Abstract
Hypoxic tumor microenvironment (HTM) promotes a more aggressive and malignant state in glioblastoma. However, little is known about the role and mechanism of CXC chemokine ligand 14 (CXCL14) in HTM‐mediated glioblastoma progression. In this study, we report that CXCL14 expression correlated with poor outcomes, tumor grade, and hypoxia‐inducible factor (HIF) expression in patients with glioblastoma. CXCL14 was upregulated in tumor cells within the hypoxic areas of glioblastoma. Hypoxia induced HIF‐dependent expression of CXCL14, which promoted glioblastoma tumorigenicity and invasiveness in vitro and in vivo. Moreover, CXCL14 gain‐of‐function in glioblastoma cells activated insulin‐like growth factor‐1 receptor (IGF‐1R) signal transduction to regulate the growth, invasiveness, and neurosphere formation of glioblastoma. Finally, systemic delivery of CXCL14 siRNA nanoparticles (NPs) with polysorbate 80 coating significantly suppressed tumor growth in vivo and extended the survival time in patient‐derived glioblastoma xenografts. Together, these findings suggest that HIF‐dependent CXCL14 expression contributes to HTM‐promoted glioblastoma tumorigenicity and invasiveness through activation of the IGF‐1R signaling pathway. CXCL14 siRNA NPs as an oligonucleotide drug can inhibit glioblastoma progression and constitute a translational path for the clinical treatment of glioblastoma patients.
Keywords: CXC chemokine ligand 14, glioblastoma, hypoxia‐inducible factor, nanoparticle, tumor hypoxia
Hypoxic tumor microenvironment (HTM) is able to largely upregulate CXC chemokine ligand 14 (CXCL14) expression in glioblastoma cells, which plays a critical role in hypoxic tumor microenvironment‐promoted glioblastoma tumorigenicity and invasiveness. Hypoxia‐inducible factor‐dependent CXCL14 activates the insulin‐like growth factor‐1 receptor signaling pathway to regulate the growth, invasiveness, and neurosphere formation of glioblastoma. CXCL14 siRNA nanoparticles with polysorbate 80 coating provide a promising therapeutic strategy in treatment of glioblastoma.
Abbreviations
- ACKR2
atypical chemokine receptor 2
- BLI
bioluminescent imaging
- CXCL14
CXC chemokine ligand 14
- CXCR4
C‐X‐C motif chemokine receptor 4
- GPR85
G protein‐coupled receptor 85
- HIF
hypoxia‐inducible factor
- HRE
hypoxia response element
- HTM
hypoxic tumor microenvironment
- IDH
isocitrate dehydrogenase
- IGF‐1R
insulin‐like growth factor‐1 receptor
- IHC
immunohistochemistry
- KD
knockdown
- Luc
luciferase
- NP
nanoparticle
- ODD
oxygen‐dependent degradation domain
- PDX
patient‐derived glioblastoma xenograft
- PS80
polysorbate 80
- Q‐PCR
real‐time quantitative PCR
- TM
tumor microenvironment
1. INTRODUCTION
CXC chemokine ligand 14 is a member of the CXC chemokine family and has a broad spectrum of biological activities. 1 CXC chemokine ligand 14 plays an important role in immune surveillance and inflammation due to the regulation of immune cell migration and differentiation. 2 CXC chemokine ligand 14 has been reported to be involved in tumorigenesis. However, its role in different cancers varies and has protumor or antitumor effects in several tumor progressions. 3 In glioblastoma, CXCL14 expression is higher in glioblastoma tissues than in normal brain tissues. 4 Survival analysis of publicly available gene expression datasets showed that CXCL14 expression was negatively correlated with the overall survival of patients with glioblastoma. 5 , 6 Moreover, it has been demonstrated in vitro that stromal CXCL14 promotes the proliferation and migration of glioblastoma cells, 5 suggesting that CXCL14 is a protumorigenic chemokine in glioblastoma. However, the impact on in vivo glioblastoma progression and their mechanisms remain poorly understood.
The HTM can regulate self‐renewal of glioma cancer stem cells and promote proliferation, angiogenesis, metastasis, and drug resistance, which play a dominant role in glioblastoma tumorigenicity, progression, and therapy resistance. 7 , 8 Although the molecular mechanisms underlying these effects are polyphyletic and complex, hypoxia‐regulated expression of chemokines and their receptors are important mediators. 9 , 10 Several chemokines and chemokine receptors, such as CXCR4‐CXCL12, CXCL10/CXCR3, and CCL3L1/CCR5 axes, are important arms of protumorigenic effects in glioma progression through the regulation of glioma cell proliferation, angiogenesis, and immune cell recruitment. 11 These chemokines and their receptors can be regulated by tumor hypoxia through direct or indirect HIF‐dependent mechanisms. 9 , 10 However, the role of HTM in CXCL14 regulation and the function of CXCL14 in HTM‐regulated glioblastoma tumorigenicity and progression are unclear.
In the present study, we hypothesized that CXCL14 might be regulated by HTM and could play a role in HTM‐promoted glioblastoma tumorigenicity and progression. We show that CXCL14 is upregulated by HTM in glioblastoma cells and activates the IGF‐1R signaling pathway, thereby promoting tumorigenicity and invasiveness of glioblastoma.
2. MATERIALS AND METHODS
Several material and methods were used in this study. These included the following: bioinformatics analysis, tissue arrays, immunohistochemistry, cell culture, in vitro hypoxic treatments, western blot analysis, Q‐PCR, ELISA, vector constructions and viral transduction, promoter analysis and luciferase assays, ChIP, co‐immunoprecipitation, His‐tag pull‐down assay, cell growth analysis, cell viability assay, cell migration and invasion assays, neurosphere formation assay, siRNA encapsulated in PS80‐coated nanoparticles, animal models, immunofluorescence imaging, isolation of implanted GL261‐lucGFP cells, flow cytometric analysis, BLI, and statistical analysis. Detailed materials and methods are provided in Appendix S1. We undertook all animal studies after obtaining permission from the Institutional Animal Care and Use Committee at China Medical University, Taiwan (CMUIACUC‐2020‐029). The Ethical Review Board of China Medical University (Taiwan) approved the clinical studies (CMUH108‐REC3‐163).
3. RESULTS
3.1. CXCL14 is the most significant chemokine‐related prognostic gene in glioma
We first investigated which chemokine networks are most appropriate for malignant progression and poor clinical outcomes in glioma. The Kaplan–Meier scan of genes involved in chemokine activity of Kyoto Encyclopedia of Genes and Genomes pathways in the R2 platform showed that CXCL14 was the only gene that overlapped in the two glioma datasets and was found to be the most significantly associated with patient overall survival (Figure S1A). High expression of CXCL14 was significantly associated with poor outcome in patients with glioma (Figure S1B). In addition, there was a significantly higher expression of CXCL14 in high‐grade gliomas (glioblastoma) than in low‐grade gliomas (Figure S1C). To further support these findings, IHC studies revealed that high expression and score of CXCL14 were observed in high‐grade glioma compared to low‐grade glioma or normal brain tissues (Figure S1D–F) in glioma tissue arrays containing 63 glioma samples. Immunofluorescence imaging assay of two human glioblastoma and their matched normal brain tissues derived from peritumoral areas also showed a significantly higher expression of CXCL14 in glioblastoma compared to that in matched normal tissues (Figure S1G). Another IHC study for CXCL14 in 32 glioblastoma samples showed that a higher level of CXCL14 gene expression was associated with poor survival (Figure S1H), indicating high CXCL14 expression is associated with poor clinical outcomes in glioblastoma. In addition, immunofluorescence imaging studies showed that CXCL14 expression colocalized with high expression of HIF‐1α or HIF‐2α in glioblastoma specimens (Figure 1A). Immunohistochemical staining of CXCL14 expression in 12 human glioblastoma samples also showed a linear correlation between CXCL14 and HIF‐1α or HIF‐2α expression in human glioblastoma samples (r 2 = 0.488, p = 0.011 for HIF‐1α; r 2 = 0.416, p = 0.021 for HIF‐2α) (Figure 1B).
FIGURE 1.
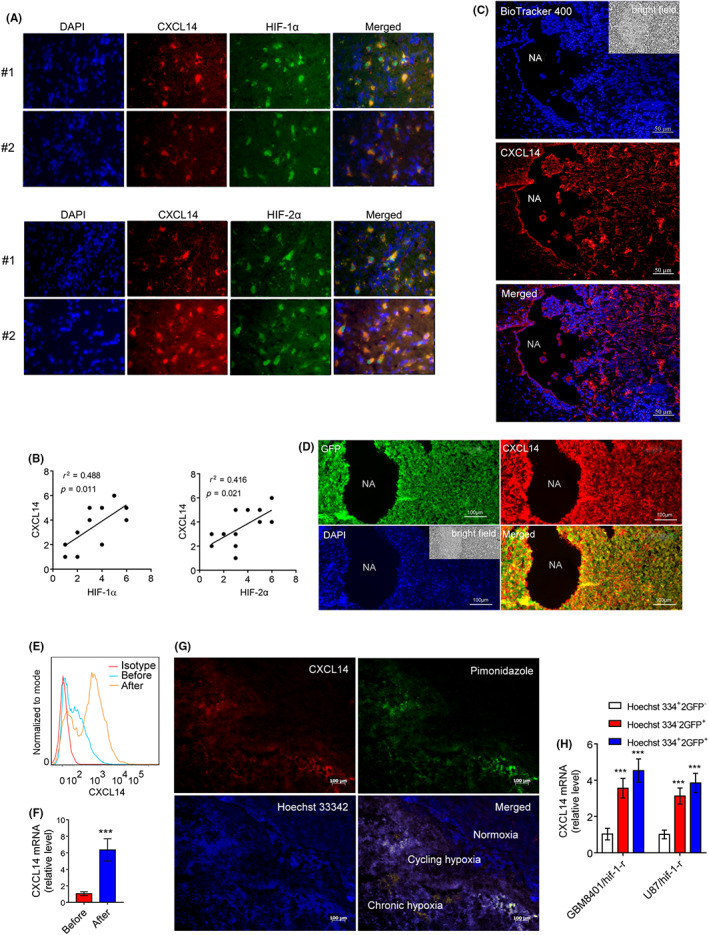
Tumor microenvironment (TM) regulates tumor CXC chemokine ligand 14 (CXCL14) expression in glioblastoma. (A) Immunofluorescence imaging of CXCL14 and hypoxia‐inducible factor‐1α (HIF‐1α) or HIF‐2α expression in human glioblastoma specimens. (B) Association between CXCL14 and HIF‐1α or HIF‐2α expression in 12 human glioblastoma samples. (C,D) Immunostaining of CXCL14 expression for BioTracker 400‐labeled glioma cells or GFP‐labeled glioma cells 2 weeks after orthotopic implantation of tumor cells in GL261 glioma. NA, necrotic area. (E,F) CXCL14 expression in mouse GFP‐labeled GL261 cells isolated from the TM by a flow sorting of GFP‐expressing cells (after) or their original cells before implantation (before). (G) Immunostaining of CXCL14 expression in hypoxic tumor subpopulations from GBM8401 xenografts. White color represents the colocalization of pimonidazole (green), Hoechst 33342 (blue), and CXCL14 (red). (H) CXCL14 mRNA levels in normoxic cells (Hoechst 3342+ and GFP−), chronic hypoxic cells (Hoechst 3342− and GFP+), and cycling hypoxic cells (Hoechst 3342+ and GFP+) isolated from disaggregated GBM8401/hif‐1‐r and U87/hif‐1‐r xenografts. Error bars, SD within triplicate experiments. ***p < 0.001, compared with normoxia
3.2. Tumor microenvironment regulates tumor CXCL14 expression in glioblastoma
Next, we determined whether the TM regulates CXCL14 in glioma cells. GL261 murine glioma cells labeled with BioTracker 400, a cell tracking dye, or dual‐labeled with luciferase and GFP were implanted into the brains of C57BL/6J mice, respectively, as a syngeneic mouse model of glioma. Two weeks later, the immunofluorescence imaging analysis showed that tumor cells in the perinecrotic areas of glioma displayed a significant increase in CXCL14 expression (Figure 1C,D). Flow cytometry and Q‐PCR assays further confirmed that the protein and mRNA levels of CXCL14 in the isolated GL261cells from the TM were higher than in the original GL261 cells before implantation (Figure 1E,F), indicating that the TM was able to upregulate CXCL14 expression in glioma cells. To verify endogenous tumor hypoxia‐mediated CXCL14 expression in glioblastoma, staining with Hoechst 33342 (a perfusion marker) and pimonidazole (a hypoxia marker), together with immunofluorescence imaging, were utilized to identify hypoxic tumor subpopulations from GBM8401 xenografts. Immunofluorescence imaging analysis showed highly heterogeneous Hoechst 33342 and pimonidazole staining in GBM8401 xenografts (Figure 1G). CXC chemokine ligand 14 staining indicated that CXCL14 expression occurs in the cycling hypoxic (Hoechst 3342+ and pimonidazole+) and chronic hypoxic (Hoechst 3342− and pimonidazole+) areas of tumors. In addition, the total RNA of the hypoxic cell subpopulations derived from disaggregated orthotopic GBM8401/HIF1‐r and U87/HIF1‐r xenografts 12 was further analyzed for CXCL14 expression by Q‐PCR. The expression of CXCL14 increased significantly in cycling and chronic hypoxic cells compared to that in normoxic cells (Figure 1H). Taken together, these results indicate that the TM upregulates CXCL14 in glioblastoma cells and suggest that tumor hypoxia might regulate CXCL14 expression.
3.3. Tumor hypoxia induces CXCL14 expression through HIF‐1α and HIF‐2α
To test whether hypoxia regulates CXCL14 in glioblastoma cells, we first analyzed the time course of CXCL14 mRNA in GBM8901 cells with or without hypoxic stress (<1% O2). After 6 h, CXCL14 mRNA levels increased significantly compared to the normoxic control (Figure 2A). Moreover, CXCL14 mRNA and protein levels were all increased in the human glioblastoma cells (U87, U251, and GBM8901) and primary glioblastoma cells (GBM04T and GBM09T) at 24 h after hypoxic treatment (<1% O2; Figure 2B–D). Hypoxia treatment also increased the concentration of CXCL14 in the medium and the percentage of CXCL14‐positive cells (Figures 2E and S2A,B). In addition, glioblastoma cells showed an increase in both intracellular and extracellular levels of CXC14 under both chronic (noninterrupted) and cycling hypoxic stress (Figure S2C,D). In an attempt to gain insight into the mechanisms of hypoxia‐induced CXCL14 induction, we examined the CXCL14 expression or secretion in glioblastoma cells with HIF‐1α or HIF‐2α inhibition under hypoxia. The silencing of HIF‐1α or HIF‐2α significantly abrogated hypoxia‐induced CXCL14 expression (Figures 2F and S2E–G). The treatment of HIF‐1α (LW6) or HIF‐2α (PT2399) inhibitor also decreased hypoxia‐mediated CXCL14 secretion (Figure 2G). Moreover, HIF‐1α or HIF‐2α overexpression in glioblastoma cells through the transfection of the HIF‐1α or HIF‐2α ODD deletion mutant plasmids significantly increased CXCL14 expression under normoxic conditions (Figure S2H,I), suggesting that HIF‐1α and HIF‐2α are crucial transcription factors for hypoxia‐mediated CXCL14 induction. To investigate the molecular mechanism by which hypoxia induces CXCL14 expression, bioinformatics analysis identified one HRE site in the human CXCL14 promoter sequence from bases −2000 to +1 (Figure 2H) and the reporter assays revealed that CXCL14 promoter reporter plasmids (pCXCL14‐Luc) responded to hypoxia stimulation or the transfection of HIF‐1α or HIF‐2α ODD deletion mutant plasmids in glioblastoma cells (Figures 2I and S2J). To pinpoint the exact binding motifs, we introduced mutations into the HRE of pCXCL14‐Luc (Figure 2I). The ablation of HRE on the CXCL14 promoter abrogated hypoxia‐mediated CXCL14 induction. The ChIP assays also confirmed the binding of HIF‐1α or HIF‐2α to the CXCL14 promoter in glioblastoma cells (Figure 2J). Collectively, these results suggest that HIF‐1α or HIF‐2α regulates CXCL14 transcription by directly binding to the CXCL14 promoter in a tumor hypoxia‐dependent manner.
FIGURE 2.
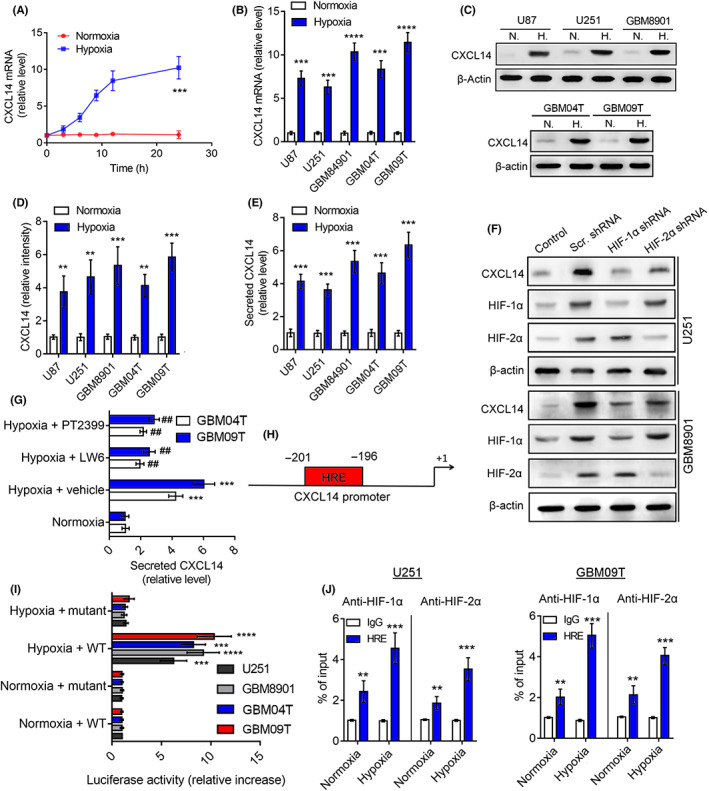
Tumor hypoxia induces CXC chemokine ligand 14 (CXCL14) expression through hypoxia‐inducible factor‐1α (HIF‐1α) and HIF‐2α. (A) Time course of CXCL14 mRNA expression after exposure of GBM8901 cells to <1% O2. (B–E) Levels of (B) CXCL14 mRNA and (C,D) cellular protein in human glioblastoma cells (U87, U251, and GBM8901) and primary glioblastoma cells (GBM04T and GBM09T) or (E) its secreted protein in medium at 24 h after normoxic (N.) and hypoxic (H.) treatment (<1% O2). (F) Protein levels of CXCL14, HIF‐1α, and HIF‐2α in U251 and GBM8901 cells with or without HIF‐1α or HIF‐2α knockdown at 24 h after hypoxic treatment (<1% O2). (G) Secreted CXCL14 protein in GBM04T and GBM09T glioblastoma cells with or without HIF‐1α (LW6) or HIF‐2α (PT2399) inhibitor at 24 h after hypoxic treatment (<1% O2). (H) One putative hypoxia response element (HRE) was identified in human CXCL14 promoter. (I) Luciferase reporter plasmids carrying the WT or mutant CXCL14 promoter regions were cotransfected with the Renilla luciferase reporter plasmid into U251, GBM8901, GBM04T, and GBM09T cells; the cells were treated with or without hypoxia (<1% O2) for 24 h. (J) ChIP followed by real‐time PCR (ChIP‐qPCR) assay of HIF‐1α or HIF‐2 α binding in CXCL14 promoter in response to hypoxia (<1% O2) for 24 h. Results are expressed as percentage of input. Error bars, SD within triplicate experiments. ***p < 0.001; ****p < 0.0001, compared with normoxia.. ## p < 0.01, compared with hypoxia + vehicle group
3.4. CXCL14 contributes to tumor hypoxia‐promoted glioblastoma tumorigenicity and progression
Next, we determined whether CXCL14 plays a role in hypoxia‐promoted cell growth, migration, invasion, and neurosphere generation of glioblastoma cells. An anti‐CXCL14 neutralizing Ab was chosen as the CXCL14 inhibitor. Glioblastoma cells were treated with a CXCL14‐specific neutralizing Ab during hypoxic treatment. The neutralization of CXCL14 significantly inhibited hypoxia‐promoted cell growth, viability, migration, invasion, and neurosphere formation (Figure S3A–I). To further determine whether hypoxia‐driven CXCL14 upregulation contributes to glioblastoma tumorigenicity and progression, we created a conditional CXCL14 KD platform using lentivirus vectors encoding 8× HRE‐driven CXCL14 shRNAs. GBM8901 cells stably expressing a dual GFP and luciferase reporter gene (GBM8901‐lucGFP) or GBM09T cells were transduced with lentivirus vectors carrying HRE‐driven CXCL14 shRNAs or scramble shRNAs. Western blotting confirmed CXCL14 KD under hypoxic conditions in glioblastoma cells (Figures 3A and S3J,K). Consistent with the CXCL14‐specific neutralization Ab studies, GBM8901 and GBM09T cells with CXCL14 KD showed a decrease in hypoxia‐elevated cell growth, viability, migration, invasion, and neurosphere formation compared with control cells with scramble shRNAs (Figure 3B–F). To investigate the impact of in vivo hypoxia‐induced tumor CXCL14 in glioblastoma tumorigenicity and progression, GBM8901‐lucGFP cells with or without hypoxia‐driven CXCL14 KD were implanted into the brains of nude mice. Western blot analysis of tumor tissue lysates indicated that GBM8901‐lucGFP xenografts with CXCL14 KD had lower expression of CXCL14 than GBM8901‐lucGFP xenografts with scramble shRNAs (Figure 3G), indicating that the endogenous HTM could silence tumor CXCL14 expression in vivo through inducing CXCL14 shRNA expression. Bioluminescent imaging showed that GBM8901‐lucGFP cells with CXCL14 KD displayed significant growth inhibition in vivo compared to control cells with scramble shRNAs (Figure 3H,I). Histological analysis of tumor sections revealed that GBM8901‐lucGFP xenografts with CXCL14 KD showed a decrease in glioblastoma cell infiltration and formation of infiltrating tumor foci compared to control xenografts with scramble shRNAs (Figure 3J,K). These results agree with the in vitro data and support the positive effect of CXCL14 on HTM‐promoted glioblastoma tumorigenicity and progression.
FIGURE 3.
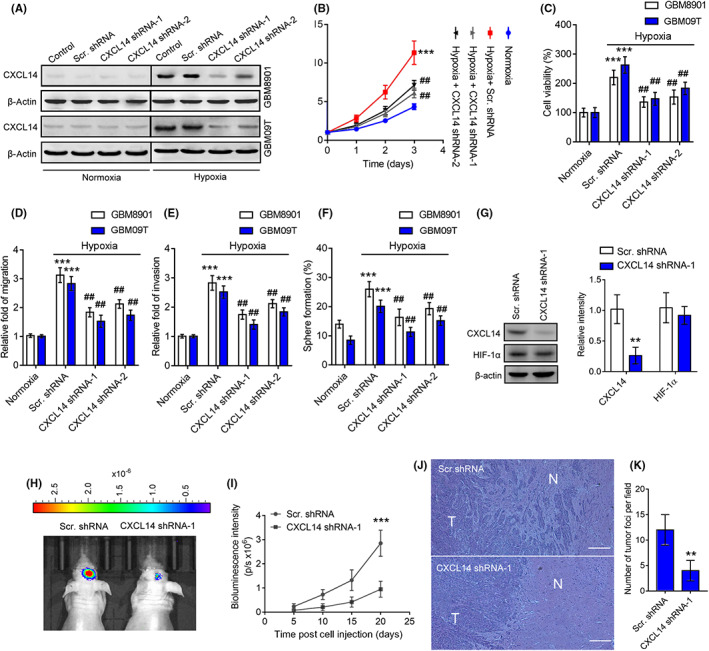
CXC chemokine ligand 14 (CXCL14) contributes tumor hypoxia‐promoted glioblastoma tumorigenicity and progression. (A) Western blot analysis of hypoxia‐induced CXCL14 knockdown in GBM8901‐lucGFP and GBM09T cells. (B–F) The cell growth (B), viability (C), migration (D), invasion (E), and neurosphere formation rates (F) of GBM8901‐lucGFP and GBM09T cells with or without hypoxia‐driven CXCL14 knockdown. Error bars, SD within triplicate experiments. ***p < 0.001, compared with normoxia. ## p < 0.01; ### p < 0.001, compared with the hypoxia‐driven scramble (Scr.) shRNA group. (G) CXCL14 and hypoxia‐inducible factor‐1α (HIF‐1α) protein levels in homogenized GBM8901‐lucGFP with or without hypoxia‐driven CXCL14 knockdown xenografts on day 15 after tumor implantation. Data are presented as means ± SD (n = 3). **p < 0.001 compared to hypoxia‐driven scramble shRNA group. (H) Bioluminescent images of GBM8901‐lucGFP xenografts with or without hypoxia‐driven CXCL14 knockdown on day 20 after tumor implantation. (I) Mean BLI values associated with longitudinal monitoring of intracranial tumor growth for each group. Data are presented as means ± SD (n = 6–8). ***p < 0.001 compared to Scr. shRNA group. (J) Representative tumors derived from GBM8901‐lucGFP xenografts with or without hypoxia‐driven CXCL14 knockdown showing tumor sections stained for H&E. N, normal tissue area; T, tumor area. (K) Quantitation of tumor foci for each group. Data are presented as means ± SD (n = 6–8). **p < 0.01 compared to Scr. shRNA group
3.5. CXCL14 gain of function increases glioblastoma tumorigenicity and progression
To determine the role of CXCL14 in glioblastoma cell progression, we supplied an additional CXCL14 recombinant protein to the medium and observed the changes in glioblastoma cell growth, viability, migration, invasion, and neurosphere formation. Supplementation with CXCL14 recombinant protein increased cell growth viability, migration, invasion, and neurosphere formation in glioblastoma cells compared to untreated cells (Figure S4A–I). We also tested whether CXCL14 overexpression in glioblastoma cells had similar effects. The dual GFP and luciferase reporter gene‐expressing GBM8401 glioblastoma cells (GBM8401‐lucGFP) derived from our previous studies 12 , 13 , 14 and GBM04T cells were stably transduced using recombinant lentiviruses expressing CXCL14 (Figure 4A,B). Compared with control lentiviral vector‐infected glioblastoma cells, CXCL14‐overexpressing glioblastoma cells also showed an increase in cell growth, viability, migration, invasion, and neurosphere formation (Figure 4C–G). The protein expression levels of stem cell markers (CD133, OCT4, and SOX2) in GBM8401 and GBM04T cells were also upregulated after CXCL14 overexpression (Figures 4H and S4J). Moreover, GBM8401‐lucGFP glioblastoma cells with or without CXCL14 overexpression were implanted into the brains of nude mice. Bioluminescent imaging was utilized to assess intracranial tumor growth. Consistent with the in vitro studies, mice implanted with CXCL14‐overexpressing GBM8401‐lucGFP had significantly better tumor growth than those implanted with control vector‐expressing GBM8401‐lucGFP glioblastoma cells (Figure 4I,J). Moreover, histochemical analysis of tumors derived from nude mice showed that CXCL14‐overexpressing GBM8401‐lucGFP xenografts displayed an increase in glioblastoma cell infiltration and formation of infiltrating tumor foci compared to control vector‐expressing xenografts (Figure 4K,L). Taken together, these results indicate that CXCL14 gain of function promotes glioblastoma tumorigenicity and progression in vitro and in vivo.
FIGURE 4.
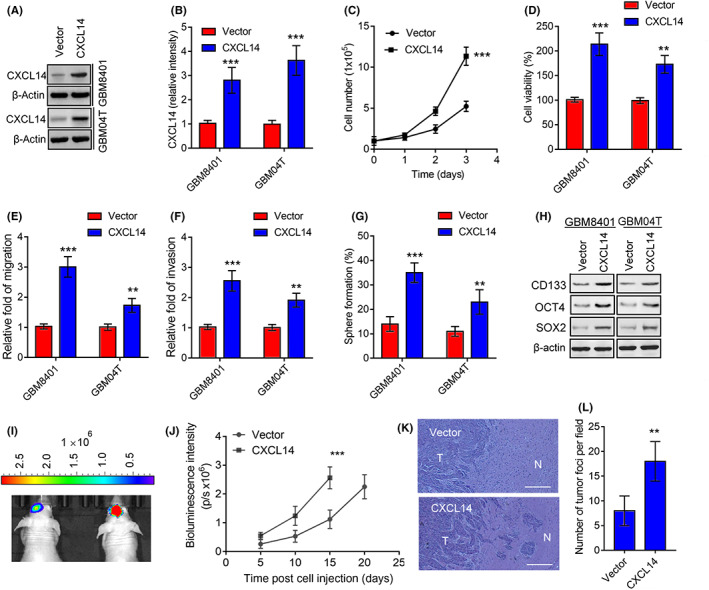
CXC chemokine ligand 14 (CXCL14) gain of function increases glioblastoma tumorigenicity and progression. (A,B) Protein levels of CXCL14 in GBM8401‐lucGFP and GBM04T cells lentivirally transduced with control vector or CXCL14‐overrexpressing vector for 3 days. Error bars, SD within triplicate experiments. ***p < 0.001, compared with vector group. (C–G) Cell growth (C), viability (D), migration (E), invasion (F), and neurosphere formation rates (G) of GBM8401‐lucGFP and GBM04T cells with or without CXCL14 overexpression. Error bars, SD within triplicate experiments. **p < 0.01; ***p < 0.001, compared with the vector group. (H) Western blot analysis of the stem cell markers (CD133, OCT4, and SOX2) in response to CXCL14 overexpression. (I) Bioluminescent images (BLI) of GBM8401‐lucGFP xenografts expressing control vector and GBM8401‐lucGFP xenografts overexpressing CXCL14 on day 15 after tumor implantation. (J) Mean BLI values associated with longitudinal monitoring of intracranial tumor growth for each group. Data are presented as means ± SD (n = 6–8). ***p < 0.001 compared to vector group. (K) Representative tumors derived from GBM8401‐lucGFP xenografts with or without CXCL14 overexpression showing tumor sections stained with H&E. N, normal tissue area; T, tumor area. (L) Quantitation of tumor foci for each group. Data are presented as means ± SD (n = 6–8). **p < 0.01 compared to the vector group
3.6. CXCL14 regulates protumorigenic effects through IGF‐1R signaling
The IGF‐1R serves as CXCL14 receptor, which mediates CXCL14‐maintained stem cell self‐renewal. 15 Moreover, IGF‐1R promotes tumor progression and therapy resistance in glioblastoma. 16 Therefore, the CXCL14‐activated IGF‐1R signaling pathway might contribute to the mechanism of CXCL14‐mediated tumor progression in glioblastoma. To test this hypothesis, we first undertook the coimmunoprecipitation and reverse pull‐down assays to verify the physical interaction between CXCL14 and IGF‐1R. Indeed, CXCL14 could bind with IGF‐1R in GBM8401 glioblastoma cells (Figure 5A,B). Moreover, western blotting further confirmed that supplementation with CXCL14 increased the phosphorylation of IGF‐1R and its downstream mediators, AKT and ERK 1/2, in GBM8401and GBM04T glioblastoma cells (Figure 5C,D), indicating CXCL14 is able to activate IGF‐1R signaling pathway in glioblastoma. Besides, the treatment of IMC‐A12 (cixutumumab), an IGF‐1R inhibitor, 17 suppressed these effects. IMC‐A12 also blocked CXCL14‐promoted cell viability, migration, invasion, and neurosphere formation in glioblastoma cells (Figure 5E–H). The blockage of IGF‐1R also inhibited CXCL14‐increased neurosphere size (Figure 5I,J). These results confirm that the CXCL14–IGF‐1R axis is crucial in the protumorigenic effects for glioblastoma.
FIGURE 5.
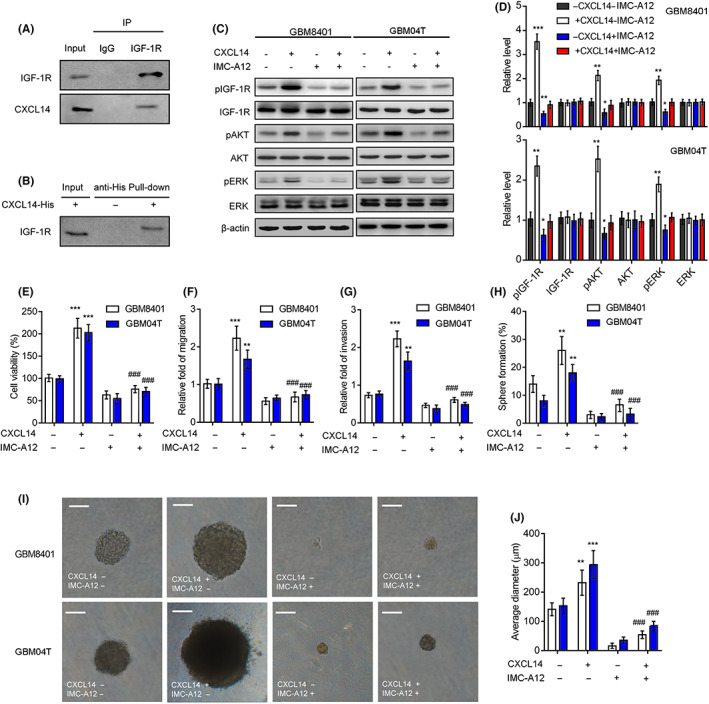
CXC chemokine ligand 14 (CXCL14) regulates the protumorigenic effects through insulin‐like growth factor‐1 receptor (IGF‐1R) signaling. (A) Coimmunoprecipitation of CXCL14 with IGF‐1R in GBM8401 cells. (B) Immunoblotting of IGF‐1R pulled down from CXCL14‐His tagged in GBM8401 cells, using Nickel magnetic beads. (C,D) Western blot analysis and quantification for detecting IGF‐1R, AKT, and ERK phosphorylation in GBM8401 and GBM04T cells treated with CXCL14 (100 ng/mL) with or without the IGF‐1R inhibitor (IMC‐A12, 100 nM) for 15 min. Error bars, SD within triplicate experiments. *p < 0.05, *p < 0.01, compared with cells without CXCL14 and IMC‐A12. (E–H) Cell viability (E), migration (F), invasion (G), and neurosphere formation rates (H) of GBM8401 and GBM04T cells treated with CXCL14 (400 ng/ml) with or without the IGF‐1R inhibitor (IMC‐A12, 100 nM) in culture medium. (I,J) Representative micrographs (I) and average diameter (J) of neurospheres developed from 10 day cultures. Error bars, SD within triplicate experiments. ***p < 0.001, compared with cells without CXCL14 and IMC‐A12. #### p < 0.0001, compared with the cells with CXCL14 alone
3.7. CXCL14 siRNA nanoparticle provides therapeutic benefits for glioblastoma
We next tested whether targeting CXCL14 with nanoparticles is able to treat glioblastoma. A PS80‐coated nanoparticle, which has recently been shown to be capable of high efficiency siRNA delivery to the brain, 18 was generated to deliver CXCL14 siRNA. Quantitative PCR and western blot analysis confirmed successful KD as manifested by significantly decreased CXCL14 expression in glioblastoma cells (Figure S5A,B). Moreover, cell growth, cell viability, migration, and invasion of glioblastoma cells were effectively inhibited when CXCL14 was silenced by treatment with CXCL14 siRNA‐loaded nanoparticles (Figure S5C–F). Before evaluating in vivo therapeutic efficacy, we determined whether CXCL14 siRNA‐loaded nanoparticles could be delivered into the brain in intact animals. Fluorescence imaging indicated that brains from nude mice injected intravenously with a 5 nmol/kg dose of Dy677‐labeled siRNA‐loaded nanoparticles showed strong fluorescence in the cortex (Figure 6A), indicating that CXCL14 siRNA‐loaded nanoparticles enter the brain upon peripheral administration. Furthermore, we tested whether the combination of PS80‐coated nanoparticles and CXCL14 siRNA chemically modified by selective incorporation of 2’OMe uridine or guanosine nucleosides can increase the in vivo KD efficiency. Western blot analysis of tumor lysates showed that systemic delivery of PS80‐coated nanoparticles loaded with native or 2’OMe‐modified CXCL14 siRNAs resulted in the silencing of CXCL14 in glioblastoma xenografts and PDX expressing the dual GFP and luciferase reporter gene (GBM8901‐lucGFP; GBM09T‐lucGFP) (Figure 6B,C). The KD efficiency of the combination of PS80‐coated nanoparticles and 2’OMe‐modified CXCL14 siRNA was higher than that of the combination of PS80‐coated nanoparticles and native CXCL14 siRNA. Moreover, 2’OMe‐modified CXCL14 siRNA‐loaded nanoparticles were able to further inhibit the phosphorylation of IGF‐1R, ARK, and ERK in GBM8901‐lucGFP and GBM09T‐lucGFP xenografts compared to 2’OMe‐modified Scr. siRNA‐loaded nanoparticles (Figure S5G,H). Therefore, 2’OMe‐modified CXCL14 siRNA‐loaded nanoparticles were chosen to further evaluate the therapeutic efficacy. Mice receiving systemic delivery of 2’OMe‐modified CXCL14 siRNA‐loaded nanoparticles showed significant inhibition of tumor growth and extension of survival time in GBM8901‐lucGFP or GBM09T‐lucGFP xenografts, compared to mice receiving treatment with 2’OMe‐modified scramble siRNA‐loaded nanoparticles (Figure 6D–F). Taken together, these results provide a preclinical proof‐of‐concept for CXCL14 blockade as a systemic therapy for glioblastoma.
FIGURE 6.
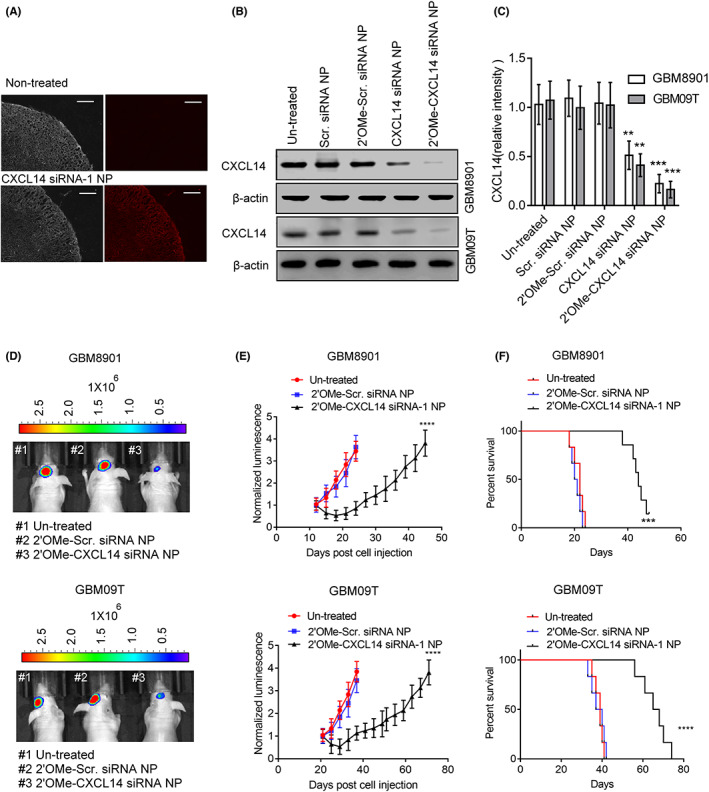
CXC chemokine ligand 14 (CXCL14) siRNA nanoparticle provides therapeutic benefits for glioblastoma. (A) Fluorescence images of brain cortex from nude mice at 1 h after intravenous injection with a 5 nmol/kg dose of Dy677‐labeled siRNA‐loaded nanoparticles. Bars, 100 μm. (B,C) CXCL14 protein levels in the homogenized GBM8901 or GBM09T xenografts with or without scramble (Scr.) siRNA nanoparticles (NP), 2’OMe‐Scr. siRNA NP, CXCL14 siRNA NP, or 2’OMe‐CXCL14 siRNA NP treatment for three consecutive days on day 12 for GBM8901 xenografts or on day 21 for GBM09T xenografts after tumor implantation. Mice received tail vein injection with Scr. siRNA NP, 2’OMe‐Scr. siRNA NP, CXCL14 siRNA NP, or 2’OMe‐CXCL14 siRNA NP at a dose of 75 nmol/kg per day. Error bars, SD within triplicate experiments. **p < 0.01, ***p < 0.001, compared with untreated group. (D) Bioluminescent images (BLI) of GBM8901‐lucGFP or GBM09T‐lucGFP xenograft‐bearing mice received 2’OMe ‐Scr. siRNA NP or 2’OMe‐CXCL14 siRNA NP treatment on day 21 for GBM8901‐lucGFP xenografts or on day 27 for GBM09T‐lucGFP xenografts after tumor implantation. Mice were injected at a dose of 75 nmol/kg per day for five consecutive days on day 12 for GBM8901‐lucGFP xenografts or on day 21 for GBM09T‐lucGFP xenografts after tumor implantation into the tail vein. (E) Mean BLI values associated with longitudinal monitoring of tumor growth for each treatment group. Data are presented as means ± SD (n = 6–8). ****p < 0.001, compared to untreated mice. (F) Corresponding survival curves of GBM8901‐lucGFP or GBM09T‐lucGFP xenograft‐bearing mice for each treatment group
4. DISCUSSION
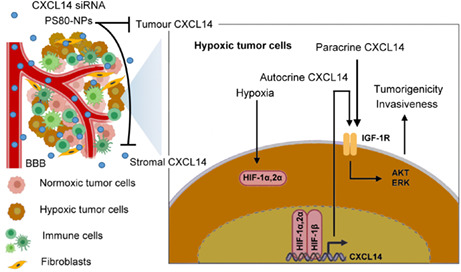
CXC chemokine ligand 14 has dual roles in cancer progression, and its antitumor role or protumor role is dependent on the cancer type. 3 In glioblastoma, emerging evidence has suggested that CXCL14 can promote the tumorigenic properties of human glioblastoma cell lines and the sphere‐forming ability of glioblastoma stem cells. 5 However, stromal CXCL14, but not tumor CXCL14, is thought to promote glioblastoma progression. 5 , 6 Here, we show that the TM, specifically hypoxia, is able to upregulate tumor CXCL14 in vitro and in vivo and that elevated CXCL14 is a predictor for poor outcomes, correlated with tumor grades and HIF‐1 expression in patients with glioblastoma. Mechanistically, hypoxia‐activated CXCL14 transcription is through direct binding of HIF‐1α or HIF‐2α to the HRE in the CXCL14 promoter. Moreover, our results provide the first evidence of hypoxia‐elevated tumor CXCL14 in promoting cell growth, migration, invasion, and neurosphere formation of glioblastoma cells in vitro, as well as tumor growth and infiltration of glioblastoma xenografts in vivo. CXC chemokine ligand 14 induced the protumorigenic effects through activation of IGF‐1R signaling transduction pathway. Additionally, we evaluated the systemic delivery of CXCL14 siRNA encapsulated in PS80‐coated nanoparticles as a potential therapeutic agent for the treatment of glioblastoma. These CXCL14 siRNA nanoparticles significantly inhibited tumor growth and increased survival time in PDX, supporting the notion that CXCL14 is a novel therapeutic target in the treatment of glioblastoma (Figure 7).
FIGURE 7.
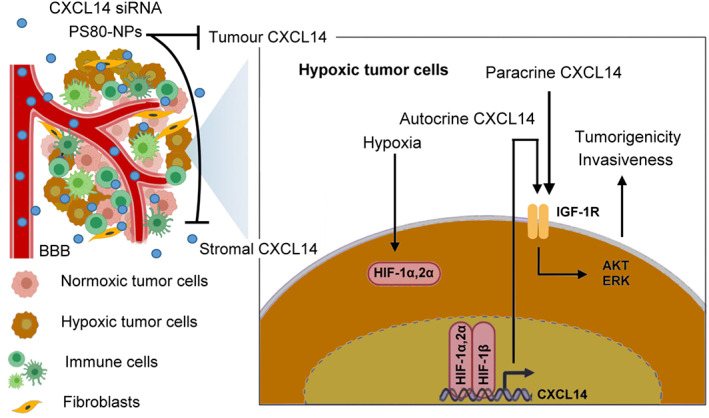
Schematic diagram illustrating the mechanisms and therapeutic impact of hypoxic tumor microenvironment (HTM)‐regulated CXC chemokine ligand 14 (CXCL14) in glioblastoma progression. HTM is able to largely upregulate CXCL14 expression in glioblastoma cells through the binding of hypoxia‐inducible factor‐1α (HIF‐1α) or HIF‐2α to the CXCL14 promoter, which plays a critical role in HTM‐promoted glioblastoma tumorigenicity and invasiveness. HIF‐dependent CXCL14 further activates the insulin‐like growth factor‐1 receptor (IGF‐1R) signaling pathway to regulate the growth, invasiveness, and neurosphere formation of glioblastoma. CXCL14 siRNA nanoparticles with polysorbate 80 (PS80) coating is able to inhibit the effects of tumor or stromal CXCL14 and provide a promising therapeutic strategy in treatment of glioblastoma
Most previous studies claimed that CXCL14 is mainly secreted by stromal cells such as cancer‐associated fibroblasts in the TM and promotes tumor growth and invasion in prostate or breast cancers. 19 , 20 In glioma, it has been reported that mRNA and protein levels of CXCL14 in glioma tissues are higher than those in peritumoral brain edema tissues. 4 , 5 , 6 In agreement with these studies, we also observed similar expression patterns in surgical samples of GBM. However, these findings raise the question of which cells in the TM express CXCL14. Although it is unclear whether glioblastoma cells in the TM are able to express and secrete CXCL14, cultured glioblastoma‐associated stromal cells or primary human astrocytes showed an increase in CXCL14 expression compared with control stromal cells or human glioblastoma cell lines. 5 , 6 These findings suggest that CXCL14 is produced and secreted in the TM, mainly by the glioblastoma stromal cells. However, these cells are not necessarily comparable, as they are derived from different patients and have been cultured under different conditions. To observe the expression of CXCL14 in glioblastoma cells in the TM, murine glioma cells were genetically labeled with GFP or pharmacologically labeled with BioTracker 400 because it lacks the specific markers to distinguish glioblastoma cells from the TM. These cells were implanted into syngenic C57/BL6 mice for in vivo TM studies. Our results clearly showed that parts of glioblastoma cells had higher expression of CXCL14 compared to their stromal cells. Most importantly, the mRNA levels of CXCL14 in isolated glioblastoma cells derived from disaggregated orthotopic glioblastoma were higher than in their original cells before implantation. These findings highlight that the TM is able to upregulate CXCL14 expression in glioblastoma cells.
Hypoxia is a hallmark of a solid TM. 7 , 21 Here, we show for the first time that hypoxia contributes to the TM by upregulating CXCL14 expression in glioblastoma. Using an approach that directly distinguishes and isolates hypoxic tumor subpopulations from solid tumors derived from our previous studies, 12 , 13 our results indicate that both cycling and chronic hypoxic areas are possibly important in TM‐mediated CXCL14 induction in glioblastoma xenografts. Moreover, the expression of CXCL14 increased significantly in isolated cycling and chronic hypoxic cells compared with that in isolated normoxic cells. In vitro studies also confirmed that hypoxic stress could stimulate glioblastoma to express and secrete CXCL14. The expression and secretion of CXCL14 are regulated by HIF‐1α and HIF‐2α in hypoxic glioblastoma cells. Both HIF‐1α or HIF‐2α directly bind to the HRE motif in the CXCL14 promoter and further increases its transactivation. CXC chemokine ligand 14 expression colocalized with high expression of HIF‐1α or HIF‐2α in human glioblastoma specimens also supports this notion. In addition, the public ChIP datasets in JBrowse (a Web‐based genome browser) also showed the binding of HIF‐1α to the CXCL14 promoter in nonglioblastoma cells. These data are in agreement with our previous study showing that CXCL14 is a hypoxia‐responsive gene. 22 Further experiments are required to investigate whether similar effects and mechanisms exist in glioblastoma‐associated stromal cells or other tumor cells. It is also interesting to determine whether IDH mutants affect CXCL14 expression and function, which further contribute to malignant progression and recurrence in glioma because IDH1 activity regulates the levels of HIF‐1α and HIF‐1α target genes. 23
Our studies clearly showed that tumor hypoxia promoted glioblastoma growth and infiltration through CXCL14 produced by tumor cells in an autocrine manner. Glioblastoma CXCL14 promotes tumor progression by binding to its receptor on tumor cells. Recently, several candidate receptors, such as CXCR4, 24 , 25 GPR85, 26 ACKR2, 27 and IGF‐1R, 15 have been shown to bind CXCL14. Unfortunately, previous studies have shown that blockage of CXCR4 did not modify CXCL14‐mediated effects on glioblastoma cells or other cells. 5 , 28 Moreover, GPR85 and ACKR2 are less expressed in glioblastoma cells, 5 suggesting that CXCL14 functions are not mediated by these receptors. Here, we revealed that binding of IGF‐1R by CXCL14 triggered the subsequent activation of IGF‐1R and its downstream mediators, ERK and AKT, involved in the protumorigenic effects for glioblastoma. However, we cannot rule out the possibility that CXCL14 has additional roles or receptors in glioblastoma progression, as it modulates the proliferation of fibroblasts or endothelial cells 20 and regulates immune cell infiltration through recruitment of several immune cell types, including dendritic cells, natural killer cells, and T cells. 2 Therefore, it is possible that CXCL14 secreted by glioblastoma cells can affect the population and function of their stromal cells through a paracrine mechanism. Related questions remain to be addressed in the future.
In this study, we utilized the nanoparticle‐mediated CXCL14 siRNA delivery system as a treatment strategy to allow for effective silencing of CXCL14 in tumor cells or stromal cells in vivo. Recently, PS80‐coated nanoparticles have been shown to deliver high‐efficiency siRNA in the brain and improve brain injuries in mice with traumatic brain injury. 18 In the present study, we further showed that the combination of PS80‐coated nanoparticles and CXCL14 siRNA chemically modified by selective incorporation of 2’OMe uridine or guanosine nucleosides could enhance in vivo KD efficiency compared to the combination of PS80‐coated nanoparticles and native CXCL14 siRNA. The mechanism could arise from the protection of systemically administered siRNA from serum nucleases and extension of siRNA in vivo half‐life, according to previous studies. 29 Our results also provide evidence that the treatment of PDX with 2’OMe‐modified CXCL14 siRNA encapsulated in PS80‐coated nanoparticles effectively suppressed tumor growth in vivo and extended the survival time in these mice. Although more work is needed to optimize the therapeutic dose and to address the problem of potential side‐effects, these findings indicate that there is clinical utility of CXCL14 siRNA targeting for the treatment of glioblastoma with high therapeutic efficiency.
FUNDING INFORMATION
Ministry of Science and Technology of the Republic of China, Grant/Award Number: MOST 110‐2628‐B‐039‐006, MOST 109‐2628‐B‐039‐005, 108‐2628‐B‐039‐002; China Medical University and Hospital, Grant/Award Number: DMR‐110‐160, DMR‐110‐246, DMR‐110‐092, DMR‐109‐149, DMR‐110‐149, DMR‐109‐114, DMR‐108‐145; China Medical University Hsinchu Hospital, Grant/Award Number: CMUHCH‐DMR‐109‐002.
CONFLICT OF INTEREST
The authors have declared no potential conflict of interest.
ETHICAL APPROVAL
Approval of the research protocol by an institutional review board: The study protocol was approved by the Ethical Review Board of China Medical University (Taiwan).
Informed consent: All patients or their guardians were given and accepted informed consent.
Registry and the registration no. of the study/trial: The clinical studies were approved by the Ethical Review Board of China Medical University (Taiwan) (registration no. CMUH108‐REC3‐163).
Animal studies: The animal experiments were approved by the Institutional Animal Care and Use Committee (IUCAC) at China Medical University, Taiwan (registration no. CMUIACUC‐2020‐029).
Supporting information
Figures S1–S5
Appendix S1
ACKNOWLEDGMENTS
Grant support was provided by the Ministry of Science and Technology of the Republic of China (Grant Nos. MOST 110‐2628‐B‐039‐006, MOST 109‐2628‐B‐039‐005, 108‐2628‐B‐039‐002), the China Medical University and Hospital (Grant Nos. DMR‐110‐160, DMR‐110‐246, DMR‐110‐092, DMR‐109‐149, DMR‐110‐149, DMR‐109‐114, DMR‐108‐145), and the China Medical University Hsinchu Hospital (CMUHCH‐DMR‐109‐002).
Wei S‐T, Chiang J‐Y, Wang H‐L, et al. Hypoxia‐induced CXC chemokine ligand 14 expression drives protumorigenic effects through activation of insulin‐like growth factor‐1 receptor signaling in glioblastoma. Cancer Sci. 2023;114:174‐186. doi: 10.1111/cas.15587
Wei and Chiang contributed equally to this work.
REFERENCES
- 1. Hara T, Tanegashima K. Pleiotropic functions of the CXC‐type chemokine CXCL14 in mammals. J Biochem. 2012;151:469‐476. [DOI] [PubMed] [Google Scholar]
- 2. Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm. 2016;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westrich JA, Vermeer DW, Colbert PL, Spanos WC, Pyeon D. The multifarious roles of the chemokine CXCL14 in cancer progression and immune responses. Mol Carcinog. 2020;59:794‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fazi B, Felsani A, Grassi L, et al. The transcriptome and miRNome profiling of glioblastoma tissues and peritumoral regions highlights molecular pathways shared by tumors and surrounding areas and reveals differences between short‐term and long‐term survivors. Oncotarget. 2015;6:22526‐22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fazi B, Proserpio C, Galardi S, et al. The expression of the chemokine CXCL14 correlates with several aggressive aspects of glioblastoma and promotes key properties of glioblastoma cells. Int J Mol Sci. 2019;20:2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeng A, Yin J, Wang Z, et al. miR‐17‐5p‐CXCL14 axis related transcriptome profile and clinical outcome in diffuse gliomas. Onco Targets Ther. 2018;7:e1510277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrova V, Annicchiarico‐Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266‐276. [DOI] [PubMed] [Google Scholar]
- 9. Korbecki J, Kojder K, Kapczuk P, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors: a review of literature. Int J Mol Sci. 2021;22:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korbecki J, Kojder K, Barczak K, et al. Hypoxia alters the expression of CC chemokines and CC chemokine receptors in a tumor: a literature review. Int J Mol Sci. 2020;21:5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groblewska M, Litman‐Zawadzka A, Mroczko B. The role of selected chemokines and their receptors in the development of gliomas. Int J Mol Sci. 2020;21:3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chou CW, Wang CC, Wu CP, et al. Tumor cycling hypoxia induces chemoresistance in glioblastoma multiforme by upregulating the expression and function of ABCB1. Neuro Oncol. 2012;14:1227‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsieh CH, Lin YJ, Wu CP, Lee HT, Shyu WC, Wang CC. Livin contributes to tumor hypoxia‐induced resistance to cytotoxic therapies in glioblastoma multiforme. Clin Cancer Res. 2015;21:460‐470. [DOI] [PubMed] [Google Scholar]
- 14. Hsieh CH, Shyu WC, Chiang CY, Kuo JW, Shen WC, Liu RS. NADPH oxidase subunit 4‐mediated reactive oxygen species contribute to cycling hypoxia‐promoted tumor progression in glioblastoma multiforme. PLoS One. 2011;6:e23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng CL, Yang SC, Lai CY, et al. CXCL14 Maintains hESC Self‐Renewal through Binding to IGF‐1R and Activation of the IGF‐1R Pathway. Cells. 2020;9:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tirro E, Massimino M, Romano C, et al. Prognostic and therapeutic roles of the insulin growth factor system in glioblastoma. Front Oncol. 2020;10:612385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC‐A12, a human IgG1 monoclonal antibody to the insulin‐like growth factor I receptor. Clin Cancer Res. 2007;13:5549s‐5555s. [DOI] [PubMed] [Google Scholar]
- 18. Li W, Qiu J, Li XL, et al. BBB pathophysiology‐independent delivery of siRNA in traumatic brain injury. Sci Adv. 2021;7:eabd6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Augsten M, Sjoberg E, Frings O, et al. Cancer‐associated fibroblasts expressing CXCL14 rely upon NOS1‐derived nitric oxide signaling for their tumor‐supporting properties. Cancer Res. 2014;74:2999‐3010. [DOI] [PubMed] [Google Scholar]
- 20. Augsten M, Hagglof C, Olsson E, et al. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi‐modal stimulator of prostate tumor growth. Proc Natl Acad Sci U S A. 2009;106:3414‐3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoogsteen IJ, Marres HA, van der Kogel AJ, Kaanders JH. The hypoxic tumour microenvironment, patient selection and hypoxia‐modifying treatments. Clin Oncol. 2007;19:385‐396. [DOI] [PubMed] [Google Scholar]
- 22. Lee HT, Liu SP, Lin CH, et al. A Crucial Role of CXCL14 for Promoting Regulatory T Cells Activation in Stroke. Theranostics. 2017;7:855‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao S, Lin Y, Xu W, et al. Glioma‐derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF‐1alpha. Science. 2009;324:261‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collins PJ, McCully ML, Martinez‐Munoz L, et al. Epithelial chemokine CXCL14 synergizes with CXCL12 via allosteric modulation of CXCR4. FASEB J. 2017;31:3084‐3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanegashima K, Suzuki K, Nakayama Y, et al. CXCL14 is a natural inhibitor of the CXCL12‐CXCR4 signaling axis. FEBS Lett. 2013;587:1731‐1735. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Weng X, Wang L, et al. HIC1 deletion promotes breast cancer progression by activating tumor cell/fibroblast crosstalk. J Clin Invest. 2018;128:5235‐5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sjoberg E, Meyrath M, Milde L, et al. A novel ACKR2‐dependent role of fibroblast‐derived CXCL14 in epithelial‐to‐mesenchymal transition and metastasis of breast cancer. Clin Cancer Res. 2019;25:3702‐3717. [DOI] [PubMed] [Google Scholar]
- 28. Otte M, Kliewer A, Schutz D, Reimann C, Schulz S, Stumm R. CXCL14 is no direct modulator of CXCR4. FEBS Lett. 2014;588:4769‐4775. [DOI] [PubMed] [Google Scholar]
- 29. Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173‐178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S5
Appendix S1


