Abstract
Because of its low critical temperature and pressure levels, supercritical carbon dioxide (scCO2) is the most widely used supercritical fluid in the supercritical fluid extraction (SFE) technique. Alizarin was extracted from madder roots (Rubia tinctorum) using scCO2 under different conditions of co-solvent ratio (0–50%), temperature (45–95 °C), pressure (150–250 bar), extraction time (15–120 min), and flow rate (5–9 mL/min). Based on alizarin recovery and minimization of environmental risk, the optimum conditions were determined. SFE was optimum at 90% CO2:10% methanol (Me), 65 °C, 250 bar, 45 min, and 9 mL/min. The alizarin recovery, and its content in R. tinctorum extract (RE) under the optimum conditions were 1.34 g/kg roots, and 6.42%, respectively. Using conventional dyeing methods, wool fabrics were dyed with RE at different concentrations (2–6%). Various types of mordants were also used in the dyeing process, including chemical and bio-mordants. Color and fastness properties of dyed wool fabrics were evaluated based on RE concentration and mordant type. A higher RE concentration and the use of mordants, specifically Punica granatum (P. granatum) peels, increased the color characteristics. RE and dyed fabrics exhibited good antibacterial activity against the tested bacterial strains, especially Pseudomonas aeruginosa and Escherichia coli.
Subject terms: Soft materials, Chemistry
Introduction
Eco-friendly approaches in the textile industry can be influenced by strict environmental regulations for textiles and garments set by countries concerned with the environment and human health1,2. Because of its wide range of functional properties and environmental benefits, sustainable dyeing with natural colorants has gained attention3,4. Synthetic dyes are toxic and cause allergic reactions; therefore, natural dyes have been chosen as sustainable sources of colorants5,6. Some natural dyes exhibit color performance comparable to that of some highly rated synthetic dyes in both natural and synthetic fabrics7–10. Anthraquinone, anthocyanin, flavonoid dyes, and polyphenolic compounds are the main components of natural dyes, which have a red shade that is relatively stable and light fast. In terms of natural dyes based on anthraquinones, R. tinctorum (madder) is one of the oldest. R. tinctorum is a perennial herbaceous plant that belongs to the Rubiaceae family. In Central Asia, Iran, and Egypt, it has been widely cultivated since 1500 BC. As a natural source of red, pink, orange, purple, gray, and brown, as well as the most valuable black and brunette shades with high color depth, R. tinctorum is widely regarded as the most popular11–16. In traditional extraction procedures, organic solvents may cause thermal degradation, oxidative changes in the components, and the generation of unacceptable residues or solvent traces17,18. These concerns have led to the development of innovative environmentally friendly processes using renewable and non-toxic solvents in response to environmental constraints, extremely strict public health legislation, and market trends toward ecology19. Natural dyes can be extracted using scCO220. As an alternative to conventional extraction methods, supercritical fluid extraction follows green chemistry principles21.
Under mild conditions (low critical pressure and temperature), extraction occurs in a closed system without oxygen22. Because of the inert atmosphere, the absence of light, and the short extraction time, this method provides significant benefits for the extraction of pigments from R. tinctorum plants. Furthermore, SFE is more selective than conventional extraction methods and strives to enhance the recovery and quality of the extracted components. CO2 is commonly used as a solvent in SFE. CO2 is a low-cost, non-flammable, and non-toxic readily available solvent that, owing to its high volatility, can be easily removed from the final extract while maintaining the biological qualities of bioactive substances as an alternative to other solvents7,23–26. The co-solvents may enhance CO2 solubilizing capacity and improve polar molecule solubility27. A considerable amount of research is being conducted worldwide on extraction under scCO228–33. As a result of the formation of complexes between the functional groups of the dye and fabrics, chemical mordants such as metallic salts are commonly used in textile dyeing to improve shade depth and fastness34. Heavy metal ions generated during the dyeing process may pose health and environmental hazards35,36. As a result, tannin-rich plants have recently become popular as bio-mordants34,37–39.
This study aimed to extract alizarin pigment from R. tinctorum using SFE technology as a green alternative to conventional extraction methods. Furthermore, various parameters, including the co-solvent ratio, temperature, pressure, extraction time, and flow rate, were optimized to maximize alizarin recovery and content. Wool fabrics were then dyed using R. tinctorum root extract obtained under optimal extraction conditions using the conventional dyeing process. In this study, the effects of extract concentration and chemical and natural mordants on the coloring properties of dyed wool fabrics were evaluated. Furthermore, RE and dyed wool fabrics were evaluated for their antibacterial properties.
Experimental sections
Materials
R. tinctorum roots were cultivated and harvested from the Faculty of Agriculture, Damietta University, Egypt. The collection of plant roots was complied with relevant institutional, national, and international guidelines and legislation. To remove impurities, they were thoroughly washed with distilled water and dried at room temperature. Using a laboratory-scale mill (M 20; IKA, Staufen, Germany), the dried roots were ground. Bleached wool fabrics (100%) were provided by Misr for the spinning and weaving Company, Mahalla El-Kobra, Egypt. The solvent used for the supercritical fluid extraction was CO2 (99.6%, supplied by NETCO Industrial Company, Cairo, Egypt), and the co-solvent was gradient-grade methanol (99.8% purity). For high-performance liquid chromatography analysis, alizarin red, HPLC-grade acetonitrile (99.8%) and ammonium acetate were used. As part of the conventional dyeing process, aluminum sulphate was used as a chemical mordant, anionic dispersing agent (Dispex, DyStar, Cairo, Egypt), and acetic acid. Chemicals were purchased from Sigma-Aldrich for this study.
Procedures
Extraction of alizarin pigment using scCO2
The supercritical CO2 extraction system consisted of a CO2 cylinder, a chiller (model Julabo FL601), a semi-preparative CO2 pump (model JASCO PU-4386), a co-solvent pump (model JASCO PU-4180), an interface box (model LC-NeII/ADC), a pressure regulator (model JASCO BP-4340), a heater controller (model HC-2068–01), a temperature and speed controller (model EYELARCX-1000 H), a 50 mL high-pressure stainless steel vessel, and a vial for extract collection (model HE-4340-01-200). The maximum flow rate of the circulation pump was 10 mL/min40.
In the extraction process, approximately 4 g of dried R. tinctorum roots was placed in the vessel. Subsequently, the system was closed. The chiller was set to − 5 °C, and CO2 was cooled to maintain liquid conditions. All the extraction parameters (composition ratio, temperature, pressure, time, and flow rate) were controlled using a software system. Liquid CO2 was preheated using a heating jacket to reach set temperature levels. The extraction procedure consists of two phases: static and dynamic. The static time used in this study was approximately 20 min. During static time, scCO2 was supplied to the extractor, while the outlet back pressure valve was closed. Static mode is the time at which the plant is exposed to a constant scCO2 amount for a certain time. This allows scCO2 to penetrate the plant matrix and dissolve target components. After the static time, the valve was adjusted to allow cooled, liquefied scCO2 to pass through the extracting vessel (dynamic conditions). In the dynamic mode, the plant matrix is regularly fed with fresh CO2, and the extracted components are moved from the extraction vessel into the collecting system41,42.
After the extraction time had expired, the temperature was reduced to room temperature and the pressure was reduced to atmospheric level. This decomposition causes scCO2 to lose its solubility and return to the gas phase. All extracts were collected in collection vials and stored in a freezer at − 20 °C until further analysis.
Determination of alizarin using high-performance liquid chromatography (HPLC)
Chromatographic measurements were performed using a high-performance liquid chromatography system (JASCO, Tokyo, Japan). This system was equipped with an SFC autosampler (model AS-4350), RHPLC pump (model PU-4180), CO2 pump (model PU-4380), interface box (model LC-NeII/ADC), UV/Vis detector (model UV-4070), column oven (model CO-4060), back-pressure regulator (model BP-4340), microsorb column-MV 100-5 C18 250 × 4.6 mm (Agilent Technologies, Santa Clara, USA), and JASCO software for determination and data analysis.
For the quantitative determination of alizarin, five standard solutions of alizarin at different concentrations (25, 50, 100, 150, and 200 μg/mL) were prepared and dissolved in a mixture of methanol and distilled water at a volume ratio of 50:50. Calibration graphs were constructed by plotting the peak areas of alizarin.
The mobile phase consisted of a mixture of acetonitrile and ammonium acetate (20 mM) at a ratio of 60:40 (v/v). Before use, the mobile phase was prepared, filtered using a micro syringe filter (0.45μm), and degassed using an ultrasonicator (model S30H, Elmasonic, Singen, Germany) for 20 min. The injection volume was 20 μL in triplicate. The elution program was performed for 15 min at 30°C. Detection was performed at an absorbance of 254 nm. The flow rate was set at 1 mL/min43.
Preparation of bio-mordants
The peels of P. granatum, flowers of Rhus coriaria (R. coriaria), and powdered leaves of Camellia sinensis (C. sinensis) were utilized as biomordants during wool dyeing. They belong to the families Lythraceae, Anacardiaceae, and Theaceae, respectively. The peels of the P. granatum and R. coriaria flowers were washed with distilled water and dried at room temperature. Dried peels and flowers were ground into a fine powder. P. granatum peel, R. coriaria flowers, and C. sinensis leaves were purchased from a local market in Damietta, Egypt.
Dyeing process
The wool fabric was scoured in a water bath containing 2 g/L non-ionic detergent (Sera Fast CRD, DyStar, Cairo, Egypt) for 15 minutes, rinsed, and dried at room temperature before mordanting. At a liquor-to-fabric ratio of 1:40, the fabric was pre-mordanted in a bath containing 50% weight of fabric (OWF) bio-mordant or 5% (OWF) aluminum sulfate as a chemical mordant. Over a period of 20 minutes, scouring wool was added to the mordanting bath at 25 °C and the temperature was gradually increased to 45 °C. At this temperature, mordanting was performed for 30 minutes. Subsequently, the samples were thoroughly rinsed and dried. The dye, RE, was dispersed by adding 2 g/L of an anionic dispersing agent. Acetic acid was used to adjust the pH to 3. Various RE concentrations of 2, 4, and 6% (OWF) were used to dye mordanted wool. Over 30 minutes, the temperature was increased from 45 °C to 90 °C, and then maintained at 90 °C for 60 minutes. The fabric was washed with cold water after dyeing. The sample was soaped with a non-ionic detergent at a concentration of 2 g/L at 70 °C for 20 min, rinsed, and dried at room temperature.
Evaluation of color characteristics and fastness properties
A spectrophotometer (Konica Minolta, CM-3600, Tokyo, Japan) was used to measure color strength (K/S values) and color coordinates in terms of CIELab (lightness [L*], redness-greenness value [a*], yellowness-blueness value [b*], chroma [C*], and hue [h]). After calibrating the instrument, each sample was assayed at three positions (left, central, and right). The color strength (K/S) values were determined in the visible region of the spectrum (400–700 nm) according to the Kubelka –Munk equation, as follows:
| 1 |
This equation describes the relationship between reflectance and absorbance, where R is the reflectance of the dyed sample, K is the absorption coefficient and S is the scattering coefficient.
The color difference between any dyed and blank sample was calculated as the square root of the squares of the corresponding L*, a*, and b* differences, as follows:
| 2 |
where ∆E is the difference between the blank wool and dyed wool fabrics, a* is the red (+)/green (-) ratio, b* is the yellow (+)/blue (-) ratio, and L* is the lightness from black (0) to white (100). The leveling properties of the dyed wool fabrics were investigated. The UV/Vis spectrum of alizarin in the dye bath was measured before and after the dyeing process using a UV/Vis spectrophotometer (Alpha-1860, Noble, IN, USA). The color fastness of the dyed wool samples was assessed according to ISO standard methods. The specific tests were as follows: (AATCC Test Method 61-1996) for wash fastness44, (AATCC Test Method 61 (2A)-1996) for durability testing45, (AATCC Test Method 16-2004) for light fastness46, and (AATCC Test Method 8-2001) for dry and wet rubbing fastness47. Color changes and staining were assessed using grayscale scores ranging from 1 (worst) to 5 (best).
Antibacterial activity
Wool fabrics dyed with RE and RE obtained via the SFE technique were evaluated according to AATCC (147-2004)48. Gram-positive (Bacillus cereus) and gram-negative (E. coli, Salmonella typhi, and P. aeruginosa) bacteria were tested for antibacterial activity. Dimethyl sulfoxide (DMSO) was used as a solvent to dissolve the extract at a concentration of 10 mg/mL to evaluate its antibacterial activity. The dyed samples were cut into squares with dimensions of 10 mm × 10 mm. To compare the antibacterial activities of standard antibiotics with those of the tested bacteria, tetracycline (30 g) and ciprofloxacin (10 g) were also tested. At 37°C, the bacterial strains were incubated for 24 hours. A growth inhibition zone diameter (mm) was determined after incubation49,50.
Statistical analysis
A Costat program version 6.311 (CoHort software, Monterey, USA) was used to analyze the data obtained from this study. Statistical analysis of variance (ANOVA) was used to compare all samples. The standard deviation (SD) was calculated, and significant variations among all means were analyzed using Duncan’s new range test at P = 0.0551–53. Furthermore, each sample was statistically analyzed three times.
Results and discussion
Design of experiment
Several experiments were conducted to study the effect of different parameters on alizarin recovery and its content in RE. Among these parameters were a solvent composition ratio of CO2: Me (50%:50%, 70%:30%, 80%:20%, 90%:10%, and 100%:0%), temperature (45, 55, 65, 75, 85, and 95°C), pressure (150, 175, 200, 225, and 250 bar), extraction time (15, 30, 45, 60, 90, and 120 min), and flow rate (5,7, and 9 mL/min). Based on alizarin recovery and minimizing environmental risk, the optimum conditions for alizarin extraction were selected. HPLC UV/Vis was used to analyze the alizarin concentration in RE.
Evaluation of the optimum conditions for alizarin extraction
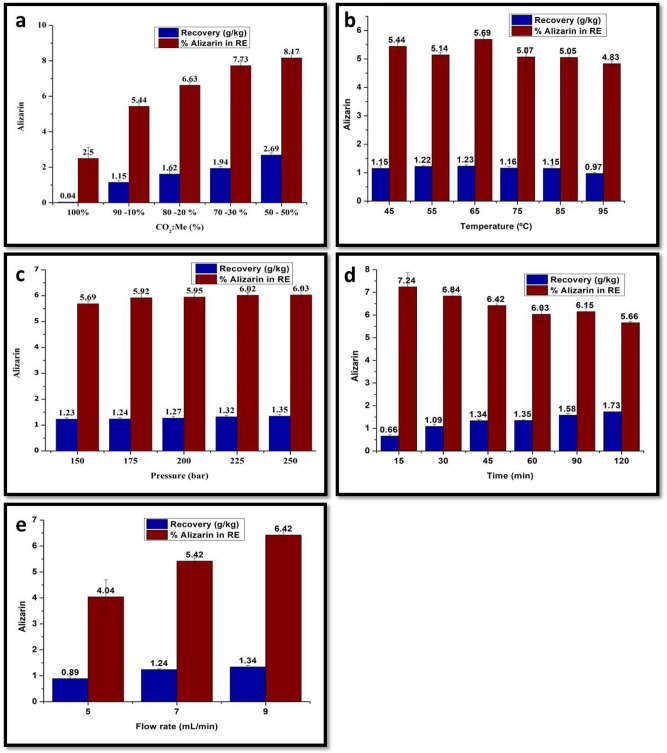
We investigated the effect of various parameters on the efficiency of alizarin extraction from R. tinctorum roots using scCO2. The optimization of extraction conditions is a crucial step in the development of the SFE method. Thus, these parameters, such as temperature, pressure, time, and flow rate, were studied and analyzed statistically, as shown in Table 1 and Fig. 1.
Table 1.
The alizarin yield and statistical analysis of different SFE conditions (a–mMeans within a column followed by the same letter(s) are not significantly different according to Duncan’s multiple range test (P = 0.05)).
| Experiment conditions | Alizarin (g per Kg roots) | Alizarin% (g per100 g extract) | |||||
|---|---|---|---|---|---|---|---|
| Composition ratio | Temperature (ºC) | Pressure (bar) | Time (min) | Flow rate (mL/min) | |||
| CO2 (%) | Me (%) | ||||||
| 100 | – | 45 ± 1 | 150 ± 0.5 | 60 | 9 | 0.04 ± 0.00 i | 2.50 ± 0.63 m |
| 90 | 10 | 1.15 ± 0.11 f. | 5.44 ± 0.20 hij | ||||
| 80 | 20 | 1.62 ± 0.19 cd | 6.63 ± 0.18 de | ||||
| 70 | 30 | 1.94 ± 0.10 b | 7.73 ± 0.32 ab | ||||
| 50 | 50 | 2.69 ± 0.13 a | 8.17 ± 0.33 a | ||||
| 90 | 10 | 45 ± 1 | 150 ± 0.5 | 60 | 9 | 1.15 ± 0.11 f. | 5.44 ± 0.20 hij |
| 55 ± 1 | 1.22 ± 0.04 ef | 5.14 ± 0.10 ijk | |||||
| 65 ± 1 | 1.23 ± 0.05 ef | 5.69 ± 0.11 ghi | |||||
| 75 ± 1 | 1.16 ± 0.06 f. | 5.07 ± 0.33 jk | |||||
| 85 ± 1 | 1.15 ± 0.10 f. | 5.05 ± 0.14 jk | |||||
| 95 ± 1 | 0.97 ± 0.03 g | 4.83 ± 0.10 k | |||||
| 90 | 10 | 65 ± 1 | 150 ± 0.5 | 60 | 9 | 1.23 ± 0.05 ef | 5.69 ± 0.11 ghi |
| 175 ± 0.5 | 1.24 ± 0.05 ef | 5.92 ± 0.20 fgh | |||||
| 200 ± 0.5 | 1.27 ± 0.06 ef | 5.95 ± 0.24 fgh | |||||
| 225 ± 0.5 | 1.32 ± 0.05 e | 6.02 ± 0.11 fg | |||||
| 250 ± 0.5 | 1.35 ± 0.03 e | 6.03 ± 0.30 fg | |||||
| 90 | 10 | 65 ± 1 | 250 ± 0.5 | 15 | 9 | 0.66 ± 0.06 h | 7.24 ± 0.63 bc |
| 30 | 1.09 ± 0.09 f. | 6.84 ± 0.31 cd | |||||
| 45 | 1.34 ± 0.06 e | 6.42 ± 0.19 def | |||||
| 60 | 1.35 ± 0.03 e | 6.03 ± 0.30 fg | |||||
| 90 | 1.58 ± 0.08 d | 6.15 ± 0.35 efg | |||||
| 120 | 1.73 ± 0.01 c | 5.66 ± 0.06 ghi | |||||
| 90 | 10 | 65 ± 1 | 250 ± 0.5 | 45 | 5 | 0.89 ± 0.13 g | 4.04 ± 0.66 l |
| 7 | 1.24 ± 0.03 ef | 5.42 ± 0.12 hij | |||||
| 9 | 1.34 ± 0.06 e | 6.42 ± 0.19 def | |||||
Figure 1.
Effects of (a) solvent composition ratio, (b) temperature, (c) pressure, (d) extraction time, (e) flow rate on alizarin recovery (g/kg roots), and alizarin content (%) in RE.
Effect of solvent composition ratio (CO2: Me)
The co-solvent in this study was methanol because it is the most selective solvent for the extraction of alizarin54. The influence of the co-solvent ratio on the extraction yield of alizarin was the most significant, as shown in Table 1. Alizarin recovery and its percentage in the RE increased as the co-solvent ratio increased. Alizarin contains hydroxyl groups that form hydrogen bonds with other methanol hydroxyl groups, resulting in this effect. Solvent polarity and density are increased by methanol. Moreover, the co-solvent causes matrix swelling, which facilitates solvent–compound contact and acts as a carrier for alizarin55. As a result of its toxic effects, an increase in the methanol ratio poses an environmental risk. Using a solvent composition ratio of 90% CO2:10% Me led to a significant increase in alizarin recovery (1.15 g/kg) over extraction without co-solvent addition (0.04 g/kg). The extract obtained at a ratio of 90% CO2:10% Me contained more alizarin in RE (5.44%) than that obtained at a ratio of 100% CO2. As the methanol ratio increased, both alizarin recovery and its content in RE increased. On the other hand, the amount of toxic organic solvent (methanol) used was reduced to 10% instead of 50% from an environmental perspective. In order to avoid the harmful effects of large amounts of organic solvents, optimal conditions of temperature, pressure, extraction time, and flow rate were investigated in order to maximize alizarin recovery at a low co-solvent ratio (10% Me).
Effect of temperature on alizarin extraction efficiency
Extraction efficiency is heavily influenced by temperature. In this study, alizarin recovery was determined at different temperatures between 45 and 95 °C under constant conditions for all other parameters. As the temperature increased above 65 °C, the recovery of alizarin decreased, and as the temperature decreased, the recovery increased. The temperature affects extraction in two ways: it reduces solvent viscosity and increases diffusivity of the compound, thereby improving extraction efficiency. The density of scCO2 can be reduced at high temperatures as a result of thermal degradation of matrix components or excessive co-extraction55. In order to minimize the degradation of thermolabile compounds, the supercritical fluid extraction temperature should be adjusted between 35 and 60 °C. As shown in Fig. 1, 65 °C was the most suitable temperature for alizarin extraction from R. tinctorum roots. For further optimization of pressure, extraction time, and flow rate parameters, the optimum temperature (65 °C) was adjusted.
Effect of pressure on the efficiency of alizarin extraction
The pressure is another parameter that affects the extraction efficiency of alizarin using the SFE technique. To study the effect of pressure on the extraction efficiency of alizarin, dried R. tinctorum roots were extracted under various pressure levels (150, 175, 200, 225, and 250 bar). The co-solvent ratio, temperature, extraction time, and flow rate were all constant at 90 CO2:10 Me, 65°C, 60 minutes, and 9 mL/min, respectively.
As pressure increased, alizarin recovery increased. Under 150 bar pressure, alizarin recovery from R. tinctorum roots was approximately 1.23 g/kg roots and increased to 1.34 g/kg under 250 bar pressure. In addition, the alizarin content in the RE With an increase in pressure from 150 bar to 250 bar, the alizarin content in the RE increased from 5.69 to 6.18% (Fig. 1 and Table 1). The density and diffusivity of CO2 increased with increasing pressure. By increasing pressure, the fluid interacts with the matrix, which improves the solubility and extraction efficiency of alizarin. Based on the optimization study of other extraction parameters, including time and flow rate, the optimum pressure level of 250 bar was selected to maximize alizarin extraction efficiency.
Effect of extraction time on the efficiency of alizarin extraction
The effect of extraction time on alizarin extraction efficiency can be used to determine the optimum time for alizarin recovery in SFE. An incomplete extraction can occur if the extraction time is too short. Conversely, if the extraction time is too long, it wastes time and solvent, resulting in a higher extraction cost56,57. At a constant flow rate of 9 mL/min, R. tinctorum roots were extracted for 15, 30, 45, 60, 90, and 120 min, with all other parameters set to their optimized conditions. As extraction time increased, alizarin recovery increased from 0.66 to 1.73 g/kg roots.
After 45 min, the rate of increase slowed down. To save time and money, we conducted the following study on flow rate optimization for 45 minutes58,59. The increased contact time between scCO2 and the sample improved extraction efficiency and maximized alizarin content in the RE. As a result of the extraction of other components from R. tinctorum roots besides alizarin, the percentage value of alizarin in the RE decreased with time (Fig. 1and Table 1).
Effect of flow rate on the efficiency of alizarin extraction
As a final step, the flow rate of the supercritical fluid was investigated in relation to the alizarin extraction efficiency, either the recovery or the content of alizarin in the RE obtained using the SFE technique. An optimization study of the flow rate determined the optimal composition ratio, temperature, pressure, and extraction time of 90% CO2:10% Me, 65°C, 250 bar, and 45 min. For alizarin extraction using SFE, flow rates of 5, 7, and 9 mL/min were tested. Increasing flow rate of supercritical fluid resulted in a significant increase in alizarin recovery from 0.89 to 1.34 g/kg roots. Additionally, the alizarin content in the extract increased with increasing flow rate, as shown in Fig. 1 and Table 1. By increasing the flow rate, the mass transfer resistance around solid particles decreases, allowing the solute to be easily transported to the supercritical fluid, leaving the extractor vessel saturated. As a result, alizarin extraction becomes more efficient56. According to the results, co-solvent ratio, flow rate, and extraction time were more effective for alizarin recovery and content than temperature and pressure. In the root samples extracted at various pressure levels, there were no significant differences in pressure, as shown in Fig. 1 and Table 1.
Testing of dyed wool fabrics
Evaluation of color and fastness properties
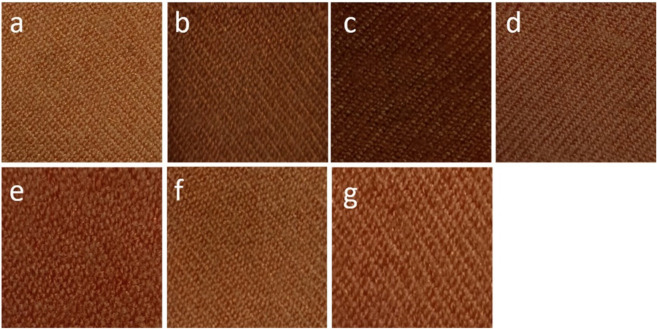
The colorimetric properties of wool dyed with RE are listed in Table 2. Alizarin (the main pigment in RE) produced rich colors on wool fabrics. Based on the results in Table 2, all dyed samples were found to be in the red-yellow zone (a > b). Depending on the RE concentration and the mordant used, the samples take on an orange hue. After adding the mordant, the shades were reddish-brown for Al2(SO4)3, heavily reddish-orange for P. granatum, brown for C. sinensis, and bright orange for R. coriaria, as shown in Fig. 2. By comparing the mordanted and unmordanted samples, it was found that the color strength (K/S) values increased, and the lightness (L*) and chroma (C*) values increased in brighter shades. This increase in K/S can be attributed to the fact that P. granatum peel, R. coriaria, and C. sinensis are rich in tannins. The phenolic hydroxyl groups of tannins form hydrogen bonds with the reactive groups in wool fabrics. These bonds improve the adsorption of alizarin on the fabric14. The color difference (ΔE) was calculated, which revealed excellent leveling characteristics.
Table 2.
Colorimetric properties of wool samples dyed with RE (Color lightness (L*), the degree of redness (+ ve) and greenness (-ve) (a*), and the degree of yellowness (+ ve) and blueness (-ve) (b*), the color strength (K/S)).
| Type | Color parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Mordant | RE concentration (%) | L* | a* | b* | ∆E* | C* | h | K/S at 360 nm |
| – | 2 | 30.06 ± 0.32 c | 5.58 ± 0.16 e | 5.87 ± 0.18 d | 56.83 ± 0.32 c | 8.1 ± 0.19 d | 46.48 ± 0.96 a | 9.97 ± 0.14 d |
| – | 4 | 31.50 ± 0.36 b | 8.58 ± 0.39 d | 7.64 ± 0.21 c | 55.73 ± 0.29 d | 11.49 ± 0.43 c | 41.68 ± 0.57 bc | 10.46 ± 0.11 c |
| – | 6 | 26.70 ± 0.48 d | 4.12 ± 0.46 f. | 4.17 ± 0.48 e | 60.23 ± 0.49 a | 5.86 ± 0.67 e | 45.36 ± 0.04 a | 11.13 ± 0.16 b |
| Al2(So4)3 | 4 | 32.47 ± 0.35 a | 9.89 ± 0.07 c | 8.90 ± 0.38 b | 54.97 ± 0.29 e | 13.30 ± 0.26 b | 41.97 ± 1.27 b | 10 ± 0.11 d |
| P. granatum | 4 | 30.44 ± 0.52 c | 12.15 ± 0.31 a | 10.18 ± 0.26 a | 57.40 ± 0.44 b | 15.85 ± 0.39 a | 39.96 ± 0.21 d | 12.11 ± 0.25 a |
| C. sinensis | 4 | 32.24 ± 0.11 a | 12.24 ± 0.21 a | 10.42 ± 0.20 a | 55.67 ± 0.15 d | 16.07 ± 0.28 a | 40.40 ± 0.16 cd | 11.06 ± 0.12 b |
| R. coriaria | 4 | 29.89 ± 0.11 c | 11.35 ± 0.40 b | 7.77 ± 0.60 c | 57.83 ± 0.06 b | 13.75 ± 0.66 b | 34.34 ± 1.19 e | 11.27 ± 0.18 b |
a–fMeans within a column followed by the same letter(s) are not significantly different according to Duncan’s multiple range test (P = 0.05).
Figure 2.
Wool fabrics dyed using (a) 2% RE without mordant, (b) 4% RE without mordant, (c) 6% RE without mordant, (d) 4% RE with Al2(SO4)3, (e) 4% RE with P. granatum, (f) 4% RE with C. sinensis, (g) 4% RE with R. coriaria.
Figure 3 depicts the absorbance of alizarin before and after exhaustion by the wool fabric in the range of 200–800 nm. The spectrum of alizarin before dyeing showed a sharp absorption band at 445 nm60,61, indicating the presence of the alizarin pigment. After dyeing, the spectrum of alizarin clearly decreased, confirming the exhaustion of alizarin by wool fabric.
Figure 3.
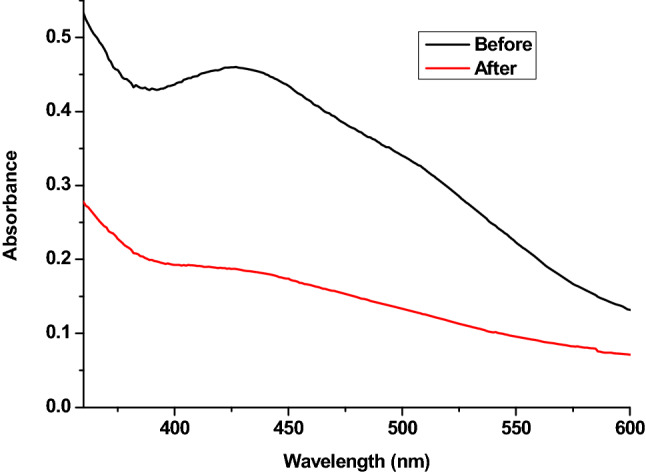
UV/ Visible spectrum of RE dyeing solution before and after dyeing.
The color fastness, including washing, light, and rubbing, was evaluated according to grayscale, and is presented in Table 3. Even after the durability test, dyed samples (without mordant and with mordant) showed excellent wash fastness (4-5 or 5). Fastness to dry and wet rubbing was good to excellent (4–5). The fastness to light ranged from good to very good.
Table 3.
Fastness properties of wool samples dyed with RE (Wash, light and rubbing fastness on gray scale: 1, poor; 2, fair; 3, moderate; 4, good; 5, excellent).
| Sample | Fastness properties of dyed wool | |||||||
|---|---|---|---|---|---|---|---|---|
| Mordant | RE concentration (%) | Wash Fastness | Durability (Up to 5 washing cycles) | Rubbing fastness | Light fastness | |||
| Color change | Staining | Color change | Staining | Wet | Dry | |||
| – | 2 | 4–5 | 5 | 4 | 4–5 | 4 | 4–5 | 4–5 |
| – | 4 | 4–5 | 5 | 4 | 4–5 | 4 | 4 | 4–5 |
| – | 6 | 4–5 | 5 | 4 | 4–5 | 4 | 4 | 4–5 |
| Al2(So4)3 | 4 | 4–5 | 5 | 4–5 | 4–5 | 4–5 | 4–5 | 4 |
| P. granatum | 4 | 4–5 | 5 | 4 | 4–5 | 4–5 | 5 | 4 |
| C. sinensis | 4 | 4–5 | 5 | 4–5 | 4–5 | 5 | 5 | 4–5 |
| R. coriaria | 4 | 4–5 | 5 | 4–5 | 4–5 | 4–5 | 4–5 | 4 |
Antibacterial activity
RE (10 mg/mL) and wool fabrics dyed with 4% RE and P. granatum peels were tested against gram-positive (B. cereus) and gram-negative (E. coli, S. typhi, and P. aeruginosa) bacteria. Under optimum conditions, the SFE extract was highly effective against P. aeruginosa and E. coli with inhibition zones of 23 and 15 mm, respectively. As shown in Table 4, it showed low antibacterial activity against B. cereus and S. typhi. Furthermore, P. aeruginosa was more sensitive to wool dyed with RE than other bacteria tested. The lowest antibacterial activity was observed against B. cereus, with a 3 mm diameter inhibition zone. A blank wool fabric showed no antibacterial activity, confirming that RE mediated the antibacterial activity of dyed fabrics. The antibacterial effect is attributed to the release of alizarin molecules, which affect the bacterial cell wall and inhibit bacterial growth13,62.
Table 4.
Diameters of inhibition zones (mm) of the extract and dyed wool fabrics at 37 °C for 24 h compared with standard compounds.
| Sample | B. cereus (G+) | E. coli (G−) | P. aeruginosa (G−) | S. typhi (G−) |
|---|---|---|---|---|
| Extract (10 mg/mL) | 7 ± 0.6 c | 15 ± 0.6 d | 23 ± 1.5 a | 8 ± 0.6 c |
| Wool fabric (blank) | 0 ± 0.0 e | 0 ± 0.0 e | 0 ± 0.0 d | 0 ± 0.0 d |
| Dyed wool fabric | 3 ± 0.6 d | 17 ± 0.6 c | 20 ± 1.5 b | 13 ± 1.0 b |
| Ciprofloxacin (10 μg) | 19 ± 1.0 a | 22 ± 1.0 a | 17 ± 1.5 bc | 15 ± 1.5 a |
| Tetracycline (30 μg) | 15 ± 1.0 b | 19 ± 2.0 b | 16 ± 2.0 c | 13 ± 0.6 b |
a–eMeans within a column followed by the same letter(s) are not significantly different according to Duncan’s multiple range test (P = 0.05).
Conclusions
By using scCO2, SFE was demonstrated to be an eco-friendly method for obtaining alizarin from R. tinctorum roots. By examining the effects of co-solvent ratio, temperature, pressure, extraction time, and flow rate, the optimal extraction conditions for alizarin were determined. The co-solvent ratio was the most important factor influencing alizarin extraction yield. Based on alizarin recovery and minimizing environmental impact, SFE conditions of 90% CO2:10% Me, 65°C, 250 bar, 45 min, and 9 mL/min were selected. The wool fabrics were dyed with RE obtained under optimal conditions at different concentrations (2–6%). Dyed fabrics with bio-mordants had better color and fastness properties than those with Al2(SO4)3. Also, dyed fabrics and RE were tested for their antibacterial properties. In comparison with other bacterial strains, dyed fabric and RE were more effective against P. aeruginosa. With SFE, it is possible to extract natural colorants with supercritical technology, minimizing the environmental impacts of organic solvents.
Supplementary Information
Author contributions
T.A. conceived the original idea of this study and participated in the design, coordination, and drafting of the manuscript. M.M. was responsible for the SFE experiments, HPLC analysis, dyeing process, and preparation of samples for antimicrobial activity testing. K.H.S. interpreted the results of SFE, alizarin analysis, and antimicrobial activities. K.H.S. and M.M. were responsible for operating the SFE and HPLC equipment in addition to statistical analysis. H.E. revised the dyeing methodology and discussed the obtained data of the color and fastness properties. All authors participated in writing and drafting the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-27110-0.
References
- 1.Abou ET, Okubayashi S, Elsisi H, Abouelenin S. Electron beam irradiation treatment of textiles materials: A review. J. Polym. Res. 2022;29:58. [Google Scholar]
- 2.Yusuf M. Synthetic dyes: A threat to the environment and water ecosystem. Textiles Cloth. 2019;2:11–26. doi: 10.1002/9781119526599.ch2. [DOI] [Google Scholar]
- 3.Yusuf M, Shabbir M, Mohammad F. Natural colorants: Historical, processing and sustainable prospects. Nat. Prod. Bioprospect. 2017;7:123–145. doi: 10.1007/s13659-017-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusuf M, et al. Eco-dyeing of wool using aqueous extract of the roots of Indian madder (Rubia cordifolia) as natural dye. J. Nat. Fibers. 2013;10:14–28. doi: 10.1080/15440478.2012.738026. [DOI] [Google Scholar]
- 5.AlAshkar A, Hassabo A. Recent use of natural animal dyes in various field. J. Textiles Coloration Polym. Sci. 2021;5:1–10. [Google Scholar]
- 6.Rather LJ, et al. Ecological dyeing of Woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. J. Environ. Chem. Eng. 2016;4:3041–3049. doi: 10.1016/j.jece.2016.06.019. [DOI] [Google Scholar]
- 7.Elnagar K, Abou ET, Raouf S. Dyeing of polyester and polyamide synthetic fabrics with natural dyes using ecofriendly technique. J. Textiles. 2014;2014:1–8. doi: 10.1155/2014/363079. [DOI] [Google Scholar]
- 8.Elmaaty TA, Kasem A, Elsalamony M, Elsisi H. A green approach for one step dyeing and finishing of wool fabric with natural pigment extracted from streptomyces thinghirensis. Egypt J. Chem. 2020;63:1999–2008. [Google Scholar]
- 9.Ren Y, et al. Dyeing and antibacterial properties of cotton dyed with prodigiosins nanomicelles produced by microbial fermentation. Dyes Pigm. 2017;138:147–153. doi: 10.1016/j.dyepig.2016.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmaaty TA, Abouelenin S, Elsisi H, Okubayashi S. Eco-friendly approach for dyeing synthetic fabrics with natural dyes using electron beam irradiation. Fibers Polym. 2022;23:759–767. doi: 10.1007/s12221-022-3144-1. [DOI] [Google Scholar]
- 11.Yusuf M, Mohammad F, Shabbir M. Eco-friendly and effective dyeing of wool with anthraquinone colorants extracted from Rubia cordifolia roots: Optimization, colorimetric and fastness assay, Coloring studies with anthraquinone colorants extracted from R. cordifolia roots on wool. J. King Saud Univ. Sci. 2017;29:137–144. doi: 10.1016/j.jksus.2016.06.005. [DOI] [Google Scholar]
- 12.Ahmed NSE, Nassar SH, El-Shishtawy RM. Novel green coloration of cotton fabric. Part I: Bio-mordanting and dyeing characteristics of cotton fabrics with madder, alkanet, rhubarb and curcumin natural dyes. Egypt J. Chem. 2020;63:1605–1617. [Google Scholar]
- 13.Agnhage T, et al. Bioactive and multifunctional textile using plant-based madder dye: Characterization of UV protection ability and antibacterial activity. Fibers Polym. 2017;18:2170–2175. doi: 10.1007/s12221-017-7115-x. [DOI] [Google Scholar]
- 14.Jahangiri A, et al. Natural dyeing of wool by madder (Rubia tinctorum L.) root extract using tannin-based biomordants: Colorimetric, fastness and tensile assay. Fibers Polym. 2018;19:2139–2148. doi: 10.1007/s12221-018-8069-3. [DOI] [Google Scholar]
- 15.Mansour R, Dhouib S, Sakli F. UV protection and dyeing properties of wool fabrics dyed with aqueous extracts of madder roots, chamomiles, pomegranate peels, and apple tree branches barks. J. Nat. Fibers. 2022;19:610–620. doi: 10.1080/15440478.2020.1758280. [DOI] [Google Scholar]
- 16.Hosseinnezhad M, Gharanjig K, Jafari R, Imani H. Green dyeing of Woolen Yarns with weld and madder natural dyes in the presences of biomordant. Prog. Color Colorants Coat. 2021;14:35–45. [Google Scholar]
- 17.Lavoine-Hanneguelle S, Périchet C, Schnaebele N, Humbert M. Development of new natural extracts. Chem. Biodivers. 2014;11:1798–1820. doi: 10.1002/cbdv.201400026. [DOI] [PubMed] [Google Scholar]
- 18.Casas MP, López-Hortas L, Díaz-Reinoso B, Moure A, Domínguez H. Supercritical CO2 extracts from Acacia dealbata flowers. J. Supercrit. Fluids. 2021;173:105223. doi: 10.1016/j.supflu.2021.105223. [DOI] [Google Scholar]
- 19.Lao F, et al. Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: Comparison to traditional aqueous and ethanolic extraction. J. Util. 2020;40:101188. doi: 10.1016/j.jcou.2020.101188. [DOI] [Google Scholar]
- 20.Ramezani N, Raji F, Rezakazemi M, Younas M. Juglone extraction from walnut (Juglans regia L.) green husk by supercritical CO2: Process optimization using Taguchi method. J. Environ. Chem. Eng. 2020;8:103776. doi: 10.1016/j.jece.2020.103776. [DOI] [Google Scholar]
- 21.Martín L, González-Coloma A, Díaz CE, Mainar AM, Urieta JS. Supercritical CO2 extraction of Persea indica: Effect of extraction parameters, modelling and bioactivity of its extracts. J. Supercrit. Fluids. 2011;57:120–128. doi: 10.1016/j.supflu.2011.03.004. [DOI] [Google Scholar]
- 22.Idham Z, et al. Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J. Util. 2021;56:101839. doi: 10.1016/j.jcou.2021.101839. [DOI] [Google Scholar]
- 23.Rezakazemi M, Heydari I, Zhang Z. Hybrid systems: Combining membrane and absorption technologies leads to more efficient acid gases (CO2 and H2S) removal from natural gas. J. Util. 2017;18:362–369. doi: 10.1016/j.jcou.2017.02.006. [DOI] [Google Scholar]
- 24.Rezakazemi M, Ebadi AA, Montazer-Rahmati MM, Ismail AF, Matsuura T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014;39:817–861. doi: 10.1016/j.progpolymsci.2014.01.003. [DOI] [Google Scholar]
- 25.Rezakazemi M, Darabi M, Soroush E, Mesbah M. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep. Purif. Technol. 2019;210:920–926. doi: 10.1016/j.seppur.2018.09.005. [DOI] [Google Scholar]
- 26.Soroush E, Mesbah M, Hajilary N, Rezakazemi M. ANFIS modeling for prediction of CO2 solubility in potassium and sodium based amino acid Salt solutions. J. Environ. Chem. Eng. 2019;7:102925. doi: 10.1016/j.jece.2019.102925. [DOI] [Google Scholar]
- 27.Sánchez-Vicente Y, Cabañas A, Renuncio JAR, Pando C. Supercritical fluid extraction of peach (Prunus persica) seed oil using carbon dioxide and ethanol. J. Supercrit. Fluids. 2009;49:167–173. doi: 10.1016/j.supflu.2009.01.001. [DOI] [Google Scholar]
- 28.Talansier E, Braga MEM, Rosa PTV, Paolucci-Jeanjean D, Meireles MAA. Supercritical fluid extraction of vetiver roots: A study of SFE kinetics. J. Supercrit. Fluids. 2008;47:200–208. doi: 10.1016/j.supflu.2008.07.018. [DOI] [Google Scholar]
- 29.Idham Z, et al. Effect of flow rate, particle size and modifier ratio on the supercritical fluid extraction of anthocyanins from Hibiscus sabdariffa (L) IOP Conf. Ser. Mater. Sci. Eng. 2020;932:1–8. doi: 10.1088/1757-899X/932/1/012031. [DOI] [Google Scholar]
- 30.Jiao G, Kermanshahi PA. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT. 2018;98:237–244. doi: 10.1016/j.lwt.2018.08.042. [DOI] [Google Scholar]
- 31.Morcelli A, Cassel E, Vargas R, Rech R, Marcílio N. Supercritical fluid (CO2+ethanol) extraction of chlorophylls and carotenoids from Chlorella sorokiniana: COSMO-SAC assisted prediction of properties and experimental approach. J. Util. 2021;51:101649. doi: 10.1016/j.jcou.2021.101649. [DOI] [Google Scholar]
- 32.Chatterjee D, Jadhav NT, Bhattacharjee P. Solvent and supercritical carbon dioxide extraction of color from eggplants: Characterization and food applications. LWT Food Sci. Technol. 2013;51:319–324. doi: 10.1016/j.lwt.2012.09.012. [DOI] [Google Scholar]
- 33.Lefebvre T, Destandau E, Lesellier E. Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from Rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A. 2021;1639:461709. doi: 10.1016/j.chroma.2020.461709. [DOI] [PubMed] [Google Scholar]
- 34.Rather LJ, et al. Adhatoda vasica in conjunction with binary combinations of metal salts and biomordants as an effective textile dye to produce novel shades on wool. J. Nat. Fibers. 2018;15:611–623. doi: 10.1080/15440478.2017.1349712. [DOI] [Google Scholar]
- 35.Montazer M, Parvinzadeh M. Effect of ammonia on madder-dyed natural protein fiber. J. Appl. Polym. Sci. 2004;93:2704–2710. doi: 10.1002/app.20880. [DOI] [Google Scholar]
- 36.Mansour HF, Heffernan S. Environmental aspects on dyeing silk fabric with sticta coronata lichen using ultrasonic energy and mild mordants. Clean. Technol. Environ. Policy. 2011;13:207–213. doi: 10.1007/s10098-010-0296-2. [DOI] [Google Scholar]
- 37.Hosseinnezhad M, Gharanjig K, Belbasi S, Saadati SHS, Saeb MR. The use of Sumac as a natural mordant in green production of Iranian Carpet. Fibers Polym. 2018;19:1908–1912. doi: 10.1007/s12221-018-7961-1. [DOI] [Google Scholar]
- 38.Yin Y, Jia J, Wang T, Wang C. Optimization of natural anthocyanin efficient extracting from purple sweet potato for silk fabric dyeing. J. Clean. Prod. 2017;149:673–679. doi: 10.1016/j.jclepro.2017.02.134. [DOI] [Google Scholar]
- 39.Hosseinnezhad M, Gharanjig K, Belbasi S, Saadati SHS. Green dyeing of silk fabrics in the presence of pomegranate extract as natural mordant. Prog. Color Colorants Coat. 2017;10:129–133. [Google Scholar]
- 40.Abou ET, Sofan M, Ayad S, Negm E, Elsisi H. Novel synthesis of reactive disperse dyes for dyeing and antibacterial finishing of cotton fabric under scCO2. J. Util. 2022;61:102053. doi: 10.1016/j.jcou.2022.102053. [DOI] [Google Scholar]
- 41.Ramezani N, Raji F, Rezakazemi M, Younas M. Juglone extraction from walnut (Juglans regia L.) green husk by supercritical CO2: Process optimization using Taguchi method. J. Environ. Chem. Eng. 2020;8:103776. doi: 10.1016/j.jece.2020.103776. [DOI] [Google Scholar]
- 42.Abou Elmaaty T, Sayed-Ahmed K, Elsisi H, Magdi M. Optimization of extraction of natural antimicrobial pigments using supercritical fluids: A review. Processes. 2022;10:2111. doi: 10.3390/pr10102111. [DOI] [Google Scholar]
- 43.Bányai P, Kuzovkina IN, Kursinszki L, Szoke É. HPLC analysis of alizarin and purpurin produced by R. tinctorum L. hairy root cultures. Chromatographia. 2006;63:111–114. doi: 10.1365/s10337-006-0792-z. [DOI] [Google Scholar]
- 44.Hu, J. Fabric Testing. (2008).
- 45.Collier, B. J. & Epps, H. H. Textile Testing and Analysis. (Prentice Hall, 1998).
- 46.American Association of Textile Chemists and Colorists, A.-2004. Colorfastness to Light. AATCC Technical Manual (2006).
- 47.Amutha, K. A Practical Guide to Textile Testing. (CRC Press, 2016).
- 48.American Association of Textile Chemists and Colorists, A. 147-2004. Antibacterial Activity Assessment of Textile Materials. (2021).
- 49.Abou ET, Sayed-Ahmed K, Mohamed Ali R, El-Khodary K, Abdeldayem SA. Simultaneous sonochemical coloration and antibacterial functionalization of leather with selenium nanoparticles (SeNPs) Polymers (Basel) 2021;14:74. doi: 10.3390/polym14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abou ET, et al. Novel antiviral and antibacterial durable polyester fabrics printed with selenium nanoparticles (SeNPs) Polymers (Basel) 2022;14:955. doi: 10.3390/polym14050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 52.Gomez KA, Gomez AA. Statistical Procedures for Agricultural Research. London: Wiley; 1984. [Google Scholar]
- 53.CoStat, V. Cohort software798 light house Ave. PMB320, Monterey, CA93940, and USA. email: info@cohort.com and Website: http://www.cohort.com. DownloadCoStatPart2.html (2005).
- 54.de Santis D, Moresi M. Production of alizarin extracts from Rubia tinctorum and assessment of their dyeing properties. Ind. Crops Prod. 2007;26:151–162. doi: 10.1016/j.indcrop.2007.02.002. [DOI] [Google Scholar]
- 55.Lu HM, Ni WD, Liang YZ, Man RL. Supercritical CO2 extraction of emodin and physcion from Polygonum cuspidatum and subsequent isolation by semipreparative chromatography. J. Sep. Sci. 2006;29:2136–2142. doi: 10.1002/jssc.200600012. [DOI] [PubMed] [Google Scholar]
- 56.Aris NA, et al. The effect of fluid flow rate and extraction time in supercritical CO2. J. Adv. Res. 2019;16:26–34. [Google Scholar]
- 57.Klein-Júnior LC, Vander Heyden Y, Henriques AT. Enlarging the bottleneck in the analysis of alkaloids: A review on sample preparation in herbal matrices. TrAC Trends Anal. Chem. 2016;80:66–82. doi: 10.1016/j.trac.2016.02.021. [DOI] [Google Scholar]
- 58.Idham Z, et al. Effect of flow rate, particle size and modifier ratio on the supercritical fluid extraction of anthocyanins from Hibiscus sabdariffa (L.) IOP Conf. Ser. Mater. Sci. Eng. 2020;932:555. doi: 10.1088/1757-899X/932/1/012031. [DOI] [Google Scholar]
- 59.Safaryan MJ, Ganjloo A, Bimakr M, Zarringhalami S. Optimization of ultrasound-assisted extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from green pea pods. Foods. 2016;5:1–15. doi: 10.3390/foods5040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang GCC, Chan SW. Photocatalytic reduction of chromium(VI) in aqueous solution using dye-sensitized nanoscale ZnO under visible light irradiation. J. Nanopart. Res. 2009;11:221–230. doi: 10.1007/s11051-008-9423-y. [DOI] [Google Scholar]
- 61.Clementi C, Nowik W, Romani A, Cibin F, Favaro G. A spectrometric and chromatographic approach to the study of ageing of madder (Rubia tinctorum L.) dyestuff on wool. Anal. Chim Acta. 2007;596:46–54. doi: 10.1016/j.aca.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 62.Kalyoncu F, Cetin B, Saglam H. Antimicrobial activity of common madder (Rubia tinctorum L.) Phytother. Res. 2006;20:490–492. doi: 10.1002/ptr.1884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].