Abstract
Infection of different strains of mice with Mycobacterium avium has revealed genetic control of the immunoglobulin isotype induced and of the balance between Th1 and Th2 cytokines. Female BALB/c or C57BL/10 mice were infected intranasally with 105 M. avium organisms. The antibody response was measured over 18 weeks by enzyme-linked immunosorbent assay and Western blotting, while numbers of cytokine-producing cells were assessed at 12 to 15 weeks by ELISPOT assay. Upon infection, C57BL/10 mice produced a clear Th1 response with strong gamma interferon (IFN-γ) production, no interleukin-4 (IL-4), and almost entirely immunoglobulin G2a (IgG2a) antibody. In contrast, BALB/c mice developed T cells producing IL-4, as well as those producing IFN-γ, while the antibody response was a mixture of IgG1 and IgG2a. Antibodies from BALB/c mice were also able to recognize a greater range of antigens than were C56BL/10 mice. B10D2 mice, which carry the BALB/c major histocompatibility complex haplotype on a C57BL/10 background, followed the C57BL/10 cytokine pattern. Mice infected with Listeria monocytogenes did not show a similar response dichotomy.
Mycobacterium avium, a widespread environmental organism, is an opportunistic pathogen of humans. Once chiefly found in individuals with underlying chronic lung disease, it is now the commonest bacterial pathogen in untreated human immunodeficiency virus infection. It has been detected in approximately 70% of patients with advanced AIDS (28). Its contribution to morbidity and mortality in AIDS is unclear, but its tendency to induce apoptosis in T cells (11, 17) may well accelerate immune deterioration.
Experimental infection of mice with M. avium can induce a chronic, progressive disease, culminating after some 40 weeks in loss of immune function and death of the animal (11). Immunity is based on activation of bactericidal function of the macrophages within which the organism largely resides. This is mediated by the production of gamma interferon (IFN-γ) by CD4+ T lymphocytes. The organism shows accelerated growth in mice lacking CD4+ T cells (5, 33), although lack of CD8+ T cells has little or no effect (4, 33). Depletion of IFN-γ (7, 33) or IL-12 (8, 34), the chief cytokine which governs IFN-γ production, exacerbates infection. These results obtained with mice are reflected in the susceptibility to M. avium infection of humans with defective IL-12 or IFN-γ receptors or deficient IL-12 production (1, 2, 9).
Thus, immunity to this organism, and also to fully virulent M. tuberculosis, is associated with a strong Th1 response (24). Nevertheless, patients with tuberculosis produce IL-10 and IL-4 (typical Th2 cytokines), as well as IFN-γ (3, 37). The Th2 component of the response is stronger is those with active disease. It is speculated, but difficult to prove, that such differences are genetically determined. The contrast between Th1 and Th2 responses is even stronger in infection with M. leprae, where there is a spectrum of disease from strongly Th1 tuberculoid leprosy to strongly Th2 lepromatous leprosy (41).
Genetic control of the Th1-Th2 balance has been most strikingly described in experimental infection with Leishmania major (31). Here, resistant C57BL mice produce a strong Th1 response which is able to limit and resolve the infection whereas BALB/c mice, which are dominated by IL-4 production, develop progressive disease. The difference between the two appears to be governed by multiple genes (31), one of which may be related to the major histocompatibility complex (MHC) (32), although mice congenic for the H-2 locus showed no MHC influence (18).
Antibody isotype is also governed by the cytokine environment and Th1-Th2 balance. IL-4 favors the production of IgG1, while IFN-γ favors the production of IgG2a (36). Although antibodies are believed not to protect against mycobacteria, they are produced during infection (26). We describe here the production of different antibody isotypes during M. avium infection, depending on the mouse strain infected. The antibody isotype was, in turn, reflected in the balance of IFN-γ and IL-4 induced during infection in the two mouse strains studied, BALB/c J and C57BL/10. Both of these strains carry the susceptibility allele of the bcg gene, which influences natural resistance to both M. bovis BCG and M. avium (22), so it is not this gene which governs the difference. Using MHC-congenic mice, an MHC haplotype influence was also ruled out as a major determinant of the balance.
MATERIALS AND METHODS
Bacteria.
The M. avium strain used was a virulent serovar 8 strain isolated from an AIDS patient at Fairfield Hospital, Melbourne, Victoria, Australia. The bacteria were grown in Middlebrook 7H9 broth with continuous stirring at 37°C for 7 to 10 days. The bacteria were pelleted by centrifugation at 12,000 × g for 20 min and washed three times in phosphate-buffered saline (PBS), and CFU were determined by plating serial dilutions on Middlebrook agar. The bacteria were stored in 1-ml aliquots at −70°C. Before use, the bacteria were thawed and sonicated for 10 s to disperse clumps. Listeria monocytogenes strain EGD was maintained by weekly subculture on horse blood agar. For infection, listeria organisms were washed from the surface of 24 h cultures and the suspension was standardized by turbidity.
M. avium antigens.
To produce an M. avium lysate, organisms grown as described above were pelleted by centrifugation at 12,000 × g for 10 min and washed extensively in PBS. The wet weight of the bacteria was estimated, and an equal weight of 0.1-mm-diameter glass beads (Daintree Industries Pty Ltd., St. Helens, Tasmania, Australia) was added. The bacteria and beads were resuspended in breaking buffer (PBS, leupeptin at 0.2 μg/ml, pepstatin at 0.2 μg/ml, 5 × 104 U of DNase [Sigma, Castle Hill, New South Wales, Australia]), aliquoted into vials, and subjected to five 20-s cycles at 5,000 rpm in a Minibead beater (Daintree Industries Pty Ltd.). The tubes were centrifuged (12,000 × g) to pellet the beads and cell debris. The supernatant was filtered through a 0.22-μm-pore-size filter and then dialyzed into PBS. The protein concentration was estimated spectrophotometrically. In addition, recombinant M. avium Hsp65 was produced as a fusion protein with glutathione S-transferase and purified by elution with reduced gluthione from glutathione S-transferase beads (V. Nagabhushanam et al., unpublished data).
Infection of mice.
C57BL/10, BALB/c J, and B10D2 mice were pedigree bred in the Department of Microbiology Animal Unit (University of Melbourne, Melbourne, Victoria, Australia). Female mice 6 to 8 weeks old were anesthetized with penthrane (Sigma), and under a biosafety hood, 50 μl containing 105 M. avium or 5 × 103 Listeria organisms was placed on their external nares to be breathed in smoothly. The dose was checked retrospectively by viable counts. To follow the course of M. avium infection, mice were sacrificed by CO2 narcosis and blood was collected by direct cardiac puncture using a 1-ml syringe with a 25-gauge needle (Terumo Pty Limited, Melbourne, Victoria, Australia). Blood samples were allowed to clot at 4°C for 1 h and then centrifuged at 12,000 × g for 10 min to separate serum. Spleens, livers, and lungs were removed aseptically, and the organs were homogenized individually in 5 ml of PBS using an Ultra Turrax tissue homogenizer (Janke & Kunkel, Breisgau, Germany). Serial 10-fold dilutions were made in sterile PBS, and aliquots were placed on Middlebrook agar plates. The plates were incubated at 37°C for 7 to 10 days, and colonies were counted to determine the number of viable bacteria.
Lymphocyte preparation for cytokine assay.
Mice were sacrificed by CO2 narcosis, and spleens or mediastinal lymph nodes were removed aseptically and dispersed into Dulbecco modified Eagle medium (DMEM) –10% fetal calf serum (FCS) through an 80 gauge/80 mesh stainless steel sieve. The cells were centrifuged at 840 × g for 7 min and red blood cells were removed by resuspension in Tris-NH4Cl and incubation at room temperature for 10 min. The cells were washed twice in DMEM–10% FCS before culture.
ELISPOT assay for detection of cytokine production.
Maxisorb plates (Nalgene Nunc International, Mt. Waverly, Victoria, Australia), 96 wells, were coated overnight at 4°C with 50 μl of HB170 (anti-IFN-γ) (15) or 100 μl of 11B11 (anti-IL-4) (14) (each at 10 μg/ml) in carbonate coating buffer (pH 9.1). The unbound antibody was flicked out, and sterile PBS was used to wash out the wells. The plates were blocked with DMEM–10% FCS at 37°C for 1 h. Cells were added to the blocked plates (2 × 105, 1 × 105, 5 × 104, and 2.5 × 104 per well in triplicate) together with M. avium lysate (MAL) antigen at 50 μg/ml or 5 × 106 heat-killed (60°C for 60 min) listeriae (HKL). They were incubated for 72 h at 37°C and 5% CO2. The cells were washed off, and bound cytokines were detected with a 1:1,000 dilution of XMG 1.2 (anti-IFN-γ) (Pharmingen, San Diego, Calif.) or BVD6 (anti-IL-4) (29) conjugated to biotin, followed by streptavidin-alkaline phosphatase. ELISPOT assays were developed using the substrate 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (Sigma). The spots were counted under bright light, and the frequency of cytokine-producing cells was calculated by averaging the number of spots for triplicate wells.
ELISA for detection of antigen-specific immunoglobulin.
Flat-bottom 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with the appropriate antigen at 5 μg/ml in carbonate buffer (pH 9.6) in 50-μl volumes and incubated at 4°C overnight. The supernatant containing unbound antigen was flicked out, and the plates were blocked with 2% FCS in PBS for 1 h at 37°C. Serum samples were titrated in two fold dilutions in PBS and incubated at 37°C for 2 h. The unbound serum was flicked out, and the plates were washed thrice with PBS–0.5% Tween 20 (BDH Chemicals, Kilsyth, Victoria, Australia). For the detection of bound immunoglubulin, sheep anti-mouse horseradish peroxidase conjugate specific for all immunoglobulin G (IgG) isotypes (Silenus, Melbourne, Victoria, Australia) or biotinylated rat anti-mouse monoclonal antibody to isotype IgG1 or IgG2a (Caltag, Burlingame, Calif.) was added in a 1:2,000 dilution, followed by incubation and washing as before. Biotin-conjugated rat anti-mouse monoclonal antibody was followed by streptavidin-peroxidase conjugate (Boehringer Mannheim, Mannheim, Germany) at a 1:1,000 dilution. The ELISA was developed using 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), and the reaction was stopped after suitable development of color with 2.5 M sulfuric acid. The optical density was read at 450 nm. The cutoff value was set at three times the optical density of the highest dilution of control serum. Titers, expressed as the reciprocal of the serum dilution, were considered positive if higher than the cutoff value.
SDS-PAGE and Western blotting.
Protein samples were mixed 1:1 (vol/vol) with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading dye (50 mM Tris · HCl [pH 6.8], 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and boiled for 3 min prior to loading onto polyacrylamide gels. SDS-PAGE for separation of proteins was carried out on a 12% polyacrylamide gel as described by Laemmli (25). Electrophoresis was performed at room temperature in Tris-glycine buffer (25 mM Tris, 250 mM glycine [pH 8.3], 0.1% SDS) at a constant voltage of 100 V using a vertical minigel system (C.B.S. Scientific Company Inc.) Del Mar, Calif. according to the manufacturer's specifications.
Western transfer was performed in trans-blot buffer (25 mM Tris, 200 mM glycine, 20% [vol/vol] methanol) as described by Towbin et al. (39) using a semidry blotting apparatus (C.B.S. Scientific Company Inc.) according to the manufacturer's specifications. Transfer of proteins was performed at a constant current of 100 mA for 45 min. Following transfer, the nitrocellulose was blocked in 5% skim milk in PBS for 1 h at room temperature. Serum from mice was added at a dilution of 1/100 in 0.5% skim milk in PBS and incubated at room temperature for 1 h. Following three washes with PBS–0.5% Tween 20 (BDH Chemicals) the membrane was incubated with a 1:1,000 dilution of a peroxidase-labeled conjugate specific for a given class of immunoglobulin for 1 h at room temperature. The blot was developed using diaminobenzidine (Sigma), and the reaction was stopped by washing in distilled water.
Statistical analysis.
Differences of mean and standard deviation between experimental groups were analyzed using the Student t test. Differences were considered significant at p < 0.05.
RESULTS
Multiple M. avium proteins are targets of the humoral response.
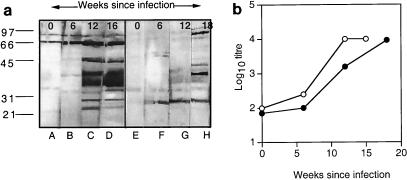
During infection with an organism as complex as mycobacteria, many antigens are presented to the immune system. To determine the major antigens recognized by B cells during an experimental infection with M. avium, BALB/c or C57BL/10 mice were infected intranasally with 105 CFU. Serum was collected from mice at 6, 12, and 16 weeks postinfection. MAL protein (20 μg per lane) was separated by SDS-PAGE and transferred to a nitrocellulose membrane. Individual lanes bearing protein were cut out using a sterile scalpel blade, and a 1:100 dilution of serum from mice at various stages of M. avium infection, as well as control serum from uninfected mice, was incubated with the individual strips. Individual proteins reacting with murine serum were detected using anti-mouse immunoglobulin conjugated to horseradish peroxidase, followed by the substrate, diaminobenzidine. As shown in Fig. 1a, M. avium infection of BALB/c mice induced the production of antibodies to a number of antigens, predominantly antigens in the 30 to 45-kDa range, the 66-kDa range, and the range below 30 kDa. Compared with BALB/c mice, C57BL/10 mice recognized fewer antigens, with the maximum intensity around the 40 to 45-kDa region and a weak response in the 66-kDa region (Fig. 1a). In each case, the intensity of the reaction increased over time with infection, reflecting an increase in antibody titer as measured by ELISA (Fig. 1b). In keeping with the Western blots, C57BL/10 mice developed a lower antibody titer during M. avium infection (Fig. 1b).
FIG. 1.
(a) MAL proteins as targets of the humoral response during M. avium infection. Serum was collected from uninfected BALB/c and C57BL/10 mice or mice infected with 105 M. avium organisms at the intervals postinfection shown. Pooled serum (1:100 dilution) from three mice per time point was tested for reactivity with MAL proteins separated on a 12% polyacrylamide gel. Lanes: A to D, BALB/c mouse serum; E to H C57BL/10 mouse serum. The positions of molecular weight (103) markers are indicated. The results shown are typical of two independent experiments. (b) Titer of antilysate antibodies during infection. The titer of antilysate antibodies in the same serum pools was determined by ELISA. Symbols: ○, BALB/c mouse serum; ●, C57BL/10 mouse serum. The results shown are typical of two independent experiments.
Antibody isotypes in BALB/c versus C57BL/10 mice.
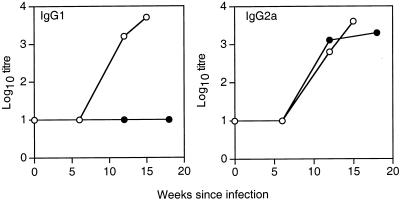
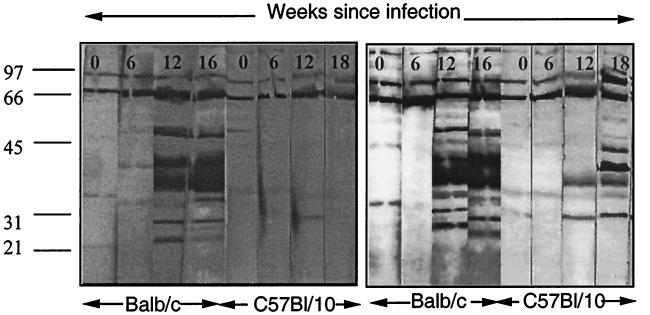
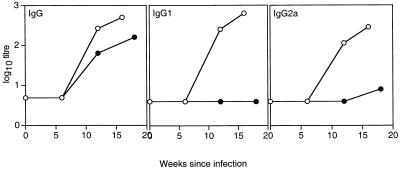
To determine the isotypes of antibody produced during M. avium infection, serum from C57BL/10 and BALB/c mice at various stages of infection was tested for reactivity with M. avium lysate protein and the isotype of the antibody was determined by ELISA. As is clear from Fig. 2, BALB/c mice produced a mixed IgG2a and IgG1 response to M. avium infection. In contrast, C57BL/10 mice developed an almost exclusively IgG2a response during M. avium infection that increased during the course of infection. Furthermore, when Western blot assays were carried out using isotype-specific antibodies (Fig. 3), it was apparent that BALB/c mice produced both IgG1 and IgG2a antibodies to the range of proteins detected by whole serum. The serum from C57BL/10 mice contained only IgG2a antibodies reacting with the more limited range of antigens detected before, mostly in the 40- to 45-kDa range. This was confirmed when antibody was titrated by ELISA against a single recombinant protein, Hsp65 of M. avium (Fig. 4). BALB/c mice produced both IgG1 and IgG2a, but C57BL/10 mice produced only IgG2a, and that appeared only late in infection and at low titer.
FIG. 2.
Antibody isotypes produced during infection. Serum was collected from BALB/c (○) and C57BL/10 (●) mice at intervals postinfection with 105 M. avium organisms. The titers of antilysate IgG1 and IgG2a were determined by ELISA. The data shown represent the titers of serum pooled from three mice per group. These results are typical of three independent experiments.
FIG. 3.
Targets of the IgG2a and IgG1 responses of BALB/c and C57BL/10 mice during M. avium infection. Serum was collected from uninfected BALB/c and C57BL/10 mice infected with 105 M. avium organisms at intervals postinfection. Pooled serum from three mice per group was tested for reactivity with MAL protein separated on a reducing 12% polyacrylamide gel, and the isotype of the reacting antibody was determined using anti-isotype antibodies. Left, Western blot assay with RMG1 (anti-IgG1); right, Western blot assay with RMG2a (anti-IgG2a). These results are typical of two independent experiments. Molecular weights (103) are shown on the left.
FIG. 4.
Antibody responses of BALB/c and C57BL/10 mice to recombinant Hsp65 during M. avium infection. Serum was collected from BALB/c (○) and C57BL/10 (●) mice infected with 105 M. avium organisms at intervals postinfection as indicated along the x axis. The titers of anti-Hsp65 IgG, IgG1, and IgG2a were determined by ELISA. The data shown represent the titers of pooled serum from three mice per group. These results are typical of three independent experiments.
Cytokine environment reflecting antibody class.
The production of IgG2a is promoted by IFN-γ, whereas IgG1 is suppressed. On the other hand, IL-4 is associated with IgG1 production (10). To determine if the in vivo cytokine environment was consistent with the difference in antibody production during M. avium infection, the frequency of IL-4- and IFN-γ-producing cells was estimated in the two genetically distinct strains of mice. As IL-4 is absorbed by B cells and rarely detected in cell culture supernatants (44), the ELISPOT technique, which utilizes cytokine-specific antibodies to capture cytokines as they are produced, was employed. Cell suspensions were prepared from the mediastinal lymph nodes from BALB/c or C57BL/10 mice at 15 weeks postinfection with 105 M. avium organisms. Because mediastinal lymph nodes from normal mice are extremely small, nodes from mice infected for 8 days with listeriae were used as a negative control. The cells were stimulated with MAL or HKL, and the frequency of IL-4- and IFN-γ-producing cells was determined by ELISPOT assay. Figure 5 shows that the stimulation of the lymphocytes was specific. MAL could only elicit cytokine production from the cells of M. avium-infected mice, while HKL elicited a response from the cells of listeria-infected mice. Both BALB/c and C57BL/10 mice produced IFN-γ in response to their specific antigens (Fig. 5, top). However, there was a striking difference in the IL-4 response between BALB/c and C57BL/10 mice infected with M. avium in that the BALB/c mice expressed a significant number of IL-4-producing cells. These were not evident in the C57BL/10 mice, nor were they present in either strain during Listeria infection. These observations were confirmed with spleen cells from similarly infected mice.
FIG. 5.
Cytokine responses of BALB/c and C57BL/10 mice to infection. Mice were infected intranasally with 105 M. avium organisms (stippled bars) or 5 × 103 listeriae (closed bars). Mediastinal lymph nodes were collected 15 weeks or 8 days later, respectively, for culture with MAL or HKL. IFN-γ- or IL-4-producing cells were assayed by ELISPOT assay. The data shown represent the means and standard deviations of groups of three mice. Culture in the absence of antigen produced <5 IFN-γ and <10 IL-4 ELISPOT. These results are typical of three independent experiments.
This difference in cytokine production did not result in increased susceptibility of BALB/c mice to M. avium infection. Both C57BL/10 and BALB/c mice carry the bcgs allele and are susceptible strains (40). At 15 weeks postinfection, the bacterial load in the lungs, livers, and spleens of the two strains of mice did not differ significantly, despite the differences in the cytokines produced in response to infection (Table 1). However, longer-term studies would determine if the mixed response (Th1-Th2) of BALB/c mice influences the outcome of infection at about 40 weeks, when C57BL/10 mice die (11).
TABLE 1.
Bacterial numbers in M. avium-infected BALB/c and C57BL/10 micea
| Tissue | No. (log10) of M. avium organisms/organ
|
|
|---|---|---|
| BALB/c | C57BL/10 | |
| Lung | 7.4 ± 0.3 | 7.7 ± 0.3 |
| Spleen | 5.9 ± 0.2 | 6.4 ± 0.2 |
| Liver | 4.1 ± 0.2 | 4.9 ± 0.4 |
Age-matched BALB/c or C57BL/10 mice were infected with 105 M. avium organisms 15 weeks before bacterial counts were made on lung, spleen, and liver tissues. The data shown represent means and standard deviations of three mice per group. These results are typical of three independent experiments.
Th1-Th2 cytokines are controlled by non-MHC genes.
The MHC haplotype is a major determining factor of the immune response, and since BALB/c and C57BL/10 mice differ at the MHC, it was clearly of interest to test the immune responses to M. avium of congenic mice which differ only at the MHC. Thus, BALB/c (H-2d), C57BL/10 (H-2b), and B10D2 (H-2d on a C57BL/10 background) mice were infected with M. avium. At 12 weeks postinfection, numbers of IFN-γ- or IL-4-producing cells were assayed by ELISPOT assay. It is clear from Table 2 that the cytokine response was not determined by the MHC haplotype, since the BALB/c and B10D2 mice, both H-2d, differed particularly in numbers of IL-4-producing cells, while C57BL/10 and B10D2 mice, sharing the B10 background, had similar responses.
TABLE 2.
Genetic control of Th1 and Th2 cytokines in M. avium infectiona
| Mouse strain | No. of ELISPOTs/106 spleen cells
|
|
|---|---|---|
| IL-4 | IFN-γ | |
| BALB/c (H-2d) | 72 ± 8 | 302 ± 42 |
| C57BL/10 (H-2b) | 12 ± 5b | 364 ± 51 |
| B10D2 (H-2d) | 10 ± 3b | 330 ± 20 |
Age-matched BALB/c, C57BL/10, or B10D2 mice were infected with 105 M. avium organisms 12 weeks before sacrifice. Splenocytes were cultured with MAL protein at 50 μg/ml or medium alone for determination of cytokine-producing cells using ELISPOT assay 72 h later. The data shown represent means and standard deviations of triplicate cultures from three mice per group. Culture without added antigen produced 8 to 11 IL-4-specific and <5 IFN-γ-specific ELISPOTs. These results are typical of two independent experiments.
BALB/c mice produced significantly more IL-4 than did C57BL/10 mice or B10D2 mice (P < 0.002). Differences in IFN-γ production were not statistically significant.
DISCUSSION
The experiments described here demonstrate a genetic basis for control of Th1-Th2 balance during experimental infection of mice with M. avium. Upon infection, C57BL/10 mice produced a clear Th1 response with strong IFN-γ, no IL-4, and almost entirely IgG2a antibody. In contrast, BALB/c mice developed T cells producing IL-4, as well as those producing IFN-γ, while their antibody response was a mixture of IgG1 and IgG2a. B10D2 mice, which carry the BALB/c MHC haplotype on a C57BL/10 background, followed the C57BL/10 cytokine pattern.
It has been easy to overlook the production of IL-4 during infection using the classical method of lymphocyte culture with antigen and measuring cytokines in the supernatant. We showed that this is due, at least in part, to the absorption of IL-4 by activated B lymphocytes (44). In the current experiments, we could not demonstrate IL-4 secretion into supernatants, even by using a sensitive ELISA (results not shown). However, we were able to overcome this by using the ELISPOT assay, where surface-bound anti-IL-4 antibody captures IL-4 as it is formed. Other ways to avoid the problem include limiting dilution of precursor clones (27, 44), intracellular cytokine staining (11, 21) and semiquantitative reverse transcription-PCR (23).
Previous studies have shown a dose-dependent influence on the Th1-Th2 response of BALB/c mice to infection with M. tuberculosis (30) or with the environmental saprophyte M. vaccae (16). Low doses of either of these organisms favored a purely Th1 response, whereas a higher dose induced a mixed Th1-Th2 response. The present experiments used just a single dose in different mouse strains. Since BALB/c and C57BL/10 mice both express the susceptibility allele bcgs, which controls innate resistance, M. avium grows similarly in the two strains (22). Therefore, our observation of different Th1-Th2 balances is not secondary to different bacterial numbers.
The pattern of the response to M. avium in BALB/c and C56BL/10 or B10D2 mice is reminiscent of their response to infection with L. major (27). BALB/c mice infected with Leishmania show a persistent IL-4 response with little IFN-γ, whereas C57BL/6 mice show a transient IL-4 response which is later replaced by IFN-γ. The basis of this difference is controversial. Some have suggested that the initiating cytokines, either IL-12 (38) or IL-4 (6), determine the subsequent differentiation of Th1 or Th2 cells. On the other hand, Hsieh et al. (19) produced strong evidence that the genetic background of the T cells themselves is the fundamental determinant of the default T-helper phenotype in vitro. Activated BALB/c T cells soon lost IL-12Rβ2 expression and became unresponsive to IL-12, whereas B10D2 cells remained responsive (13). This could be overridden by early addition of IL-12. In this context, it was interesting that in our experiments, infection with L. monocytogenes induced IFN-γ-producing T cells and only background levels of IL-4 in either BALB/c or C57BL/10 mice. Listeria is a remarkably strong IL-12 stimulus (20, 43) and may well be able to override any deficiency in BALB/c mice.
That the difference observed here is the same genetically or mechanistically as that observed in leishmaniasis, while likely, is only speculative. It is clear that it does not relate to the known determinant of innate resistance or susceptibility to some mycobacteria, the bcg or Nramp-1 gene, since both of the strains studied carry the susceptibility allele (40). Very early studies showed that C57BL mice stand out from other strains in being subject to early death following infection with high doses of virulent M. tuberculosis (12). This correlated with a strong delayed-type hypersensitivity response (what we would now call a Th1 response), and death was accompanied by severe lung pathology. The other side of this coin is the superior ability of BCG to immunize C57BL/10 mice compared to BALB/c mice (42). These workers attributed the difference to functions of macrophages. Bone marrow-derived macrophages from C57BL/10 mice produced more IL-12 in response to BCG and expressed more inducible nitric oxide synthase in response to IFN-γ.
Since the discovery of immune response genes within the MHC, there has been considerable interest in linking disease susceptibility to the MHC haplotype. Somewhat surprisingly, given the multitude of antigens produced by any bacterium, some such linkages have been found. Notably, although susceptibility to leprosy is determined by expression of a non-MHC gene, the progression to mild tuberculoid leprosy (Th1 response) or severe lepromatous leprosy (Th2 response) is associated with genes of the MHC (35). Using congenic mice, we found no MHC effect on the Th1-Th2 balance. However, in leishmaniasis, congenic mice also showed no effect of the MHC haplotype on Th1-Th2 bias (18). Nevertheless, using sophisticated gene mapping techniques, the MHC gene was revealed as one of a number of genes contributing to resistance to this disease in different mouse strains (32). If the Th1-Th2 bias in mycobacterial infection were to be linked to the same gene within the MHC, this would suggest not a classical immune response gene reflecting variation in presentation of epitopes by MHC molecules, since such epitopes are unlikely to be shared between two such disparate organisms but some mechanism as yet unknown for the control of T-cell differentiation.
ACKNOWLEDGMENT
This work was supported by grant 980639 from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Altare F, Durandy A, Lammas D, Emile J F, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, Jeppsson O, Gollob J A, Meinl E, Segal A W, Fischer A, Kumararatne D, Casanova J L. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 2.Altare F, Lammas D, Revy P, Jouanguy E, Doffinger R, Lamhamedi S, Drysdale P, Scheel-Toellner D, Girdlestone J, Darbyshire P, Wadhwa M, Dockrell H, Salmon M, Fischer A, Durandy A, Casanova J L, Kumararatne D S. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J Clin Investig. 1998;102:2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes P F, Shuzhuang L, Abrams J S, Wang E, Yamamura M, Modlin R L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Petrofsky M. Host defense against Mycobacterium avium does not have an absolute requirement for major histocompatibility complex class I-restricted T cells. Infect Immun. 1999;67:3108–3111. doi: 10.1128/iai.67.6.3108-3111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caruso A M, Serbina N, Klein E, Triebold K, Bloom B R, Flynn J L. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 6.Chatelain R, Varkila K, Coffman R L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 7.Doherty T M, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 8.Doherty T M, Sher A. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol. 1998;160:5428–5435. [PubMed] [Google Scholar]
- 9.Dorman S E, Holland S M. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Investig. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 11.Gilbertson B, Zhong J, Cheers C. Anergy, IFN-gamma production, and apoptosis in terminal infection of mice with mycobacterium avium. J Immunol. 1999;163:2073–2080. [PubMed] [Google Scholar]
- 12.Gray D F, Affleck M N. Relationship of allergy to gross lung disease and culturable bacilli in tuberculous mice. Am Rev Tuberc. 1958;78:226. doi: 10.1164/artpd.1958.78.2.226. [DOI] [PubMed] [Google Scholar]
- 13.Guler M L, Jacobson N G, Gubler U, Murphy K M. T cell genetic background determines maintenance of IL-12 signaling: effects on BALB/c and B10.D2 T helper cell type 1 phenotype development. J Immunol. 1997;159:1767–1774. [PubMed] [Google Scholar]
- 14.Haak-Frendscho M, Brown J F, Iisawa Y, Wagner R D, Czuprynski C J. Administration of anti-IL-4 monoclonal antibody 11B11 increases resistance of mice to Listeria monocytogenes infection. J Immunol. 1992;148:3978–3985. [PubMed] [Google Scholar]
- 15.Havell E A. Purification and further characterization of an anti-murine interferon-gamma monoclonal neutralizing antibody. J Interferon Res. 1986;6:489–497. doi: 10.1089/jir.1986.6.489. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiromatsu K, Aoki Y, Makino M, Matsumoto Y, Mizuochi T, Gotoh Y, Nomoto K, Ogasawara J, Nagata S, Yoshikai Y. Increased Fas antigen expression in murine retrovirus-induced immunodeficiency syndrome, MAIDS. Eur J Immunol. 1994;24:2446–2451. doi: 10.1002/eji.1830241028. [DOI] [PubMed] [Google Scholar]
- 18.Howard J G, Hale C, Chan-Liew W L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980;2:303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh C S, Macatonia S E, O'Garra A, Murphy K M. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh B, Schrenzel M D, Mulvania T, Lepper H D, DiMolfetto-Landon L, Ferrick D A. In vivo cytokine production in murine listeriosis. Evidence for immunoregulation by gamma delta+ T cells. J Immunol. 1996;156:232–237. [PubMed] [Google Scholar]
- 22.Hubbard R D, Flory C M, Collins F M. T cell immune responses in Mycobacterium avium-infected mice. Infect Immun. 1992;60:150–153. doi: 10.1128/iai.60.1.150-153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iizawa Y, Brown J F, Czuprynski C J. Early expression of cytokine mRNA in mice infected with Listeria monocytogenes. Infect Immun. 1992;60:4068–4073. doi: 10.1128/iai.60.10.4068-4073.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann S H E. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–342. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Mahadevan S. Clinical utility of serodiagnosis of tuberculosis. Indian J Pediatr. 1997;64:97–103. doi: 10.1007/BF02795786. [DOI] [PubMed] [Google Scholar]
- 27.Morris L, Troutt A B, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–2721. [PubMed] [Google Scholar]
- 28.Nightingale S D, Byrd L T, Southern P M, Jockusch J D, Cal S X, Wynne B A. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 29.Ohara J, Paul W E. Production of a monoclonal antibody to and molecular characterisation of B-cell stimulatory factor-1. Nature. 1985;315:333. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 30.Power C A, Wei G, Bretscher P A. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66:5743–5750. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 32.Roberts L J, Baldwin T M, Curtis J M, Handman E, Foote S J. Resistance to Leishmania major is linked to the H2 region on chromosome 17 and to chromosome 9. J Exp Med. 1997;185:1705–1710. doi: 10.1084/jem.185.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders B M, Cheers C. Inflammatory response following intranasal infection with Mycobacterium avium complex:role of T-cell subsets and gamma interferon. Infect Immun. 1995;63:2282–2287. doi: 10.1128/iai.63.6.2282-2287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders B, Zhan Y, Cheers C. Endogenous IL-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–4015. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schurr E, Malo D, Radzioch D, Buschman E, Morgan K, Gros P, Skamene E. Genetic control of innate resistance to mycobacterial infections. Immunol Today. 1991;12:A42–45. doi: 10.1016/S0167-5699(05)80012-X. [DOI] [PubMed] [Google Scholar]
- 36.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 37.Surcel H-M, Troye-Blomberg M, Paulie S, Anderson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine responses of blood leukocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 38.Sypek J P, Chung C L, Mayor S E, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon J. Immunoblotting in the clinical laboratory. J Clin Chem Clin Biochem. 1989;27:495–501. [PubMed] [Google Scholar]
- 40.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 41.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida A, Koide Y, Uchijima M, Yoshida T O. Dissection of strain difference in acquired protective immunity against Mycobacterium bovis Calmette-Guerin bacillus (BCG). Macrophages regulate the susceptibility through cytokine network and the induction of nitric oxide synthase. J Immunol. 1995;155:2057–2066. [PubMed] [Google Scholar]
- 43.Zhan Y, Cheers C. Control of IL-12 and IFN-γ production in response to live or dead bacteria by TNF and other factors. J Immunol. 1998;161:1447–1453. [PubMed] [Google Scholar]
- 44.Zhan Y, Kelso A, Cheers C. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect Immun. 1995;63:969–975. doi: 10.1128/iai.63.3.969-975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]