible patients were to have undergone or were scheduled to undergo BRACAnalysis. The timing of gBRCAm testing and tBRCAm/HRD testing was not controlled by the study protocol. The status of gBRCAm was obtained from the result of the Myriad BRACAnalysis test, undertaken for patients at each site in usual practice. This means that the timings of Myriad BRACAnalysis tests varied among patients, which could have caused a variation in the results of the gBRCAm analysis (e.g., variant first judged as “VUS” might have been changed to “deleterious” or “suspected deleterious” after the Myriad BRACAnalysis test). Additionally, a change of mutation status between two gene tests within a patient could not be analyzed in this study. However, samples for gBRCAm testing and for tBRCAm/HRD testing were collected from January 2019 to November 2020; therefore, testing was not separated by more than 2 years. We did not evaluate genetic sequences from gBRCA. Thus, the concordance of the mutated sequence between gBRCA and tBRCA was not evaluated. In addition, this study did not measure the presence of homologous recombination repair mutations or promoter hypermethylation of BRCA1 or RAD51C, which can cause HRD. Further studies will be necessary to investigate these aspects of ovarian cancer in Japan.
In this study, tBRCAm was observed in 55 (26.8%) out of 205 Japanese patients with FIGO stage III and IV ovarian cancer, gBRCAm was observed in 44 (21.5%), and HRD‐positive status was observed in 123 (60.0%). The prevalence of sBRCAm was 6.3%. Notably, one in four to five patients with ovarian cancer and tBRCAm could be positive for sBRCAm. Furthermore, most HRD‐positive patients were not identified by gBRCA testing. These results suggest gBRCA testing alone cannot clearly identify the best course of treatment, highlighting the importance of sBRCA testing in Japan. The present results also suggest that testing for tBRCA and HRD should be encouraged in advanced ovarian cancer patients to drive precision medicine.
AUTHOR CONTRIBUTIONS
All authors contributed to the conceptualization, methodology, and writing, reviewing and editing the manuscript. Katsutoshi Oda, Daisuke Aoki, Hitoshi Tsuda, Hiroshi Nishihara, Muneaki Shimada, and Takayuki Enomoto contributed to data presentation and visualization. Hisanori Aoyama contributed to project administration and writing the original draft. Hyoe Inomata contributed to funding acquisition. Takayuki Enomoto supervised the research planning and execution.
FUNDING INFORMATION
This study was supported by AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., who are codeveloping olaparib.
DISCLOSURE
Hiroshi Nishihara reports remuneration from Social Medical Corporation Seiwakai and SECOM General Insurance Co., Ltd. for his role as an officer or advisor, and from NLAC Co., Ltd. to his family members, research funding from Mitsubishi Space Software Co., Ltd. and NanoCarrier Co., Ltd., and scholarship endowments from Sanofi K.K., Princess Takamatsu Cancer Research Fund, and Social Medical Corporation Koseikai Kizawa Memorial Hospital. Katsutoshi Oda reports lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and research funding from AstraZeneca K.K. and Konica Minolta Japan, Inc. Daisuke Aoki reports lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and research funding from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and MSD K.K. Takayuki Enomoto reports lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and MSD K.K. Hisanori Aoyama and Hyoe Inomata are employees of AstraZeneca K.K. The other authors have no conflicts of interest to disclose. AstraZeneca K.K. funded the study and was involved in the study design; data collection, analysis, and interpretation; and in the review of the manuscript. The authors had full access to the data and take responsibility for the decision to publish the manuscript. Katsutoshi Oda, Hitoshi Tsuda, and Hiroshi Nishihara are board members of Cancer Science.
ETHICS STATEMENT
Approval of the research by an institutional review board: This study was approved by the ethics committee of each participating institution as well as a central independent ethics committee (Non‐Profit Organization MINS Institutional Review Board, approval ID: MINS‐REC‐190248) and performed in accordance with the Declaration of Helsinki, the ethical guidelines specified in the International Conference on Harmonization Guideline for Good Clinical Practice, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and the Ethical Guidelines for Human Genome and Genetic Analysis Research.
Informed consent: All participants gave informed consent prior to enrollment.
Registry and registration no. of the study: UMIN Clinical Trial Registry (Identifier: UMIN000039226).
Animal studies: N/A.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors wish to thank the investigators who participated in this study (Table S6), and Yuko Sasajima, MD, from the Department of Diagnostic Pathology, Teikyo University Hospital and Hiroshi Yoshida, MD, from the Department of Diagnostic Pathology, National Cancer Center Hospital for their support as central reviewers. The authors also wish to thank Keyra Martinez Dunn, MD, of Edanz, Japan, for providing medical writing support, which was funded by AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., who are codeveloping olaparib, through EMC K.K., Japan, in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Oda K, Aoki D, Tsuda H, et al. Japanese nationwide observational multicenter study of tumor BRCA1/2 variant testing in advanced ovarian cancer. Cancer Sci. 2023;114:271‐280. doi: 10.1111/cas.15518
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Lee JY, Kim S, Kim YT, et al. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer. 2018;18:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RFPM. No improvement in long‐term survival for epithelial ovarian cancer patients: a population‐based study between 1989 and 2014 in The Netherlands. Eur J Cancer. 2018;88:31‐37. [DOI] [PubMed] [Google Scholar]
- 4. Wu SG, Wang J, Sun JY, He ZY, Zhang WW, Zhou J. Real‐world impact of survival by period of diagnosis in epithelial ovarian cancer between 1990 and 2014. Front Oncol. 2019;9:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population‐based cancer registries for the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884‐891. [DOI] [PubMed] [Google Scholar]
- 6. Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 2009;3:138‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280‐304. [DOI] [PubMed] [Google Scholar]
- 8. Japan Society of Gynecologic Oncology . The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer (in Japanese). Japan, 2020. Accessed October 21, 2021. https://jsgo.or.jp/guideline/ransou/2020/pdf/ransou02.pdf [DOI] [PMC free article] [PubMed]
- 9. Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation‐positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654‐2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobi CE, van Ierland Y, van Asperen CJ, et al. Prediction of BRCA1/2 mutation status in patients with ovarian cancer from a hospital‐based cohort. Genet Med. 2007;9:173‐179. [DOI] [PubMed] [Google Scholar]
- 11. Malander S, Ridderheim M, Måsbäck A, et al. One in 10 ovarian cancer patients carry germ line BRCA1 or BRCA2 mutations: results of a prospective study in southern Sweden. Eur J Cancer. 2004;40:422‐428. [DOI] [PubMed] [Google Scholar]
- 12. Risch HA, McLaughlin JR, Cole DEC, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin‐cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694‐1706. [DOI] [PubMed] [Google Scholar]
- 13. Soegaard M, Kjaer SK, Cox M, et al. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population‐based series of ovarian cancer cases from Denmark. Clin Cancer Res. 2008;14:3761‐3767. [DOI] [PubMed] [Google Scholar]
- 14. Enomoto T, Aoki D, Hattori K, et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross‐sectionaL approach to Ovarian cancer geneTic TEsting of BRCA (CHARLOTTE). Int J Gynecol Cancer. 2019;29:1043‐1049. [DOI] [PubMed] [Google Scholar]
- 15. Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pothuri B. BRCA1‐ and BRCA2‐related mutations: therapeutic implications in ovarian cancer. Ann Oncol. 2013;24(Suppl 8):viii22‐viii27. [DOI] [PubMed] [Google Scholar]
- 17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27:1449‐1455. [DOI] [PubMed] [Google Scholar]
- 18. Callens C, Vaur D, Soubeyran I, et al. Concordance between tumor and germline BRCA status in high‐grade ovarian carcinoma patients in the phase III PAOLA‐1/ENGOT‐ov25 trial. J Natl Cancer Inst. 2021;113:917‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cunningham JM, Cicek MS, Larson NB, et al. Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci Rep. 2014;4:4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390:1949‐1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dougherty BA, Lai Z, Hodgson DR, et al. Biological and clinical evidence for somatic mutations in BRCA1 and BRCA2 as predictive markers for olaparib response in high‐grade serous ovarian cancers in the maintenance setting. Oncotarget. 2017;8:43653‐43661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum‐sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154‐2164. [DOI] [PubMed] [Google Scholar]
- 23. Yates MS, Timms K, Daniels MS, et al. Evaluation of BRCA1/2 and homologous recombination defects in ovarian cancer and impact on clinical outcomes. J Clin Oncol. 2017;35(15_Suppl):5511. [Google Scholar]
- 24. Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ray‐Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first‐line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416‐2428. [DOI] [PubMed] [Google Scholar]
- 26. Myriad Genetics, Inc . Myriad genetics receives additional reimbursement for myChoice® diagnostic system in Japan [press release]. Accessed February 13, 2022. https://www.globenewswire.com/news‐release/2021/03/24/2198946/15459/en/Myriad‐Genetics‐Receives‐Additional‐Reimbursement‐for‐myChoice‐Diagnostic‐System‐in‐Japan.html
- 27. Telli ML, Timms KM, Reid J, et al. Homologous recombination deficiency (HRD) score predicts response to platinum‐containing neoadjuvant chemotherapy in patients with triple‐negative breast cancers. Clin Cancer Res. 2016;22:3764‐3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. Int J Gynaecol Obstet. 2003;83:135‐166. [DOI] [PubMed] [Google Scholar]
- 29. The Japan Society of Obstetrics and Gynecology . Annual patients report 2018. Acta Obstet Gynaecol Jpn. 2020;72:800‐856. [Google Scholar]
- 30. Kanchi KL, Johnson KJ, Lu C, et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat Commun. 2014;5:3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hennessy BTJ, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570‐3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. González‐Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391‐2402. [DOI] [PubMed] [Google Scholar]
- 34. Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first‐line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403‐2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takaya H, Nakai H, Sakai K, et al. Intratumor heterogeneity and homologous recombination deficiency of high‐grade serous ovarian cancer are associated with prognosis and molecular subtype and change in treatment course. Gynecol Oncol. 2020;156:415‐422. [DOI] [PubMed] [Google Scholar]
- 36. National Cancer Center/Cancer Control Information Center . Cancer treatment cooperation base hospitals, etc. In‐hospital cancer registration 2019 national aggregate report (in Japanese). Japan, 2021. Accessed October 21, 2021. https://ganjoho.jp/public/qa_links/report/hosp_c/pdf/2019_report.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


