Abstract
Lung cancer is one of the leading causes of death among cancer patients worldwide. Carbon‐ion radiotherapy is a radical nonsurgical treatment with high local control rates and no serious adverse events. N6‐methyladenosine (m6A) modification is one of the most common chemical modifications in eukaryotic messenger RNA (mRNA) and has important effects on the stability, splicing, and translation of mRNAs. Recently, the regulatory role of m6A in tumorigenesis has been recognized more and more. However, the dysregulation of m6A and its role in carbon‐ion radiotherapy of non‐small‐cell lung cancer (NSCLC) remains unclear. In this study, we found that the level of methyltransferase‐like 3 (METTL3) and its mediated m6A modification were elevated in NSCLC cells with carbon‐ion radiotherapy. Knockdown of METTL3 in NSCLC cells impaired proliferation, migration, and invasion in vitro and in vivo. Moreover, we found that METTL3‐mediated m6A modification of mRNA inhibited the decay of H2A histone family member X (H2AX) mRNA and enhanced its expression, which led to enhanced DNA damage repair and cell survival.
Keywords: carbon‐ion radiotherapy, H2AX, methyltransferase‐like 3, N6‐methyladenosine, non‐small‐cell lung cancer
The level of methyltransferase‐like 3 (METTL3) and its mediated N6‐methyladenosine (m6A) modification were elevated in non‐small‐cell lung cancer (NSCLC) cells with carbon‐ion radiotherapy. Knockdown of METTL3 in NSCLC cells impaired proliferation, migration, and invasion in vitro and in vivo. METTL3‐mediated m6A modification of messenger RNA (mRNA) inhibited the decay of H2AX mRNA and enhanced its expression, which led to enhanced DNA damage repair and cell survival.

1. INTRODUCTION
Lung cancer is one of the leading causes of death among cancer patients worldwide. 1 , 2 Unfortunately, the survival rate is extremely low due to chemo‐resistance and metastasis. 3 Although ionizing radiation is recognized as the standard radiation therapy for non‐small‐cell lung cancer (NSCLC), many reports show an increase in malignant characteristics after gamma irradiation. However, these limitations have been overcome in hadronic therapy, particularly the use of carbon ions (12C), which has been established as a promising approach for the treatment of NSCLC. 4 , 5 , 6 , 7
Carbon‐ion radiotherapy is a radical nonsurgical treatment with high local control rates and no serious adverse events. 8 Due to the Bragg peak, carbon ions have good dose localization, which can reduce the radiation dose to the surrounding normal tissues. In addition, the carbon‐ion beam has high bioavailability and is beneficial for tumor control. 9 Previously, the safety and efficacy of carbon‐ion radiotherapy in the treatment of early and locally advanced lung cancer have been reported. 10 However, whether or not NSCLC cells have developed resistance to carbon ion radiotherapy has not been reported.
Biochemical evidence from 40 years ago indicated that mammalian messenger RNA (mRNA) contains N6‐methyl‐adenosine (m6A), occurring mainly near the 3′ end of mRNA. Recent localization of the m6A site in mammalian cell transcriptome has shown that thousands of mRNAs are modified on a consistent sequence motif, with the m6A peak located on the mRNA genomic body, usually near the termination codon. 11 , 12 , 13 , 14 , 15 These methylation markers can be dynamically regulated, and the m6A pattern varies among cell types. Methyltransferase‐like 3 (METTL3) was initially identified as the methyltransferase responsible for m6A modification. 16 , 17 , 18 In line with the dynamic control of m6A RNA modification, two specific demethylases also localized to nuclear spots, fat mass and obesity‐associated protein (FTO) and ALKB homolog 5 (ALKBH5), have been identified. In addition, as the most important component of the “writer” complex, METTL3 plays a very important role in the regulation of gene expression by affecting RNA stability, mRNA degradation, and translation. 19 Therefore, when METTL3 is dysfunctional, it can lead to the development and progression of human cancers. Some studies have shown that m6A mRNA methylation leads to many mammalian tumors and diseases by regulating cell differentiation, tissue development, and tumorigenesis by METTL3. For example, the deletion of the METTL3 gene leads to the termination of early embryonic development, indicating that m6A methylation modification plays an important role in the development of mammalian embryos. 20 Recently, it has been reported that METTL3 promotes tumor growth, metastasis, and drug/radiotherapy resistance in human cancers. 21 , 22 , 23 , 24 , 25 However, for NSCLC, its biological roles in carbon‐ion radiotherapy need to be further explored.
In this study, we found that the level of METTL3 and its mediated m6A modification were elevated in NSCLC cells with carbon‐ion radiotherapy. Knockdown of METTL3 in NSCLC cells impaired proliferation, migration, and invasion in vitro and in vivo. Moreover, we found that METTL3‐mediated m6A modification mRNA inhibited the decay of H2AX mRNA and enhanced its expression, which led to enhanced DNA damage repair and cell survival.
2. MATERIAL AND METHODS
2.1. Cell lines and culture
Human NSCLC cell lines A549 and H1975 were purchased from American Type Culture Collections. They were cultivated in DMEM medium supplemented with 10% FBS (Gibco) and penicillin/streptomycin (100 mg/ml; Gibco). Culture dishs were maintained at 37°C in a humid incubator with 5% CO2.
2.2. Carbon‐ion irradiation
Human NSCLC cell lines A549 and H1975 cells were plated in T25 flasks (Corning). The IONTRIS intensity‐modulated raster scan system with energy 333.82 MeV/u was used to irradiate the flasks at the Shanghai Proton and Heavy Ion Center (SPHIC) Shanghai, China, as previously described. The linear energy transfer (LET) was approximately 29.1351 keV/μm for carbon‐ion radiation. The beam line was horizontal, and the Bragg peak of carbon‐ion radiation was adjusted to the solid surface where cell attached. The irradiation doses were all physical doses. All the cells, including the mock‐irradiated ones, were then washed with fresh medium and incubated at 37°C with 5% CO2.
2.3. RNA m6A methylation quantification
An m6A RNA Methylation Assay Kit (ab185912; Abcam) was used to evaluate the content of m6A in total RNA as previously reported. 24
2.4. Dot blot assay
Dot blot assays were performed as previously reported. 11 The poly(A) enriched RNAs (400 ng) were double‐diluted and spotted onto a nylon membrane (GE Healthcare). Briefly, the membranes were then UV crosslinked, blocked, incubated with m6A antibody and horseradish peroxidase‐conjugated anti‐rabbit IgG, and finally detected with a 3,3′‐diaminobenzidine peroxidase substrate kit. The same 400 ng poly(A) + RNAs were spotted on the membrane, stained with 0.02% methylene blue in 0.3 M sodium acetate for 2 h, and washed with ribonuclease‐free water for 5 h.
2.5. Quantitative real‐time PCR
Total RNA was lysed using TRIzol reagent and used for the synthesis of cDNA with a One‐Step RT‐PCR Kit (Thermo Fisher Scientific). Quantitative real‐time PCR (qRT‐PCR) was performed using the ABI ViiA 7 system (Applied Biosystems). GAPDH was used as a housekeeping gene. Relative gene expression was calculated by the 2−△△CT cycle threshold method.
2.6. Cell transfection and stable cell lines construction
To establish stable METTL3 knockdown models, the lentivirus vector containing shMETTL3 (Lv‐shMETTL3) was amplified and cloned by Simobio. To obtain stable METTL3 overexpression cell lines, METTL3 cDNA was amplified and subcloned into the lentiviral vector (Simobio). Recombinant lentiviruses expressing METTL3 or NC, shMETTL3 or shNC were obtained from Simobio. The human NSCLC cell lines A549 and H1975 cells were transfected with concentrated lentiviruses, and stable cell lines were selected by treatment with puromycin for 2 weeks. 26
2.7. Colony formation assay
NSCLC cell lines (A549 and H1975) were seeded into plates at a density of 200 cells per well and cultured at 37°C for about 1 week until visible colonies formed. The colonies were fixed with 4% paraformaldehyde for 30 min, followed by staining with 0.1% crystal violet for 30 min. The assay was independently conducted three times.
2.8. Xenograft experiments
For in vivo studies, 4–6‐week‐old male BALB/c nude mice were purchased from the Shanghai Laboratory Animal Center of China. A549 cells (3 × 105 cells in 200 μl PBS) were subcutaneously injected into the nude mice to establish tumors. When the tumors had grown to almost 100 mm3, a 5‐GyE dosage of carbon‐ion irradiation was applied and the mice were examined every 3 days, then sacrificed 30 days after the injection. Tumor size was measured using digital calipers, and tumor volume was calculated with the formula volume = 0.5 × width2 × length.
2.9. Immunohistochemistry
Immunohistochemistry (IHC) assay was performed to evaluate METTL3 and H2AX expression in the tumor tissues. Briefly, paraffin‐embedded tumor slides were dried at 90°C for 4 h, dewaxed in xylene, and then rehydrated in graded ethanol solutions. Cooled tissue sections were immersed in 0.3% hydrogen peroxide solution for 15 min to block endogenous peroxidase activity, followed by rinsing with PBS for 5 min and blocking with 3% BSA solution at room temperature for 30 min. After washing with PBS, sections were incubated with the primary antibody (rabbit anti‐human METTL3 [ab195352, 1:200; Abcam] and H2AX [ab229914, 1:200; Abcam]) monoclonal antibodies at 4°C overnight. The next day, sections were washed with PBS and incubated with HRP‐labeled secondary antibody at 37°C for 30 min. Sections were then dehydrated, cleared, and mounted. Notably, diaminobenzene was used as the chromogen and hematoxylin was used as the nuclear counterstain.
2.10. Statistical analysis
Data were expressed as the mean ± SEM. The unpaired, two‐tailed t‐test was used for comparisons between two groups. For multiple comparisons, ANOVA or repeated ANOVA followed by the Bonferroni post hoc test was used with GraphPad Prism version 6.0 software. A P value <0.05 was considered statistically significant.
3. RESULT
3.1. Carbon‐ion radiation dose‐dependently enhanced the METTL3 mediated m6A methylation in A549 cells.
To evaluate the effect of carbon‐ion radiation exposure on m6A RNA modification, human NSCLC cell line A549 was mock‐irradiated (0 Gy) or subjected to carbon‐ion radiation at 2 Gy (C2Gy) and carbon‐ion radiation at 4 Gy (C4Gy), respectively. The global m6A quantification showed that carbon‐ion radiation dose‐dependently elevated the level of total m6A‐modified RNAs (Figure 1A), which was further verified by dot blot assay (Figure 1B). To further investigate the crucial m6A methyltransferases/demethylases in carbon‐ion radiation exposure on m6A RNA modification, we detected the mRNA levels of major m6A methyltransferases (METTL3, METTL14, RBM15, Wilms' tumor 1 associated protein [WTAP] and VIRMA) and demethylases (FTO and ALKBH5) in the mock‐irradiated (0 Gy) and carbon‐ion radiated groups (C2Gy and C4Gy). As shown in Figure 1C, m6A methyltransferase METTL3 was remarkably upregulated whereas demethylase FTO was significantly decreased in the carbon‐ion radiated groups (C2Gy and C4Gy) compared to the mock‐irradiated group (0 Gy). However, no significant difference was observed in the mRNA levels of METTL14, RBM15, WTAP, and VIRMA (Figure 1C). Considering the catalytic ability of these m6A regulators and the increased m6A modification in the carbon‐ion radiated groups, the important active component of m6A methyltransferase METTL3 was selected as the candidate molecule for aberrant m6A modification in carbon‐ion radiation.
FIGURE 1.

Carbon‐ion radiation dose‐dependently enhanced METTL3‐mediated m6A methylation in A549 cells. (A) The level of total m6A‐modified RNA in human NSCLC cell line A549 with mock‐irradiated (0 Gy) or subjected to carbon‐ion radiation at 2 Gy (C2Gy) and carbon‐ion radiation at 4 Gy (C4Gy) was analyzed by global m6A quantification. (B) The level of total m6A‐modified RNA was verified by dot blot assy. (C) The mRNA levels of major m6A methyltransferases (METTL3, METTL14, RBM15, WTAP, and VIRMA) and demethylases (FTO and ALKBH5) in the mock‐irradiated (0 Gy) and carbon‐ion radiated (C2Gy and C4Gy) groups were detected by real‐time PCR. *P < 0.05.
3.2. METTL3 knockdown inhibits the cell proliferation, migration, and invasion of NSCLC cells
To determine the role of METTL3‐mediated m6A modification in the regulation of the biological function of NSCLC cells, we knocked down the level of METTL3 with specific short interfering (siRNA). As shown in Figure 2A, METTL3 knockdown significantly impaired the cell proliferation of A549 and H1975 cells. Colony formation assay further confirmed the inhibition of METTL3 knockdown on the cell proliferation of A549 and H1975 cells (Figure 2B). Moreover, the migration (Figure 2C) and invasion (Figure 2D) abilities of NSCLCs, indicated by wound healing assay and transwell assay, respectively, were also inhibited by METTL3 knockdown. Consistently, the level of phosphorylation of the histone variant H2AX, which produced γH2AX, was significantly increased in METTL3 knocked down A549 and H1975 cells (Figure 2E). Furthermore, METTL3 knockdown impaired the epithelial‐mesenchymal transition (EMT) phenotype of A549 and H1975 cells, including elevated protein levels of E‐cadherin protein and decreased protein levels of Vimentin and Snail1 (Figure 2F). These results demonstrate that METTL3 may contribute to cell survival and metastasis in carbon‐ion irradiated NSCLC cells.
FIGURE 2.

METTL3 knockdown inhibits the cell proliferation, migration, and invasion of NSCLC cells. (A) The effect of METTL3 knockdown on cell viability was analyzed by CCK‐8 assay. (B) The effect of METTL3 knockdown on colony formation ability was evaluated by colony formation assay. (C) The effect of METTL3 knockdown on migration ability was analyzed by wound healing assay. (D) The effect of METTL3 knockdown on invasion ability was determined by transwell assay. (E) The level of γH2AX was analyzed by ELISA. (F) The protein levels of EMT markers, including E‐cadherin, Vimentin, and Snail1, were analyzed by Western blot. *P < 0.05.
3.3. METTL3 overexpression promotes the cell proliferation, migration, and invasion of NSCLC cells
To further verified the oncogene role of METTL3 on NSCLC cells, we generated two METTL3 stably overexpressed NSCLC cells (A549 and H1975) by lentivirus. Consistently, METTL3 overexpression dramatically enhanced the cell proliferation of A549 and H1975 cells (Figure 3A), which was further confirmed by colony formation assay (Figure 3B). Furthermore, the migration (Figure 3C) and invasion (Figure 3D) abilities of NSCLCs, indicated by wound healing assay and transwell assay, respectively, were also promoted by METTL3 overexpression. Notably, the level of γH2AX was significantly decreased in METTL3 overexpressed A549 and H1975 cells (Figure 3E). Meanwhile, METTL3 overexpression improved the EMT phenotype of A549 and H1975 cells, including decreased protein levels of E‐cadherin protein and elevated protein levels of Vimentin and Snail1 (Figure 3F). Collectively, these results suggest that METTL3 may contribute to cell survival and metastasis in carbon‐ion irradiated NSCLC cells.
FIGURE 3.

METTL3 overexpression promotes the cell proliferation, migration, and invasion of NSCLC cells. (A) The effect of METTL3 overexpression on cell viability was analyzed by CCK‐8 assay. (B) The effect of METTL3 overexpression on colony formation ability was evaluated by colony formation assay. (C) The effect of METTL3 overexpression on migration ability was analyzed by wound healing assay. (D) The effect of METTL3 overexpression on invasion ability was determined by transwell assay. (E) The level of γH2AX was analyzed by ELISA. (F) The protein levels of EMT markers, including E‐cadherin, Vimentin, and Snail1, were analyzed by Western blot. *P < 0.05.
3.4. METTL3 regulates the expression of H2AX via m6A modification of H2AX mRNA
To investigate the mechanism by which METTL3 regulates the behavior of NSCLC cells, we conducted gene set enrichment analysis (GSEA) to explore the potential downstream pathways of METTL3. We noticed that H2AX was significantly upregulated on carbon‐ion radiation in NSCLC cells. Notably, recent studies have shown that phosphorylated H2AX (‐H2AX) plays an important role in the recruitment and/or retention of DNA repair and checkpoint proteins such as BRCA1, MRE11/RAD50/NBS1 complex, MDC1, and 53BP1, which promotes cancer cell survival after radiotherapy. Thus, we assumed that METTL3 regulates cell behavior by upregulating H2AX. To verify this hypothesis, m6A‐labeled mRNA levels of H2AX in METTL3 overexpressed NSCLC cells were analyzed. As shown in Figure 4A, the m6A‐labeled mRNA of H2AX was significantly elevated in METTL3 overexpressed A549 and H1975 cells. Next, we generated a mutated METTL3 (W397A) construct with disordered enzymatic activity, as described previously, and found that mutant METTL3 failed to elevate the m6A methylated level of H2AX mRNA in A549 and H1975 cells (Figure 4B). Consistently, METTL3 knockdown remarkably decreased the m6A‐methylated level of H2AX mRNA (Figure 4B). Furthermore, a similar change in H2AX mRNA expression and decay rate was confirmed by real‐time PCR in A549 and H1975 cells with METTL3 overexpression or knockdown (Figure 4C,D). Collectively, these results demonstrate that METTL3 epigenetically elevates the level of H2AX.
FIGURE 4.

METTL3 regulates the expression of H2AX via m6A modification of H2AX mRNA. (A) The effect of METTL3 overexpression on m6A‐methylated H2AX mRNA was evaluated by Me‐RIP‐qPCR. (B) The effect of METTL3 knockdown or mutant on m6A‐methylated H2AX mRNA was evaluated by Me‐RIP‐qPCR. (C) The effect of METTL3 knockdown or mutant on the level of total H2AX mRNA was evaluated by qPCR. (D) The decay rate of H2AX mRNA was detected by transcript inhibition assay. *P < 0.05.
3.5. METTL3‐mediated H2AX m6A modification decreased the sensitivity of carbon‐ion radiotherapy on NSCLCs
To verify the role of METTL3‐mediated H2AX m6A modification on the sensitivity of carbon‐ion radiation to NSCLC cells, we stably knocked down the METTL3 on A549 cells with or without H2AX overexpression. These A549 cells were subcutaneously injected to nude mice. When the tumor volume had grown to approximately 100 mm3, carbon‐ion radiation was performed. As shown in Figure 5A–C, METTL3 knockdown dramatically inhibited tumor volume and weight after carbon‐ion radiation (C4Gy). However, this inhibition was reversed by H2AX overexpression. Moreover, the efficiency of METTL3 knockdown and H2AX overexpression in the tumors was verified by IHC (Figure 5D). On the contrary, we stably overexpressed METTL3 on A549 cells. These A549 cells were subcutaneously injected to nude mice. When the tumor volume had grown to approximately 100 mm3, carbon‐ion radiation was performed. As shown in Figure 6A–C, METTL3 overexpression significantly increased tumor volume and weight after carbon‐ion radiation (C4Gy). These results demonstrate that METTL3‐mediated H2AX m6A modification enhances the sensitivity of carbon‐ion radiotherapy on NSCLC in vivo.
FIGURE 5.
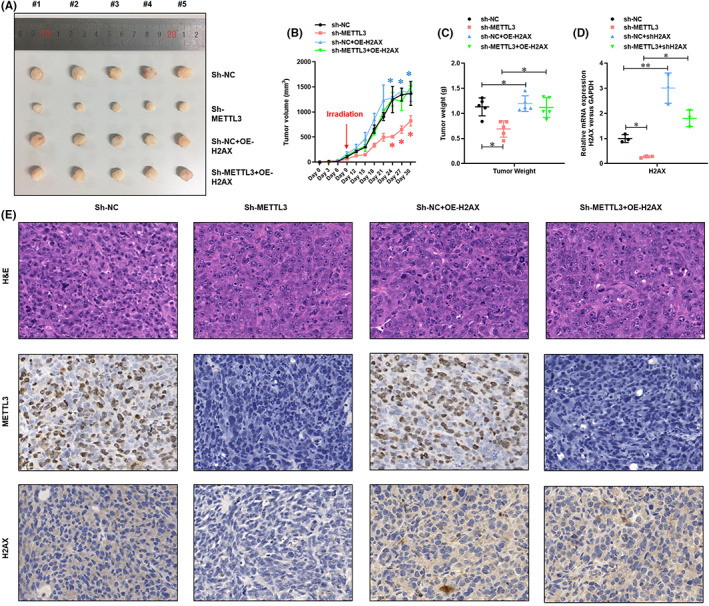
Deletion of METTL3‐mediated H2AX m6A modification enhanced the sensitivity of carbon‐ion radiotherapy on NSCLCs. (A) Image of xenograft mice‐generated tumors from METTL3 knockdown with or without H2AX overexpression A549 cells. (B) The tumor volume of xenograft mice‐generated tumors from METTL3 knockdown with or without H2AX overexpression A549 cells. (C) The tumor weight of xenograft mice‐generated tumors from METTL3 knockdown with or without H2AX overexpression cells. (D) Real‐time PCR analysis of the expression of H2AX mRNA in the generated tumors. (E) Histological and IHC analysis of the expression of METTL3 and H2AX in the generated tumors. *P < 0.05.
FIGURE 6.

METTL3‐mediated H2AX m6A modification enhanced the resistance of carbon‐ion radiotherapy on NSCLCs. (A) Image of xenograft mice‐generated tumors from METTL3 overexpressed A549 cells. (B) The tumor volume of xenograft mice‐generated tumors from METTL3 overexpressed A549 cells. (C) The tumor weight of xenograft mice‐generated tumors from METTL3 overexpressed cells. *P < 0.05, **P < 0.01.
4. DISCUSSION
Carbon ions radiation belong to the high LET radiation, which shows higher ionization density and DNA damage rate under the direct action of radiation. 4 Carbon ions mostly cause double‐strand breaks in DNA, which are difficult to repair and lead to cell death. The patterns of cell death and inactivation induced by carbon ions include apoptosis, necrosis, autophagy, premature senescence, accelerated differentiation, delayed reproductive death of progeny cells, and bystander cell death. 5 , 27 In addition to good local effects, carbocation therapy may also inhibit the potential of metastasis of cancer cells. 7 , 10 , 28 Herein, we demonstrated that the level of METTL3 and its mediated m6A modification were elevated in NSCLC cells with carbon‐ion radiotherapy. Knockdown of METTL3 in NSCLC cells impaired proliferation, migration, and invasion in vitro and in vivo. Moreover, we found that METTL3‐mediated m6A modification mRNA inhibited the decay of H2AX mRNA and enhanced its expression, which led to enhanced DNA damage repair and cell survival.
There is growing evidence that m6A modification and its regulatory proteins also play an important role in a variety of cancers, including leukemia, brain tumors, breast cancer, and lung cancer. 29 , 30 , 31 , 32 It was observed that the expression of METTL3 was upregulated in lung adenocarcinoma and played a cancer‐promoting role in the growth, survival, and invasion of human lung cancer cells. 25 Notably, METTL3 has been shown to promote the translation of target mRNA transcripts (such as epidermal growth factor receptor (EGFR) and transcriptional coactivator with PDZ‐binding motif (TAZ)) in a manner independent of its methyltransferase activity by interacting with translation initiation mechanisms. However, the biological function of METTL3 in lung cancer cells is determined only by knockdown of METTL3 expression, and it is unclear whether loss of METTL3 mutant methyltransferase activity can play an equal role in carcinogenesis for wild‐type METTL3 in promoting the growth, survival, and invasion of human lung cancer cells. 16 , 17 Therefore, systematic functional acquisition studies of constructed wild‐type and mutant METTL3 in the absence of endogenous MELLT3 expression deletion are necessary to determine the functional importance of METTL3 as a cytoplasmic m6A reader in the pathogenesis of lung and other types of cancer. In this study, we found that carbon‐ion radiotherapy enhanced the level of METTL3 and its mediated m6A modification in A549 cells. Furthermore, overexpression of METTL3 in NSCLC cells promoted proliferation, migration, and invasion.
DNA double‐strand breaks (DSBs) pose a threat to the stability of the genome because their faulty repair can lead to chromosomal rearrangements, deletions, or other potentially damaging mutations. 33 , 34 DSB repair defects are closely related to cancer susceptibility, aging, neurodegeneration, and immune deficiency. H2AX plays a key role in DNA double‐stranded fracture repair and genomic stability. Chromosome double‐strand breaks cause extensive reactions of adjacent chromatin, characterized by the phosphorylation of histone H2AX on its C‐terminal serine 139 (forming “Thra2ax”). 35 , 36 DSBs caused by ionizing radiation, biologically programmed breaks characterized by normal immune cell development, and pathological exposure of DNA ends caused by telomere dysfunction can be repaired in a variety of situations. 34 , 37 , 38 In this study, we found that METTL3‐mediated m6A modification mRNA inhibited the decay of H2AX mRNA and enhanced its expression, which led to enhanced DNA damage repair and cell survival.
Collectively, we demonstrated that carbon‐ion radiotherapy elevated the level of METTL3 and its mediated m6A modification in NSCLC cells. Loss‐of‐function of METTL3 in NSCLC cells impaired proliferation, migration, and invasion in vitro and in vivo. Moreover, we found that METTL3‐mediated m6A modification mRNA inhibited the decay of H2AX mRNA and enhanced its expression, which led to enhanced DNA damage repair and cell survival.
ACKNOWLEDGEMENT
This work was supported by the Science and Technology Development Fund of Shanghai Pudong New Area (Grant no. PKJ2017‐Y53) and the Shanghai Shen Kang Hospital Development Center New Frontier Technology Joint Project of Municipal Hospital (Grant no. SHDC12017114).
CONFLICT OF INTEREST
The authors have no conflict of interest.
ETHICAL APPROVAL
The experiments with animals in this study were approved by the Animal Care Use Committee of Fudan University Shanghai Cancer Center (Shanghai, China).
ANIMAL STUDIES
The experiments with animals in this study were conducted according to the principles of the Declaration of Helsinki and National Institutes of Health guidelines.
Xu X, Zhang P, Huang Y, et al. METTL3‐mediated m6A mRNA contributes to the resistance of carbon‐ion radiotherapy in non‐small‐cell lung cancer. Cancer Sci. 2023;114:105‐114. doi: 10.1111/cas.15590
Xiaofeng Xu and Peiru Zhang contributed equally to this work.
Contributor Information
Jian Chen, Email: fathomer@163.com.
Renquan Lu, Email: lurenquan@126.com.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Demizu Y, Fujii O, Iwata H, Fuwa N. Carbon ion therapy for early‐stage non‐small‐cell lung cancer. Biomed Res Int. 2014;2014:727962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayashi K, Yamamoto N, Nakajima M, et al. Clinical outcomes of carbon‐ion radiotherapy for locally advanced non‐small‐cell lung cancer. Cancer Sci. 2019;110(2):734‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang Y, Dong Y, Zhao J, Zhang L, Kong L, Lu JJ. Comparison of the effects of photon, proton and carbon‐ion radiation on the ecto‐calreticulin exposure in various tumor cell lines. Ann Transl Med. 2019;7(20):542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwata H, Murakami M, Demizu Y, et al. High‐dose proton therapy and carbon‐ion therapy for stage I nonsmall cell lung cancer. Cancer. 2010;116(10):2476‐2485. [DOI] [PubMed] [Google Scholar]
- 8. Klein C, Dokic I, Mairani A, et al. Overcoming hypoxia‐induced tumor radioresistance in non‐small cell lung cancer by targeting DNA‐dependent protein kinase in combination with carbon ion irradiation. Radiat Oncol. 2017;12(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakajima M, Yamamoto N, Hayashi K, et al. Carbon‐ion radiotherapy for non‐small cell lung cancer with interstitial lung disease: a retrospective analysis. Radiat Oncol. 2017;12(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shirai K, Kubota Y, Ohno T, et al. Carbon‐ion radiotherapy for isolated lymph node metastasis after surgery or radiotherapy for lung cancer. Front Oncol. 2019;9:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J, Zhang YC, Huang C, et al. M(6)a regulates neurogenesis and neuronal development by modulating histone methyltransferase Ezh2. Genomics Proteomics Bioinformatics. 2019;17:154‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dominissini D, Moshitch‐Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature. 2012;485(7397):201‐206. [DOI] [PubMed] [Google Scholar]
- 13. Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361(6409):1346‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia G, Fu Y, Zhao X, et al. N6‐methyladenosine in nuclear RNA is a major substrate of the obesity‐associated FTO. Nat Chem Biol. 2011;7(12):885‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ke S, Pandya‐Jones A, Saito Y, et al. M(6)a mRNA modifications are deposited in nascent pre‐mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31(10):990‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Han Y, Zhang H, et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479‐485. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Harada BT, He C. Regulation of gene expression by N(6)‐methyladenosine in cancer. Trends Cell Biol. 2019;29:487‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathiyalagan P, Adamiak M, Mayourian J, et al. FTO‐dependent N(6)‐methyladenosine regulates cardiac function during remodeling and repair. Circulation. 2019;139(4):518‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan Y, Ma P, Liu Y, Li W, Shu Y. Multiple functions of m(6)a RNA methylation in cancer. J Hematol Oncol. 2018;11(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6‐methyladenosine methyltransferase. Cell Res. 2014;24(2):177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi H, Zhang X, Weng YL, et al. M(6)a facilitates hippocampus‐dependent learning and memory through YTHDF1. Nature. 2018;563(7730):249‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song H, Feng X, Zhang H, et al. METTL3 and ALKBH5 oppositely regulate m(6)a modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation‐treated cardiomyocytes. Autophagy. 2019;15:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Hu X, Huang M, et al. Mettl3‐mediated mRNA m(6)a methylation promotes dendritic cell activation. Nat Commun. 2019;10(1):1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Chai P, Jia R, Jia R. Novel insights on m(6)a RNA methylation in tumorigenesis: a double‐edged sword. Mol Cancer. 2018;17(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu YL, Li HY, Zhao XP, Jiao JY, Tang DX, Yan LJ, Wan Q, Pan CB. Mesenchymal stem cell‐derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017;108(5):897‐909. 10.1111/cas.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayashi K, Yamamoto N, Karube M, et al. Feasibility of carbon‐ion radiotherapy for re‐irradiation of locoregionally recurrent, metastatic, or secondary lung tumors. Cancer Sci. 2018;109(5):1562‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamoto N, Nakajima M, Tsujii H, Kamada T. Carbon ion radiotherapy for oligo‐recurrence in the lung. Pulm Med. 2013;2013:219746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Ishfaq M, Xu L, Xia C, Chen C, Li J. METTL3/m(6)a/miRNA‐873‐5p attenuated oxidative stress and apoptosis in colistin‐induced kidney injury by modulating Keap1/Nrf2 pathway. Front Pharmacol. 2019;10:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weng H, Huang H, Wu H, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)a modification. Cell Stem Cell. 2018;22(2):191‐205 e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winkler R, Gillis E, Lasman L, et al. M(6)a modification controls the innate immune response to infection by targeting type I interferons. Nat Immunol. 2019;20(2):173‐182. [DOI] [PubMed] [Google Scholar]
- 32. Zhuang M, Li X, Zhu J, et al. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Res. 2019;47(9):4765‐4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mota MBS, Carvalho MA, Monteiro ANA, Mesquita RD. DNA damage response and repair in perspective: Aedes aegypti, Drosophila melanogaster and Homo sapiens . Parasit Vectors. 2019;12(1):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scully R, Xie A. Double strand break repair functions of histone H2AX. Mutat Res. 2013;750(1–2):5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinner A, Wu W, Staudt C, Iliakis G. Gamma‐H2AX in recognition and signaling of DNA double‐strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36(17):5678‐5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double‐strand break repair pathway choice. Cell Res. 2008;18(1):134‐147. [DOI] [PubMed] [Google Scholar]
- 37. Weyemi U, Redon CE, Bonner WM. H2AX and EMT: deciphering beyond DNA repair. Cell Cycle. 2016;15(10):1305‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang D, Tang B, Xie X, Xiao YF, Yang SM, Zhang JW. The interplay between DNA repair and autophagy in cancer therapy. Cancer Biol Ther. 2015;16(7):1005‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


